Abstract
In this study, we characterized a putative peroxidase Prx1 of Candida albicans by: 1) demonstrating the thioredoxin-linked peroxidase activity with purified proteins, 2) examining the sensitivity to several oxidants and the accumulation of intracellular reactive oxygen species with a null mutant (prx1Δ), a mutant (C69S) with a point mutation at Cys69, and a revertant, and 3) subcelluar localization. Enzymatic assays showed that Prx1 is a thioredoxin-linked peroxidase which reduces both hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (t-BOOH). Compared with two other strong H2O2 scavenger mutants for TSA1 and CAT1, prx1Δ and C69S were less sensitive to H2O2, menadione and diamide at all concentrations tested, but were more sensitive to low concentration of t-BOOH. Intracellular reactive oxygen species accumulated in prx1Δ and C69S cells treated with t-BOOH but not H2O2. These results suggest that peroxidase activity of Prx1 is specified to t-BOOH in cells. In both biochemical and physiological cases, the evolutionarily conserved Cys69 was found to be essential for the function. Immunocytochemical staining revealed Prx1 is localized in the cytosol of yeast cells, but is translocated to the nucleus during the hyphal transition, though the significances of this observation are unclear. Our data suggest that PRX1 has a thioredoxin peroxidase activity reducing both t-BOOH and H2O2, but its cellular function is specified to t-BOOH.
Keywords: hydrogen peroxide, peroxidase, PRX1, tert-butyl hydroperoxide, translocalization
INTRODUCTION
Prxs (peroxiredoxins), a large family of thiol-specific antioxidant proteins including thioredoxin peroxidases and alkyl hydroperoxide reductases, are observed across all kingdoms (Kang et al., 2005; Wood et al., 2003) six Prxs (PrxI-VI) were identified in mammalian cells (Lim et al., 1994; Rhee et al., 2001; Seo et al., 2000) and five Prxs (TSA1/cTPxI, TSA2/cTPxII, AHP1/cTPxIII, PRX1/mTPx, DOT5/nTPx) in Saccharomyces cerevisiae (Park et al., 2000; Wong et al., 2004). Prxs exert their protective antioxidant role in a cell through their peroxidase activity (ROOH + 2e− + 2H+ → ROH + H2O), whereby hydrogen peroxide and a wide range of organic hydroperoxides (ROOH) are reduced and detoxified (Rhee et al., 2001) with the use of electrons provided by thioredoxin (Trx) (Bryk et al., 2000; Hofmann et al., 2002; Jacobson et al., 1989; Peshenko and Shichi, 2001). The activity of Prxs is dependent upon an absolutely conserved peroxidatic cysteine that is essential for the hydroperoxide reduction step (Choi et al., 1998; Ellis and Poole, 1997). The Prx superfamily can be divided into three sub-groups, typical 2-Cys Prx, atypical 2-Cys Prx and 1-Cys Prx according to the number of conserved cysteinyl residues directly involved in catalysis (Chae et al., 1994a; 1999).
Candida albicans, the most prevalent human fungal pathogen, can cause diverse forms of candidiasis through systemic or life-threatening infections, especially in immunocompromised patients and reveals its pathogenecity through adhesion, morphological transition and secretion of proteases during evasion from the host immune system (Calderone and Fonzi, 2001). C. albicans, like other organisms, is suspected to have a variety of antioxidant enzymes, such as catalase, superoxide dismutase, glutathione peroxidase, thiol-specific antioxidant 1 (TSA1), to respond to the stress. No report on the characterization of Prx of C. albicans prompted us to identify a gene homologous with the PRX1 gene of S. cerevisiae. The identified gene, CaPRX1, was found to encode a putative thioredoxin peroxidase, which is localized in the cytoplasm in yeast cells, while is translocated to the nucleus in hyphae. Hereafter, the two-letter prefix ‘Ca’ or ‘Ca’ is no longer used for genes or their protein products originated from C. albicans, while used for other species, for example, ‘Sc’ or ‘Sc’ for S. cerevisiae.
