Abstract
The activation of transglutaminase 2 (TG2), an enzyme that catalyzes post-translational modifications of proteins, has been implicated in apoptosis, cell adhesion and inflammatory responses. We previously reported that intracellular TG2 is activated under oxidative stress conditions, such as ultraviolet irradiation, ischemia-reperfusion, and hypoxia. In this study, we examined the effect of genotoxic stress on the intracellular activity of TG2 using doxorubicin which generates reactive oxygen species that lead to double-strand breakage of DNA. We demonstrated that doxorubicin elicits the persistent activation of TG2. Doxorubicin-induced TG2 activity was suppressed by treatment with caffeine at the early phase, N-acetylcysteine at the mid-phase, and EGTA at the late phase. However, treatment with a blocking antibody against TGFβ or toll-like receptor 2 showed no effect on TG2 activity, indicating that at least three different signaling pathways may be involved in the process of TG2 activation. In addition, using MEF cells defective for TG2 and cells overexpressing an activesite mutant of TG2, we revealed that doxorubicin-induced cell death is inversely correlated with TG2 activity. Our findings indicate that the persistent activation of TG2 by doxorubicin contributes to cell survival, suggesting that the mechanism-based inhibition of TG2 may be a novel strategy to prevent drug-resistance in doxorubicin treatment.
Keywords: activation, doxorubicin, intracellular activity, transglutaminase 2
INTRODUCTION
Transglutaminase 2 (TG2) is a calcium-dependent enzyme that catalyzes an acyl transfer reaction between a protein glutamyl residue and either a lysyl residue of another protein or a polyamine, thereby producing crosslinked or polyaminated proteins (Iismaa et al., 2009). TG2 activity has been known to be involved in the regulation of apoptotic, inflammatory and fibrogenic processes via the post-translational modifications of a number of proteins including caspase 3, IκB and fibronectin (Oh et al., 2011; Sohn et al., 2003; Yamaguchi and Wang, 2006).
TG2 is inactive in the intracellular environment, therefore the protein level of TG2 does not correlate with its intracellular activity (Jeon et al., 2003). We have previously reported that TG2 is activated under conditions of oxidative stress, such as ultraviolet irradiation (Shin et al., 2004), ischemia-reper-fusion (Shin et al., 2008a), and hypoxia (Jang et al., 2010). At a high level of oxidative stress, TG2 is activated through the increase of intracellular calcium, whereas at a low level of oxidative stress, reactive oxygen species (ROS) induce the activation of the TGFβ signaling pathway, which is responsible for TG2 activation (Shin et al., 2008b).
TG2 activity has been proposed to induce cell death by apoptotic stimulation through the crosslinking of cellular proteins, thereby preventing the release of intracellular proteins and DNA from the dying cells (Fesus and Szondy, 2005). However, recent studies showed that TG2 protects the cells from various stresses such as treatment with N-(4-hydroxyphenyl) retinamide or TNF-α or serum deprivation (Antonyak et al., 2003; Fesus and Szondy, 2005; Kweon et al., 2004). In addition, TG2 inhibits thapsigargin-induced apoptosis by crosslinking caspase 3 (Yamaguchi and Wang, 2006), and HIF-1-induced TG2 protects cells from hypoxia by modulating NF-κB activity (Jang et al., 2010). Therefore, the role of TG2 in apoptosis may depend on the type of cell or stress.
Doxorubicin is an anthracycline antibiotic that has been used as a first line chemotherapy for a variety of solid and hematological tumors. Doxorubicin induces cell death by intercalating between DNA base pairs resulting in the interference of strand elongation, and by inhibiting topoisomerase II and RNA polymerase leading to double-strand break and the suppression of protein synthesis (Müller et al., 1998). Doxorubicin also induces membrane and mitochondrial damage by producing complexes with iron or copper, and generating hydrogen peroxide and hydroxyl radicals (Gewirtz, 1999). In previous studies, an increased expression of TG2 has been observed in cells treated with doxorubicin: TG2 expression was up-regulated in breast cancer (MCF-7) cells and lung cancer (PC14) cells when those cancer cells that are resistant to doxorubicin were selected (Han and Park, 1999; Herman et al., 2006). In addition, epidermal growth factor (EGF), which promotes the expression of TG2, inhibited the doxorubicin-induced apoptosis in breast cancer cells (Antonyak et al., 2004). Moreover, the suppression of TG2 expression by siRNA inhibited the fibronectin-mediated cell attachment and cell survival in doxorubicin-resistant cells (Herman et al., 2006). These observations suggested that the increased protein level of TG2 plays a role in the survival of cancer cell treated with doxorubicin.
Doxorubicin generates oxygen free radicals (Gewirtz, 1999), and a previous study has shown that latent TG2 is activated in response to oxidative stress (Shin et al., 2004). Thus, we hypothesized that TG2 activation by doxorubicin may be a prerequisite for the survival of cancer cells under doxorubicin-treatment conditions. In the present study, we found that doxorubicin induces the sustained activation of TG2 through at least three different signaling pathways and that the persistent activity of TG2 is critical for the survival of doxorubicin-treated cells.
