Abstract
The biological functions of Myc are to regulate cell growth, apoptosis, cell differentiation and stem-cell self-renewal. Abnormal accumulation of c-Myc is able to induce excessive proliferation of normal cells. von Hippel-Lindau protein (pVHL) is a key regulator of hypoxia-inducible factor1α (HIF1α), thus accumulation and hyperactivation of HIF1α is the most prominent feature of VHL-mutated renal cell carcinoma. Interestingly, the Myc pathway is reported to be activated in renal cell carcinoma even though the precise molecular mechanism still remains to be established. Here, we demonstrated that pVHL locates at the c-Myc promoter region through physical interaction with Myc. Furthermore, pVHL reinforces HDAC1/2 recruitment to the Myc promoter, which leads to the auto-suppression of Myc. Therefore, one possible mechanism of Myc auto-suppression by pVHL entails removing histone acetylation. Our study identifies a novel mechanism for pVHL-mediated negative regulation of c-Myc transcription.
Keywords: c-Myc, histone deacetylase (HDAC), renal cell carcinoma (RCC), von Hippel-Lindau (VHL)
INTRODUCTION
Renal cell carcinoma (RCC) is the major type of kidney cancer which accounts for 2–3% of malignant adult diseases (Lam et al., 2005). Moreover, 20% of clear cell RCC cases have mutations in the von Hippel-Lindau (VHL) gene or hypermethylated promoter resulting in inactivation of its function (Banks et al., 2006; Cohen, 1999). The most well-known function of pVHL is to recognize hydroxylated HIF1α, followed by proteasome-dependent degradation of HIF1α under normoxic conditions (Bruick and McKnight, 2001; Epstein et al., 2001; Ivan et al., 2001; Jaakkola et al., 2001; Maxwell et al., 1999). Under hypoxic conditions, however, HIF1α is accumulated due to lack of hydroxylation and initiates the transcription of many target genes including VEGF, PDK1 and GLUTs (Semenza, 1999; Wykoff et al., 2004). Thus, VHL diseases, caused by lack of VHL gene function, are characterized by highly vascularized and metastatic cancer, making it resistant with conventional chemotherapy (Costa and Drabkin, 2007; Kawasaki et al., 1999; Lowe, 1995).
Meanwhile, it has been questioned whether dysregulation of HIF1α alone is enough to cause RCC since stabilized HIF1α is not tumor-prone in mice (Kondo et al., 2002; Mack et al., 2003; Maranchie et al., 2002; Zimmer et al., 2004). Recently, the involvement of pVHL in cell cycle, apoptosis, differentiation and extracellular matrix assembly has been gradually revealed (Guo et al., 2009; Roe and Youn; 2006; Roe et al., 2006; 2011a; Semenza, 2006). Notably, abnormal cooperation between HIF1α and c-Myc is reported to induce metabolic switching (Dang et al., 2008).
c-Myc, a proto-oncogene, regulates 15% of all protein expression of genes covering the cell cycle, apoptosis, development and signal transduction (Cho et al., 2010; Facchini and Penn, 1998; Thompson, 1998). However, in comparison with its importance, the precise mechanism by which the negative auto-regulatory loop of c-Myc works has not been fully elucidated. Up-regulation of c-Myc is one of the prevailing phenomena in many types of cancers such as breast cancer, colon cancer and uterine cancer and thus, it is often used as a marker for metastasis (Aoyama et al., 1998; Liao and Dickson, 2000; Masramon et al., 1998). Likewise, several groups found unusual accumulation of c-Myc mRNA and protein in RCC, as well as its diverse target genes including cyclinD1, mina53 and TERT(Bindra et al., 2002; Ishizaki et al., 2007; Rohde et al., 2000; Tang et al., 2009). Even though growing evidence indicate the critical correlation between c-Myc and pVHL, the precise mechanism has not been studied so far.
Here, in this study we found that pVHL cooperates with c-Myc and induces suppression of c-Myc gene transcription. Along with c-Myc, pVHL binds to the c-Myc promoter, then exerts transcriptional repression of the c-Myc gene. Interestingly, pVHL-mediated suppression of c-Myc requires HDAC-mediated histone deacetylation. Coincidently, VHL null-type RCC4 cells show increased c-Myc gene transcription, originating from decreases in histone H3 deacetylation. Finally, we propose a novel mechanism by which pVHL maintains cellular homeostatsis through c-Myc regulation. Since a c-Myc autos-uppressive mechanism is disrupted in many cancer cell lines, introduction of pVHL could be a therapeutic strategy for treatment of cancers.
