Abstract
CLCA proteins (calcium-activated chloride channel regulators) have been linked to diseases involving secretory disorders, including cystic fibrosis (CF) and asthma. They have been shown to modulate endogenous chloride conductance, possibly by acting as metalloproteases. Based on the differential processing of the subunits after post-translational cleavage, two subgroups of CLCA proteins can be distinguished. In one subgroup, both subunits are secreted, in the other group, the carboxy-terminal subunit possesses a transmembrane segment, resulting in shedding of only the amino-terminal subunit. Recent data on the post-translational cleavage and proteolytic activity of CLCA are limited to secreted CLCA. In this study, we characterized the cleavage of mCLCA6, a murine CLCA possessing a transmembrane segment. As for secreted CLCA, the cleavage in the endoplasmic reticulum was not observed for a protein with the E157Q mutation in the HEXXH motif of mCLCA6, suggesting that this mutant protein and secreted CLCA family members share a similar autoproteolytic cleavage mechanism. In contrast to secreted CLCA proteins with the E157Q mutation, the uncleaved precursor of the mCLCA6E157Q mutant reached the plasma membrane, where it was cleaved and the amino-terminal subunit was shed into the supernatant. Using crude membrane fractions, we showed that cleavage of the mCLCA6E157Q protein is zinc-dependent and sensitive to metalloprotease inhibitors, suggesting secondary cleavage by a metalloprotease. Interestingly, anchorage of mCLCA6E157Q to the plasma membrane is not essential for its secondary cleavage, because the mCLCA6Δ™E157Q mutant still underwent cleavage. Our data suggest that the processing of CLCA proteins is more complex than previously recognized.
Keywords: CLCA protein, HEXXH zinc-binding amino-acid motif, metalloprotease
INTRODUCTION
The CLCA protein family, originally termed chloride channels, calcium activated, has been shown to modulate calcium-activated chloride-conductance and thus has been proposed to play a role in diseases involving secretory dysfunction, such as cystic fibrosis (CF) (Kamada et al., 2004; Ritzka et al., 2004). The mechanism of action is still elusive, but the HEXXH amino acid motif present in CLCA proteins, typical of metalloproteases (Pawlowski et al., 2006), and the autoproteolytic activity of CLCA family members (Bothe et al., 2011) strongly suggest that these proteins function as proteases.
In each species analyzed to date, four to eight CLCA family members are predominantly expressed on the surfaces of mucous membranes and the skin (Braun et al., 2010; Loewen and Forsyth, 2005; Patel et al., 2009; Plog et al., 2009). All CLCA proteins are post-translationally cleaved into two subunits (Patel et al., 2009). CLCA proteins can be divided into phylogenetic subgroups based on whether the whole protein is secreted as a heterodimer or only the amino-terminal subunit undergoes ectodomain shedding, with the carboxy-terminal subunit remaining anchored to the plasma membrane by a transmembrane segment (Bothe et al., 2008; Elble et al., 2006; Gibson et al., 2005; Mundhenk et al., 2006). Recently, we have shown that the secreted murine mCLCA3 protein possesses zinc-dependent autoproteolytic activity (Bothe et al., 2011), supporting the hypothesis that CLCA proteins act as metalloproteases (Pawlowski et al., 2006). However, the cleavage characteristics of a transmembrane CLCA protein have not been investigated previously.
In previous cleavage studies, the introduction of the E157Q site-directed mutation in the HEXXH motif of secreted CLCA proteins prevented the cleavage of the precursor molecule of different secreted CLCA proteins (Bothe et al., 2011; Pawlowski et al., 2006). In this study, we introduced this mutation into the murine mCLCA6 protein, a transmembrane CLCA protein expressed in non-goblet cell enterocytes, and investigated the cellular processing and cellular transport of the mutant protein mCLCA6E157Q.
MATERIALS AND METHODS
Generation of truncated mCLCA6Δ™ and site-directed mutagenesis of wild-type and truncated mCLCA6
A truncated form of mCLCA6 without the transmembrane domain (named mCLCA6Δ™) was generated by PCR using 5′atg gct ttc tcc aga ggg cct gtt ttc3′ as the forward primer and 5′ga gtc cgt ctc gga ctg gga cta ctt act3′ as the reverse primer, which included a stop codon (underlined). The wild-type mCLCA6 sequence cloned into the pcDNA3.1 vector (Bothe et al., 2008) was used as the template DNA. The PCR product was cloned into pcDNA3.1 by standard T/A ligation. The E157Q mutation was introduced into mCLCA6 and mCLCA6Δ™ using the Phusion® site-directed mutagenesis protocol (Finnzymes) as previously described (Bothe et al., 2011). The plasmids encoding mCLCA6Δ™, mCLCA6E157Q and mCLCA6Δ™ E157Q were verified by sequencing.