MATERIALS AND METHODS
Strains and cultures
C. albicans strains used in this study are described in Table 1. Cells were routinely grown at 30μC in liquid YPD medium (1% yeast extract, 2% peptone and 2% glucose). For hyphal induction, yeast cells grown to mid-log phase at 30μC were transferred to RPMI-1640 medium (Gibco-BRL, USA) or YPD supplemented with 10% fetal bovine serum (FBS) (Welgene, Korea) and further grown at 37μC for 2 h or at 37μC for 8 h to obtain germ tube or hyphal culture, respectively. The wild-type (wt) strain was SC5314 (Fonzi and Irwin, 1993). CAI4 was used as a parental strain to construct a prx1 null mutant (prx1Δ). All prx1 disruptants were selected on synthetic dextrose (SD) medium composed of 0.67% yeast nitrogen base without amino acids (Difco, USA), 2% glucose, 0.06% uracil drop-out mix and 2% agar if needed. Uridine (80 mg/L) was added to liquid and solid media when culturing uracil auxotrophs. Escherichia coli strain DH5α was used to amplify all plasmids.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SC5314 | Wild-type | Fonzi and Irwin (1993) |
| CAI4 | ura3Δ::imm434/ura3Δ::imm434 | Fonzi and Irwin (1993) |
| tsa1Δ/ tsa1Δ | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG tsa1Δ::HIS1/tsa1Δ::hisG tsa1Δ::ARG4/tsa1Δ::hisG-URA3-hisG | Shin et al. (2005) |
| cat1Δ/ cat1Δ | ura3Δ::imm434/ura3Δ::imm434 cat1Δ::dpl200/cat1Δ::URA3-dpl200 | Nasution et al. (2008) |
| CPD1 | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG-URA3-hisG/PRX1 | This work |
| CPD1-U | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/PRX1 | This work |
| CPD2 | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/ prx1Δ:: URA3-dpl200 | This work |
| CPD2-U (prx1Δ) | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/prx1Δ::dpl200 | This work |
| CPD2+U | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/prx1Δ::dpl200 iro1::URA3 | This work |
| PRX1-R (CPD2-R) | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/prx1Δ::dpl200 iro1::PRX1-URA3 | This work |
| C69S | ura3Δ::imm434/ura3Δ::imm434 prx1Δ::hisG/prx1Δ::dpl200 iro1::PRX1C69S-URA3 | This work |
Molecular methods
Plasmid preparation, poly(A)+ RNA preparation, cloning, and nucleic acid and protein blotting analysis were performed as previously described (Sambrook and Russell, 2001). The nucleotide sequences were confirmed, if necessary.
Cloning of PRX1
After a sequence with highest similarity to ScPRX1 was searched through BLAST, DNA fragments containing the open reading frame (ORF) of PRX1 or ORF plus flanking sequences (or alternatively full gene; FG for short) were amplified from the genomic DNA of SC5314 with primer pairs 5′-GAATTCG ATGAGAGACAAAAAACAAAC-3′ (the initiation codon in bold)/5′-GAATTCTTACTTGTCTTCCTTTTCCA-3′ (the termination codon in bold), and 5′-GAATTCGAAGCTACGGAGAAGATT CG-3′/5′-GAATTCAATCAGTTCAAAGAATAATT-3′, and subcloned into vectors pGEM-T Easy (Promega, USA) and pCR 2.1-TOPO vector (Invitrogen, USA), respectively, yielding T-PRX1ORF and TOPO-PRX1FG.
Construction of PRX1-related strains
For determination of possible physiological and biochemical properties of Prx1, we constructed a strain in which PRX1 was nullified, C69S mutant in which Cys69 was replaced by serine, and a revertant. Since two alleles of PRX1 were found to be present in the genome of C. albicans (designated ‘orf19.5180’ according to the Candida Genome Database), two sequential disruptions were necessary to obtain a PRX1-null mutant. The presence or absence of PRX1 allele was confirmed by Southern, Northern, and Western blot analyses (Supplementary Fig. 1).
For the first disruption of PRX1 allele, the 4.0 kb hisG-URA3-hisG (URA Blaster) cassette from KpnI digestion of p5922 (Fonzi and Irwin, 1993) was blunt-ended with Klenow and then ligated into T-PRX1ORF which was digested with BsmBI and blunt-ended with Klenow. The resulting plasmid was designated pJM. The PRX1 disruption cassette was obtained by AatII/BsgI digestion of pJM and was transformed into strain CAI4. URA+ transformants were selected on a uracil-deficient medium to yield CPD1. For the second disruption of PRX1 allele, CPD1-U was obtained by culturing CPD1 on SD medium containing 5-fluoroorotic acid (5-FOA; 625 mg/ml) and uridine (80 mg/L). Separately, the disruption cassette (URA3-dpl200) of pDDB57 (kindly provided by Dr. D. Davis) (Wilson et al., 2000) was amplified with primers containing 65 nucleotides of PRX1 at the 5′ end (sense, 5′-GACGTTCTCCCCCCCTAGACCAATCGAAAGCCGTGGTATTATTCCGGGCTTTGAAGAAAAGTCTTTCTTTTTTCCCAGTCACGACCTT-3′; antisense, 5′-CCTTAAACAATAAAAAAAAAACGAGATCTTAAAAAATAAACTCTTCGTAAATTTAAGGCACACGAAGTGGGTGGAATTGTGCGGATA-3′). CPD2, in which both alleles of PRX1 are disrupted, was obtained by transformation of amplified URA3-dpl200 cassette into CPD1-U and selection on a uracil-deficient medium.
