Abstract
Ginsenoside, one of the active ingredients of Panax ginseng, has a variety of physiological and pharmacological actions in various organs. However, little is known about the effects of ginsenosides on gastrointestinal (GI) motility. We studied the modulation of pacemaker potentials by ginsenoside in the interstitial cells of Cajal (ICCs) using the whole-cell patch clamp technique in the current clamp mode. Among ginsenosides, we investigated the effects of ginsenoside Rb1, Rg3 and Rf. While externally applied Rb1 and Rg3 had no effects on pacemaker potentials, Rf caused membrane depolarization. The application of flufenamic acid or niflumic acid abolished the generation of pacemaker potentials and inhibited the Rf-induced membrane depolarization. Membrane depolarization induced by Rf was not inhibited by intracellular application of guanosine 5′-[β-thio]diphosphate trilithium salt. Pretreatment with a Ca2+-free solution, thapsigargin, a Ca2+-ATPase inhibitor of the endoplasmic reticulum, U-73122, a phospholipase C inhibitor, or 2-APB, an IP3 receptor inhibitor, abolished the generation of pacemaker potentials and suppressed Rf-induced actions. However, treatment with chelerythrine and calphostin C, protein kinase C inhibitors, did not block Rf-induced effects on pacemaker potentials. These results suggest that ginsenoside Rf modulates the pacemaker activities of ICCs and therby regulates intestinal motility.
Keywords: gastrointestinal (GI) motility, ginsenoside, interstitial cells of Cajal (ICCs)
INTRODUCTION
Ginseng, the root of Panax ginseng C. A. Meyer, is a well-known folk medicine that has been used as a tonic agent. The molecular components primarily responsible for the actions of ginseng are ginsenosides, also known as ginseng saponins. Ginsenosides are derivatives of triterpenoid dammarane with molecular structures consisting of 30 carbon atoms (Nah, 1997). Ginsenosides have a 4-ring, steroid-like structure, with attached sugar moieties. About 30 different ginsenosides have been isolated and identified from the Panax ginseng root (Kim et al., 2007). In a study using purified compounds from ginsenosides, ginsenosides Rb1, Rg3 and Rf were found to exert a number of biological activities that affected central and peripheral nervous systems, cardiovascular, and immune systems (Chen, 1996; Gillis, 1997; Kim et al., 1998; Saito et al., 1977). Interestingly, many studies have demonstrated that ginsenosides have effects on gastrointestinal (GI) motility. Ginseng accelerated mouse small intestinal movement (Furukawa et al., 1995), stimulated relaxation of the circular muscle of the gastric body, and longitudinal muscle contraction in the ileum and distal colon in isolated guinea pig GI tract tissues (Hashimoto et al., 2001). In rabbit intestine, ginseng had stimulatory effects on intestinal motility (Murata et al., 2001). Furthermore, Kim et al. (2007) suggested that ginseng total saponins (GTS) modulated the pacemaker activity of interstitial cells of Cajal (ICCs) in the GI tract. However, it is still unclear which ginsenoside types exert pharmacological or physiological effects on intestinal ICCs.
Many regions of the tunica muscularis of the GI tract display spontaneous contractions. These are mediated by the periodic generation of electrical slow waves (Szurszewsik, 1987). Recent studies have shown that ICCs act as the pacemakers and conductors of the electrical slow waves in GI smooth muscles (Huizinga et al., 1995; Langton et al., 1989; Ordog et al., 1999; Sanders, 1996; Ward et al., 1994). Slow waves propagate within ICC networks, conduct into smooth muscle cells through gap junctions, and initiate phasic contractions by activating Ca2+ entry through L-type Ca2+ channels. Pacemaker activity in the murine small intestine is due mainly to periodic activation of nonselective cation channels (NSCC) (Kim et al., 2008; Koh et al., 2002) or Cl− channels (Huizinga et al., 2002; Zhu et al., 2009). Moreover there is evidence that endogenous agents such as neurotransmitters, hormones and paracrine substances modulate intestinal motility by influencing ICCs (Jun et al., 2004a; 2004b; 2005; Kim et al., 2010; So et al., 2009). The pacemaker mechanism of ICCs has been shown to involve rhythmic oscillations in the intracellular calcium concentrations and Ca2+ release from D-myo-inositol 1,4,5-trisphosphate (IP3) receptor-operated stores. Uptake of Ca2+ activates voltage-independent, Ca2+-inhibited, NSCCs (Torihashi et al., 1999).
Since exposure to ginsenosides alters GI motility, and since ICCs are the pacemaking cells of intestinal muscle, in the present study we examined which ginsenosides have the ability to alter the electrical properties of cultured ICC clusters derived from murine small intestine.