Cell culture experiments
If not indicated otherwise, HEK293 cells were grown in 6-well dishes in DMEM supplemented with 10% (v/v) fetal calf serum at 37°C in a 5% CO2 humidified atmosphere. Cells were transiently transfected with the plasmids using TurboFect™ (Fermentas) according to the manufacturer’s protocol. Twenty-four hours after transfection, the cells were washed three times with PBS and incubated in AEM (Invitrogen) for 6 h. Supernatants and cell lysates were harvested as described previously (Bothe et al., 2011) and subjected to immunoblot analysis with αm6-N-1ap or αm6-C-1b antibody (Bothe et al., 2008). Cell lysates were incubated with Endo H or PNGase F (New England Biolabs) prior to SDS-PAGE as described previously (Bothe et al., 2008).
Surface biotinylation
Transfected cells were surface biotinylated as described by Elble et al. (2006) with minor modifications. Briefly, transfected cells were washed three times with PBS, incubated for 30 min at 4°C with 0.5 mg/ml sulfonated biotin (LHS-SS-Long Arm, Pierce), and treated with 50 mM ammonium chloride containing 0.1% bovine serum albumin to quench the reaction. Cells were scraped, lysed, and subjected to standard immunoprecipitation with High Capacity Streptavidin Agarose Resin (Pierce). Immunoprecipitated samples were analyzed by immunoblotting using the αm6-N-1ap antibody. The immature precursor of wild-type mCLCA6, which only reaches the endoplasmic reticulum, served as an internal negative control for surface-specific labeling.
Peripheral membrane protein analysis
Twenty-four hours after transfection, HEK293 cells transfected with the plasmid encoding mCLCA6E157Q and grown on a 10 cm plate were washed three times with PBS and split in two equal parts. The release of non-covalently bound protein by treatment with a solution of pH 2.5 was performed as described previously (Bothe et al., 2008; Elble et al., 2006). In brief, cells were pelleted by centrifugation; resuspended in either PBS, pH 7.5, or 0.9% NaCl equilibrated to pH 2.5 with acetic acid; and incubated for 20 min at 4°C. Cells and supernatant were separated by brief centrifugation. The supernatants were spun at 40,000 × g for 30 min at 4°C and precipitated overnight by standard ethanol precipitation. Precipitates were lysed in 40 μl standard lysis buffer and subjected to immunoblot analysis.
Membrane preparation and membrane activity assay
Membrane fractions were prepared as described previously (Bothe et al., 2011). To test the effects of various metal cations, the membrane pellet was first resuspended in 200 μl PBS and split into aliquots of 20 μl. Each aliquot was supplemented with 1 mM MgCl2 (Mg2+), 1 mM CaCl2 (Ca2+), 1 mM ZnCl2 (Zn2+) or a combination of these cations or was left untreated. To test the effects of protease inhibitors, the membrane pellet was resuspended in 100 μl PBS supplemented with 1 mM Mg2+, Ca2+ and Zn2+, split into aliquots of 20 μl and supplemented with either 1 mM EDTA (AppliChem), 1 mM EGTA (AppliChem), 1 mM 1,10-phenanthroline (AppliChem), 100 nM TPEN (Sigma-Aldrich), 1× ProteoBlock™ (100 mM AEBSF, 80 uM aprotinin, 5 mM bestatin, 1.5 mM E-64, 2 mM leupeptin, and 1 mM pepstatin A; Fermentas) or 1 mM marimastat (Merck) or left untreated. To determine the necessity of membrane anchorage for the cleavage process, one aliquot was supplemented with 1% (v/v) Triton X-100. To test the pH dependence of the cleavage process, extracted membranes were split into aliquots after the first centrifugation step, spun at 2,600 × g for 15 min and resuspended in 20 μl PBS supplemented with 1 mM Zn2+ at a pH ranging from 2.5 to 10.5. In every experiment, the final volume of each sample was adjusted to 25 μl with PBS, and samples were incubated for 6 h at 37°C, boiled in 5× Laemmli loading buffer and analyzed by immunoblotting. The ideal incubation time of 6 h was determined previously using a time course ranging from 10 min to 48 h.