We also constructed a strain complemented with PRX1 by using URA3 as a selection marker, URA3 was again excised from the genome of CPD2 by FOA selection as above, generating CPD2-U (or alternatively prx1Δ). Separately, the 1.9 kb SpeI/HindIII fragment of TOPO-PRX1FG was cloned into the SpeI and HindIII site of URA3-containing pLUX (kindly provided by Dr. W. Fonzi) to yield pLUX-PRX1. pLUX-PRX1 was linearized by NheI and transformed into CPD2-U to integrate the functional PRX1 gene into the IRO1 locus. One of colonies selected on a uracil-deficient medium was designated CPD2-R (or alternatively PRX1-R). A strain (CPD2+U) with the plain pLUX integrated into the IRO1 locus was also constructed to use as a control for the effect of the URA3 copy present in the IRO1 locus in the case of CPD2-R.
To construct C69S, PCR-mediated site-directed mutagenesis was performed with complementary primers (5′- GCTGCCCA CACCAGTGTGAGTAGCACCGAGCTTTCTG-3′ and 5′- CAGAAAGCTCGGTGCTACTCACACTGGTGTGGGCAGC-3′), which contain a 1-base mismatch (bold) that converts the codon for 69th residue cysteine to serine, and pLUX-PRX1 as a template, yielding pLUX-PRX1C69S. The integration into the IRO1 locus followed the same procedures as done for CPD2-R.
Preparation of Prx1 proteins
To produce wild type (Prx1wt) and mutant proteins (Prx1C69S), the Prx1-encoding DNA fragments were PCR-amplified with primers 5′-CGC GCTAGC AGA GAC AAA AAA CAA ACA AAA AAA AAA AA-3′ containing a NheI site (underlined) and 5′- GC CTCGAG TTA CTT GTC TTC CTT TTC CAA CGG-3′ containing both an XhoI site (underlined) and the stop codon (bold) and either T-PRX1ORF or pLUX-PRX1C69S for template. Purified PCR products were digested with NheI and XhoI, and cloned into the pET-28b vector. The constructed plasmids were transformed into E. coli BL21 (DE3) to express Prx1 proteins via IPTG induction. Recombinant proteins were purified using Ni-chelating Sepharose column after sonication as manufacturer’s recommendation (Amersham Biosciences). The fractions containing His-Prx1 protein were pooled and dialyzed against 20 mM Tris-HCl (pH 7.6), which were subsequently used for either antibody production (with the case of Prx1wt) or enzyme assay (with both Prx1wt and Prx1c69s).
Determination of peroxidase activity of CaPrx1
Yeast thioredoxin reductase 1 and thioredoxin 1 were purchased from Ab Frontier. A peroxidase reaction was carried out in a 200 μl reaction mixture containing 50 mM HEPES (pH 7.0), 5 mM DTT, and 0.1 mM H2O2 or 0.1 mM t-BOOH in the absence or presence of 2 μM CaPrx1 at room temperature. The glutathione-dependent peroxidase activity was assayed with the reaction mixture containing glutathione instead of DTT. At the appropriate time, 5 μl of the reaction mixture was added to 0.2 ml of FOX1 reagent, incubated at room temperature for 30 min, and then the remaining amount of peroxide was monitored by measuring the absorbance at 560 nm and calculated from the standard curve (Rhee et al., 2010). The FOX1 reagent contained 100 μM xylenol orange, 250 μM ferrous ammonium sulfate, 100 mM sorbitol, and 25 mM H2SO4; the iron salt was dissolved directly in acid, since ferrous ions are prone to autoxidation at physiological pH (Rhee et al., 2010).
Spot assay
Aliquots (10 μl) containing approximately 103 cells of an overnight culture were spotted on YPD plates containing oxidants at indicated concentrations. Plates were observed after 2–3 days of incubation at 30μC (Lee et al., 1999).
Intracellular ROS assay
The relative amount of intracellular ROS was measured using CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester) (Molecular Probe, USA) as described previously (Bae et al., 1997).
Anti-CaPrx1 antibody production
The CaPRX1 ORF was cloned into pET-30b (Novagen, USA) and transformed into E. coli strain BL21 (DE3). Fusion proteins were purified according to the manufacturer and used for anti-CaPrx1 antiserum in the mouse.
Subcellular localization of Prx1
Immunostaining was performed as described previously (Shin et al., 2005), with using anti-Prx1 antiserum and rhodamine-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, USA). Nuclei were counterstained by DAPI. Fluorescent images were captured with a LSM510 Meta confocal microscope (Carl Zeiss, Germany) at excitation 545 nm and emission 560 nm for rhodamine and at excitation 364 nm and emission BP 385–470 nm for DAPI.
