Abstract
We recently observed that lipoteichoic acid (LTA) isolated from Lactobacillus plantarum inhibited endotoxin-mediated inflammation of the immune cells and septic shock in a mouse model. Here, we examined the inhibitory role of L. plantarum LTA (pLTA) on the inflammatory responses of intestinal epithelial cells (IEC). The human colon cell line, HT-29, increased interleukin (IL)-8 expression in response to recombinant human tumor necrosis factor (TNF)-alpha, but not in response to bacterial ligands and interferon (IFN)-gamma. TNF-α also increased the production of inducible nitric oxide synthase (iNOS), nitric oxide (NO), and intercellular adhesion molecule 1 (ICAM-1) through activation of p38 mitogen-activated protein kinase (MAPK) from HT-29 cells. However, the inflammatory response of HT-29 on TNF-α stimulation was significantly inhibited by pLTA treatment. This pLTA-mediated inhibition accompanied the inhibition of nuclear factor (NF)-kappa B and MAPKs. Our data suggest that pLTA regulates cytokine-mediated immune responses and may be a good candidate for maintaining intestinal homeostasis against excessive inflammation.
Keywords: colon, cytokine, HT-29, inflammation, lipoteichoic acid
INTRODUCTION
Interleukin (IL)-8 is a chemokine produced by many cell types, including macrophages, endothelial cells, and epithelial cells. IL-8 protein is a member of the CXC chemokine family that is encoded by the IL8 gene in humans (Modi et al., 1990). IL-8 acts as a chemotactic factor that attracts neutrophils and basophils to the lesion. In addition, it plays important roles in angiogenesis, metastasis, and inflammation (Singh et al., 2010). IL-8 binds to both the CXCR1 and CXCR2 receptors with similar affinity and initiates down-stream signaling cascades. CXCR1 and CXCR2 are expressed on cancer cells, endothelial cells, neutrophils, and tumor-associated macrophages. After IL-8 binds to its receptors, signals activate phosphatidylinositol-3-kinase or phospholipase C to promote calcium mobilization and the activation of Akt and PKC. Alternatively, IL-8 signaling activates mitogen-activated protein kinase (MAPK) signaling cascades (Waugh and Wilson, 2008). In epithelial cells, IL-8 secretion is up-regulated by Chlamydia trachomatis infection (Buchholz and Stephens, 2008) through signaling pathways that include the extracellular signal-regulated kinase (ERK) signaling pathway (Buchholz and Stephens, 2007; Fernandes et al., 2009). In addition, lipopolysaccharide (LPS), IL-1α, IL-1β, IL-10, IFN-γ, and TNF-α regulate IL-8 production in various cell types (Cassatella, 1995). Its secretion is also up-regulated by chemoattractants. In human monocytes, IL-8 production is controlled by autocrine regulation (Browning et al., 2000). Cell wall components from Gram-negative and Gram-positive bacteria, such as LPS and lipoteichoic acid (LTA), increase IL-8 secretion from intestinal epithelial cells through the activation of IL-1 receptor associated kinase (IRAK) and MAP kinases (Otte et al., 2004). However, the effects of LTA isolated from probiotics on IL-8 secretion in intestinal epithelial cells have not been well studied. HT-29 cells produce IL-8 after stimulation by TNF-α and IL-1β, but not by IFN-γ, IL-10, or IL-13. TNF-α and nitric oxide (NO) trigger colonic inflammation and carcinogenesis in a Helicobacter hepaticus-infected mouse model, indicating that inflammation mediated by elevated TNF-α and NO causes colon carcinogenesis (Erdman et al., 2009; Kolios et al., 1996). Adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), are also implicated in cancer progression (O’Hanlon et al., 2002; Rosette et al., 2005).
LTA is found within the cell walls of Gram-positive bacteria, and is considered analogous to the LPS of Gram-negative bacteria because they share many biochemical and physiological properties (Ginsburg, 2002). Although both pathogenic and probiotic Gram-positive bacteria express LTA (Neuhaus and Baddiley, 2003), the immunomodulatory properties of the two types are very different. LTAs from pathogenic Gram-positive bacteria such as S. aureus, S. pneumonia, and S. epidermidis, efficiently activate monocytes and macrophages through the secretion of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 (Ellingsen et al., 2002; Mattsson et al., 1993; Standiford et al., 1994). In contrast, LTA from the probiotic L. plantarum (pLTA) only minimally induces TNF-α production when compared to that of S. aureus (aLTA). In addition, pLTA effectively inhibits aLTA- or LPS-triggered TNF-α secretion and suppresses the septic shock caused by aLTA or LPS stimulation (Kim et al., 2008a; 2008b). The distinct immunological activities of aLTA and pLTA might contribute to the different physiological effects of S. aureus and L. plantarum on the human innate immune response.