MATERIALS AND METHODS
Cells
HEK293 cells were obtained from ATCC. VHL null-type RCC4 cells were obtained from Dr. B. Bruene (Johann Wolfgang Goethe-University, Germany). RCC4/VEC and RCC4/HA-pVHL stable cells were selected using G418 (1 mg/ml) from RCC4 cells that were transiently transfected with pCDNA3.0 and pCR3-HA-VHL plasmids. Stable colonies were picked and maintained in G418 (800 μg/ml). HEK293 cells were cultured in DMEM, containing 5% (v/v) fetal bovine serum, 5% (v/v) calf serum, and 50 U/ml penicillin/streptomycin. The media for RCC4 cells were supplemented with 10% (v/v) fetal bovine serum and 1.0 mg/L ciprofloxacin (Sigma).
DNA and transfection
The expression vectors for full-length c-Myc were generated by inserting c-Myc PCR fragments into pCDNA3.0-HA and pCDNA3.0-Flag. Mammalian expression vectors for full-length or truncated pVHL mutants were generated as described previously (Roe et al., 2006). Detailed information about the DNA that was used in this study is available on request. DNA transfection was performed using Lipofectamine according to the manufacturer’s instruction (Invitrogen).
Purification of recombinant proteins
Recombinant GST-fused full-length or deletion mutants of pVHL were obtained as described previously (Roe et al., 2006).
Immunoprecipitation and immunoblotting
Cells were lysed with lysis buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% (v/v) NP-40, 1X protease inhibitor cocktail (Roche)] and cell lysates were incubated with the appropriate antibody and protein A/G beads (Santa Cruz Biotechnology). Immunoprecipitates were boiled with SDS sample buffer, loaded onto SDS-PAGE gels, transferred to nitrocellulose membranes and probed with the suitable antibodies. The following antibodies were used in this study: anti-β-actin mAb and anti-Flag mAb were purchased from Sigma; anti-HA mAb from Covance; anti-HDAC1 (ab7028) pAb from Abcam; anti-HDAC2 (51–5100) pAb from Invitrogen; anti-c-Myc (N-262) pAb, anti-IgG mAb and anti-IgG pAb from Santa Cruz Biotechnology. Rabbit polyclonal anti-pVHL antibody was generated as described previously (Roe et al., 2011).
In vivo ubiquitination analysis
HEK293 cells were transfected with different combinations of plasmids. Transfected cells were treated with 5 μM MG132 (Calbiochem) for 16 h and lysed with lysis buffer. Supernatants were immunoprecipitated with the proper antibody and washed immunoprecipitates were separated by SDS-PAGE and probed with an anti-HA antibody.
RNA purification and quantitative real-time RT-PCR (qRT-PCR)
Total RNA from cells was prepared using TRI Reagents (Molecular Research Center). For synthesis of cDNA, total RNA was reverse-transcribed with reverse transcriptase (AMV-XL reverse transcriptase, Takara) using random-hexamer (Takara). Quantitative real-time RT-PCR (qRT-PCR) was performed as described in the manufacturer’s instructions (DyNAmo HS SYBR Green qPCR Kit, Finnzymes). The accumulations of specific products in a reaction were continuously monitored by iCycler Real-Time PCR (Bio-Rad).
RNA interference
For viral-mediated RNA interference of VHL, lentiviral vector that contained VHL-targeting sequences [pLKO.1-shVHL (RHS3979-9607016)] was used. Lentiviral backbone plasmid was co-transfected with vector helper plasmids pLP1, pLP2 and pLP/VSV-G into 293FT packaging cells. As a control, pLKO.1-scrambled was used for the lentiviral backbone plasmid. These lentiviral vectors were purchased from Open Biosystems and helper plasmids were purchased from Invitrogen.
Statistical analysis
All assays were repeated at least three times and reported as means ± S.D.
RESULTS
c-Myc activity is up-regulated in RCC4 cells
Aberrant c-Myc regulation was previously reported in tissues from RCC patients (Tang et al., 2009), but whether this dysregulation coincides with cellular mRNA and protein abnormalities has not been determined. First, we generated RCC4 stable cells which harbor empty vector (RCC4/VEC) or wild type HA-pVHL (RCC4/HA-pVHL) by introducing each construct into VHL-null RCC4 cells. Successful introduction of wild type VHL was confirmed by reduced protein levels of HIF1α, along with PDK1, a well established target of HIF1α (Fig. 1A). Notably, aberrant accumulation of cellular c-Myc protein was observed in RCC4/VEC cells when it was compared to RCC4/HA-pVHL cells (Fig. 1A). By acquiring confocal microscopic images of both RCC4/VEC and RCC4/HA-pVHL, increment of c-Myc protein (red) was also observed in RCC/VEC cells, consistent with immunoblots in Fig. 1A (Fig. 1B). In particular, pVHL located in the nucleus was found to have a role in c-Myc down-regulation.