MATERIALS AND METHODS
Preparation of cells
Balb/C mice (8–13 d old) of either sex were anesthetized with ether and sacrificed by cervical dislocation. All experiments were performed according to the Guiding Principles for the Care and Use of Animals and were approved by the Ethics Committee of Chosun University and Pusan National University. The small intestine from 1 cm below the pyloric ring to the cecum were removed, opened along the mesenteric border, and the luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa was removed by sharp dissection. Small strips of intestinal muscle were equilibrated in Ca2+-free Hank’s solution containing KCl 5.36 mM, NaCl 125 mM, NaOH 0.336 mM, Na2HCO3 0.44 mM, glucose 10 mM, sucrose 2.9 mM, and HEPES 11 mM, adjusted to pH 7.4 with Tris for 30 min. After incubation for 15 min at 37°C in an enzyme solution containing collagenase (1.3 mg/ml; Worthington Biochemical, USA), bovine serum albumin (2 mg/ml; Sigma, USA), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml), the cells were dispersed. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 mg/ml; Falcon/BD Biosciences, USA) in 35 mm culture dishes. The cells were then cultured at 37°C in a 5% CO2 incubator in smooth muscle growth medium (Clonetics Corp., USA), supplemented with 2% antibiotics/antimycotics (Gibco, USA) and murine stem cell factor (5 ng/ml; SCF, Sigma). ICCs were identified immunologically with a monoclonal antibody for Kit protein (ACK2) and were labeled with Alexa Fluor 488 (Molecular Probes, USA).
Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane potentials (current clamp) from cultured ICC clusters. Potentials were amplified using Axopatch 1-D (Axon Instruments, USA). A command pulse was applied using an IBM compatible personal computer and pClamp software (version 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on an oscilloscope, computer monitor, and pen recorder (Gould 2200, Gould, USA). The results were analyzed using pClamp and Graph Pad Prism (version 2.01) software. All experiments were performed at 30°C.
Solutions and drugs
The cells were bathed in a solution containing KCl (5 mM), NaCl (135 mM), CaCl2 (2 mM), glucose (10 mM), MgCl2 (1.2 mM), and HEPES (10 mM) adjusted to pH 7.2 with Tris. The pipette solution contained K-aspartate (120 mM), KCl (20 mM), MgCl2 (5 mM), K2ATP (2.7 mM), Na2GTP (0.1 mM), creatine phosphate disodium (2.5 mM), EGTA (0.1 mM), and HEPES (5 mM), adjusted to pH 7.2 with Tris. The drugs used were guanosine 5′-[β-thio]diphosphate trilithium salt (GDP-β-S), U-73122, calphostin C, chelerythrine, and thapsigargin. All drugs were purchased from Sigma. Flufenamic acid and niflumic acid were purchased from Calbiochem (USA). The ginsenosides Rb1, Rg3, and Rf were provided by AMBO Institute (Korea).
Statistical analysis
Data are expressed as the mean ± standard errors. Statistical differences in the data were evaluated using Student’s t-tests. A p value less than 0.05 was considered to be statistically significant. n values reported in the text refer to the number of cells used in patch-clamp experiments.
RESULTS
Effect of ginsenosides on pacemaker potentials of cultured ICC clusters
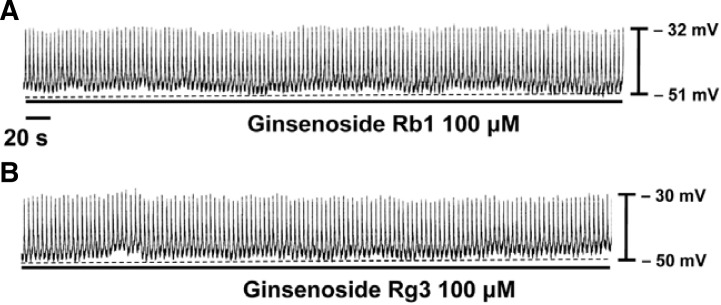
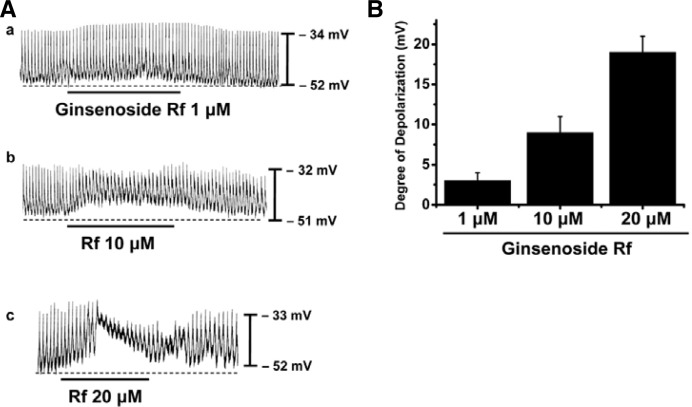
The patch-clamp technique was tested on ICCs that formed network-like structures (clusters) in culture (typically within 2–4 days). Spontaneous rhythms were routinely recorded from cultured ICC clusters under current-clamp conditions, and ICCs within clusters displayed more robust electrical rhythms. Tissue-like spontaneous slow waves have previously been recorded from these cells (Koh et al., 1998). Recordings from the cultured ICC clusters under the current clamp mode (I = 0) revealed spontaneous pacemaker potentials. The resting membrane potential was −51 ± 3 mV and the amplitude was 21 ± 3 mV. Ginsenoside Rb1 (100 μM) was without effect on the pacemaker potentials (n = 4; Fig. 1A), as was Rg3 (100 μM) (n = 4; Fig. 1B). However, in the presence of Rf (1–20 μM), the resting membrane potentials were depolarized. The corresponding resting membrane depolarizations were 3 ± 1 mV at 1 μM, 9 ± 2 mV at 10 μM, and 19 ± 1 mV at 20 μM (n = 5; Fig. 2A). A bar graph of Rf effects on pacemaker potentials is shown in Fig. 2B.