RESULTS
Amino acid sequence analysis of PRX1
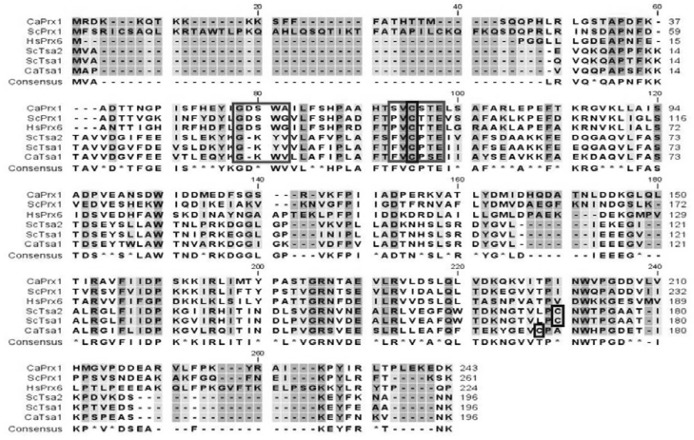
When Blast was performed from the Candida Genome Database [Stanford Genome Technology Center (http://sequence-www.stanford.edu/group/candida)] with the amino acid sequence of ScPRX1 (YBL064C) as a query, PRX1 (GenBank accession number: XP_717002) was found to have highest similarity (55%) among C. albicans homologs (Fig. 1). The deduced amino acid sequence revealed that Prx1 has only one conserved cysteine (Cys69), corresponding to Cys47 of ScTsa1, thus, it can be classified as a member of 1-Cys Prx family. In fact, Prx1 showed around 30% similarity with 2-Cys Prx members Tsa1 and ScTsa1 (Pedrajas et al., 2000), but 46% similarity with phylogenically distant human PrxVI, a 1-Cys Prx member (Chae et al., 1994b) (Fig. 1). Interestingly, Prx1 has no organelle-targeted sequence other than the nuclear localization signal (NLS) (8KKKK11) at the N-terminus, contrasting the mitochondrial localization signal of ScPrx1 (2FSRICSAQLKRT13) at the N-terminal region. In addition, the presence of a single putative lipase motif (52GDSWA56) represents Ca2+-independent phospholipase A2 activity (Nevalainen, 2010), which functions to protect cell membrane phospholipid against oxidative damage.
Fig. 1.
Amino acid sequence alignment. Amino acid sequences of PRX1 and TSA1 of C. albicans, three TPxs of S. cerevisiae, and mammalian PrxVI were aligned according to priority of higher similarity. Conserved Cys in blue box. Peroxidase motif (PVCTTE) in red box. Lipase motif (GDSWA) in green box.
Thioredoxin peroxidase activity of Prx1
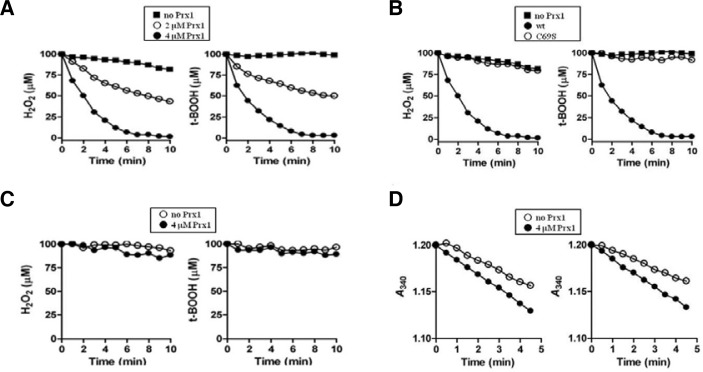
To test whether Prx1 has a peroxidase activity, Prx1wt and Prx1C69S were included in the reaction mixture containing DTT as a reducing equivalent and naturally occurring hydroperoxide H2O2 or t-BOOH as a substrate. Prx1wt removed both H2O2 and t-BOOH at a similar rate, which was dependent on Prx1 concentration (Fig. 2A), whereas Prx1C69S resulted in complete loss of the enzymatic activity (Fig. 2B). These data demonstrates that Prx1 is a thiol-linked peroxidase and that conserved Cys69 is essential for the peroxidase activity.
Fig. 2.