MATERIALS AND METHODS
Cell culture and treatment
Wild-type and TG2-null mouse embryonic fibroblasts (MEFs) were established using a mouse embryo on day E13.5, as follows. The mouse embryos were chopped and minced in the presence of trypsin (Invitrogen), and the cells were washed with serum-free DMEM and sub-cultured in 10% fetal bovine serum (FBS)-DMEM medium. The wild-type and TG2-null MEFs of the same passage (earlier than passage 4) were employed in this experiment. HEK293 cells that overexpress TG2 (293-TG2) and activity-defective mutant TG2 (293-C277S) were established as described previously (Jeon et al., 2003). All of the cells were cultured in DMEM containing 10% FBS and 100 units/ml penicillin and streptomycin.
To determine the doxorubicin-induced cell death and TG2 activation, all of the cells were treated with 0–1 μg/ml doxorubicin (Dong-A Pharm. Co.). To identify the mediators of doxorubicin-induced TG2 activation, the cells were pre-treated for 1 h with 1 mM of N-acetylcysteine (NAC; Sigma), 20 μM BAPTA-AM (Molecular probes), 2 mM EGTA (Amresco), 30 μg/ml transforming growth factor β (TGFβ)-neutralizing antibody (R&D Systems), 10 μg/ml toll-like receptor 2 (TLR2)-neutralizing antibody (R&D Systems) or 0–20 mM caffeine (Sigma) before the doxorubicin treatment.
Trypan blue exclusion analysis
The cultured cells were harvested by centrifugation (1,000 × g for 5 min at 4°C) and resuspended in 500 μl DMEM. Both the floating dead cells in the medium and the cells that remained attached to the plates were collected. Following the addition of trypan blue solution (0.4%, Invitrogen), the stained cells were counted using a hematocytometer. The percentage of dead cells was plotted versus the total number of cells.
In situ TG activity assay
The in situ TG activity was assayed by measuring the amount of 5-biotinamidopentylamine (BP; Pierce) that is incorporated into the proteins. Both the floating and attached cells were incubated together for 1 h with 1 mM BP and were harvested by centrifugation. Cell extracts were prepared by sonication, followed by centrifugation (14,000 × g, 10 min at 4°C). The cell extracts (10 μg/well) in coating buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 5 mM EGTA, and 5 mM EDTA) were added to each well of a 96-well microtiter plate (Nunc). The BP products were probed with horseradish peroxidase (HRP)-conjugated streptavidin (Zymed Laboratories), followed by reaction with o-phenylenediamine dihydrochloride (Sigma). The absorbance at 490 nm was measured using a microplate spectrophotometer (Molecular Devices).
Western blot analysis
The cells were lysed in a buffer containing 50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% Triton X-100 and protease inhibitor cocktail (Roche) and centrifuged at 14,000 × g for 10 min at 4°C; the protein concentration of the supernatant was determined using the BCA method. Each sample was resolved by SDS-PAGE and transferred onto nitrocellulose membranes. After treatment for 1 h with 5% skim milk in Tris-buffered saline, the membranes were incubated separately with antibodies against TG2 (Jeon et al., 2003), caspase 3 (Cell Signaling) and actin (Sigma) for 2 h. The membranes were subsequently washed, incubated with HRP-conjugated secondary antibody, and developed using a chemiluminescence substrate solution, as instructed by the manufacturer (Pierce). For the visualization of the in situ TG activity, BP-incorporated proteins were probed with HRP-conjugated streptavidin, followed by chemiluminescence detection.
Statistical analysis
Differences between two variables were assessed using an unpaired Student’s t-test, and differences between multiple variables were assessed using one-way ANOVA with Tukey’s multiple comparison test. The differences were considered significant when the p-value was < 0.05. All of the statistical calculations were performed using Prism 4.0 (GraphPad).
RESULTS
Persistent activation of intracellular TG2 by doxorubicin
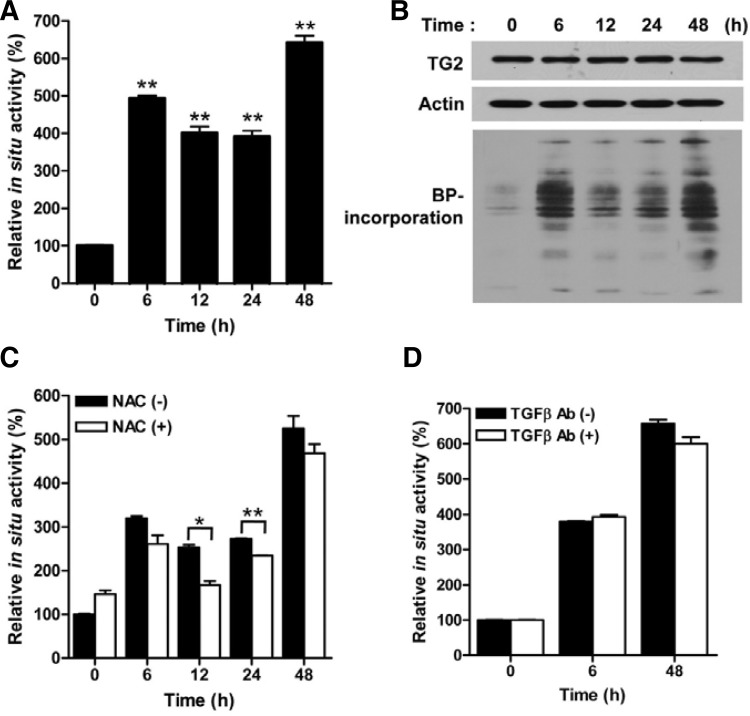
Intracellular TG2 is inactive when cells are cultured under normal physiological conditions. To determine whether TG2 could be activated by doxorubicin treatment, we monitored the intracellular TG2 activity in HeLa cells, which exhibit a high level of TG2 protein activated by extracellular stimuli (Shin et al., 2004). The HeLa cells were treated with doxorubicin, and the intracellular TG2 activity was determined by measuring the amount of BP incorporated into the cellular proteins. The TG activity was increased approximately 4 to 6 fold over a time period of 48 h and apparently peaked at 6 h and 48 h after the doxorubicin treatment (Figs. 1A and 1B). Under the same experimental conditions, however, no significant increase in the protein level of TG2 was observed (Fig. 1B), showing that doxorubicin induces the persistent activation of TG2.