Fig. 1.
Effects of ginsenoside Rb1 and Rg3 on pacemaker potentials recorded in cultured ICC clusters from murine small intestine. Rb1 (100 μM) (A) and Rg3 (100 μM) (B) had no effects on pacemaker potentials.
Fig. 2.
Effects of Rf on pacemaker potentials recorded in cultured ICC clusters from murine small intestine. (A) Pacemaker potentials of ICCs exposed to various concentrations of Rf (1, 10, and 20 μM) in the current-clamp mode (I = 0). Responses to Rf are summarized in (B). The bars represent mean values ± S.E.
Effects of a nonselective cation channel blocker and a Cl− channel blocker on Rf-induced responses in cultured ICC clusters
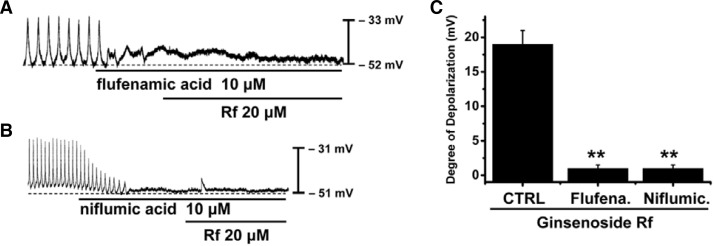
The pacemaker activity of murine small intestine is due mainly to periodic activation of NSCCs (Kim et al., 2008; Koh et al., 2002) or Cl− channels (Huizinga et al., 2002; Zhu et al., 2009). To determine the characteristics of pacemaker potentials induced by Rf, flufenamic acid, an NSCC blocker, or niflumic acid, a Cl− channel blocker, were used. In the presence of flufenamic acid (10 μM) or niflumic acid (10 μM), spontaneous potentials were abolished and subsequent application of Rf (20 μM) was without effect (Fig. 3). The resting membrane potential induced by Rf was −52 ± 1 mV in the presence of flufenamic acid and −51 ± 2 mV in the presence of niflumic acid, and these values were not significantly different when compared to control conditions (n = 4; Fig. 3). The resting membrane depolarizations were 19 ± 2 mV under control conditions, 1 ± 0.5 mV in the presence of flufenamic acid, and 1 ± 0.5 mV in the presence of niflumic acid (n = 5; Figs. 3A and 3B). A bar graph of Rf effects on pacemaker potentials in the presence of flufenamic acid or niflumic acid is shown in Fig. 3C.
Fig. 3.
Effects of flufenamic acid or niflumic acid on Rf-induced responses on pacemaker potentials in cultured ICC clusters from murine small intestine. (A) Application of flufenamic acid (10 μM) abolished the generation of pacemaker potentials. Under these conditions, Rf did not induce membrane depolarizations. (B) Niflumic acid (10 μM) also abolished the generation of pacemaker potentials. Under these conditions, Rf did not induce membrane depolarizations. Responses to Rf in the presence of flufenamic acid or niflumic acid are summarized in (C). The bars represent mean values ± S.E. **Significantly different from untreated controls (p < 0.01).