RESULTS
Cleavage of mCLCA6E157Q is reduced but not absent compared to the cleavage of wild-type mCLCA6
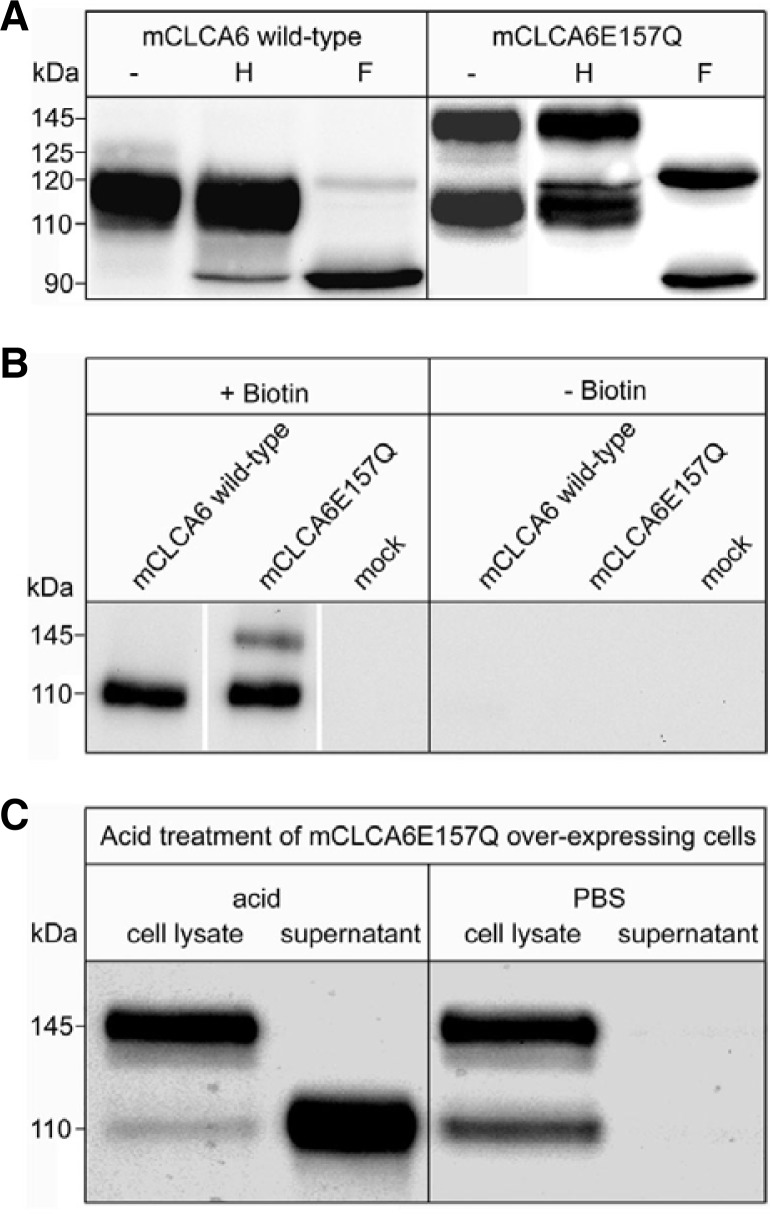
The self-cleavage of secreted CLCA proteins is inhibited in proteins with an E157Q mutation in the HEXXH zinc-binding motif (Bothe et al., 2011; Pawlowski et al., 2006). To determine whether this is also true for CLCA proteins possessing a transmembrane segment, we introduced the E157Q mutation into murine mCLCA6, an integral membrane protein (Bothe et al., 2008). Surprisingly, the level of cleavage of the mCLCA6E157Q mutant was reduced but not completely absent when expressed in HEK293 cells (Fig. 1). Antibodies directed against the amino- or the carboxy-terminal subunit of the wild-type mCLCA6 protein detected two variants of the uncleaved precursor of mCLCA6E157Q mutant: a strong band of 145 kDa, which was not detectable for the wild-type protein, and a faint band of 125 kDa, consistent with the size of the precursor of wild-type mCLCA6, as described previously (Bothe et al., 2008). The antibody directed against the amino-terminal subunit detected the amino-terminal subunit at a size of 110 kDa, whereas the antibody directed against the carboxy-terminal subunit detected several additional bands representing glycosylated forms of the carboxy-terminal subunit (Bothe et al., 2008) at approximately 35 kDa.
Fig. 1.
Reduction but not elimination of the cleavage of the precursor of the mutant protein mCLCA6E157Q. HEK293 cells were transfected with plasmids expressing wild-type mCLCA6 or the mCLCA6E157Q mutant. Cells were lysed after 24 h and analyzed by immunoblotting with antibodies directed against the aminoterminal (αm6-N-1ap) or carboxy-terminal (αm6-C-1b) subunit of the mCLCA6 protein, respectively. Asterisk (*) = immature precursor molecule.
The mCLCA6E157Q mutant is cleaved at the plasma membrane instead of the endoplasmic reticulum
In the following experiments, we analyzed the cellular transport of the mCLCA6E157Q mutant using glycosidase treatment, surface biotinylation and acid release (Fig. 2). In the deglycosylation experiments with Endo H and PNGase F, all proteins were sensitive to PNGase F but differed in their sensitivity to Endo H treatment as follows. The precursor molecule of wild-type mCLCA6, detected with the αm6-N-1ap antibody as a faint band of 125 kDa in cell lysates of transiently transfected HEK293 cells, was sensitive to Endo H, representing a high-mannose-glycan bearing (immature) protein, as shown previously (Bothe et al., 2008). The band shifted from 125 kDa to 120 kDa, where upon treatment with Endo H, it was obscured by the band of the amino-terminal subunit. The amino-terminal subunit was partially Endo H-sensitive and shifted from 110 kDa to 90 kDa. The glycosylation patterns of the immature wild-type mCLCA6 precursor and the mCLCA6 subunits indicate that the protein is cleaved in a pre-Golgi compartment, most likely the endoplasmic reticulum. In contrast, the uncleaved precursor of mCLCA6E157Q had a different response to Endo H treatment. The faint band at 125 kDa was sensitive to Endo H and shifted to 120 kDa, as did the immature precursor of wild-type mCLCA6. The strong band at 145 kDa was Endo H resistant. It shifted from 145 kDa to 120 kDa, thus representing the complex-glycosylated (mature) variant of the uncleaved precursor of mCLCA6E157Q. Interestingly, in contrast to the wild-type protein, the amino-terminal subunit of mCLCA6E157Q was exclusively detected in an Endo H-resistant form, thus representing a mature glycoprotein. This result suggests that this mutant must be cleaved in a cellular compartment after the protein has passed through the Golgi apparatus. The same glycosidase sensitivity pattern was observed after detection with the antibody directed against the carboxy-terminal subunit (data not shown).