Fig. 1.
Doxorubicin induces the persistent activation of intracellular TG2 in HeLa cells. (A) The intracellular TG activity of HeLa cells treated with doxorubicin (1 μg/ml) was monitored at 0, 6, 12, 24 and 48 h. (B) Western blot analysis for TG2 expression and incorporation of 5-biotinamidopentylamine to substrates (BP-incorporation) in doxorubicin (1 μg/ml) treated cells. (C, D) The intracellular TG activity was measured in HeLa cells after the exposure to doxorubicin (1 μg/ml) for the indicated times in the presence of N-acetylcysteine (NAC; 1 mM) or TGFβ-neutralizing antibody (30 μg/ml). The data represent the mean values ± standard deviations based on 3 independent experiments. *, p < 0.05; **, p < 0.01.
To understand the mechanism of the persistent activation, we determined the TG2 activation pathway in cells treated with doxorubicin. Because doxorubicin generates oxygen free radicals, and ROS are known to activate TG2 through the TGFβ signaling pathway (Shin et al., 2008b), we examined the effect of NAC on the doxorubicin-induced TG activity. When treated together with doxorubicin, NAC inhibited the TG activity only during a time range between 12 h and 24 h (Fig. 1C). In addition, the treatment with a blocking antibody against TGFβ in the culture medium showed no effect on the activity of TG2 (Fig. 1D), indicating that a signaling pathway activated by ROS other than the TGFβ pathway mediates the activation of TG2 during this time period.
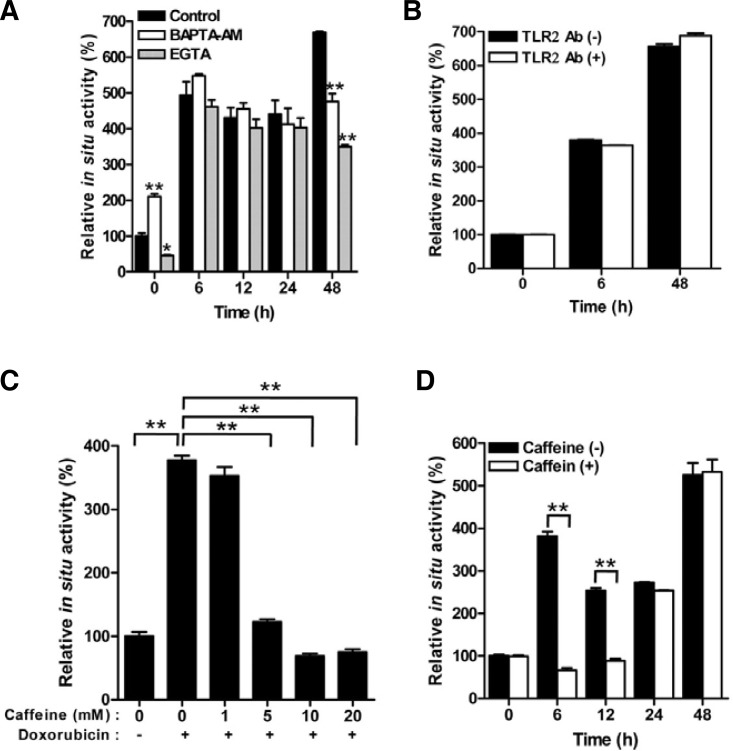
Doxorubicin inhibits calcium-ATPases, thereby leading to the depletion of calcium stores (Arai et al., 2000). Because TG2 is a calcium-dependent enzyme (Yi et al., 2006), we tested the effect of calcium chelators on the doxorubicin-induced TG activity. Both BAPTA-AM and EGTA significantly inhibited the TG activity, as measured after 48 h of doxorubicin treatment, whereas no effect of calcium chelators on the TG activity was determined at 6 h, 12 h, or 24 h (Fig. 2A). Moreover, EGTA was more effective than BAPTA-AM in the inhibition of doxorubicin-induced TG activation. These results indicate that TG2 is activated by an increase of intracellular calcium, mainly due to the influx from the medium at the late phase.
Fig. 2.
Doxorubicin-induced TG2 activation is inhibited by calcium chelators and caffeine. (A, B) The effect of BAPTA-AM (20 μM), EGTA (2 mM), or TLR2-neutralizing antibody (10 μg/ml) on TG activity after exposure to doxorubicin (1 μg/ml) was monitored in HeLa cells. (C) Dose-dependent effect of caffeine. The TG activity was measured in HeLa cells after 6 h of doxorubicin treatment (1 μg/ml). (D) Time-dependent effect of caffeine. TG activity in the presence of caffeine (10 mM) was monitored in doxorubicin (1 μg/ml)-treated HeLa cells. The data represent the mean values ± standard deviations based on 3 independent experiments. **, p < 0.01.