Peroxidase activity of Prx1. Mean values of triplicate experiments are shown. (A) DTT-dependent removal of H2O2 (left panel) and t-BOOH (right panel) by Prx1. Peroxidase reaction was carried out at room temperature in 200 μl of reaction mixture containing 50 mM HEPES (pH 7.0), 5 mM DTT, 100 μM H2O2 or 100 μM t-BOOH, and in the absence (closed square) or presence of Prx1 (closed circle, 2 μM; closed triangle, 4 μM). At the indicated times, the remaining peroxides were measured using FOX1 reagent as described in Materials and methods. (B) Peroxidase reaction was carried as in (A) with 100 μM H2O2 (left panel) or 100 μM t-BOOH (right panel) in the absence (closed square) or presence of 4 μM Prx1 (wild type, closed circle; C69S, closed triangle). (C) Peroxidase reaction was performed using 4 μM Prx1 as in (A), except the presence of GSH instead of DTT as a reducing equivalent: closed square, without Prx1; closed triangle, with Prx1. (D) Peroxidase activity linked to NADPH oxidation with S. cerevisiae Trx system was monitored by decreases of A340 in a 200-μl reaction mixture containing 50 mM HEPES (pH 7.0), 0.2 mM NADPH, 2 μM ScTrr1, 5 μM ScTrx1, 4 μM Prx1, and either 0.1 mM H2O2 (left panel) or 0.1 mM t-BOOH (right panel): closed square, without Prx1; closed triangle, with Prx1.
As an attempt to find a physiological reducing equivalent for Prx1, its peroxidase activity toward H2O2 and t-BOOH was assessed in the presence of GSH or S. cerevisiae thioredoxin system, which includes NADPH, thioredoxin reductase and thioredoxin 1 of S. cerevisiae (Ab Frontier, USA). The peroxidase activity of Prx1 was barely supported by GSH (Fig. 2C), whereas the S. cerevisiae thioredoxin system marginally provided electrons to Prx1 (Fig. 2D). Prx1 is a thiol-dependent peroxidase and the results suggest that Prx1 is likely a thioredoxin-dependent peroxidase. However, further study is necessary to confirm this possibility.
Sensitivity to oxidants
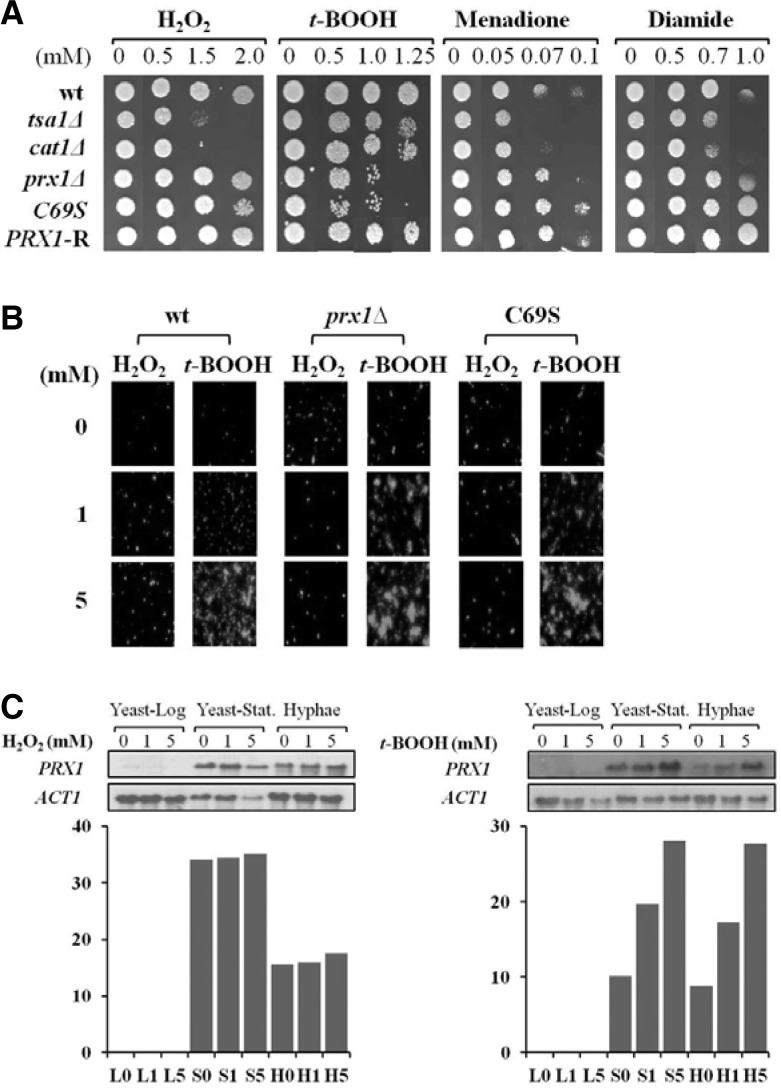
After confirming that there was no position effect of the URA3 copy of CPD2+U and CPD2-R (or PRX1-R) (data not shown), we examined the sensitivity of PRX1-related strains (prx1Δ, C69S, PRX1-R) to H2O2, t-BOOH, and two artificial oxidants diamide and menadione by exploiting spot assay. For comparison, wt and null mutants (tsa1Δ and cat1Δ) for known antioxidant genes TSA1 and CAT1, respectively, were also used. As shown in Fig. 3A, prx1Δ and C69S were less sensitive to H2O2, menadione and diamide at all concentrations tested, but more sensitive to low concentration of t-BOOH than tsa1Δ and cat1Δ (Fig. 3A). Meanwhile, both cat1Δ and tsa1Δ were highly sensitive to menadione, suggesting a role in detoxification of H2O2 formed by sequential reduction of superoxide anion generated by menadione (Jamieson, 1992). These data suggest that Prx1 is specialized in reducing t-BOOH but not H2O2in vivo. Additionally, the strain C69S exhibited a similar sensitivity pattern to that of prx1Δ, demonstrating that Cys69 is essential for the biological activity of Prx1.