Fig. 2.
The uncleaved mCLCA6E157Q precursor passes through the Golgi and undergoes cleavage in a post-Golgi-compartment, and the amino-terminal subunit is shed into the supernatant. (A) The lysates of HEK293 cells transfected with an expression vector encoding mCLCA6 wild-type or the mCLCA6E157Q mutant were treated with Endo H (H) or PNGase F (F) or were left untreated (−). (B) HEK293 cells transiently transfected with an expression vector encoding wild-type mCLCA6 or mCLCA6E157Q or with the pcDNA3.1 vector alone (mock) were subjected to surface biotinylation, lysed and precipitated using high-capacity streptavidin beads. Precipitates were analyzed by immunoblotting using the anti-amino-terminal mCLCA6 antibody. (C) Cells were incubated at pH 2.5 to release non-covalently associated proteins from the plasma membrane into the supernatant. Incubation with PBS served as a negative control.
To further establish the site of secondary cleavage, surface biotinylation of wild-type mCLCA6- and the mCLCA6E157Q-expressing cells was performed. After precipitation of the biotinylated surface proteins with high-capacity streptavidin agarose beads, the amino-terminal subunit was detected at a size of 110 kDa. As expected, the immature precursor molecule of the wild-type protein, which is cleaved after reaching the endoplasmic reticulum, was not detected at the cell surface, whereas the mature 145 kDa variant of the precursor of mCLCA6E157Q was detected, as was the 110 kDa amino-terminal subunit. No protein was precipitated from non-biotinylated cells. Therefore, we conclude that the mature variant of the uncleaved precursor of mCLCA6E157Q reaches the plasma membrane and is cleaved either at the plasma membrane or during endosomal recycling.
To determine whether or not the amino-terminal subunit of mCLCA6E157Q is shed into the supernatant similarly to wild-type mCLCA6 (Bothe et al., 2008), we released the peripheral proteins from the cells under acidic conditions. Proteins or subunits possessing a transmembrane segment cannot be released by low pH treatment. Accordingly, there was no release of the carboxy-terminal subunit of wild-type mCLCA6 (Bothe et al., 2008) or the carboxy-terminal subunit of the mCLCA6E157Q mutant (data not shown). The amino-terminal subunit of the mCLCA6E157Q mutant protein was released from the cells by low pH treatment, as was the amino-terminal subunit of the wild-type protein (Bothe et al., 2008). As expected, the mature precursor form of mCLCA6E157Q could not be removed by acid treatment due to its transmembrane domain. Furthermore, the amino-terminal subunit of the mCLCA6E157Q mutant protein was not released by PBS treatment, which demonstrates the specificity of the acid release.
Based on these studies, we conclude that the precursor molecule of mCLCA6E157Q is no longer cleaved in the endoplasmic reticulum. Instead, it passes through the Golgi apparatus without cleavage and is transported to the plasma membrane as a complex glycosylated, mature protein. At the plasma membrane, cleavage occurs, and the amino-terminal subunit is shed into the supernatant.