No involvement of G proteins on Rf-induced responses in cultured ICC clusters
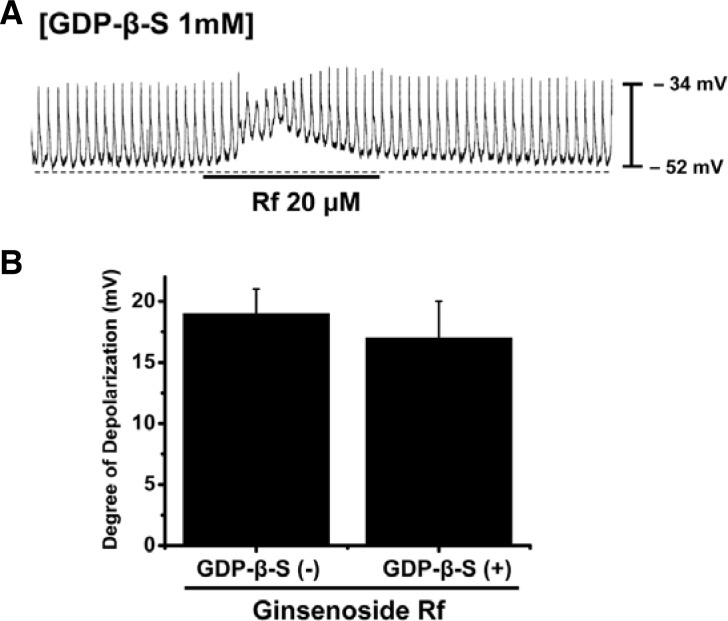
The effects of guanosine 5′-[β-thio]diphosphate trilithium salt (GDP-β-S), a non-hydrolyzable guanosine 5′-diphosphate analogue that permanently inactivates GTP-binding proteins were examined to determine whether G-proteins have a role in Rf-induced effects in ICC clusters. When GDP-β-S (1 mM) was present in the pipette, Rf still induced membrane depolarizations (Fig. 4A). When GDP-β-S was present in the pipette, resting membrane depolarizations were 17 ± 3 mV (n = 4; Fig. 4B). This result indicates that G proteins are not involved in Rf-induced effects on pacemaker potentials in ICC clusters.
Fig. 4.
Effects of GDP-β-S on Rf-induced pacemaker potentials in cultured ICC clusters from murine small intestine. (A) Pacemaker potentials from ICCs exposed to Rf in the presence of GDP-β-S (1 mM) in the pipette solution. Pacemaker potentials and membrane depolarizations induced by Rf remained unchanged by internally applied GDP-β-S (1 mM). The effects of Rf in the presence of GDP-β-S are summarized in (B). Bars represent mean values ± S.E. Rf-induced pacemaker potentials in the presence of GDP-β-S were not significantly different from those evoked by Rf alone.
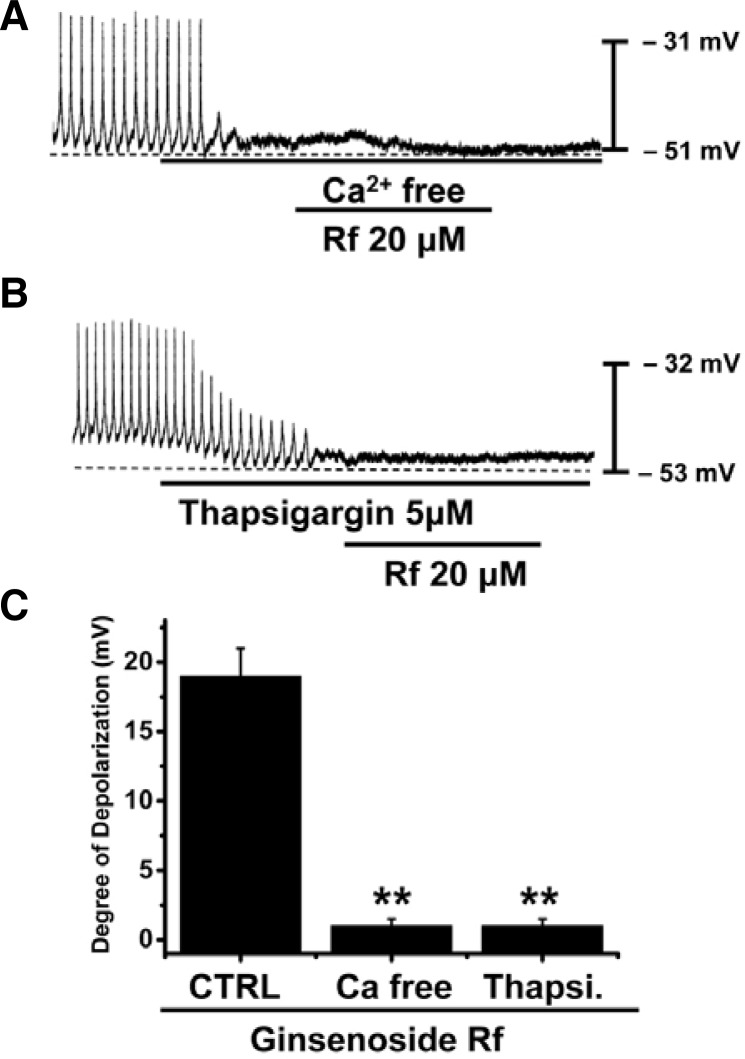
Effects of external Ca2+-free solution and an endoplasmic reticulum Ca2+-ATPase inhibitor on Rf-induced responses in cultured ICC clusters
To investigate the role of external or internal Ca2+, effect of Rf were investigated under external Ca2+-free conditions or in the presence of thapsigargin, an endoplasmic reticulum Ca2+-ATPase inhibitor. Pacemaker potentials were completely abolished in external Ca2+-free solution (n = 5; Fig. 5A), and Rf had no effects on membrane depolarization under these conditions. The degree of depolarization was significantly different when compared with Rf in the normal Ca2+ solution (Fig. 5C). Furthermore, Rf-induced depolarizations were inhibited by pretreatment with thapsigargin (n = 5, Fig. 5B). In the presence of thapsigargin (5 μM), the membrane depolarizations produced by Rf had no effects. The degree of depolarization was significantly different when compared with Rf in the absence of thapsigargin (Fig. 5C).