Becuase neither NAC, TGFβ-blocking antibody nor calcium chelators inhibited the TG activity at 6 h after the doxorubicin treatment, we evaluated other mechanisms of doxorubicin action. Doxorubicin is a natural product isolated from Streptomyces spp. and is known to be a ligand for TLR2 provoking cardiotoxicity (Nozaki et al., 2004). We tested the association between the doxorubicin-induced TG2 activity and TLR2 signaling, and found that the pretreatment with a TLR2-neutralizing antibody did not affect the TG2 activity (Fig. 2B). Doxorubicin also results in double-stranded DNA breaks through the inhibition of topoisomerase II (Müller et al., 1998). Double-stranded DNA breaks elicit the activation of phosphoinositide 3-kinase (PI3K)-related protein kinase (PIKK) family members, including ATM, ATR and DNA-PK (Lavin, 2008). We tested whether this signaling pathway may be involved in TG2 activation. When the cells were treated with caffeine, a well-known inhibitor of a number of kinases, the intracellular TG activity at 6 h after doxorubicin treatment was suppressed in a dose-dependent manner (Fig. 2C), and the inhibitory effect of caffeine lasted until 12 h after the doxorubicin treatment (Fig. 2D). Taken together, these results indicate that doxorubicin increases the TG2 activity through the sequential activation of the DNA damage response, ROS and calcium signaling pathways, leading to the persistent activation of TG2.
Suppression of doxorubicin-induced cell death by TG2 activity
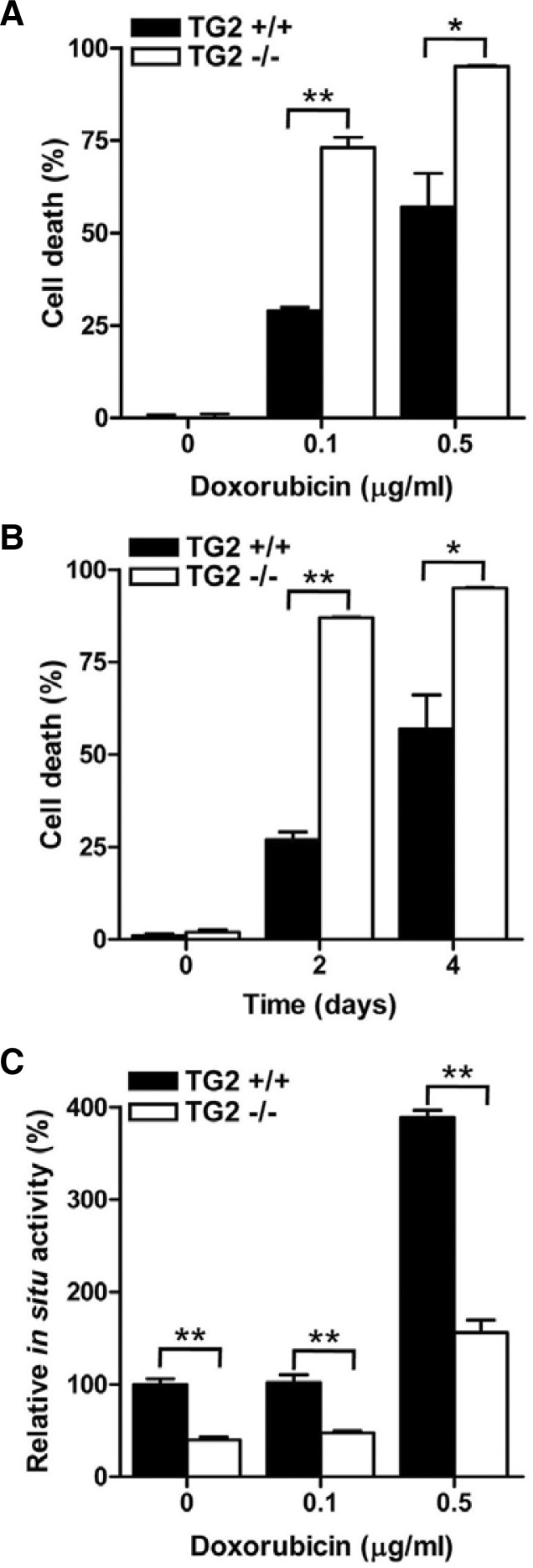
Previous studies have suggested that the increased expression of TG2 is associated with doxorubicin resistance (Antonyak et al., 2004; Han and Park, 1999; Herman et al., 2006). To confirm the relationship between the level of TG2 expression and doxorubicin resistance, we examined the cell death of TG2-deficient MEFs after doxorubicin treatment. The percentages of dead cells in the TG2-deficient MEFs were approximately increased by 2-fold compared to the wild-type MEFs at the tested doxorubicin concentrations (Fig. 3A) and at different time points (Fig. 3B). Additionally, an inverse correlation between the TG activity and cell death was observed in the MEFs (Fig. 3C).
Fig. 3.
TG2 expression promotes the survival of doxorubicin-treated MEFs. (A) Wild type (TG2+/+) and TG2-null (TG2−/−) MEFs were treated with 0.1 and 0.5 μg/ml of doxorubicin for 4 days. (B) MEFs were treated with 0.5 μg/ml doxorubicin for 2 and 4 days. The extent of cell death was evaluated by trypan blue exclusion staining. Cell death was expressed as the percentage of dead cells out of the total number of cells. (C) MEFs were treated with 0.1 and 0.5 μg/ml of doxorubicin for 24 h. Each sample was analyzed for the intracellular activity of TG2. The data represent the mean values ± standard deviations based on 3 independent experiments. *, p < 0.05; **, p < 0.01.