Fig. 1.
Reintroduction of pVHL into RCC4 cells down-regulates c-Myc activity (A, B). Down-regulation of c-Myc in RCC4 cells harboring pVHL, assessed by Western blot (A) and confocal microscopy (B). Both HIF-1α and PDK1 represent positive-controls for pVHL restoration in (B). (C) Transcripts of c-Myc, as well as c-Myc itself, decreased upon pVHL restoration. (D) Ectopically overexpressed pVHL into HEK293 cells leads to c-Myc down-regulation. (E) Lentiviral shRNA-mediated knockdown of pVHL in HEK293 cells increases c-Myc protein expression.
Since pVHL is a component of E3 ubiquitin ligase, we needed to test whether pVHL-mediated ubiquitination causes the down-regulation of c-Myc. However, the in vivo ubiquitination assay and cycloheximide chase experiment showed that decrease in c-Myc protein was not related to pVHL-mediated ubiquitination (Supplementary Fig. S1). Meanwhile, c-Myc mRNA was up-regulated in RCC4/VEC cells concurrent with protein increases (Fig. 1C). In addition, decreased mRNA level of various c-Myc target genes (CCND1, CCNA and CDK4) in RCC4/HA-pVHL implicated that VHL-restoration into RCC4/VEC represses abnormal transactivation of c-Myc. Next, forced expression of HA-pVHL to HEK293 cells resulted in down-regulation of endogenous c-Myc (Fig. 1D). Moreover, using c-Myc response element harboring luciferase reporters, an increasing amount of pVHL led to gradual down-regulation of c-Myc transcriptional activation (Supplementary Fig. S2). To further determine the effect of VHL on c-Myc transactivation, we knocked down V HL u sing a s hRNA l enti-virus system w h ich targets mRNA of VHL. We found that VHL knock-down induces upregulation of c-Myc protein (Fig. 1E). This result was in agreement with increased c-Myc levels in RCC4/VEC cells, indicating that pVHL might act as a negative regulator for c-Myc.
c-Myc interacts with β-domain of pVHL
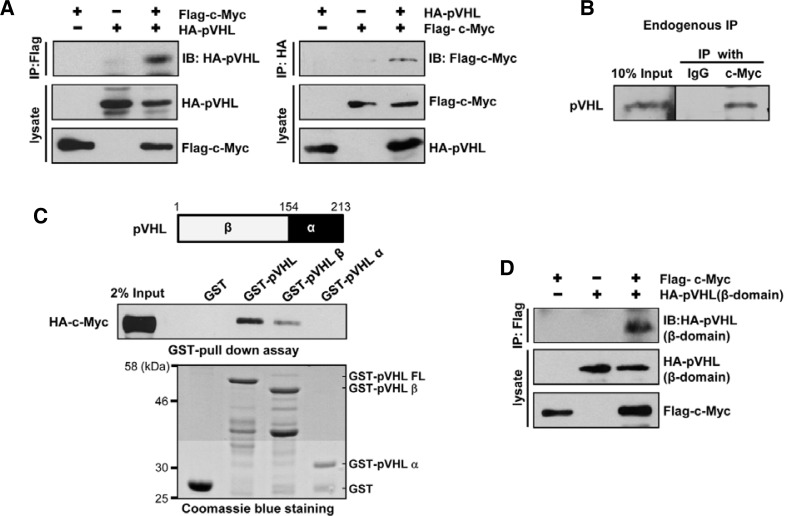
Above results raised the possibility whether pVHL may physically interact with c-Myc. To address this, we carried out a co-immunoprecipitation assay using HA-pVHL and Flag-c-Myc expressing HEK293 cell lysates and we concluded that pVHL associates with c-Myc (Fig. 2A). To verify endogenous interaction between pVHL and c-Myc, endogenous pVHL prepared from HEK293 cells was co-immunoprecipitated with anti-pVHL antibody and we successfully confirmed pVHL complex harbors c-Myc protein (Fig. 2B).
