Abstract
Members of the nuclear receptor superfamily function as transcription factors involved in innate and adaptive immunity as well as lipid metabolism. These highly conserved proteins participate in ligand-dependent or -independent regulatory mechanisms that affect gene expression. Peroxisome proliferator-activated receptors (PPARs), which include PPARα, PPARβ/δ, and PPARγ, are a group of nuclear receptor proteins that play diverse roles in cellular differentiation, development, and metabolism. Each PPAR subfamily is activated by different endogenous and synthetic ligands. Recent studies using specific ligand treatments and cell type-specific PPAR knockout mice have revealed important roles for these proteins in T-cell-related autoimmune diseases. Moreover, PPARs have been shown to regulate T-cell survival, activation, and CD4+ T helper cell differentiation into the Th1, Th2, Th17, and Treg line-ages. Here, we review the studies that provide insight into the important regulatory roles of PPARs in T-cell activation, survival, proliferation, differentiation, and autoimmune disease.
Keywords: autoimmune disease, nuclear receptor, PPAR, T cell
INTRODUCTION
Nuclear receptors belong to a family of structurally conserved transcription factors that regulate a diversity of cellular processes by ligand-dependent and -independent activation or repression of gene expression (Chawla et al., 2001; Mangelsdorf et al., 1995). The most extensively characterized nuclear receptor subfamily is the classical steroid hormone receptor family, which includes the receptors for glucocorticoids, estrogen, androgen, and vitamin D. Orphan receptors, for which a ligand has yet to be identified, comprise another subfamily; examples include the small heterodimeric partner (SHP), NUR77, and RAR-related orphan receptors (RORs). The third class of nuclear receptors, adopted orphan receptors, were initially classified as orphan receptors until their ligands were identified; retinoid X receptors (RXRs) and peroxisome proliferator-activated receptors (PPARs) are members of this subfamily (Glass and Ogawa, 2006; Glass and Saijo, 2010).
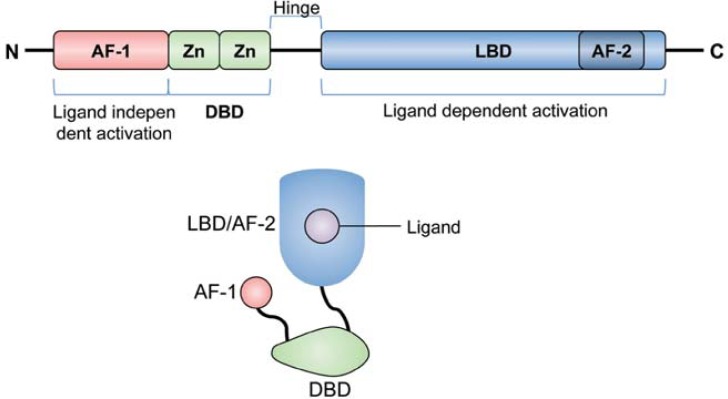
PPARs exist as three isoforms, namely PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3). The role of PPARs in lipid metabolism and inflammatory disease was elucidated by studying the effects of their respective ligands on gene expression (Varga et al., 2011). Similar to other nuclear receptors, PPARs have a conserved structure that includes an N-terminal ligand-independent activation domain, a highly conserved DNA-binding domain, a C-terminal ligand-binding domain, and a C-terminal ligand-dependent activation domain (Fig. 1) (Chan et al., 2010; Zieleniak et al., 2008). Single amino acid mutations within these functional domains result in severe defects in PPAR function that affect lipid metabolism and insulin resistance (Agostini et al., 2006; Barroso et al., 1999; Hegele et al., 2002; Ristow et al., 1998). PPAR heterodimerization with RXRs leads to binding of the peroxisome proliferator response element independent of ligand. Nevertheless, PPARs bind DNA response elements with greater affinity and stability when associated with their ligands (Moras and Gronemeyer, 1998; Renaud et al., 1995).
Fig. 1.
Structure of PPARs. PPARs have a conserved domain structure consisting of a ligand-independent activation domain (AF1), DNA-binding domain (DBD), ligand-binding domain (LBD), and ligand-dependent activation domain (AF2).