Fig. 3.
Antioxidant activity of PRX1. (A) Spot assay. Ten-fold dilutions of SC5314 (wt), tsa1Δ, cat1Δ, prx1Δ, C 69S, and PRX1-R cells were exposed to H2O2, t-BOOH, diamide, and menadione on YPDA plates at given concentrations. (B) Intracellular ROS assay. wt, prx1Δ, and C69S cells were treated with H2O2 or t-BOOH for 1 h and assayed for intracellular ROS by fluorescence. (C) Northern analysis of PRX1 in response to H2O2 and t-BOOH. wt was cultured in YPD until mid-log phase and stationary phase. Hyphae were differentiated with 10% serum in YPD for 6 h. RNAs were prepared from cells treated with H2O2 and t-BOOH at given concentrations for 1 h and subjected to Northern analysis.
The spot assay results were further evidenced by measuring intracellular ROS in wt, prx1Δ, and Cys69 cells treated with H2O2 or t-BOOH with a fluorescent probe H2DCFDA, which increases intensity when oxidized to DCF by ROS. If Prx1 acts as an antioxidant and the presence of Cys69 is an important factor for the function, ROS might be accumulated in prx1Δ as well as in C69S mutant. Figure 3B shows that fluorescence intensity was significantly increased in prx1Δ and C69S compared with that in wild type strain under normal aerobic condition. However, when exogenous H2O2 (1 mM) was challenged to the cells, the fluorescence level of mutants decreased to a similar level with wt and even below the level of wt under high concentration of H2O2 (5 mM). When mutant cells were treated with t-BOOH, however, the fluorescence was dramatically increased to much more level than that of H2O2 treatment, again indicating that Prx1 reduce t-BOOH but not H2O2 and that Cys69 is an essential residue for Prx1 activity. The results of either the spot or ROS assays imply that the major biological substrate of Prx1 is t-BOOH not H2O2. The discrepancy between biochemical (Figs. 2A and 2B) and physiological data (Figs. 3A and 3B) is probably due to that the activity of Prx1 for removing H2O2 was compensated by two strong H2O2 scavenger Cat1 and Tsa1 in cells.
It was of our concern whether the PRX1 transcript is influenced by the increased concentration of either H2O2 or t-BOOH during the growth. When poly(A)+ RNA samples prepared from exponentially growing yeast cells were subjected to Northern blot analysis, no messages were detected (Fig. 3C). So, we continued to examine the expression of PRX1 in cells of stationary phase and hyphae. It was shown that PRX1 was expressed relatively high by both H2O2 and t-BOOH, but inducible in a dose-dependent manner by t-BOOH only (Fig. 3C). Although the biological significance of phase-specific expression of PRX1 is unclear, these data show that cells at the stationary phase during yeast growth and the hyphal phase respond to increased t-BOOH but H2O2 doesn’t increase the RNA level.
Sub-cellular localization of Prx1
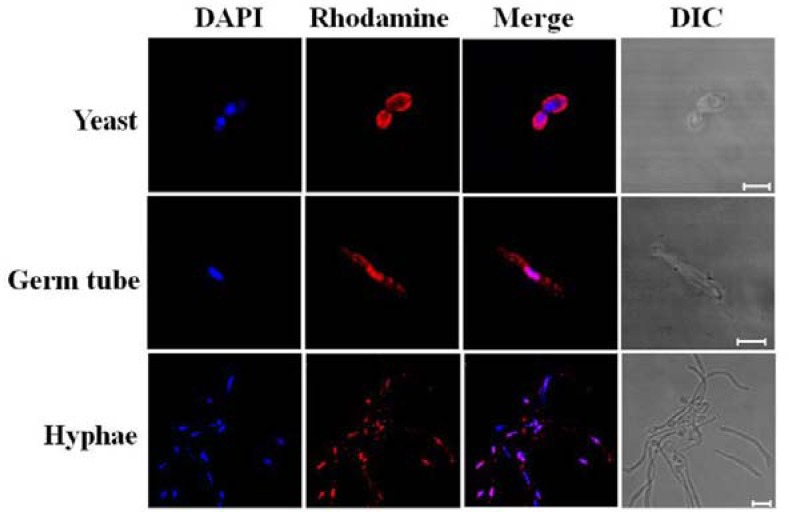
Five isoforms of thioredoxin peroxidases of S. cerevisiae are classified according to their sub-cellular localizations; cTPxI, cTPxII, and cTPxIII in the cytoplasm, mTPx (also called ScPrx1) in the mitochondria, and nTPx in the nucleus (Park et al., 2000). As mentioned earlier, Prx1 has a nuclear localization signal at the N-terminus instead of a mitochondria targeting sequence shown in ScPrx1. When immunocytochemical staining was performed with Prx1-specific antibody (Fig. 4), Prx1 was apparently localized in the cytoplasm (first row). However, it was translocated to the nucleus in both germ tubes and hyphal cells induced by 10% serum (second and third rows), suggesting that the localization of Prx1 is controlled by signal transduction. Thus, Prx1 behaves differently from ScPrx1 with highest amino acid similarity. The biological significance of nuclear translocation of Prx1 remains to be further clarified.