Fig. 5.
Effects of external Ca2+-free solution or thapsigargin on Rf-induced effects on pacemaker potentials in cultured ICC clusters from murine small intestine. (A) External Ca2+-free solution abolished the generation of pacemaker potentials. Under these conditions, Rf-induced depolarizations were blocked. (B) Thapsigargin (5 μM) also abolished the generation of pacemaker potentials and blocked the Rf-induced depolarizations. Responses to Rf in external Ca2+-free solution and in the presence of thapsigargin are summarized in (B). Bars represent mean values ± S.E. **Significantly different from untreated controls (p < 0.01).
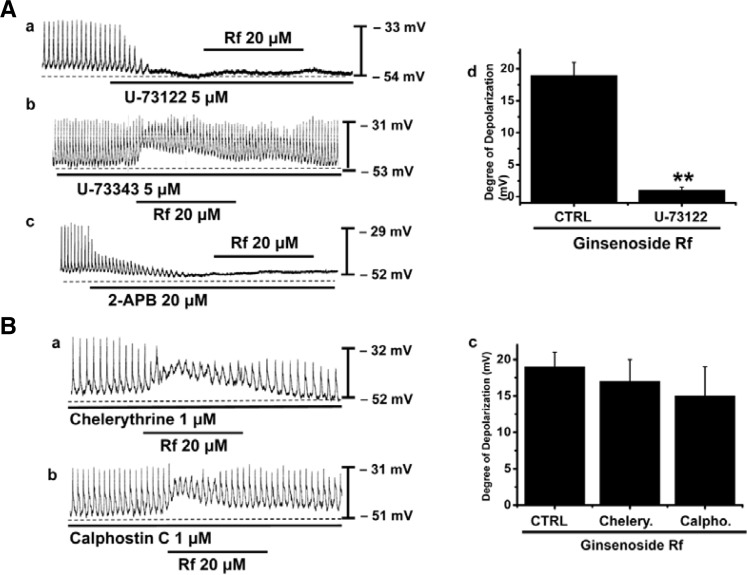
Effects of a phospholipase C inhibitor on Rf-induced responses in cultured ICC clusters
Since membrane depolarizations produced by Rf treatment were related to intracellular Ca2+ mobilization, it is likely that these effects also require phospholipase C (PLC) activation. To test this possibility, Rf-induced depolarizations were measured in the presence and absence of U-73122, a PLC inhibitor. Pacemaker potentials were completely abolished by application of U-73122 (5 μM) (n = 5; Fig. 6Aa) and Rf had no effects on membrane depolarizations in the presence of the PLC inhibitor. The extent of depolarizations was significantly different when compared to Rf treatment in the absence of U-73122 (n = 5; Fig. 6Ac). However, U-73343 (an inactive analog of U-73122) had no effect on basal pacemaker potentials and did not alter pacemaker potentials induced by Rf (n = 3; Fig. 6Ab). To determine the involvement of 1,4,5-inositol triphosphate (IP3) receptors in mediating membrane depolarizations induced by Rf, we used 2-APB, an antagonist of IP3 receptors. Pacemaker potentials were completely abolished by application of 2-APB (20 μM) (n = 5; Fig. 6Ac) and Rf had no effects on membrane depolarizations in the presence of the IP3 receptor blocker. This data show that the PLC-IP3 pathway is involved in Rf-induced depolarizations in ICC clusters.
Fig. 6.
Effects of U-73122, chelerythrine and calphostin C on Rf-induced actions on pacemaker potentials in cultured ICC clusters from murine small intestine. (A) (a) U-73122 (5 μM) abolished the generation of pacemaker potentials and blocked Rf -induced depolarizations. (b) U-73343 (inactive analog of U-73122) did not alter pacemaker potentials induced by Rf. (c) 2-APB (IP3 receptor inhibitor) abolished the generation of pacemaker potentials and blocked Rf-induced depolarizations. Responses to Rf are summarized in (d). The bars represent mean values ± S.E. The effects of U-73122 on Rf-induced pacemaker potentials were significantly different to those induced by Rf alone. (B) (a,b) Pacemaker potentials of ICCs exposed to Rf in the presence of chelerythrine (1 μM) or calphostin C (1 μM). Under these conditions, Rf-induced membrane depolarizations were unaltered. Responses to Rf in the presence of chelerythrine and calphostin C are summarized in (c). The bars represent mean values ± S.E. **Significantly different from untreated controls (p < 0.01).