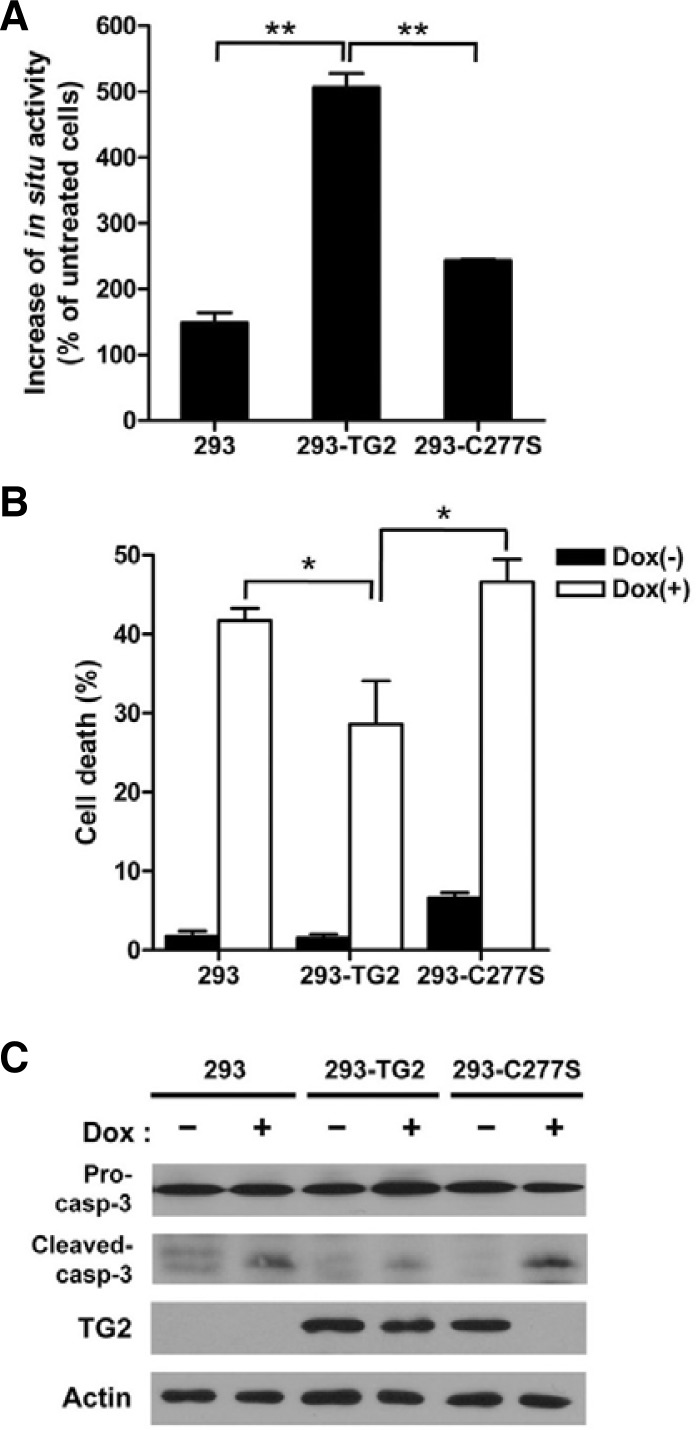
Next, we assessed the involvement of TG2 activity in the development of doxorubicin-resistance. To this end, we generated HEK293 cells overexpressing TG2 (293-TG2) and an activity-defective mutant TG2 (293-C277S) in which the cysteine residue of the active site was replaced with a serine residue. When the cells were treated with doxorubicin, the 293-TG2 cells showed approximately 5-fold increased intracellular TG activity, whereas the HEK293 and 293-C277S cells showed similar TG activities (Fig. 4A). Under the same experimental conditions, we compared the cell death between the HEK293 cells and the derivative cell lines. The percentage of dead cells in the 293-TG2 cell line was decreased compared with the HEK293 cell line. In contrast, the percentage of dead cells in the 293-C277S cell line was similar to that in the HEK293 cell line (Fig. 4B), indicating that the transamidation activity of TG2 is critical for cell survival in response to doxorubicin treatment. To confirm further the involvement of TG2 activity in cell survival, we examined the protein level of caspase 3, which can be inhibited by the TG2-mediated crosslinking reaction. Our Western blot analysis showed that the level of cleaved caspase-3 was decreased in the 293-TG2 cells compared with the HEK293 and 293-C277S cells (Fig. 4C). Interestingly, when the cells were treated with doxorubicin, the activity-defective mutant of TG2 expressed in the HEK293 cells rapidly disappeared, suggesting that the activity-defective mutant may be more susceptible to calcium-mediated ubiquitination than the wild-type TG2, as previously observed (Jeong et al., 2009). Taken together, these results indicate that doxorubicin induces the persistent activation of TG2, preventing doxorubicin-induced cell death.
Fig. 4.