PPARs can be activated by numerous fatty acid metabolites. For instance, leukotriene B4 (LTB4) has been shown to activate PPARα. 13-hydroxyoctadecadienoic acid (13-HODE) and 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2), which activate PPARγ, are well-characterized endogenous ligands that regulate a wide spectrum of cellular processes, including inflammation (Yang et al., 2010). In this review, we focus on recent studies that further our knowledge of the roles of PPARs in T-cell functions such as activation, survival, proliferation, and differentiation. The involvement of PPARs in T-cell-related autoimmune diseases is also discussed.
PPAR LIGANDS
Primarily found within the nucleus without their ligand, PPARs localize to target gene promoters with either co-activator or co-repressor complexes (Akiyama et al., 2002; Guan et al., 2005). Studies have identified many ligands that activate and modulate PPAR functions (Berger et al., 1999; Forman et al., 1995; Itoh et al., 2008; Kliewer et al., 1997; Sanderson et al., 2009; Varga et al., 2011). These ligands are summarized in Table 1. For example, endogenous lipid metabolites from saturated or unsaturated fatty acids can bind to nuclear receptors and activate or repress gene expression. Another group of PPAR ligands consists of lipid metabolites from essential fatty acids, such as arachidonic acid derived from lipoxygenase or cyclooxygenase activity. In particular, the best-characterized endogenous ligands known to stimulate PPARα are the eicosanoids LTB4 and 8-hydroxyeicosatetraenoic acids (8(S)-HETE), while 15d-PGJ2 and 13-HODE activate PPARγ. Other essential fatty acid metabolites, such as 15-HETE, have been suggested to activate PPARβ/δ.
Table 1.
Ligands for PPAR subfamily
| Receptors | Endogenous ligands | Synthetic agonists | Synthetic antagonists |
|---|---|---|---|
| PPARα | LTB4 | Gemfibrozil | GW-6471 |
| 8-HETE | Fenofibrate | ||
| Ciprofibrate | |||
| Wy-14643 | |||
| PPARβ/δ | 15-HETE | GW-0742 | GSK-0660 |
| GW-501516 | GSK-3787 | ||
| L-165041 | |||
| PPARγ | 15d-PGJ2 | Rosiglitazone | GW-9662 |
| 15-HETE | Pioglitazone | ||
| 9-HODE | Troglitazone | ||
| 13-HODE | Ciglitazone |
PPARs also have synthetic ligands that have been studied for potential use as therapeutics in treating atherosclerosis and diabetes (Forman et al., 1997; Lehmann et al., 1995; Oliver et al., 2001). Unlike endogenous ligands, which are less specific and cross-reactive, synthetic ligands are highly specific agonists for each PPAR. One potent synthetic ligand for PPARα is Wy-14643. The best characterized ligands for PPARγ are members of the thiazolidinedione (TZD) group, which includes troglitazone, pioglitazone, ciglitazone, and rosiglitazone. GW-501516 is a synthetic agonist for PPARβ/δ, while GW-9662 is a specific synthetic antagonist for PPARγ. Studies focused on elucidating the effect of PPAR ligands in vitro and in vivo have been challenging and controversial because ligand-dependent and -independent mechanisms exist that can cross-react, and therefore obscure, effects induced by other PPAR family members. Nevertheless, investigation of PPAR ligands in various metabolic and inflammatory diseases provides insight into their potential therapeutic effect. For instance, rosiglitazone and pioglitazone have been approved for treating Type 2 diabetes (Gervois et al., 2007).