Fig. 4.
Subcellular localization of Prx1. In situ hybridization with anti-Prx1 antibody was performed in yeast cells, germ tubes, and hyphae. Scale bars, 5 μm.
DISCUSSION
A search of the Candida Genome Database reveals six putative peroxidases including AHP1, AHP2, DOT5, TRP99, TSA1, and orf19.5180 (PRX1). TSA1 and PRX1 are homologs of cTPxI and mTPx of S. cerevisiae respectively. DOT5 is homologous with ScDOT5/nTPx and AHP1, AHP2, and TRP99 are homologous with ScAHP1/cTPxIII. Of those, only TSA1 has been characterized as an antioxidant (Shin et al., 2005; Urban et al., 2005), while the rest have remained uncharacterized. From this study, PRX1 becomes a second Prx member proven to have peroxidase activity in C. albicans. Using the pSORT II program (http://www.psort.org/), DOT5, AHP1 and AHP2, and TRP99 were localized to the nucleus, cytoplasm, and mitochondria, respectively. In this regard, ScPRX1/mTPx used for a BLAST query to search PRX1 indicates a close relationship with TRP99. Thus, in C. albicans, it is highly possible that three major fractionated compartments (nucleus, cytoplasm, and mitochondria) possess their own Prxs.
The translocation of Prx1 from the cytosol to the nucleus in hyphal cells (Fig. 4) is an interesting observation. As typically exemplified by I-κB/NF-κB and steroid receptors, nuclear translocation of proteins is induced by extra/intracellular signals and mostly results in gene activation. It is unlikely that the nuclear translocation of Prx1 is simply aimed at reducing nuclear H2O2, since DOT5 is predicted to be present in the nucleus. However, the doubling time of the prx1Δ was almost same as the wild-type, and no apparent physiological changes were observed under the hyphae-inducing condition (data not shown). The only effect of PRX1 deletion was some sensitivity to oxidative stresses. Whether the nuclear translocation of Prx1 is a cause or a consequence of hyphal differentiation remains to be answered.
The prx1 null mutant showed weak sensitivity toward H2O2 and hypersensitivity toward t-BOOH (Fig. 2A), suggesting that Prx1 may act as a peroxidase for organic peroxides in cells. This was consistent with the results of the ROS assay (Fig. 2B). In contrast, the high sensitivity of tsa1Δ and cat1Δ to menadione and diamide suggests that Tsa1 and Cat1 are antioxidants for H2O2 detoxification and also might be involved in the major stress responses, as diamide has pleiotropic effects and can elicit a wide range of cellular damage (Trotter and Grant, 2002). In contrast to the inducibility of PRX1 expression with t-BOOH (Figs. 3A and 3B), however, recombinant Prx1 protein displayed peroxidase activity for both H2O2 and t-BOOH with a similar rate (Fig. 3A). These data indicate that an unidentified regulation system may control the function of different antioxidants in C. albicans. ScYap1, a bZIP transcription factor of S. cerevisiae, regulates the cellular response to oxidative stress, and Cap1 of C. albicans is a functional homologue with ScYap1 (Zhang et al., 2000). In the upstream region of the PRX1 ORF there are two Cap1-binding sequence: TTACTAA (Zhang et al., 2000) and TTAGTGA (Pedrajas et al., 2000) at −430 and −319 from the start codon, respectively. Furthermore, seven stress response elements of a consensus sequence AGGGG are also found. Thus, PRX1 could respond to a range of stresses through interaction with different transcription factors and genes. A detailed analysis for the cell protective function and molecular mechanism such as redox cycle of Prx1 is needed.