Fig. 2.
β-domain of pVHL interacts with c-Myc. (A) pVHL associates with c-Myc in vivo. Indicated proteins were expressed in HEK293 cells and subjected to immnunoprecipitation with appopriate antibodies. (B) Endogenous interaction between pVHL and c-Myc. Whole-cell lysates from HEK293 cells were immnunoprecipitated with anti-IgG rabbit or anti-c-Myc antibody. (C) GST pull-down assay shows that the β-domain of pVHL is required for interaction. (D) Truncated pVHL mutant which expresses β-domain only can interact with c-Myc.
Furthermore, bacterially purified glutathione S-transferase (GST) - fusion proteins of full length, β-domain (amino acid 1-154) and α-domain (amino acid 155-213) of pVHL were subjected to a GST-pull-down assay to determine the c-Myc interacting domain. As a result, β-domain of pVHL was found to be required for the association with c-Myc (Fig. 2C). The β-domain of pVHL is considered as indispensable region for its tumor suppressive function and also known as a substrate binding region of HIF-1α, p53 and microtubules (Hergovich et al., 2003; Roe et al., 2006). The α-domain of pVHL, however, binds directly to elongin B/C and is required for ubiquitin ligase function of pVHL. Consistent with the above result, HA-pVHL truncated mutant, α-domain of pVHL which is completely deleted from wild-type pVHL, was enough to binds to Flag-c-Myc (Fig. 2D).
pVHL auto-suppresses c-Myc transcription through binding to the c-Myc promoter
Several groups found that irregular amounts of c-Myc protein cause down-regulation of endogenous c-Myc transcripts by binding to its own promoter (Goodliffe et al., 2005; Kim and Carroll, 2004; Luo et al., 2004; Sivak et al., 1997). This negative auto-regulation of c-Myc is considered as an important feedback circuit keeping cellular homeostasis (Facchini et al., 1997; Penn et al., 1990). Therefore, trans-acting factors are speculated to take part in c-Myc auto-regulation, but the critical factor has not been identified yet. Therefore, we tested whether pVHL plays a role in c-Myc auto-suppression by binding to the c-Myc promoter, thus reducing its transcription as a result.
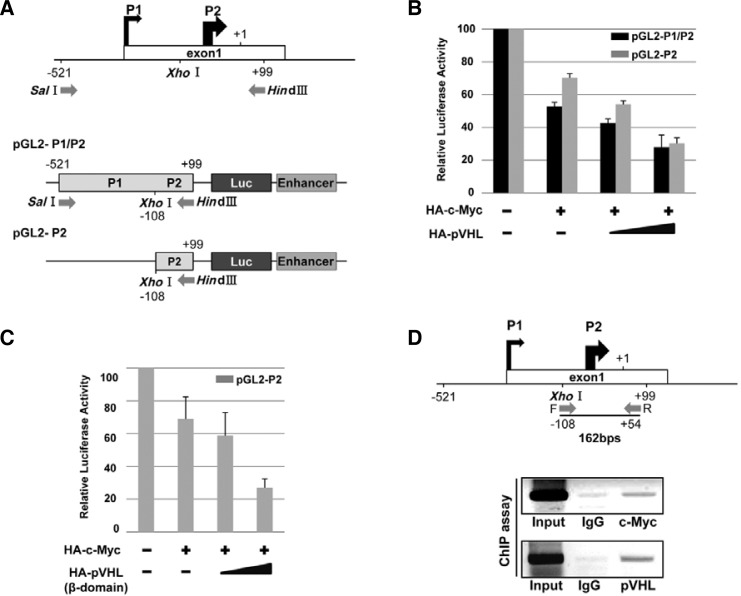
To evaluate whether pVHL represses c-Myc transcription, the pGL2-luciferase reporters containing P1/P2 or P1 promoter regions of c-Myc were generated and transfected into HEK293 cells to measure c-Myc transcriptional activity (Fig. 3A). It has been reported that P1 and P2 promoters account for 10%–25% and 75–90%, respectively of total c-Myc transcripts among well-established promoter regions of c-Myc from the P0 to P3 promoter (Wierstra and Alves, 2008). Moreover, the P2 promoter has been known to contain direct c-Myc protein binding regions (Penn et al., 1990). Given this information, we postulated that the cooperation of c-Myc with pVHL may down-regulate c-Myc transcripts by binding to the P2 promoter. We found that c-Myc-mediated auto-suppression at the P1/P2 promoter was gradually accelerated by an increasing amount of transfected pVHL (Fig. 3B). Besides, the P2 promoter region of c-Myc was sufficient for pVHL-mediated c-Myc regulation. Coincident with our previous result that the β-domain of pVHL is required for the association with c-Myc, the β-domain of pVHL alone is sufficient to down-regulate luciferase activity on the P2 minimal promoter (Fig. 3C). Next, we investigated whether endogenous pVHL binds to the c-Myc promoter using a chromatin-immunoprecipiation (ChIP) assay (Fig. 3D). We observed that pVHL as well as c-Myc binds to the P2 promoter. Taken together, we suggested that pVHL along with c-Myc directly binds to the c-Myc promoter and takes part in the auto-suppression of c-Myc transcription.