PPARS AND INFLAMMATION
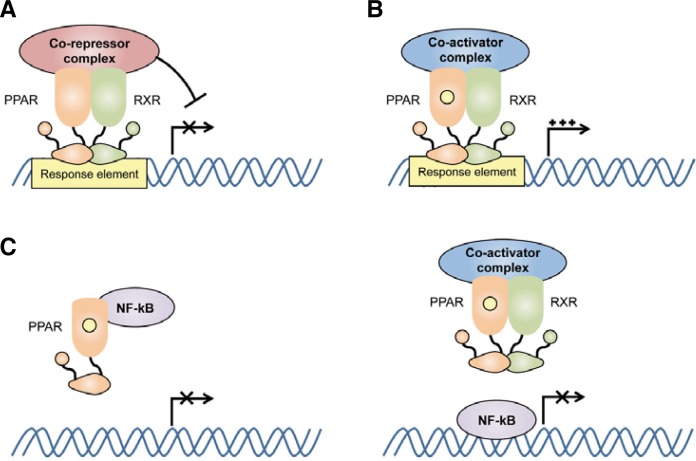
PPARs are expressed widely throughout the body with relatively low levels present in lymphoid organs (Su et al., 1999b). Previous studies demonstrated that PPARs are not only expressed in adipose tissue but also in macrophages (Jiang et al., 1998; Ricote et al., 1998), dendritic cells (Gosset et al., 2001), B cells (Setoguchi et al., 2001), and T cells (Jones et al., 2002). PPARs have been shown to play an important role in regulating inflammation mediated by nuclear factor-kappa B (NF-κB). All PPAR subfamilies exhibit anti-inflammatory effects in vitro and in vivo via several different molecular mechanisms (Fig. 2). For instance, PPARs have been shown to regulate gene expression in the presence or absence of ligand. PPARs heterodimerize with RXRs to suppress the transcription of target genes in the absence of ligand and their molecular structure has recently investigated (Chandra et al., 2008) (Fig. 2A). This mechanism involves the recruitment of co-repressor complexes such as NCoR and SMRT. Similarly, ligand-dependent transactivation has been shown to involve the recruitment of co-activator complexes that participate in assembling the transcription machinery (McKenna and O’Malley, 2002) (Fig. 2B).
Fig. 2.
Mechanism of transcriptional regulation by PPARs. PPARs heterodimerize with retinoid X receptors (RXRs) in the presence or absence of their ligands. (A) Ligand-independent inhibition of target gene transcription. PPARs bind to response elements in the absence of their ligand and recruit co-repressor complexes for transcriptional regulation. (B) Ligand-dependent activation of target gene transcription. PPARs bind to response elements in the presence of their ligand and recruit co-activator complexes for transcriptional activation. (C) Ligand-dependent inhibition of transcription factors. PPARs can inhibit other transcription factors, such as nuclear factor-kappa B (NF-κB), by direct interaction or by outcompeting them for co-activator complexes that are required for their activation.
PPARs can also modulate gene expression by influencing transcription factor activity. For example, ligand binding can lead to PPAR-mediated inhibition of NF-κB and activator protein 1 (AP-1) family members, which are critical transcription factors involved in pro-inflammatory responses (Daynes and Jones, 2002; Delerive et al., 1999; Ricote et al., 1998). PPARs can inhibit NF-κB through direct interaction as well as by outcompeting NF-κB for co-activator complexes required for its activation (Fig. 2C). PPARγ ligands have also been shown to inhibit gene expression upon TLR4 signaling by preventing NCoR turnover, which promotes NCoR-SMART-mediated repression of gene expression in macrophages (Pascual et al., 2005). This mechanism results in sumoylation of the ligand binding domain of PPARγ by protein inhibitor of activated STAT1 (PIAS1), a SUMO E3 ubiquitin ligase.
Besides their involvement in the macrophage for anti-inflammatory response, activated PPARs play an important role in the production of retinoic acid, which regulates dendritic cell function and maturation, and the induction of CD4+ T-cell anergy (Klotz et al., 2007; Szatmari et al., 2006; 2007). While pro-inflammatory cytokines inhibit PPARγ activity, interleukin (IL)-4 stimulates PPARγ interaction with STAT6 to promote gene expression in macrophages and dendritic cells (Szanto et al., 2010).