TG2 activity is required for the survival of doxorubicin-treated cells. (A) HEK293 cells and HEK293 cells stably transfected with wild-type TG2 (293-TG2) or an active-site mutant of TG2 (293-C277S) were treated with doxorubicin (1 μg/ml) for 24 h. Each sample was analyzed for intracellular TG2 activity. (B) The HEK293, 293-TG2 and 293-C277S cells were treated with doxorubicin (1 μg/ml) for 48 h, and the extent of cell death was evaluated by trypan blue exclusion staining. The data represent the mean values ± standard deviations based on 3 independent experiments. (C) Cells were treated with 1 μg/ml doxorubicin for 48 h, and the TG2 expression and cleavage of caspase-3 were subsequently determined by Western blot analysis. *, p < 0.05; **, p < 0.01.
DISCUSSION
The results of the present study show that doxorubicin induces the persistent activation of TG2 through signaling pathways involving the DNA damage response, ROS and calcium, and that the transamidation activity of TG2 is required for cell survival under genotoxic stress conditions, suggesting that TG2 activation is one of the mechanisms for the development of doxorubicin resistance.
At present, the exact mechanism(s) that regulates the activation of intracellular TG2 is not fully understood. Previously, we reported that oxidative stress activates TG2 through calcium or the TGFβ signaling pathway depending on the level of stress (Shin et al., 2004; 2008b). As shown in the present study, the doxorubicin-treated cells exhibited a sustained activation of TG2. Because doxorubicin generates high level of ROS, TG2 might be activated by ROS-triggered signaling pathways. It was reported that ROS causes a rapid increase in the calcium concentration in the cytoplasm, due to both calcium release from cellular stores and calcium import from the extracellular space (Ermak and Davies, 2002). Indeed, doxorubicin induces a supramicromolar elevation of intracellular calcium, usually after 24–48 h of exposure (Tombal et al., 2002). Thus, it is possible to speculate that the ROS generated by doxorubicin increases the calcium that activates TG2. Consistently, our data showed that calcium chelators inhibited TG2 activity only after 48 h of exposure to doxorubicin. Therefore, ROS-induced calcium is responsible for the later phase of TG2 activation. However, our results revealed that the treatment with a TGFβ-neutralizing antibody had no effect on the activity of TG, whereas the NAC treatment resulted in a decrease of TG activity between 12 and 24 h after the exposure to doxorubicin. Hence, the TGFβ signaling pathway is probably not involved in the mid-phase of TG2 activation.
Doxorubicin induces double-stranded DNA breaks, which rapidly activate signaling pathways for cell-cycle control and DNA repair. In these responses, members of the PIKK family, such as ATM, ATR and DNA-PK, play principal roles through the phosphorylation of several substrates (Lavin, 2008). We showed that the inhibition of kinases with caffeine led to the suppression of activation of TG2 for up to 12 h after the exposure to doxorubicin, thereby indicating that DNA damage signaling is responsible for the early phase of TG2 activation. These findings have implications for the regulation of TG2 by phosphorylation and the role of TG2 as a downstream effector of DNA damage responses. Previous report showed that ATM, ATR and DNA-PK exhibited different sensitivities to caffeine, with the half maximal inhibitory concentration (IC50) estimated to be 0.2, 1.1, and 10 mM, respectively (Sarkaria et al., 1999). We found that caffeine inhibited the doxorubicin-induced activation of TG2 in a dose-dependent manner (Fig. 2C), and the 50% inhibition of the in situ TG activity was estimated to be approximately 4.3 mM, suggesting that ATR is most likely involved in the activation of TG2. However, in addition to the inhibition of ATM, ATR and DNA-PK, caffeine has several other effects, including the inhibition of other PIKKs, the prevention of drug binding to DNA, and the inhibition of adenosine receptors (Sabisz and Skladanowski, 2008). Therefore, the exact mechanism of TG2 activation in response to DNA damage requires further investigation.
Our results showed that TG2 is activated sequentially by DNA damage signaling, ROS and calcium in cells treated with doxorubicin. This finding is partly due to the nature of each cellular pathway. DNA damage signaling is mediated through the immediate phosphorylation of several mediator proteins. The phosphorylation of p53, which is one of markers of DNA damage signaling, was detected within 2 h after DNA damage and was usually sustained until 12 h after the damage (Kurz et al., 2004; Wittlinger et al., 2007). Conversely, the ROS generated by doxorubicin elicits a broad range of responses, ranging from the immediate oxidation of macromolecules to the late alterations of gene expression through the activation of several signaling pathways, including changes in the activity and expression of calcium channels (Dalton et al., 1999). In fact, the elevation of intracellular calcium occurred after 24–48 h of doxorubicin treatment (Tombal et al., 2002). Thus, the sequential activation of TG2 by three different mechanisms may result in its sustained activity.
The protein expression of TG2 has been associated with doxorubicin-sensitivity. TG2 was highly expressed in doxorubicin-resistant breast and lung cancer cells (Han and Park, 1999; Herman et al., 2006), and TG2 expression was correlated with cell survival factors such as NF-κB activity and the expression of BCL-2 and BCL-xL (Jang et al., 2010; Kim et al., 2009; Park et al., 2009). Although conflicting evidence has been reported on the role of TG2, our results obtained from experiments using TG2-deficient MEFs and cells transfected with an active-site mutant TG2 show that TG2 activity is required for cell survival under genotoxic stress conditions. Previous studies also support the anti-apoptotic role of TG2 activity. TG2 modifies pRB, which is resistant to caspase-induced degradation in a transamidation-dependent manner (Boehm et al., 2002). TG2 inactivates caspase 3 by a forming crosslinked polymer, thereby suppressing thapsigargin-induced cell death (Yamaguchi and Wang, 2006). However, we could not detect a crosslinked polymer of caspase 3 in our experiments (data not shown). In addition, specific inhibitors for TG2 can promote cell death and therefore enhance drug-sensitivity in the chemotherapy for glioblastomas (Yuan et al., 2005). Moreover, the calcium-binding defective mutants of TG2, which exhibit decreased TG activity, showed increased doxorubicin cytotoxicity compared to the wild-type TG2 (Datta et al., 2006). Therefore, the activation of intracellular TG2 might be one of the cytoprotective mechanisms that respond to several stress conditions.