As an effective molecule for several immune cells, including monocytes, macrophages, dendritic cells, and T-cells, LTA activates or inhibits the immune system through the TLR1-TLR2 signaling pathway (Ellingsen et al., 2002; Meron-Sudai et al, 2008; Son et al., 2008). However, the role of LTA in colon epithelial cells has not been well investigated. In particular, studies regarding the effects of LTA on TNF-α-induced epithelial inflammation are needed. In this study, we have demonstrated that pLTA efficiently inhibits TNF-α-induced inflammation in a colon cell line. The inhibition mechanism was concomitant with the down-regulation of NF-κB and MAPKs. In addition, pLTA inhibited the adhesion of monocytes to HT-29 cells through the down-regulation of adhesion molecules, which was increased by TNF-α. Therefore, our data suggests that pLTA plays important roles in the maintenance of intestinal homeostasis.
MATERIALS AND METHODS
Materials and reagents
Whole cell bacteria and subcellular bacterial fractions were prepared from S. flexneri (KCTC 2517), L. plantarum K8 (KCTC10887BP), and S. aureus (KCTC 1621) as previously described (Kim et al., 2007a). P. gingivalis LPS (PGLPS), muramyl dipeptide (MDP), Pam3CSK4, and monophosphoryl lipid A from S. minnesota (MPLA) were purchased from Invivogen (USA). Lipopolysaccharide from Escherichia coli 055:B5 was purchased from Sigma (USA). Seung Hyun Han, a professor of Department of Oral Microbiology and Immunology at Seoul National University in Korea, provided Actinobacillus actinomycetemcomitans LPS (AALPS). The biochemical inhibitors SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), PD98059 (ERK1/2 inhibitor), IKK inhibitor, NF-κB activation inhibitor, Akt inhibitor, and Wortmannin (PI3K inhibitor) were obtained from Calbiochem (USA). Anti-human TLR2 (clone: TL2.1) and anti-human ICAM-1 (clone: BBIG-I1) neutralizing antibodies were purchased from eBioscience (USA) and R & D Systems (USA), respectively.
Cell culture
The human colon cancer cell line, HT-29, was cultured in Dulbecco’s minimal essential medium (DMEM, Gibco) supplemented with 10% decomplemented fetal bovine serum (FBS) in a humid atmosphere under 5% CO2 at 37°C. Cells were plated at a density of 105 cells per well in 12-well plates 24 h before treatment. Each well was treated with bacterial ligands and cytokines. At the end of the incubation period, supernatants and cells were collected from each plate and the amounts of pro-inflammatory cytokine and mRNA were quantified by enzyme linked immunosorbent assay (ELISA) and reverse transcriptase polymerase chain reaction (RT-PCR).
ELISA
An indirect sandwich ELSIA method was used to quantify IL-8 secretions into the supernatants of both control and treated HT-29 cells according to the manufacturer’s instructions. ELISA was performed using monoclonal mouse IgG1 clone #6217 for capture and biotinylated human IL-8-specific polyclonal goat IgG (R&D Systems, USA). Colorimetric results were read in an ELx800 microplate reader (Biotek Instruments, USA) at a wavelength of 450 nm in 96-well high-binding Stripwell Costar EIA microplates (Costar 2592). Substrate Reagent Pack (Catalog # DY999, R&D Systems, USA) was used for all streptavidin-HRP reactions.
Western blot analysis
Proteins in cell lysates were resolved by SDS-PAGE, transferred onto nitrocellulose membrane, and blotted with the appropriate primary antibodies. Positive signals were detected using a peroxidase-conjugated secondary antibody. Protein bands were visualized by enhanced chemiluminescence with the Super Signal West Detection Kit (Thermo Chemical Company, USA). Cellular β-actin was similarly detected to compare the protein load in each lane.