Supplementary Material
Acknowledgments
This work was supported by Korea Science and Engineering Foundation grant funded by the Korea government (Ministry of Science and Technology) (No. 2006-0063-2), the Pioneer Research Center Program (No. 2009-0081512) and Bio R&D Program Grant M10642040002-07N4204-00210 (to W.J.) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology. K.S. and J.K. were recipients of Ewha Global Partnership Program 2006 and the Brain Korea 21 Scholars Program, respectively.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Bae Y.S., Kang S.W., Seo M.S., Baines I.C., Tekle E., Chock P.B., Rhee S.G. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- Bryk R., Griffin P., Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- Calderone R.A., Fonzi W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Chae H.Z., Chung S.J., Rhee S.G. Thioredoxin-dependent peroxide reductase from yeast. J. Biol. Chem. 1994a;269:27670–27678. [PubMed] [Google Scholar]
- Chae H.Z., Robison K., Poole L.B., Church G., Storz G., Rhee S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA. 1994b;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H.Z., Kim H.J., Kang S.W., Rhee S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Kang S.W., Yang C.H., Rhee S.G., Ryu S.E. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat. Struct. Biol. 1998;5:400–406. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- Ellis H.R., Poole L.B. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997;36:15013–15018. doi: 10.1021/bi972191x. [DOI] [PubMed] [Google Scholar]
- Fonzi W.A., Irwin M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Hecht H.J., Flohe L. Peroxiredoxins. J. Biol. Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- Jacobson F.S., Morgan R.W., Christman M.F., Ames B.N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 1989;264:1488–1496. [PubMed] [Google Scholar]
- Jamieson D.J. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 1992;174:6678–6681. doi: 10.1128/jb.174.20.6678-6681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.W., Rhee S.G., Chang T.S., Jeong W., Choi M.H. 2-Cys peroxiredoxin function in intracellular signal transduction therapeutic implications. Trends Mol. Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Spector D., Godon C., Labarre J., Toledano M.B. A new antioxidant with alkyl hydroperoxide defense properties in yeast. J. Biol. Chem. 1999;274:4537–4544. doi: 10.1074/jbc.274.8.4537. [DOI] [PubMed] [Google Scholar]
- Lim Y.S., Cha M.K., Kim H.K., Kim I.H. The thiol-specific antioxidant protein from human brain: gene cloning and analysis of conserved cysteine regions. Gene. 1994;140:279–284. doi: 10.1016/0378-1119(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Nasuition O., Srinivasa K., Kim M., Kim Y.J., Kim W., Jeong W., Choi W. Hydrogen peroxide induces hyphal differentiation in Candida albicans. Eukarypt. Cell. 2008;7:2008–2011. doi: 10.1128/EC.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T.J. 1-Cysteine peroxiredoxin: a dual-function enzyme with peroxidase and acidic Ca2+-independent phospholipase A2 activities. Biochimie. 2010;92:638–644. doi: 10.1016/j.biochi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Park S.G., Cha M.K., Jeong W., Kim I.H. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:5723–5732. doi: 10.1074/jbc.275.8.5723. [DOI] [PubMed] [Google Scholar]
- Pedrajas J.R., Miranda-Vizuete A., Javanmardy N., Gustafsson J.A., Spyrou G. Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J. Biol. Chem. 2000;275:16296–16301. doi: 10.1074/jbc.275.21.16296. [DOI] [PubMed] [Google Scholar]
- Peshenko I.V., Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic. Biol. Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Chang T.S., Jeong W., Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D.W. Molecular cloning: a laboratory manual. 3rd eds. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Seo M.S., Kang S.W., Kim K., Baines I.C., Lee T.H., Rhee S.G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- Shin D.H., Jung S., Park S.J., Kim Y.J., Ahn J.M., Kim W., Choi W. Characterization of thiol-specific antioxidant 1 (TSA1) of Candida albicans. Yeast. 2005;22:907–918. doi: 10.1002/yea.1283. [DOI] [PubMed] [Google Scholar]
- Trotter E.W., Grant C.M. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 2002;46:869–878. doi: 10.1046/j.1365-2958.2002.03216.x. [DOI] [PubMed] [Google Scholar]
- Urban C., Xiong X., Sohn K., Schroppel K., Brunner H., Rupp S. The moonlighting protein Tsa1p is implicated in oxidative stress response and in cell wall biogenesis in Candida albicans. Mol. Microbiol. 2005;57:1318–1341. doi: 10.1111/j.1365-2958.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- Wilson R.B., Davis D., Enloe B.M., Mitchell A.P. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wong C.M., Siu K.L., Jin D.Y. Peroxiredoxin-null yeast cells are hypersensitive to oxidative stress and are genomically unstable. J. Biol. Chem. 2004;279:23207–23213. doi: 10.1074/jbc.M402095200. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schroder E., Robin Harris J., Poole L.B. Structure mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Zhang X., De Micheli M., Coleman S., Sanglard D., Moye-Rowley W. Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol. Microbiol. 2000;36:618–629. doi: 10.1046/j.1365-2958.2000.01877.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.