PPARS IN T CELLS AND AUTOIMMUNITY
In addition to their role in the anti-inflammatory response of innate immune cells, PPARs are involved in mediating the adaptive immune responses of T and B cells. PPARγ exists as two isoforms. PPARγ1 is expressed in most tissues while PPARγ2 is found predominantly in adipose tissue and intestine (Fajas et al., 1997). PPARγ has been studied more extensively in both mouse and human T cells than the other PPAR sub-families. PPAR expression changes with T-cell activation and proliferation following T-cell receptor (TCR) stimulation. Interestingly, PPARγ negatively regulates T-cell activation following TCR stimulation by inhibiting nuclear factor of activated T-cells (NFAT) and subsequent IL-2 production (Clark et al., 2000; Yang et al., 2000). Treatment of mouse T cells with 15d-PGJ2 or ciglitazone inhibited IL-2 production induced by CD3 stimulation (Clark et al., 2000). Likewise, human T cells showed reduced IL-2 production and proliferation upon phytohaemaglutinin (PHA) stimulation in the presence of these PPARγ ligands (Yang et al., 2000). Rosiglitazone treatment of mouse splenocytes decreased phorbol 12-myristate 13-acetate (PMA) and ionomycin-induced interferon-gamma (IFN-γ) production. Previous studies have demonstrated that PPARγ agonists such as PGJ2 or TZDs strongly inhibited T-cell activation and inflammatory disease (Desreumaux et al., 2001; Su et al., 1999a). Different colitis animal models, including dextran sulfate sodium (DSS)-induced colitis and adoptive CD4+CD25−CD45RBhigh T-cell transfer into RAG−/− mice, have indicated that PPARγ agonists can inhibit inflammatory bowl disease (IBD). Moreover, PPARγ deficiency in T cells led to increased disease susceptibility and severity (Hontecillas and Bassaganya-Riera, 2007). PPARγnull CD4+ T cells from MMTV-Cre/PPARγfl/fl mice proliferate following a recall response to ovalbumin (OVA) in OVA-immunized mice and produce IFN-γ upon TCR stimulation with increased disease susceptibility to IBD (Hontecillas and Bassaganya-Riera, 2007). PPARγ deficiency leads to decreased numbers of CD4+Foxp3+ T cells and increased CD4+IFN-γ+ cells, suggesting that PPARγ plays a role in regulatory T-cell (Treg) survival and regulation of effector T-cell functions. Similarly, T-cell-specific PPARγ-deficient mice showed reduced Treg recruitment to mesenteric lymph nodes following DSS challenge and increased expression of apoptosis-related genes (Guri et al., 2010). In addition, ciglitazone or PGE2 treatment of naïve CD4+ T cells enhanced induction of Foxp3+ inducible regulatory T cells (Baratelli et al., 2005; Wohlfert et al., 2007), suggesting that PPARγ may contribute to the quality and quantity of Treg functions in vivo.
The role of PPARγ in T-cell survival versus apoptosis remains controversial. One study demonstrated that treatment with high doses of TZDs enhanced T-cell apoptosis (Harris and Phipps, 2001). However, PPARγ activation also resulted in increased survival under conditions of cytokine withdrawal or serum starvation (Jo et al., 2006; Wang et al., 2002). Recent studies have more precisely analyzed the function of PPARγ in CD4 T-cell-targeted PPARγ-deficient mice. Expression of IL-7Rα, which is important for survival and homeostatic proliferation, in PPARγ-deficient CD4+ T cells was slightly diminished in a lymphopenic autoimmunity model, possibly suggesting a pro-survival effect in T cells (Housley et al., 2011).
Recent studies have also focused on the important role of PPARs in T-cell differentiation. T-cell-specific PPARγ−/− mice were reported to exhibit enhanced disease severity in experimental autoimmune encephalomyelitis (EAE) with increased infiltration of Th17 cells into the central nervous system (Klotz et al., 2009). Interestingly, pioglitazone treatment alleviated the disease severity of EAE with selective inhibition of RORγt and Th17, but not Th1, differentiation. Naïve CD4 T cells lacking PPARγ are also prone to Th17 differentiation in vitro, suggesting an important regulatory role for PPARγ in helper T-cell differentiation. PPARγ activation also regulates allo-reactive T-cell proliferation in vitro. This is further supported by the fact that 15d-PGJ2, ciglitazone, and pioglitazone inhibited the allo-reactive response to grafted human artery in mice by inhibiting intima formation, CD45RO+ memory T-cell infiltration, and inflammatory cytokine production (Tobiasova et al., 2011). Altogether these data demonstrate the clinical potential of PPARγ activation in regulating immune responses to autoimmune disease or graft rejection.