Fig. 3.
Transcriptional repression of c-Myc resulted from chromatin-bound pVHL. (A) Construction of luciferase reporter plasmids for c-Myc promoter. (B) c-Myc transcription activity is suppressed by pVHL as shown in the c-Myc promoter. (C) β-domain of pVHL is enough to suppress c-Myc transcription. (D) Chromatin bound c-Myc and pVHL for the c-Myc promoter, as assessed by chromatin immunoprecipitation (ChIP) assay using indicated antibodie
pVHL is involved in HDAC1/2-mediated repression of c-Myc on its promoter
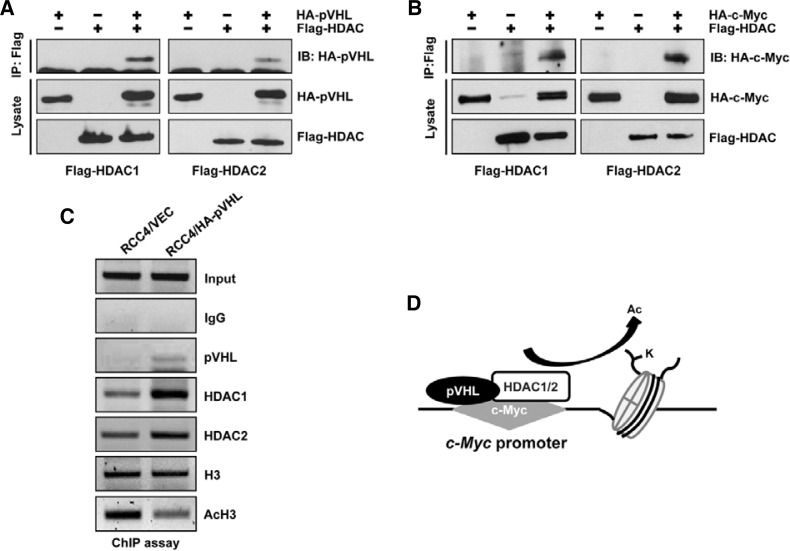
It has been reported that pVHL functions as a component of the transcriptional co-repressor complex that inhibits HIF-1α transactivation by recruiting HDACs and pVHL which strengthen the interaction between HDACs with HIF-1α (Mahon et al., 2001). We could also confirm the association of pVHL with HDAC 1/2 (Fig. 4A). In addition, it has been shown that HDACs associate with c-Myc, thus mediating the repression of c-Myc activity (Kim and Carroll, 2004; Kurland and Tansey, 2008; Marshall et al., 2010; Satou et al., 2001). Therefore, we hypothesized that HDACs are indispensable for pVHL-mediated repressive function on the c-Myc promoter. To test this hypothesis, we confirmed the association between HDACs and c-Myc using the immunoprecipitation assay (Fig. 4B). These results raised the possibility whether pVHL may affect histone acetylation status on the c-Myc promoter in VHL-null RCC4 or HA-pVHL restored RCC4 cells. Interestingly, ChIP assays demonstrated pVHL occupancy binding to the c-Myc promoter, together with HDAC1 and HDAC2 (Fig. 4C). Importantly, restoration of pVHL significantly down-regulated acetylated histone H3 association with the c-Myc promoter, where there was an approximately 60% decrease in the amount (Fig. 4C). A clear decrease in histone H3 acetylation on the c-Myc promoter in RCC4/HA-pVHL cells supports our idea that recruitment of HDACs by pVHL plays an important role in negative auto-regulation of c-Myc (Fig. 4D).
Fig. 4.