Cleavage at the plasma membrane is zinc dependent
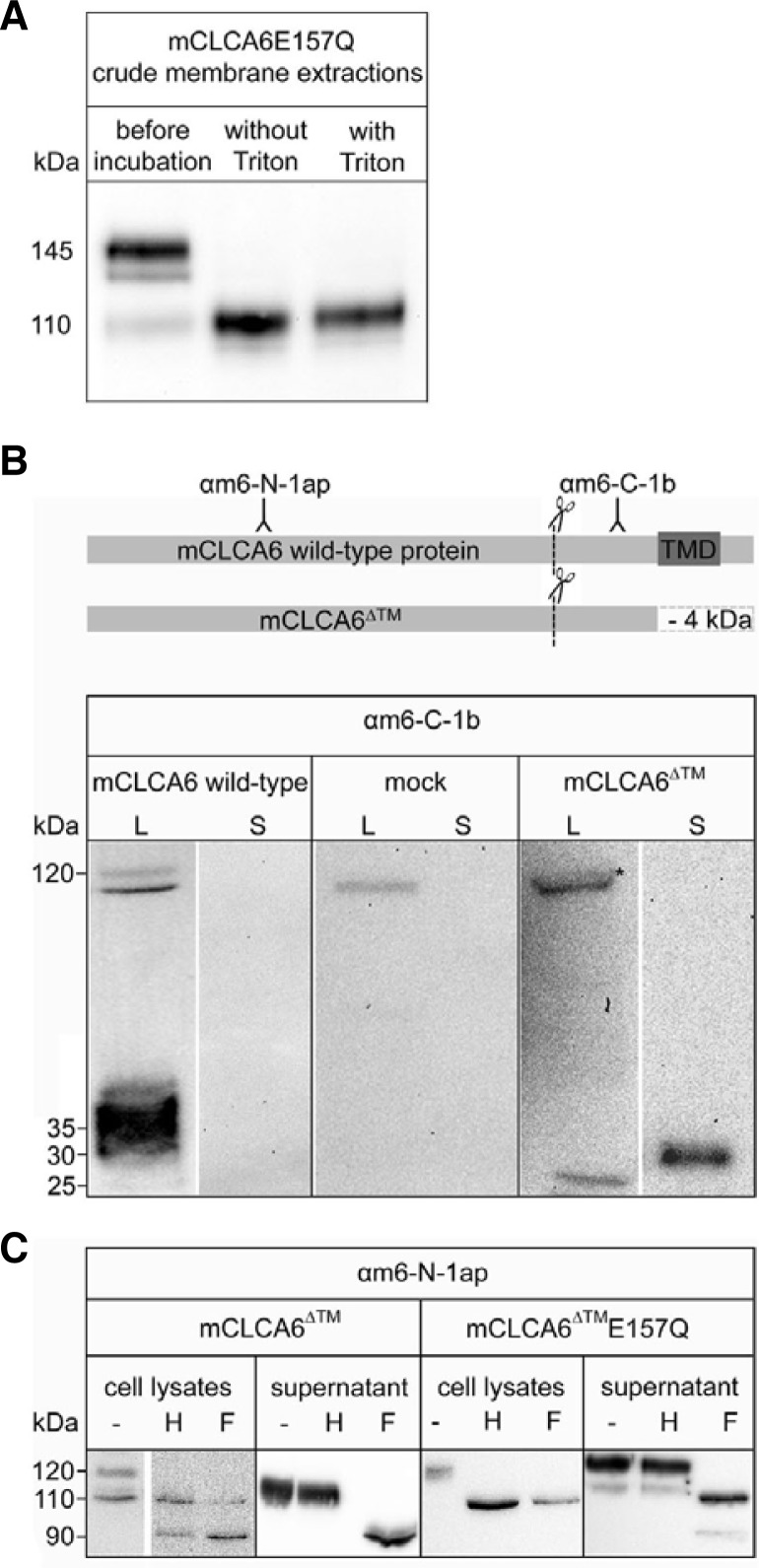
For mCLCA3, a secreted homologue of mCLCA6, the cleavage process has been shown to be zinc dependent (Bothe et al., 2011). To test the zinc dependence of the cleavage of mCLCA6 E157Q at the plasma membrane, we performed a membrane activity assay using total membrane extracts of HEK293 cells expressing mCLCA6E157Q and different metal cations. After incubation for 6 h, no significant reduction in the intensity of the mature precursor bands was observed for cells not incubated with ions relative to the band intensities before incubation (Fig. 3A). The addition of Mg2+ and Ca2+ alone or in combination did not result in significant reductions in the band intensity. In contrast, after the addition of Zn2+ alone or in combination with Mg2+ or Ca2+, the precursor band was absent. Thus, the cleavage of mCLCA6E157Q is zinc dependent, indicating either zinc-dependent autoproteolysis or the involvement of a zinc-dependent metalloprotease.
Fig. 3.
Cleavage of the mature mCLCA6E157Q precursor is zinc dependent. (A) The cleavage of the mCLCA6E157Q precursor in membrane preparations upon incubation with 1.0 mM magnesium (Mg2+), calcium (Ca2+), zinc (Zn2+) or a combination of these ions was analyzed by immunoblotting. (B) Immunoblot analysis of the cleavage of the mCLCA6E157Q precursor in membrane preparations was inhibited by various protease inhibitors in the presence of Mg2+, Ca2+ and Zn2+. Incubation in the presence of Mg2+, Ca2+ and Zn2+ without inhibitor served as a negative control.
To determine whether chelating agents such as EDTA, EGTA, 1,10 phenanthroline (Kaup et al., 2002) and TPEN (Howes et al., 2007) inhibit the cleavage of mCLCA6E157Q, we incubated the total membrane extracts in the presence of Mg2+, Ca2+ and Zn2+ with different chelating agents (Fig. 3B). After incubation without any inhibitor, the protein band representing the mature precursor was absent. The cleavage was inhibited by EDTA, EGTA, 1,10-phenanthroline and TPEN but not by ProteoBlock™, a combination of broad-spectrum protease inhibitors for serine, acidic and cysteine proteases, indicating that a zinc-dependent metalloprotease cleaves the mature precursor. Zinc-dependent metalloproteases are a large group of proteases that includes several families (Hooper, 1994). Among these families are matrix metalloproteases (MMPs), which show structural similarities to CLCA proteins (Pawlowski et al., 2006). To exclude the involvement of MMPs in the cleavage of the mature mCLCA6E157Q precursor, we tested the effect of marimastat, a broad-spectrum inhibitor of MMPs, in the membrane activity assay. Treatment with marimastat failed to inhibit the cleavage of the mCLCA6E157Q precursor (Fig. 3B).