There are seven TG isoenzymes identified in addition to TG2. The wild-type MEFs and TG2-overexpressing cells (293-TG2 cells) showed significantly higher TG activities after treatment with doxorubicin than did the TG2-deficient MEFs or minimally expressing cells (HEK293 cells; Figs. 3C and 4A), indicating that TG2 is undoubtedly activated by doxorubicin treatment and probably a major contributor to the doxorubicin-induced TG activation. Because HeLa cells express a high level of TG2 protein (Fig. 1B), TG2 is possibly responsible for a significant part of the TG activity that is increased by doxorubicin treatment. However, doxorubicin did increase the TG activity, albeit slightly, in the TG2-deficient MEFs and minimally expressing cells (Figs. 3C and 4A). Therefore, TG isoenzymes other than TG2 appear to also be involved in the increase of TG activity to some extent, which is an observation that requires further investigation.
Doxorubicin is one of the most effective agents in the treatment of several tumors; however, one of the obstacles limiting its use is drug resistance, which leads to an unsuccessful outcome in many patients (Smith et al., 2006). Therefore, an understanding of the molecular mechanisms for the development of doxorubicin resistance is an important issue for effective treatment in cancer chemotherapy. Several mechanisms have been suggested for doxorubicin resistance, including an increased drug efflux mediated by ATP-binding cassette transporters, the down-regulation of topoisomerase II, a decreased susceptibility to ROS, and the activation of protein kinase C (Tsuruo et al., 2003). In this study, we show that the persistent activation of intracellular TG2 by doxorubicin through three different signaling pathways contributes to the development of doxorubicin resistance. Therefore, the mechanism-based inhibition of intracellular TG2 activity can be a potent strategy for the effective treatment of cancers.
Acknowledgments
We thank Dr. Y.D. Kim for critical comments on the manuscript. This work was supported by grants from the National Research Foundation of Korea (2010-0014684 and 2010-0019472). J.L and J.H.L were supported by the Brain Korea 21 graduate program of the Korean Ministry of Education, Science and Technology.
REFERENCES
- Antonyak M.A., McNeill C.J., Wakshlag J.J., Boehm J.E., Cerione R.A. Activation of the Ras-ERK pathway inhibits retinoic acid-induced stimulation of tissue transglutaminase expression in NIH3T3 cells. J. Biol. Chem. 2003;278:15859–15866. doi: 10.1074/jbc.M300037200. [DOI] [PubMed] [Google Scholar]
- Antonyak M.A., Miller A.M., Jansen J.M., Boehm J.E., Balkman C.E., Wakshlag J.J., Page R.L., Cerione R.A. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J. Biol. Chem. 2004;279:41461–41467. doi: 10.1074/jbc.M404976200. [DOI] [PubMed] [Google Scholar]
- Arai M., Yoguchi A., Takizawa T., Yokoyama T., Kanda T., Kurabayashi M., Nagai R. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca2+-ATPase gene transcription. Circ. Res. 2000;86:8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- Boehm J.E., Singh U., Combs C., Antonyak M.A., Cerione R.A. Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J. Biol. Chem. 2002;277:20127–20130. doi: 10.1074/jbc.C200147200. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Shertzer H.G., Puga A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- Datta S., Antonyak M.A., Cerione R.A. Importance of Ca2+-dependent transamidation activity in the protection afforded by tissue transglutaminase against doxorubicin-induced apoptosis. Biochemistry (Mosc) 2006;45:13163–13174. doi: 10.1021/bi0606795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Fesus L., Szondy Z. Transglutaminase 2 in the balance of cell death and survival. FEBS Lett. 2005;579:3297–3302. doi: 10.1016/j.febslet.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Han J.A., Park S.C. Reduction of transglutaminase 2 expression is associated with an induction of drug sensitivity in the PC-14 human lung cancer cell line. J. Cancer Res. Clin. Oncol. 1999;125:89–95. doi: 10.1007/s004320050247. [DOI] [PubMed] [Google Scholar]
- Herman J.F., Mangala L.S., Mehta K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene. 2006;25:3049–3058. doi: 10.1038/sj.onc.1209324. [DOI] [PubMed] [Google Scholar]
- Iismaa S.E., Mearns B.M., Lorand L., Graham R.M. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol. Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- Jang G.Y., Jeon J.H., Cho S.Y., Shin D.M., Kim C.W., Jeong E.M., Bae H.C., Kim T.W., Lee S.H., Choi Y., et al. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene. 2010;29:356–367. doi: 10.1038/onc.2009.342. [DOI] [PubMed] [Google Scholar]
- Jeon J.H., Kim C.W., Shin D.M., Kim K., Cho S.Y., Kwon J.C., Choi K.H., Kang H.S., Kim I.G. Differential incorporation of biotinylated polyamines by transglutaminase 2. FEBS Lett. 2003;534:180–184. doi: 10.1016/s0014-5793(02)03836-x. [DOI] [PubMed] [Google Scholar]