Another PPAR family member expressed in immune cells is PPARα, which is expressed in macrophages, granulocytes, and lymphocytes (Jones et al., 2002; Kliewer et al., 1994). The endogenous ligands for PPARα are dietary fatty acids, implying that diet can influence the immune system by activating PPARα-mediated gene expression (Varga et al., 2011). PPARα may suppress autoimmune diseases by regulating Th2 cytokine production. Studies showed that treatment with gemfibrozil inhibited the severity of EAE in mice by enhancing IL-4 production. Furthermore, ligand activation of PPARα was demonstrated to regulate the expression of IL-4- and IL-5-induced target genes in murine T cells (Gocke et al., 2009). Expression of PPARα in male T cells is greater than in females. In fact, PPARα−/− males displayed greater susceptibility to developing EAE than females, exhibiting increased production of IFN-γ, TNF-α, and IL-2, but not IL-17 (Dunn et al., 2007). These differences in autoimmune susceptibility between the genders may be explained partly by the fact that PPARα-expressing male mice are less prone to developing Th1-mediated autoimmune diseases. In addition, PPARα−/− T cells are hyper-responsive to TCR stimulation, inducing cytokine secretion and proliferation. These findings indicate the importance of PPARα in T cells as a negative regulator, especially in males. In colitis models, fenofibrate treatment inhibited IFN-γ and IL-17 production, as well as decreased disease severity in IL-10−/− mice (Lee et al., 2007). The synthetic ligand WY-14643 also showed reduced inflammatory cytokine production and disease severity in the DSS-induced colitis model (Azuma et al., 2010), suggesting that this ligand is a potential therapeutic for treating autoimmune diseases.
PPARβ/δ is the least studied subfamily of PPARs. However, recent studies showed that GW-0742, which is a specific agonist for, reduced EAE disease severity in a receptor- dependent manner (Polak et al., 2005). In addition, GW-501516 and L-165041 can inhibit EAE by reducing IFN-γ and IL-17 secretion, suggesting a possible regulatory function for PPARβ/δ in T-cell differentiation (Kanakasabai et al., 2010). The critical role of PPARβ/δ in T cells is further confirmed by the observation that PPARβ/δ−/− mice exhibited higher EAE severity than wild type mice concomitant with increased IFN-γ- and IL-17-producing CD4+ T cells (Dunn et al., 2010). PPARβ/δ−/− naïve CD4+ T cells are more prone to Th17 differentiation and hyper-responsive to TCR stimulation, as evidenced by the increased proliferation observed in these cells. Interestingly, PPARβ/δ has been hypothesized to bind the transcription factor B cell lymphoma 6 (BCL6) and repress inflammation-related gene expression (Barish et al., 2008; Takata et al., 2008; Varga et al., 2011). Collectively, these studies demonstrate the clinical importance of PPAR ligands in treating inflammatory diseases by regulating immune cells.
CONCLUSIONS
The PPAR nuclear receptors are fatty acid-activated transcription factors with diverse roles in innate and adaptive immune responses as well as lipid metabolism. Ligands for PPARs exhibit potent anti-inflammatory effects related to innate and adaptive immunity in various disease models, including IBD and EAE. However, use of PPAR ligands as therapeutics is limited by their adverse side effects (Peraza et al., 2006). Combinations of PPAR and RXR agonists may produce synergistic effects with reduced side effects (Ogawa et al., 2005). Administration of cell-permeable recombinant proteins may prove to be a promising approach to increasing PPAR activity in cells with minimal chemical toxicity (Choi et al., 2006; 2010). Recent studies suggest that PPARα, PPARβ/δ, and PPARγ play important roles in T-cell survival, activation, and differentiation into Th1 or Th17 cells, implying their therapeutic potential as drug targets for treating autoimmune diseases or graft rejection. The intrinsic role of PPARs as a regulatory molecule that determines T-cell fate has also been demonstrated. Moreover, the expression of PPAR subfamilies has been shown to differ in males and females. Further research is required to investigate how the relationship between dietary fatty acid-mediated PPAR activation and T-cell-related autoimmune diseases differs between genders.