Reverse-transcriptase polymerase chain reaction and quantification
The total RNA from the controls and pLTA tolerant HT-29 cells was extracted using the guanidium thiocyanate-acid phenolchloroform method. cDNA synthesis was conducted using the Improm-II™ Reverse Transcription System (Promega, USA). RT-PCR was performed in a total reaction volume of 20 μl containing 5 U of Taq polymerase, 1X PCR buffer, 50 mM MgCl2, 10 mM dNTP mixture, 10 μM of each oligonucleotide primer, and 1 μl of cDNA. Amplification was performed with an initial denaturation at 95°C for 10 min followed by 25 to 35 cycles at 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Amplification was finalized at 72°C for 10 min. After completing the cycling process, PCR products were separated by 0.8% agarose gel electrophoresis and band quantity was analyzed using the Image J program. The forward and reverse primers were as follows: 5′-GTCTTCACCACCATGGAGAA-3′ and 5′-AGTGAGG GTCTCTCTCTTCC-3′ for GAPDH, 5′-ACCCTAGGGGAAAC ATCTCT-3′ and 5′-GCATCCTCACAGGCTGAAT-3′ for TLR2, 5′-TTTCTAAAGCGCGTCGATGC-3′ and 5′-CAGCGCTAGATT CTGGATGG-3′ for CD14, 5′-GATTCTGACGAAGCCAGAGG-3′ and 5′-CATTATGACTGCGGCTGCTA-3′ for I-CAM1, 5′-AAGATGGTCGTGATCCTTGG-3′ and 5′-TTCTTGCAGCTTT GTGGATG-3′ for V-CAM1, and 5′-CGCTTCAGAAAACCAC CTCA-3′ and 5′-CAAAGTTGGGACAGTCACCG-3′ for TNFRSF1A, 5′-ACTTACCCCAGCCAGTGTCC-3′ and 5′-TCTCCA GCTGTGACCGAAG-3′ for TNFRSF1B.
Statistical analysis
Results are expressed as the mean ± SD and statistical analyses were performed by two-tailed unpaired Student’s t-tests with the GraphPad Prism 5 program (GraphPad, USA). A p value of < 0.05 was considered statistically significant.
RESULTS
rhTNF-α increased IL-8 production in HT-29 cells
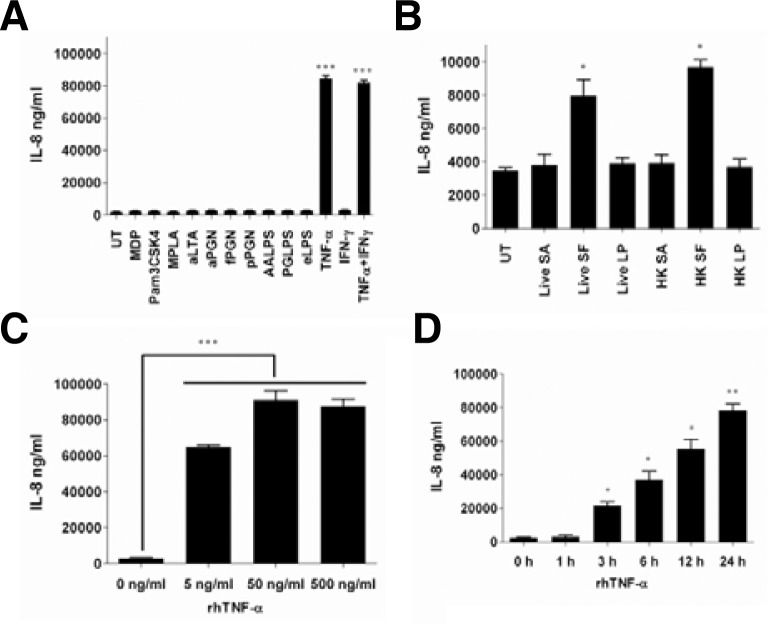
To examine the effects of various ligands on IL-8 induction, HT-29 cells were treated with isolated bacterial cell wall components, synthetic TLR ligands, cytokines, and whole cell bacteria. Bacterial cell wall components such as lipoteichoic acid (LTA), lipopolysaccharide (LPS; 055:B5), peptidoglycan (PGN), and synthetic ligands such as monodipeptide (MDP), Pam3CSK4, and monophosphoryl lipid A (MPLA) did not induce IL-8 expression (Fig. 1A). Both live and heat-killed whole cell bacteria from Shigella flexneri (SF) exhibited increased IL-8 expression compared to unstimulated cells (Fig. 1B). However, neither Staphylococcus aureus (SA) nor Lactobacillus plantarum (LP) exhibited increased IL-8 expression. Interestingly, IL-8 expression in colon cells significantly increased as a result of treatment with recombinant human (rh) TNF-α, but not with rhIFN-γ (Fig. 1A). The synergic effect of both rhTNF-α and rhIFN-γ was not shown, indicating that IFN-γ does not affect the inflammation of intestinal epithelial cells while TNF-α modulates intestinal inflammation. These results are consistent with previously reported data (Erdman et al., 2009; Kolios et al., 1996). HT-29 cells stimulated with rhTNF-α increased IL-8 expression in a dose-dependent manner (Fig. 1C) and reached the highest level at 24 h of incubation (Fig. 1D).