- Jeong E.M., Kim C.W., Cho S.Y., Jang G.Y., Shin D.M., Jeon J.H., Kim I.G. Degradation of transglutaminase 2 by calcium-mediated ubiquitination responding to high oxidative stress. FEBS Lett. 2009;583:648–654. doi: 10.1016/j.febslet.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Kim D.S., Park K.S., Kim S.Y. Silencing of TGase 2 sensitizes breast cancer cells to apoptosis by regulation of survival factors. Front. Biosci. 2009;14:2514–2521. doi: 10.2741/3394. [DOI] [PubMed] [Google Scholar]
- Kurz E.U., Douglas P., Lees-Miller S.P. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J. Biol. Chem. 2004;279:53272–53281. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- Kweon S.M., Lee Z.W., Yi S.J., Kim Y.M., Han J.A., Paik S.G., Ha S.S. Protective role of tissue transglutaminase in the cell death induced by TNF-alpha in SH-SY5Y neuroblastoma cells. J. Biochem. Mol. Biol. 2004;37:185–191. doi: 10.5483/bmbrep.2004.37.2.185. [DOI] [PubMed] [Google Scholar]
- Lavin M.F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Müller I., Niethammer D., Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int. J. Mol. Med. 1998;1:491–494. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- Nozaki N., Shishido T., Takeishi Y., Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation. 2004;110:2869–2874. doi: 10.1161/01.CIR.0000146889.46519.27. [DOI] [PubMed] [Google Scholar]
- Oh K., Park H.B., Byoun O.J., Shin D.M., Jeong E.M., Kim Y.W., Kim Y.S., Melino G., Kim I.G., Lee D.S. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J. Exp. Med. 2011;208:1707–1719. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.S., Kim D.S., Jeong K.C., Kim S.Y. Increase in transglutaminase 2 expression is associated with NF-kappaB activation in breast cancer tissues. Front. Biosci. 2009;14:1945–1951. doi: 10.2741/3354. [DOI] [PubMed] [Google Scholar]
- Sabisz M., Skladanowski A. Modulation of cellular response to anticancer treatment by caffeine: inhibition of cell cycle checkpoints, DNA repair and more. Curr. Pharm. Biotechnol. 2008;9:325–336. doi: 10.2174/138920108785161497. [DOI] [PubMed] [Google Scholar]
- Sarkaria J.N., Busby E.C., Tibbetts R.S., Roos P., Taya Y., Karnitz L.M., Abraham R.T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Shin D.M., Jeon J.H., Kim C.W., Cho S.Y., Kwon J.C., Lee H.J., Choi K.H., Park S.C., Kim I.G. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J. Biol. Chem. 2004;279:15032–15039. doi: 10.1074/jbc.M308734200. [DOI] [PubMed] [Google Scholar]
- Shin D.M., Kang J., Ha J., Kang H.S., Park S.C., Kim I.G., Kim S.J. Cystamine prevents ischemia-reperfusion injury by inhibiting polyamination of RhoA. Biochem. Biophys. Res. Commun. 2008a;365:509–514. doi: 10.1016/j.bbrc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Shin D.M., Jeon J.H., Kim C.W., Cho S.Y., Lee H.J., Jang G.Y., Jeong E.M., Lee D.S., Kang J.H., Melino G., et al. TGFβ mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008b;22:2498–2507. doi: 10.1096/fj.07-095455. [DOI] [PubMed] [Google Scholar]
- Smith L., Watson M.B., O’Kane S.L., Drew P.J., Lind M.J., Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer. Ther. 2006;5:2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- Sohn J., Kim T.I., Yoon Y.H., Kim J.Y., Kim S.Y. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J. Clin. Invest. 2003;111:121–128. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombal B., Denmeade S.R., Gillis J.M., Isaacs J.T. A supramicromolar elevation of intracellular free calcium ([Ca2+]i) is consistently required to induce the execution phase of apoptosis. Cell Death Differ. 2002;9:561–573. doi: 10.1038/sj.cdd.4400999. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Naito M., Tomida A., Fujita N., Mashima T., Sakamoto H., Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittlinger M., Grabenbauer G.G., Sprung C.N., Sauer R., Distel L.V. Time and dose-dependent activation of p53 serine 15 phosphorylation among cell lines with different radiation sensitivity. Int. J. Radiat. Biol. 2007;83:245–257. doi: 10.1080/09553000701275432. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Wang H.G. Tissue transglutaminase serves as an inhibitor of apoptosis by cross-linking caspase 3 in thapsigargin-treated cells. Mol. Cell. Biol. 2006;26:569–579. doi: 10.1128/MCB.26.2.569-579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S.J., Kim K.H., Choi H.J., Yoo J.O., Jung H.I., Han J.A., Kim Y.M., Suh I.B., Ha K.S. [Ca2+]-dependent generation of intracellular reactive oxygen species mediates maitotoxin-induced cellular responses in human umbilical vein endothelial cells. Mol Cells. 2006;21:121–128. [PubMed] [Google Scholar]
- Yuan L., Choi K., Khosla C., Zheng X., Higashikubo R., Chicoine M.R., Rich K.M. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Mol. Cancer Ther. 2005;4:1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]