Effects of a protein kinase C inhibitor in the Rf-induced responses in cultured ICC clusters
We examined the effects of chelerythrine or calphostin C, inhibitors of protein kinase C, to investigate whether Rf-induced pacemaker potentials are mediated by the activation of protein kinase C. Chelerythrine (1 μM) or calphostin C (1 μM) did not alter pacemaker potentials induced by Rf (Figs. 6Ba and 6Bb). The degree of depolarization induced by Rf was not significantly different when compared to that obtained in the absence of chelerythrine or calphostin C (n = 5; Fig. 6Bc).
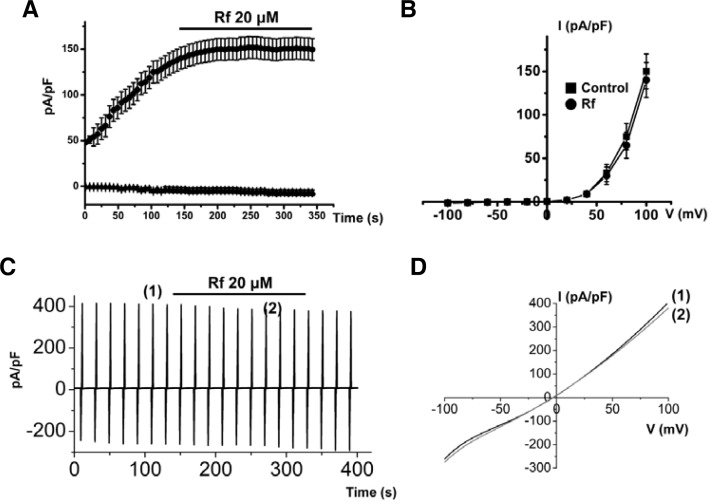
Effects of Rf in the TRPM7 and Ca2+ activated Cl− channels
Since the pacemaking activity of ICCs has been shown to be normally initiated by transient receptor potential (TRP) melastatin 7 (TRPM7) (Kim et al., 2005) or Ca2+ activated Cl− channels (Zhu et al., 2009), we examined the effects of Rf on these channels. However, we found that Rf did not alter the activity of either TRPM7 or Ca2+ activated Cl− channels (n = 4; Fig. 7). Therefore, Rf-induced effects, similar to neurotransmitters, were due to the stimulation of depolarization.
Fig. 7.
Effects of Rf on TRPM7 and Ca2+ activated Cl− channels. (A) Representative trace of Rf actions on TRPM7. Rf did not affect TRPM7 activity. (B) Representative I–V relationships of (A). (C) Representative trace of Rf actions on Ca2+ activated Cl− channels. Rf did not affect Ca2+ activated Cl− channels. (D) Representative I–V relationships of (C).
DISCUSSION
Ginsenosides, the active ingredients of Panax ginseng, have been widely used as invigorating agents and many reports have described a variety of physiological and pharmacological effects in various organs and tissues (Attle et al., 1999). However, there are only a few reports on the effects of ginsenosides on GI motility. In the present study, we found that ginsenosides regulate intestinal motility by modulating the pacemaker potentials of ICCs. The effects of ginsenosides are mediated by activation of both NSCC and Cl− channels, through a mechanism involving intracellular Ca2+ mobilization, but which is independent of protein kinase C.
ICCs are the pacemaking cells of GI muscle that generate rhythmic oscillations in membrane potentials known as slow waves (Huizinga et al., 1995; Sanders, 1996; Ward et al., 1994). Slow waves propagate within ICC networks, conduct into smooth muscle cells through gap junctions, and initiate phasic contractions by activating Ca2+ entry through L-type Ca2+ channels. Pacemaker activity in the murine small intestine is due mainly to periodic activation of NSCCs (Kim et al., 2008; Koh et al., 2002) or Cl− channels (Huizinga et al., 2002; Zhu et al., 2009). ICCs also mediate or transduce inputs from the enteric nervous system. Due to their central role in GI motility, loss of ICCs would be extremely detrimental. Research into the biology of ICCs provides exciting new opportunities to understand the etiology of diseases that have long eluded understanding. In the present study, we found that ICCs produced spontaneous pacemaker potentials under the current-clamp mode and that application of Rf evoked membrane depolarizations in these cells. Our results also showed that flufenamic acid or niflumic acid abolished the generation of pacemaker potentials and that Rf-induced depolarizations were blocked by flufenamic acid or niflumic acid. These findings suggest that both NSCCs and Cl− channels are involved in generation of pacemaker potentials in ICCs, and that ginsenosides have a role to play in regulating pacemaker potentials through modulation of these channels.