Acknowledgments
This work is supported by Hanyang University grant (HY-2010-00000000219).
REFERENCES
- Agostini M., Schoenmakers E., Mitchell C., Szatmari I., Savage D., Smith A., Rajanayagam O., Semple R., Luan J., Bath L., et al. Non-DNA binding, dominant-negative human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T.E., Baumann C.T., Sakai S., Hager G.L., Gonzalez F.J. Selective intranuclear redistribution of PPAR iso-forms by RXR alpha. Mol. Endocrinol. 2002;16:707–721. doi: 10.1210/mend.16.4.0797. [DOI] [PubMed] [Google Scholar]
- Azuma Y.T., Nishiyama K., Matsuo Y., Kuwamura M., Morioka A., Nakajima H., Takeuchi T. PPARalpha contributes to colonic protection in mice with DSS-induced colitis. Int. Immunopharmacol. 2010;10:1261–1267. doi: 10.1016/j.intimp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Baratelli F., Lin Y., Zhu L., Yang S.C., Heuze-Vourc’h N., Zeng G., Reckamp K., Dohadwala M., Sharma S., Dubinett S.M. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Barish G.D., Atkins A.R., Downes M., Olson P., Chong L.W., Nelson M., Zou Y., Hwang H., Kang H., Curtiss L., et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl. Acad. Sci. USA. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso I., Gurnell M., Crowley V.E., Agostini M., Schwabe J.W., Soos M.A., Maslen G.L., Williams T.D., Lewis H., Schafer A.J., et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Berger J., Leibowitz M.D., Doebber T.W., Elbrecht A., Zhang B., Zhou G., Biswas C., Cullinan C.A., Hayes N.S., Li Y., et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J. Biol. Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- Chan M.M., Evans K.W., Moore A.R., Fong D. Peroxisome proliferator-activated receptor (PPAR) balance for survival in parasitic infections. J. Biomed. Biotechnol. 2010;2010:828951. doi: 10.1155/2010/828951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T.P., Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A., Repa J.J., Evans R.M., Mangelsdorf D.J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Ahn M.H., Chae W.J., Jung Y.G., Park J.C., Song H.M., Kim Y.E., Shin J.A., Park C.S., Park J.W., et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat. Med. 2006;12:574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Shin J.H., Sohn M.H., Harding M.J., Park J.H., Tobiasova Z., Kim D.Y., Maher S.E., Chae W.J., Park S.H., et al. Cell-permeable Foxp3 protein alleviates autoimmune disease associated with inflammatory bowel disease and allergic airway inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:18575–18580. doi: 10.1073/pnas.1000400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.B., Bishop-Bailey D., Estrada-Hernandez T., Hla T., Puddington L., Padula S.J. The nuclear receptor PPAR gamma and immunoregulation PPAR gamma mediates inhibition of helper T cell responses. J. Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- Daynes R.A., Jones D.C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Delerive P., De Bosscher K., Besnard S., Vanden Berghe W., Peters J.M., Gonzalez F.J., Fruchart J.C., Tedgui A., Haegeman G., Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- Desreumaux P., Dubuquoy L., Nutten S., Peuchmaur M., Englaro W., Schoonjans K., Derijard B., Desvergne B., Wahli W., Chambon P., et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 2001;193:827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S.E., Ousman S.S., Sobel R.A., Zuniga L., Baranzini S.E., Youssef S., Crowell A., Loh J., Oksenberg J., Steinman L. Peroxisome proliferator-activated receptor (PPAR) alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S.E., Bhat R., Straus D.S., Sobel R.A., Axtell R., Johnson A., Nguyen K., Mukundan L., Moshkova M., Dugas J.C., et al. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J. Exp. Med. 2010;207:1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L., Auboeuf D., Raspe E., Schoonjans K., Lefebvre A.M., Saladin R., Najib J., Laville M., Fruchart J.C., Deeb S., et al. The organization, promoter analysis and expression of the human PPARgamma gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B. M., Evans R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P., Fruchart J.C., Staels B. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:145–156. doi: 10.1038/ncpendmet0397. [DOI] [PubMed] [Google Scholar]