Fig. 1.
rhTNF-α increased IL-8 expression in HT-29 cells. (A) HT-29 cells were stimulated with muramyl dipeptide (MDP), Pam3CSK4, monophosphoryl lipid A (MPLA), S. aureus LTA (aLTA), S. aureus peptidoglycan (aPGN), S. flexneri peptidoglycan (fPGN), L. plantarum peptidoglycan (pPGN), A. actinomycetemcomitans LPS (AALPS), P. gingivalis (PGLPS), E. coli LPS, rhTNF-α, and rhIFN-γ for 6 h. HT-29 cells were stimulated with live and heat-killed S. aureus, S. flexneri, and L. plantarum (B), with different doses of rhTNF-α (C) for 6 h, and with 50 ng/ml of rhTNF-α for the indicated time points (D). In all experiments, culture supernatants were collected and IL-8 expression was analyzed using ELISA. UT indicates untreated cells. Data are given as means ± standard deviations for triplicate samples in a single experiment. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to UT (A, B, and C) or 0 h stimulation (D).
LTA inhibited rhTNF-α-induced IL-8 production
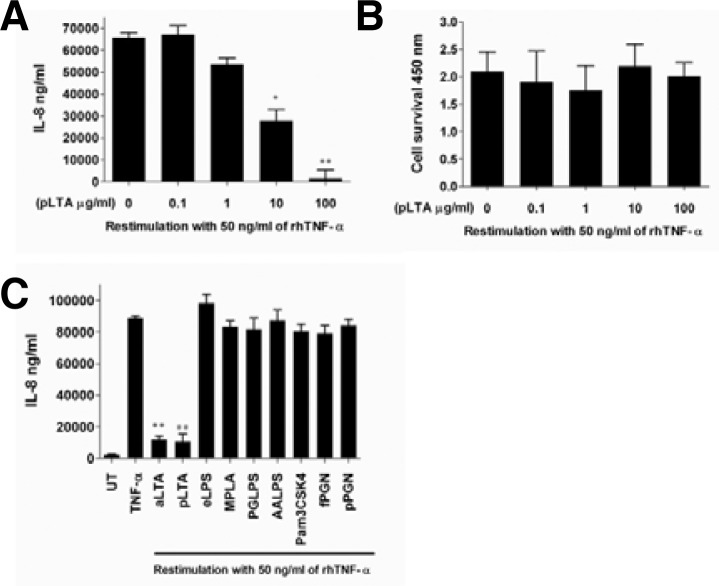
Previously, we have shown that pLTA inhibited LPS- and aLTA-induced TNF-α production in THP-1 cells (Kim et al., 2008a; 2008b). In this study, we examined the inhibitory effect of pLTA against rhTNF-α in HT-29 cells. As shown in the figures, HT-29 cells inhibited IL-8 expression after stimulation with pLTA followed by rhTNF-α. This inhibition increased in a pLTA dose-dependent manner (Fig. 2A), and no significant cytotoxicity was seen as a result of pLTA and rhTNF-α (Fig. 2B) under the same conditions. This result suggests that IL-8 inhibition occurred as a result of treatment with pLTA, and not by cell death. To test whether other bacterial ligands inhibit rhTNF-α-induced IL-8 expression, HT-29 cells were pretreated with diverse ligands from Gram-negative and Gram-positive bacteria cell wall components. These included LPS, LTA, and PGN, and synthetic ligands such as MPLA and Pam3CSK4. After re-treating with 50 ng/ml of rhTNF-α, IL-8 expression was reduced in LTA treated cells, but other ligands did not reduce IL-8 expression (Fig. 2C).