Several reports have shown that ginsenosides share a common G-protein-coupled signaling pathway with well-defined neurotransmitters and are endogenous agents for their pharmacological or physiological actions (Choi et al., 2001; Kim et al., 2007; Nah et al., 1995; Yoshimura et al., 1998). For example, it has been shown that ginsenosides inhibit voltage-dependent Ca2+ channels in sensory neurons through activation of pertussis toxin-sensitive G-proteins to a similar degree as opiates and that they also regulate Ca2+ channels through G protein interactions in rat chromaffin cells. However, in the current study, when GDP-β-S was present in the pipette, Rf-induced membrane depolarizations were not altered. This indicates that the effects of ginsenosides on the electrical activity of ICCs may not be related to G-proteins. Influx of external Ca2+ is necessary for GI smooth muscle contraction and also to generate pacemaker currents in ICCs (Tokutomi et al., 1995). In the present study, Rf-induced depolarizations were suppressed when Ca2+ was removed from the external solution. This shows that influx of external Ca2+ is necessary for ginsenoside-induced membrane depolarizations. The influx pathway for external Ca2+ might be through Ca2+ activated Cl− channels or Ca2+ depleted Ca2+ channels. However, the exact mechanisms involved need to be investigated.
Some reports demonstrated that ginsenosides interact with unidentified membrane components to induce mobilization of Ca2+ from 1,4,5-inositol triphosphate (IP3)-sensitive intracellular stores via a PLC-dependent pathway in Xenopus oocytes (Choi et al., 2001a; 2001b; Kim et al., 2007). It has also been suggested that extracellular Ca2+, IP3 receptor activation, and PLC activation are important components of the molecular pathway underlying ginsenoside-induced activation of store operated Ca2+ entry in Xenopus oocytes (Jeong et al., 2004). These observations suggest that ginsenosides may exert biological activity through mobilization of intracellular Ca2+ and activation of PLC. In the present study, thapsigargin, a potent endoplasmic reticulum Ca2+-ATPase inhibitor, and U-73122, a PLC inhibitor, suppressed Rf-induced membrane depolarizations, suggesting that ginsenoside-induced release of Ca2+ from internal stores and activation of PLC are essential for the effects of these agents on membrane depolarization. However, chelerythrine and calphostin C, specific and potent protein kinase C inhibitors, did not block Rf-induced effects, suggesting that protein kinase C is not involved in ICC responses to ginsenosides.
Kim et al. (2007) suggested that GTS modulates pacemaker activity in ICCs. However, GTS consists of a mixture of all ginsenosides and it was not clear which ginsenosides were responsible for this effect. In the present study, we showed that among ginsenosides, Rf is a good candidate for regulating pacemaker activity in intestinal ICCs.
In summary, we have shown that by virtue of its ability to modulate the pacemaker activity of intestinal ICCs. Ginsenoside Rf may be useful for regulating intestinal motility.
Acknowledgments
This work was supported by the Bio-Scientific Research Grant funded by the Pusan National University (PNU, Bio-Scientific Research Grant) (PNU-2010-101-258).
REFERENCES
- Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin. Exp. Pharmacol. Physiol. 1996;2:728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Choi S., Kim H.J., Ko Y.S., Jeong S.W., Kim Y.I., Simonds W.F., Oh J.W., Nah S.Y. Gαq/11 coupled to mammalian PLCβ3-like enzyme mediates the ginsenoside effect on Ca2+-activated Cl− current in the Xenopus oocytes. J. Biol. Chem. 2001a;276:48797–48802. doi: 10.1074/jbc.M104346200. [DOI] [PubMed] [Google Scholar]
- Choi S., Rho S.H., Jung S.Y., Kim S.C., Park C.S., Nah S.Y. A novel activation of Ca2+-activated Cl− channel in Xenopus oocytes by Ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca(2+) mobilization. Br. J. Pharmacol. 2001b;132:641–648. doi: 10.1038/sj.bjp.0703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Shiga Y., Hanyu N., Hashimoto Y., Mukai H., Nishikawa K., Nakamura T. Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction. Jpn. J. Gastroenterol. Surg. 1995;28:956–960. [Google Scholar]
- Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem. Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Satoh K., Kase Y., Ishige A., Kubo M., Sasaki H., Nishikawa S., Kurosawa S., Yakabi K., Nakamura T. Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med. 2001;67:179–181. doi: 10.1055/s-2001-11513. [DOI] [PubMed] [Google Scholar]