- Glass C.K., Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat. Rev. Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- Glass C.K., Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Gocke A.R., Hussain R.Z., Yang Y., Peng H., Weiner J., Ben L.H., Drew P.D., Stuve O., Lovett-Racke A.E., Racke M.K. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-{ alpha} agonists in autoimmune disease. J. Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosset P., Charbonnier A.S., Delerive P., Fontaine J., Staels B., Pestel J., Tonnel A.B., Trottein F. Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur. J. Immunol. 2001;31:2857–2865. doi: 10.1002/1521-4141(2001010)31:10<2857::aid-immu2857>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Guan H.P., Ishizuka T., Chui P.C., Lehrke M., Lazar M.A. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guri A.J., Mohapatra S.K., Horne W.T., 2nd, Hontecillas R., Bassaganya-Riera J. The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterol. 2010;10:60. doi: 10.1186/1471-230X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.G., Phipps R.P. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur. J. Immunol. 2001;31:1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hegele R.A., Cao H., Frankowski C., Mathews S.T., Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- Hontecillas R., Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J. Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- Housley W.J., Adams C.O., Vang A.G., Brocke S., Nichols F.C., LaCombe M., Rajan T.V., Clark R.B. Peroxisome proliferator-activated receptor gamma is required for CD4+ T cell-mediated lymphopenia-associated autoimmunity. J. Immunol. 2011;187:4161–4169. doi: 10.4049/jimmunol.1101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Fairall L., Amin K., Inaba Y., Szanto A., Balint B.L., Nagy L., Yamamoto K., Schwabe J.W. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Jo S.H., Yang C., Miao Q., Marzec M., Wasik M.A., Lu P., Wang Y.L. Peroxisome proliferator-activated receptor gamma promotes lymphocyte survival through its actions on cellular metabolic activities. J. Immunol. 2006;177:3737–3745. doi: 10.4049/jimmunol.177.6.3737. [DOI] [PubMed] [Google Scholar]
- Jones D.C., Ding X., Daynes R.A. Nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J. Biol. Chem. 2002;277:6838–6845. doi: 10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- Kanakasabai S., Chearwae W., Walline C.C., Iams W., Adams S.M., Bright J.J. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;130:572–588. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S.A., Forman B.M., Blumberg B., Ong E.S., Borgmeyer U., Mangelsdorf D.J., Umesono K., Evans R.M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S.A., Sundseth S.S., Jones S.A., Brown P.J., Wisely G.B., Koble C.S., Devchand P., Wahli W., Willson T.M., Lenhard J.M., et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L., Dani I., Edenhofer F., Nolden L., Evert B., Paul B., Kolanus W., Klockgether T., Knolle P., Diehl L. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J. Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- Klotz L., Burgdorf S., Dani I., Saijo K., Flossdorf J., Hucke S., Alferink J., Nowak N., Beyer M., Mayer G., et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Bajwa P.J., Carson M.J., Jeske D.R., Cong Y., Elson C.O., Lytle C., Straus D.S. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133:108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N.J., O’Malley B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Moras D., Gronemeyer H. The nuclear receptor ligand-binding domain structure and function. Curr. Opin. Cell Biol. 1998;10:384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lozach J., Benner C., Pascual G., Tangirala R.K., Westin S., Hoffmann A., Subramaniam S., David M., Rosenfeld M.G., et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver W.R., Jr, Shenk J.L., Snaith M.R., Russell C.S., Plunket K.D., Bodkin N.L., Lewis M.C., Winegar D.A., Sznaidman M. L., Lambert M.H., et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G., Fong A.L., Ogawa S., Gamliel A., Li A.C., Perissi V., Rose D.W., Willson T.M., Rosenfeld M.G., Glass C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza M.A., Burdick A.D., Marin H.E., Gonzalez F.J., Peters J.M. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol. Sci. 2006;90:269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- Polak P.E., Kalinin S., Dello Russo C., Gavrilyuk V., Sharp A., Peters J.M., Richardson J., Willson T.M., Weinberg G., Feinstein D.L. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Renaud J.P., Rochel N., Ruff M., Vivat V., Chambon P., Gronemeyer H., Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- Ricote M., Li A.C., Willson T.M., Kelly C.J., Glass C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Ristow M., Muller-Wieland D., Pfeiffer A., Krone W., Kahn C.R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N. Engl. J. Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- Sanderson L.M., Degenhardt T., Koppen A., Kalkhoven E., Desvergne B., Muller M., Kersten S. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 2009;29:6257–6267. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi K., Misaki Y., Terauchi Y., Yamauchi T., Kawahata K., Kadowaki T., Yamamoto K. Peroxisome proliferator-activated receptor-gamma haploinsufficiency enhances B cell proliferative responses and exacerbates experimentally induced arthritis. J. Clin. Invest. 2001;108:1667–1675. doi: 10.1172/JCI13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C.G., Wen X., Bailey S.T., Jiang W., Rangwala S.M., Keilbaugh S.A., Flanigan A., Murthy S., Lazar M.A., Wu G.D. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 1999a;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J.L., Winegar D.A., Wisely G.B., Sigel C.S., Hull-Ryde E.A. Use of a PPAR gamma-specific monoclonal antibody to demonstrate thiazolidinediones induce PPAR gamma receptor expression in vitro. Hybridoma. 1999b;18:273–280. doi: 10.1089/027245799315934. [DOI] [PubMed] [Google Scholar]
- Szanto A., Balint B.L., Nagy Z.S., Barta E., Dezso B., Pap A., Szeles L., Poliska S., Oros M., Evans R.M., et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I., Pap A., Ruhl R., Ma J.X., Illarionov P.A., Besra G.S., Rajnavolgyi E., Dezso B., Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I., Torocsik D., Agostini M., Nagy T., Gurnell M., Barta E., Chatterjee K., Nagy L. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110:3271–3280. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]
- Takata Y., Liu J., Yin F., Collins A.R., Lyon C.J., Lee C.H., Atkins A.R., Downes M., Barish G.D., Evans R.M., et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc. Natl. Acad. Sci. USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiasova Z., Zhang L., Yi T., Qin L., Manes T.D., Kulkarni S., Lorber M.I., Rodriguez F.C., Choi J.M., Tellides G., et al. Peroxisome proliferator-activated receptor-gamma agonists prevent in vivo remodeling of human artery induced by allo-reactive T cells. Circulation. 2011;124:196–205. doi: 10.1161/CIRCULATIONAHA.110.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L., Frauwirth K.A., Rangwala S.M., Lazar M.A., Thompson C.B. Thiazolidinedione activation of peroxi-some proliferator-activated receptor gamma can enhance mitochondrial potential and promote cell survival. J. Biol. Chem. 2002;277:31781–31788. doi: 10.1074/jbc.M204279200. [DOI] [PubMed] [Google Scholar]
- Wohlfert E.A., Nichols F.C., Nevius E., Clark R.B. Peroxisome proliferator-activated receptor gamma (PPAR gamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and - independent mechanisms. J. Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- Yang X.Y., Wang L.H., Chen T., Hodge D.R., Resau J.H., Da Silva L., Farrar W.L. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J. Biol. Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lovett-Racke A.E., Racke M.K. Regulation of immune responses and autoimmune encephalomyelitis by PPARs. PPAR Res. 2010;2010:104705. doi: 10.1155/2010/104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieleniak A., Wojcik M., Wozniak L.A. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch. Immunol. Ther. Exp (Warsz) 2008;56:331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]