Auto-suppression of c-Myc requires HDAC1/2 recruitment by pVHL. (A, B) pVHL (A) and c-Myc (B) associate with HDAC1/2. (C) ChIP assays shows pVHL enhances HDAC1/2 recruitment to c-Myc, along with pVHL induced deacetylation of histone H3 bound to the c-Myc promoter. (D) Schematic model for pVHL containing c-Myc negative feedback regulation.
DISCUSSION
The proto-oncogene c-Myc functions as a pivotal transcription factor to regulate metabolism, apoptosis, cell cycle regulation, differentiation, cell adhesion and tumorigenesis (Facchini and Penn, 1998; Thompson, 1998). Transcription of c-Myc is auto-suppressed by binding of c-Myc to its own promoter. However, its molecular mechanism is still unclear. It has been shown that c-Myc and its regulated genes are dysregulated in pVHL-mutated or deleted renal cell carcinomas (Bindra et al., 2002; Ishizaki et al., 2007; Rohde et al., 2000; Tang et al., 2009). This fact attempted us to study the relationship of pVHL with c-Myc. Here, we demonstrated the association of c-Myc with pVHL. Moreover, pVHL also associates with HDAC1/2 together with c-Myc and works as a co-repressor for c-Myc auto-suppression.
Recently, it has been suggested that pVHL can generate renal cell carcinoma independently of HIF1α (Roe and Youn, 2006; Roe et al., 2006; 2011b; Semenza, 2006). Even though pVHL usually is located in both cytoplasm and nucleus, the nuclear form of pVHL showed the anti-tumor properties (Lewis and Roberts, 2003). Previously, we showed that pVHL interacts and forms stable complexes with p53/p300/ATM that respond to DNA damage, leading to transcriptional activation of p53 (Roe et al., 2006). In addition, we recently reported that DNA-damage dependent phosphorylation of pVHL by checkpoint kinase-2 (Chk2) is a prerequisite for p53 regulation to cope with genotoxic stress (Roe et al., 2011b). Thus, nuclear function of pVHL is thought to be another aspect of tumor suppression. Therefore, our present study which showed that the auto-suppression of c-Myc was augmented by chromatin associated pVHL-HDACs providing a rationale for revealing a novel nuclear function of pVHL.
Since the auto-regulatory feedback mechanism of c-Myc is disrupted in many cancer cell lines including renal cell carcinoma, it is important to unveil precise molecular mechanisms underlying the c-Myc feedback loop. For example, MM1-mediated recruitment of a transcriptional co-repressor complex is thought to be important for c-Myc inhibition (Satou et al., 2001). Recently miR-185-3p, a novel c-Myc target gene, was identified as an auto-suppressive miRNA which can directly inhibit c-Myc protein translation (Liao and Lu, 2011). Together with the above reports, our results showing that the pVHL containing co-repressor complex is involved in in maintaining the proper level of c-Myc expression could broaden the existing paradigm for c-Myc regulation. Even though we cannot exclude the possibility that the other oncogenic factors might cooperate with c-Myc and lead to the development of cancer, targeting pVHL could be a powerful approach for renal cancer treatment, given the essential roles for c-Myc in ccRCC proliferation.
Acknowledgments
This work was supported by National Research Foundation (NRF) grant (KRF-C00257) to E. J. Cho.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Aoyama C., Peters J., Senadheera S., Liu P., Shimada H. Uterine cervical dysplasia and cancer: identification of c-myc status by quantitative polymerase chain reaction. Diagn. Mol. Pathol. 1998;7:324–330. doi: 10.1097/00019606-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Banks R.E., Tirukonda P., Taylor C, Hornigold N., Astuti D., Cohen D., Maher E.R., Stanley A.J., Harnden P., Joyce A., et al. Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res. 2006;66:2000–2011. doi: 10.1158/0008-5472.CAN-05-3074. [DOI] [PubMed] [Google Scholar]
- Bindra R.S., Vasselli J.R., Stearman R., Linehan W.M., Klausner R.D. VHL-mediated hypoxia regulation of cyclin D1 in renal carcinoma cells. Cancer Res. 2002;62:3014–3019. [PubMed] [Google Scholar]
- Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Cho H.J., Oh Y.J., Kwon J., Kwon J.Y., Kim K.S., Kim H. c-Myc stimulates cell invasion by inhibiting FBX8 function. Mol. Cells. 2010;30:355–362. doi: 10.1007/s10059-010-0134-8. [DOI] [PubMed] [Google Scholar]
- Cohen H.T. Advances in the molecular basis of renal neoplasia. Curr. Opin. Nephrol. Hypertens. 1999;8:325–331. doi: 10.1097/00041552-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Costa L.J., Drabkin H.A. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist. 2007;12:1404–1415. doi: 10.1634/theoncologist.12-12-1404. [DOI] [PubMed] [Google Scholar]
- Dang C.V., Kim J.W., Gao P., Yustein J. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O’Rourke J., Mole D.R., Mukherji M., Metzen E., Wilson M.I., Dhanda A., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Facchini L.M., Chen S., Marhin W., Lear J.N., Penn L.Z. The Myc negative autoregulation mechanism requires Myc-Max association and involves the c-myc P2 minimal promoter. Mol. Cell. Biol. 1997;17:100–114. doi: 10.1128/mcb.17.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini L.M., Penn L.Z. The molecular role of Myc in growth and transformation: recent discoveries lead to new in-sights. FASEB J. 1998;12:633–651. [PubMed] [Google Scholar]
- Goodliffe J.M., Wieschaus E., Cole M.D. Polycomb mediates Myc autorepression and its transcriptional control of many loci in Drosophila. Genes Dev. 2005;19:2941–2946. doi: 10.1101/gad.1352305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Schoell M.C., Freeman R.S. The von Hippel-Lindau protein sensitizes renal carcinoma cells to apoptotic stimuli through stabilization of BIM (EL) Oncogene. 2009;28:1864–1874. doi: 10.1038/onc.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A., Lisztwan J., Barry R., Ballschmieter P., Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- Ishizaki H., Yano H., Tsuneoka M., Ogasawara S., Akiba J., Nishida N., Kojiro S., Fukahori S., Moriya F., Matsuoka K., et al. Overexpression of the myc target gene Mina53 in advanced renal cell carcinoma. Pathol. Int. 2007;57:672–680. doi: 10.1111/j.1440-1827.2007.02156.x. [DOI] [PubMed] [Google Scholar]
- Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J.M., Lane W.S., Kaelin W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Bilim V., Takahashi K., Tomita Y. Infrequent alteration of p53 pathway in metastatic renal cell carcinoma. Oncol. Rep. 1999;6:329–333. doi: 10.3892/or.6.2.329. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Carroll W.L. Autoregulation of the N-myc gene is operative in neuroblastoma and involves histone deacetylase 2. Cancer. 2004;101:2106–2115. doi: 10.1002/cncr.20626. [DOI] [PubMed] [Google Scholar]
- Kondo K., Klco J., Nakamura E., Lechpammer M., Kaelin W.G. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Kurland J.F., Tansey W.P. Myc-mediated transcriptional repression by recruitment of histone deacetylase. Cancer Res. 2008;68:3624–3629. doi: 10.1158/0008-5472.CAN-07-6552. [DOI] [PubMed] [Google Scholar]
- Lam J.S., Leppert J.T., Figlin R.A., Belldegrun A.S. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology. 2005;66:1–9. doi: 10.1016/j.urology.2005.06.112. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Roberts B.J. Role of nuclear and cytoplasmic localization in the tumour-suppressor activity of the von Hippel-Lindau protein. Oncogene. 2003;22:3992–3997. doi: 10.1038/sj.onc.1206683. [DOI] [PubMed] [Google Scholar]
- Liao D.J., Dickson R.B. c-Myc in breast cancer. Endocr. Relat. Cancer. 2000;7:143–164. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- Liao J.M., Lu H. Auto-regulatory suppression of c-Myc by miR-185-3p. J. Biol. Chem. 2011;286:33901–33909. doi: 10.1074/jbc.M111.262030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S.W. Cancer therapy and p53. Curr. Opin. Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Luo Q., Li J., Cenkci B., Kretzner L. Autorepression of c-myc requires both initiator and E2F-binding site elements and cooperation with the p107 gene product. Oncogene. 2004;23:1088–1097. doi: 10.1038/sj.onc.1207225. [DOI] [PubMed] [Google Scholar]