Anchorage to the plasma membrane is not essential for the cleavage of mCLCA6E157Q
To characterize the role of anchorage to the plasma membrane in the cleavage of mCLCA6E157Q, we performed a membrane activity assay in the presence of Triton X-100, which solubilizes cell membranes. Triton X-100 had no inhibitory effect on the cleavage of the mature precursor of mCLCA6E157Q (Fig. 4A), suggesting that a correct membrane arrangement is not important for cleavage. To verify that anchorage in the plasma membrane is not essential for the cleavage of mCLCA6E157Q, we generated a truncated form of mCLCA6 (mCLCA6Δ™) that no longer bears a transmembrane domain. Transfection of HEK293 cells with an expression vector encoding the wild-type mCLCA6 protein results in the detection of the precursor (125 kDa) and the carboxy-terminal subunit (approximately 35 kDa) in the cell lysate but not in the supernatant (Fig. 4B). After transfection of HEK293 cells with an expression vector encoding the truncated mCLCA6Δ™, the precursor of mCLCA6Δ™ (approximately 120 kDa) and the carboxy-terminal subunit of mCLCA6Δ™ (approximately 25 kDa) were detected with the anti-mCLCA6 carboxy-terminal antibody. In contrast to the wild-type protein, the carboxy-terminal subunit of mCLCA6Δ™ was shed into the supernatant, where it was detected at a size of approximately 30 kDa (Fig. 4B). The mass shift of the carboxy-terminal subunit was due to mannose-rich glycosylation in the cell lysate versus complex glycosylation in the supernatant (data not shown). Deglycosylation of both the cell lysates and supernatants of cells transfected with an expression vector encoding mCLCA6Δ™ or mCLCA6Δ™E157Q revealed that in the lysates of cells expressing mCLCA6Δ™, the precursor and the amino-terminal subunit were sensitive to Endo H and PNGase F treatment (Fig. 4C). These sensitive proteins represent the immature, mannose-rich glycosylated forms that reached the endoplasmic reticulum. Only the amino-terminal subunit was shed into the supernatant, where it was Endo H resistant and PNGase F sensitive. This glycosylation pattern of the precursor and the subunit is consistent with previous findings reported for the fully secreted mCLCA3 (Mundhenk et al., 2006). In cells transfected with an expression vector encoding mCLCA6Δ™E157Q, only the precursor molecule could be detected in the cell lysate, where it was sensitive to both Endo H and PNGase F, representing a mannose-rich glycosylated protein located in the endoplasmic reticulum (Fig. 4C). Neither the amino-terminal (Fig. 4C) nor the carboxy-terminal subunit (data not shown) appeared in the cell lysate. In the supernatant, the precursor molecule was resistant to Endo H and sensitive to PNGase F treatment, indicating that the precursor molecule of mCLCA6Δ™E157Q is not cleaved during cellular transport. Instead, the precursor passes through the Golgi apparatus and is shed into the supernatant. There, an additional band, representing an Endo H-resistant and PNGase F-sensitive form of the amino-terminal subunit, was detected. The carboxy-terminal subunit of mCLCA6Δ™E157Q was also found in the supernatant as a very faint band (data not shown). This result indicates that in contrast to the secreted mCLCA3E157Q (Bothe et al., 2011), cleavage of the precursor is not eliminated in the secreted mCLCA6Δ™E157Q protein. Instead, cleavage appears to take place in the extracellular space instead of an intracellular compartment.
Fig. 4.
Anchorage of mCLCA6E157Q with a transmembrane segment to the plasma membrane is not essential for the cleavage of mCLCA6E157Q. (A) The cleavage of the mCLCA6E157Q precursor in crude membrane extracts upon incubation with 1.0 mM magnesium (Mg2+), calcium (Ca2+) and zinc (Zn2+) with or without the addition of Triton X-100 (Triton) to solubilize membranes was analyzed by immunoblotting. (B) A truncated mCLCA6 protein lacking the transmembrane domain (mCLCA6Δ™) was generated and expressed in HEK293 cells. Samples were analyzed by immunoblotting using the anti-mCLCA6-carboxy-terminal antibody αm6-C-1b. Asterisk (*) = truncated precursor of mCLCA6Δ™ (approximately 120 kDa). (C) Deglycosylation of mCLCA6Δ™ and mCLCA6Δ™ E157Q expressed by HEK293 cells in the cell lysates and in the supernatant. Samples were analyzed by immunoblotting using the anti-mCLCA6-amino-terminal antibody αm6-N-1ap.