- Huisinga J.D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H.B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–352. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Zhu Y., Ye J., Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- Jeong S.M., Lee J.H., Kim S., Rhim H., Lee B.H., Kim J.H., Oh J.W., Lee S.M., Nah S.Y. Ginseng saponins induce store-operated calcium entry in Xenopus oocytes. Br. J. Pharmacol. 2004;142:585–593. doi: 10.1038/sj.bjp.0705797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.Y., Choi S., Yeum C.H., Chang I.Y., Park C.K., Kim M.Y., Kong I.D., So I., Kim K.W., You H.J. Noradrenaline inhibits pacemaker currents through stimulation of beta 1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br. J. Pharmacol. 2004a;141:670–677. doi: 10.1038/sj.bjp.0705665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.Y., Choi S., Yeum C.H., Chang I.Y., You H.J., Park C.K., Kim M.Y., Kong I.D., Kim M.J., Lee K.P., et al. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur. J. Pharmacol. 2004b;495:35–42. doi: 10.1016/j.ejphar.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Jun J.Y., Choi S., Chang I.Y., Yoon C.K., Jeong H.G., Kong I.D., So I., Kim K.W., You H.J. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br. J. Pharmacol. 2005;144:242–251. doi: 10.1038/sj.bjp.0706074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.C., Kim S.R., Markelonis G.J., Oh T.H. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate induced neurodegeneration. J. Neurosci. Res. 1998;53:426–432. doi: 10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S., So I., Stanfield P.R., Kim K.W. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Parajuli S.P., Yeum C.H., Park J.S., Jeong H.S., So I., Kim K.W., Jun J.Y., Choi S. Effects of ginseng total saponins on pacemaker currents of interstitial cells of Cajal from the small intestine of mice. Biol. Pharm. Bull. 2007;30:2037–2042. doi: 10.1248/bpb.30.2037. [DOI] [PubMed] [Google Scholar]
- Kim S., Nah S.Y., Rhim H. Neuroprotective effects of ginseng saponins against L-type Ca2+ channel-mediated cell death in rat cortical neurons. Biochem. Biophys. Res. Commun. 2008;365:399–405. doi: 10.1016/j.bbrc.2007.10.048. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Chae H., Kwon Y.K., Choi S., Jun J.Y., Jeon J.H., So I., Kim S.J. Effects of imatinib mesylate in interstitial cells of Cajal from murine small intestine. Biol. Pharm. Bull. 2010;33:993–997. doi: 10.1248/bpb.33.993. [DOI] [PubMed] [Google Scholar]
- Koh S.D., Sanders K.M., Ward S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J. Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S.D., Jun J.Y., Kim T.W., Sanders K.M. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J. Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P., Ward S.M., Carl A., Nerell M.A., Sanders K.M. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc. Natl. Acad. Sci USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata P., Hayakawa T., Satoh K., Kase Y., Ishige A., Sasaki H. Effects of Dai-kenchu-to, a herbal medicine, on uterine and intestinal motility. Phytother. Res. 2001;23:29–41. doi: 10.1002/ptr.745. [DOI] [PubMed] [Google Scholar]
- Nah S.Y. Ginseng: recent advances and trends. Korean J. Ginseng. Sci. 1997;21:1–12. [Google Scholar]
- Nah S.Y., Park H.J., McCleskey E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci USA. 1995;92:8739–8743. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordog T., Ward S.M., Sanders K.M. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J. Physiol. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tsuchiya M., Naka S., Takagi K. Effects of Panax Ginseng root on conditioned avoidance response in rats. J. Pharmacol. Soc. 1977;27:509–516. doi: 10.1254/jjp.27.509. [DOI] [PubMed] [Google Scholar]
- Sanders K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- So K.Y., Kim S.H., Sohn H.M., Choi S.J., Parajuli S.P., Choi S., Yeum C.H., Yoon P.J., Jun J.Y. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009;27:525–531. doi: 10.1007/s10059-009-0076-1. [DOI] [PubMed] [Google Scholar]
- Szurszewsik J.H. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Prostaglandins and the Gastrointestinal Tract. Raven Press; New York: 1987. p. 383. [Google Scholar]
- Torihashi S., Nishi K., Tokutomi Y., Nishi T., Ward S., Sanders K.M. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–148. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- Tokutomi N., Maeda H., Tokutomi Y., Sato D., Sugita M., Nishigawa S., Nakao J., Imamura T., Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit-positive cells. Pflügers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Ward S.M., Burns A.J., Torihashi S., Sanders K.M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 1994;480:91–102. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H., Watanabe K., Ogawa N. Psychotropic effects of ginseng saponins on agonistic behavior between resident and intruder mice. Eur. J. Pharmacol. 1998;146:291–297. doi: 10.1016/0014-2999(88)90305-6. [DOI] [PubMed] [Google Scholar]
- Zhu M.H., Kim T.W., Ro S., Yan W., Ward S.M., Koh S.D., Sanders K.M. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]