- Mack F.A., Rathmell W.K., Arsham A.M., Gnarra J., Keith B., Simon M.C. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranchie J.K., Vasselli R., Riss J., Bonifacino J.S., Linehan W.M., Klausner R.D. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Marshall G.M., Gherardi S.N., Xu N., Neiron Z., Trahair T., Scarlett C.J., Chang D.K., Liu P.Y., Jankowski K., Iraci N., et al. Transcriptional upregulation of histone deacetylase 2 promotes Myc-induced oncogenic effects. Oncogene. 2010;29:5957–5968. doi: 10.1038/onc.2010.332. [DOI] [PubMed] [Google Scholar]
- Masramon L., Arribas R., Tórtola S., Perucho M., Peinado M.A. Moderate amplifications of the c-myc gene correlate with molecular and clinicopathological parameters in colorectal cancer. Br. J. Cancer. 1998;77:2349–2356. doi: 10.1038/bjc.1998.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., Wykoff C.C., Pugh C.W., Maher E.R., et al. The tumor suppressor protein VHL targets hypoxiainducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Penn L.J., Brooks M.W., Laufer E.M., Land H. Negative autoregulation of c-myc transcription. EMBO J. 1990;9:1113–1121. doi: 10.1002/j.1460-2075.1990.tb08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J.S., Youn H.D. The positive regulation of p53 by the tumor suppressor VHL. Cell Cycle. 2006;5:2054–2056. doi: 10.4161/cc.5.18.3247. [DOI] [PubMed] [Google Scholar]
- Roe J.S., Kim H.S., Lee S.M., Kim S.T., Cho E.J., Youn H.D. p53 stabilization and transactivation by a von Hippel-Lindau protein. Mol. Cell. 2006;22:395–405. doi: 10.1016/j.molcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Roe J.S., Kim H.R., Hwang I.Y., Cho E.J., Youn H.D. von Hippel-Lindau protein promotes Skp2 destabilization on DNA damage. Oncogene. 2011a;30:3127–3138. doi: 10.1038/onc.2011.40. [DOI] [PubMed] [Google Scholar]
- Roe J.S., Kim H.R., Hwang I.Y., Ha N.C., Kim S.T., Cho E.J., Youn H.D. Phosphorylation of von Hippel-Lindau protein bycheckpoint kinase 2 regulates p53 transactivation. Cell Cycle. 2011b;10:3920–3928. doi: 10.4161/cc.10.22.18096. [DOI] [PubMed] [Google Scholar]
- Rohde V., Sattler H.P., Bund T., Bonkhoff H., Fixemer T., Bachmann C., Lensch R., Unteregger G., Stoeckle M., Wullich B. Expression of the human telomerase reverse transcriptase is not related to telomerase activity in normal and malignant renal tissue. Clin. Cancer Res. 2000;6:4803–4809. [PubMed] [Google Scholar]
- Satou A., Taira T., Iguchi-Ariga S.M., Ariga H. A novel transrepression pathway of c-Myc. Recruitment of a transcriptional corepressor complex to c-Myc by MM-1, a c-Myc-binding protein. J. Biol. Chem. 2001;276:46562–46567. doi: 10.1074/jbc.M104937200. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Ann. Rev. Cell Dev. Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. VHL and p53: tumor suppressors team up to prevent cancer. Mol. Cell. 2006;22:437–439. doi: 10.1016/j.molcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sivak L.E., Tai K.F., Smith R.S., Dillon P.A., Brodeur G.M., Carroll W.L. Autoregulation of the human N-myc oncogene is disrupted in amplified but not single-copy neuroblastoma cell lines. Oncogene. 1997;15:1937–1946. doi: 10.1038/sj.onc.1201363. [DOI] [PubMed] [Google Scholar]
- Tang S.W., Chang W.H., Su Y.C., Chen Y.C., Lai Y.H., Wu P.T., Hsu C.I., Lin W.C., Lai M.K., Lin J.Y. MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett. 2009;273:35–43. doi: 10.1016/j.canlet.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Thompson E.B. The many roles of c-Myc in apoptosis. Annu. Rev. Physiol. 1998;60:575–600. doi: 10.1146/annurev.physiol.60.1.575. [DOI] [PubMed] [Google Scholar]
- Wierstra I., Alves J. The c-myc promoter: still MysterY and challenge. Adv. Cancer Res. 2008;99:113–333. doi: 10.1016/S0065-230X(07)99004-1. [DOI] [PubMed] [Google Scholar]
- Wykoff C.C., Sotiriou C., Cockman M.E., Ratcliffe P.J., Maxwell P., Liu E., Harris A.L. Gene array of VHL mutation and hypoxia shows novel hypoxia-induced genes and that cyclin D1 is a VHL target gene. Br. J. Cancer. 2004;90:1235–1243. doi: 10.1038/sj.bjc.6601657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Doucette D., Iliopoulos N., Siddiqui O. Inhibition of hypoxia-inducible factor is sufficient for growth suppression of VHL−/− tumors. Mol. Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]