DISCUSSION
All characterized CLCA proteins are post-translationally cleaved into two subunits (Pauli et al., 2000). All murine CLCA proteins investigated to date are cleaved in the endoplasmic reticulum (Bothe et al., 2008; 2010; Mundhenk et al., 2006). This cleavage appears to be crucial; CLCA proteins possess a HEXXH zinc-binding amino acid motif, and murine mCLCA3, a fully secreted CLCA protein of goblet cells, has recently been shown to be capable of intermolecular autoproteolysis, both indicating a possible function as metalloprotease (Bothe et al., 2011; Pawlowski et al., 2006). In this study, we investigated the cleavage of murine mCLCA6, a partially secreted CLCA protein expressed in non-goblet cell enterocytes, to compare the cleavage processes of the secreted and membrane-bound CLCA proteins.
The primary difference in terms of the cleavage of mCLCA3 and mCLCA6 is the postponed cleavage of the mCLCA6E157Q mutant protein (Fig. 5). Instead of cleavage in the endoplasmic reticulum, as occurs for wild-type mCLCA6 (Bothe et al., 2008), the mCLCA6E157Q mutant is cleaved in a post-Golgi compartment, resulting in ectodomain shedding of the aminoterminal subunit. As shown by surface biotinylation, the mature precursor molecule reaches the plasma membrane. It is therefore reasonable to assume that cleavage occurs at the plasma membrane or during endosomal recycling.
Fig. 5.
Schematic comparison of the cleavage processes of the wild-type and E157Q mutant forms of mCLCA3 and mCLCA6.
For secreted CLCA proteins, cleavage is fully inhibited for family members bearing an E157Q mutation of the HEXXH motif (Bothe et al., 2011; Pawlowski et al., 2006). Cleavage in the endoplasmic reticulum, as occurs for wild-type mCLCA6 (Bothe et al., 2008), is abolished for the mCLCA6E157Q mutant, which undergoes postponed cleavage at the plasma membrane. Similar to the autoproteolytic cleavage of mCLCA3 in the endoplasmic reticulum, cleavage of the mCLCA6E157Q mutant is also zinc dependent and is inhibited by chelating agents. The change in location of the cleavage process raises the question as to whether ectodomain shedding may be due to extant autocatalytic activity of the mCLCA6E157Q protein or whether an additional protease may cleave the protein.
Substitution of the glutamic acid (E) in the HEXXH motif with glutamine (Q) has repeatedly been shown to eliminate or at least reduce catalytic activity of the mutated protease (Cha and Auld, 1997; Fushimi et al., 1999; Li et al., 2000). Thus, we cannot fully exclude the possibility that the protein undergoes conformational changes that allow delayed self-cleavage and thus autoproteolytic ectodomain shedding at the plasma membrane.
The zinc dependence of the mCLCA6E157Q cleavage at the plasma membrane suggests the involvement of a metalloprotease in the cleavage process. Interestingly, the mutated truncated mCLCA6Δ™E157Q protein, which lacks the transmembrane segment and represents a fully secreted protein, is cleaved in the supernatant instead of an intracellular compartment. Therefore, the putative proteolytic agent is hypothesized to be present extracellularly, i.e., at the plasma membrane or in the supernatant, or, more unlikely, two distinct proteases cleave the membrane-bound mCLCA6E157Q protein and the secreted mCLCA6Δ™E157Q protein. The identification of the putative metalloprotease involved in the cleavage of the mCLCA6E157Q mutant will be the subject of future studies.
We have investigated the cleavage mechanism of murine mCLCA6, an integral transmembrane protein expressed in non-goblet cell enterocytes. After introduction of the E157Q mutation in the HEXXH motif of mCLCA6, cleavage in the endoplasmic reticulum is eliminated, as occurs for the secreted homologue mCLCA3. Instead of cleavage in the endoplasmic reticulum, the mCLCA6E157Q protein undergoes cleavage at the plasma membrane, a distinct cellular compartment. Interestingly, the transmembrane domain of mCLCA6 is not crucial for this distinct cleavage. Our data show that the proteolytic cleavage of CLCA proteins is more complex than the recently detected autoproteolysis of its homologue mCLCA3 and may be important for the function of CLCA proteins.
Acknowledgments
We thank Ursula Kobalz and Tine Schildt for excellent technical support, Wallace B. Thoreson, Department of Ophthalmology and Pharmacology, University of Nebraska, for helpful discussion and the German Research Foundation (DFG MU 3015/1-1), the Dahlem Research School and the Mukoviszidose e.V. for financial support.
REFERENCES
- Bothe M.K., Braun J., Mundhenk L., Gruber A.D. Murine mCLCA6 is an integral apical membrane protein of non-goblet cell enterocytes and co-localizes with the cystic fibrosis transmembrane conductance regulator. J. Histochem. Cytochem. 2008;56:495–509. doi: 10.1369/jhc.2008.950592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe M.K., Mundhenk L., Kaup M., Weise C., Gruber A.D. The murine goblet cell protein mCLCA3 is a zinc-dependent metalloprotease with autoproteolytic activity. Mol Cells. 2011;32:535–541. doi: 10.1007/s10059-011-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Bothe M.K., Mundhenk L., Beck C.L., Gruber A.D. Murine mCLCA5 is expressed in granular layer keratinocytes of stratified epithelia. Histochem. Cell Biol. 2010;133:285–299. doi: 10.1007/s00418-009-0667-0. [DOI] [PubMed] [Google Scholar]
- Cha J., Auld D.S. Site-directed mutagenesis of the active site glutamate in human matrilysin: investigation of its role in catalysis. Biochemistry. 1997;36:16019–16024. doi: 10.1021/bi972223g. [DOI] [PubMed] [Google Scholar]
- Elble R.C., Walia V., Cheng H.C., Connon C.J., Mundhenk L., Gruber A.D., Pauli B. U. The putative chloride channel hCLCA2 has a single C-terminal transmembrane segment. J. Biol. Chem. 2006;281:29448–29454. doi: 10.1074/jbc.M605919200. [DOI] [PubMed] [Google Scholar]
- Fushimi N., Ee C.E., Nakajima T., Ichishima E. Aspzincin, a family of metalloendopeptidases with a new zinc-binding motif. Identification of new zinc-binding sites (His(128), His(132), and Asp(164)) and three catalytically crucial residues (Glu(129), Asp(143), and Tyr(106)) of deuterolysin from Aspergillus oryzae by site-directed mutagenesis. J. Biol. Chem. 1999;274:24195–24201. doi: 10.1074/jbc.274.34.24195. [DOI] [PubMed] [Google Scholar]
- Gibson A., Lewis A.P., Affleck K., Aitken A.J., Meldrum E., Thompson N. hCLCA1 and mCLCA3 are secreted non-integral membrane proteins and therefore are not ion channels. J. Biol. Chem. 2005;280:27205–27212. doi: 10.1074/jbc.M504654200. [DOI] [PubMed] [Google Scholar]
- Hooper N.M. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- Howes J.M., Theakston R.D., Laing G.D. Neutralization of the haemorrhagic activities of viperine snake venoms and venom metalloproteinases using synthetic peptide inhibitors and chelators. Toxicon. 2007;49:734–739. doi: 10.1016/j.toxicon.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kamada F., Suzuki Y., Shao C., Tamari M., Hasegawa K., Hirota T., Shimizu M., Takahashi N., Mao X.Q., Doi S., et al. Association of the hCLCA1 gene with childhood and adult asthma. Genes Immun. 2004;5:540–547. doi: 10.1038/sj.gene.6364124. [DOI] [PubMed] [Google Scholar]
- Kaup M., Dassler K., Weise C., Fuchs H. Shedding of the transferrin receptor is mediated constitutively by an integral membrane metalloprotease sensitive to tumor necrosis factor alpha protease inhibitor-2. J. Biol. Chem. 2002;277:38494–38502. doi: 10.1074/jbc.M203461200. [DOI] [PubMed] [Google Scholar]
- Li L., Binz T., Niemann H., Singh B.R. Probing the mechanistic role of glutamate residue in the zinc-binding motif of type A botulinum neurotoxin light chain. Biochemistry. 2000;39:2399–2405. doi: 10.1021/bi992321x. [DOI] [PubMed] [Google Scholar]
- Loewen M.E., Forsyth G.W. Structure and function of CLCA proteins. Physiol. Rev. 2005;85:1061–1092. doi: 10.1152/physrev.00016.2004. [DOI] [PubMed] [Google Scholar]
- Mundhenk L., Alfalah M., Elble R.C., Pauli B.U., Naim H.Y., Gruber A.D. Both cleavage products of the mCLCA3 protein are secreted soluble proteins. J. Biol. Chem. 2006;281:30072–30080. doi: 10.1074/jbc.M606489200. [DOI] [PubMed] [Google Scholar]
- Patel A.C., Brett T.J., Holtzman M.J. The role of CLCA proteins in inflammatory airway disease. Annu. Rev. Physiol. 2009;71:425–449. doi: 10.1146/annurev.physiol.010908.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli B.U., Abdel-Ghany M., Cheng H.C., Gruber A.D., Archibald H.A., Elble R.C. Molecular characteristics and functional diversity of CLCA family members. Clin. Exp. Pharmacol. Physiol. 2000;27:901–905. doi: 10.1046/j.1440-1681.2000.03358.x. [DOI] [PubMed] [Google Scholar]
- Pawlowski K., Lepisto M., Meinander N., Sivars U., Varga M., Wieslander E. Novel conserved hydrolase domain in the CLCA family of alleged calcium-activated chloride channels. Proteins. 2006;63:424–439. doi: 10.1002/prot.20887. [DOI] [PubMed] [Google Scholar]
- Plog S., Mundhenk L., Klymiuk N., Gruber A.D. Genomic, tissue expression, and protein characterization of pCLCA1, a putative modulator of cystic fibrosis in the pig. J. Histochem. Cytochem. 2009;57:1169–1181. doi: 10.1369/jhc.2009.954594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzka M., Stanke F., Jansen S., Gruber A.D., Pusch L., Woelfl S., Veeze H.J., Halley D.J., Tummler B. The CLCA gene locus as a modulator of the gastrointestinal basic defect in cystic fibrosis. Hum. Genet. 2004;115:483–491. doi: 10.1007/s00439-004-1190-y. [DOI] [PubMed] [Google Scholar]