Fig. 2.
pLTA inhibited rhTNF-α-mediated IL-8 expression in HT-29 cells. (A) HT-29 cells were stimulated with the indicated doses of pLTA for 20 h followed by restimulation with 50 ng/ml of rhTNF-α for 24 h. IL-8 expression was analyzed from culture supernatant using ELSIA. (B) After stimulation with 50 ng/ml of rhTNF-α following pLTA pretreatment at the indicated dose, the cell survival rate was analyzed by a Premix WST-1 cell proliferation assay system (Takara Bio Inc.). (C) Cells were pretreated with the indicated ligands for 20 h and 50 ng/ml of rhTNF-α was retreated for 24 h. IL-8 expression was analyzed with supernatant using ELISA. The data are given as means ± standard deviations for triplicate samples in a single experiment. *, p < 0.05; **, p < 0.01 compared to 0 μg/ml of pLTA treatment (A) or rhTNF-α treatment only (C). UT indicates untreated cells.
LTA inhibited cytokine-mediated NO production
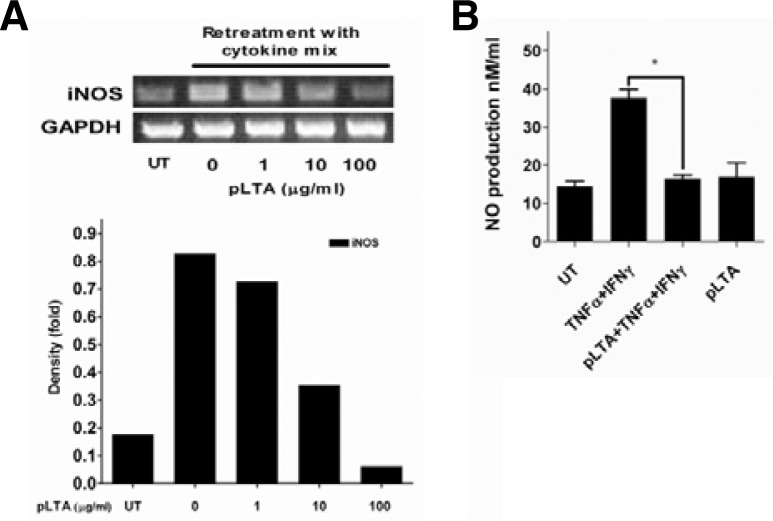
Inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production trigger colonic inflammation and carcinogenesis (Erdman et al., 2009). To examine the inhibitory effect of pLTA on the production of iNOS and NO, HT-29 cells were pretreated with pLTA. They were then re-treated with a cytokine cocktail including recombinant human TNF-α, IFN-γ, and IL-1α for 24 to 48 h (Kolios et al., 1995). The levels of iNOS mRNA and NO production were determined by reverse transcriptase polymerase chain reaction (RT-PCR) and a nitro oxide detection kit, respectively. Cytokine cocktail-mediated iNOS production was inhibited in pLTA-treated cells in a dose-dependent manner (Fig. 3A, upper panel). The iNOS level increased approximately 4 fold following incubation of HT-29 cells with cytokine cocktail, while a ∼2 fold reduction in iNOS level was observed with a high dose of pLTA pretreatment (Fig. 3A, lower panel). In addition, pLTA pretreatment inhibited cytokine-induced NO production compared to untreated cells (Fig. 3B). Interestingly, pLTA itself did not induce NO production.
Fig. 3.
pLTA inhibited rhTNF-α-mediated iNOS and NO production. (A) HT-29 cells were treated with the indicated dose of pLTA for 20 h and then restimulated with cytokine cocktail (100 ng/ml of rhTNF-α, 300 U/ml of IFN-γ, and 10 ng/ml of IL-1α) for 48 h. Total RNA was extracted, cDNA was synthesized, and PCR was performed with specific primers for iNOS and GAPDH (upper panel). The iNOS mRNA quantity was normalized with GAPDH after density analysis using Image J software (lower panel). Experiments were replicated at least three times. One representative data point is displayed. (B) HT-29 cells were preincubated with pLTA for 20 h and retreated with cytokine cocktail for 24 h. NO production was analyzed with a Nitric Oxide Colorimetric Assay Kit (Abcam) according to the manufacturer’s instruction. Data are given as means ± standard deviations for triplicate samples in a single experiment. *, p < 0.05. UT indicates untreated cells.
LTA inhibited rhTNF-α-mediated THP-1 cell adhesion to HT-29
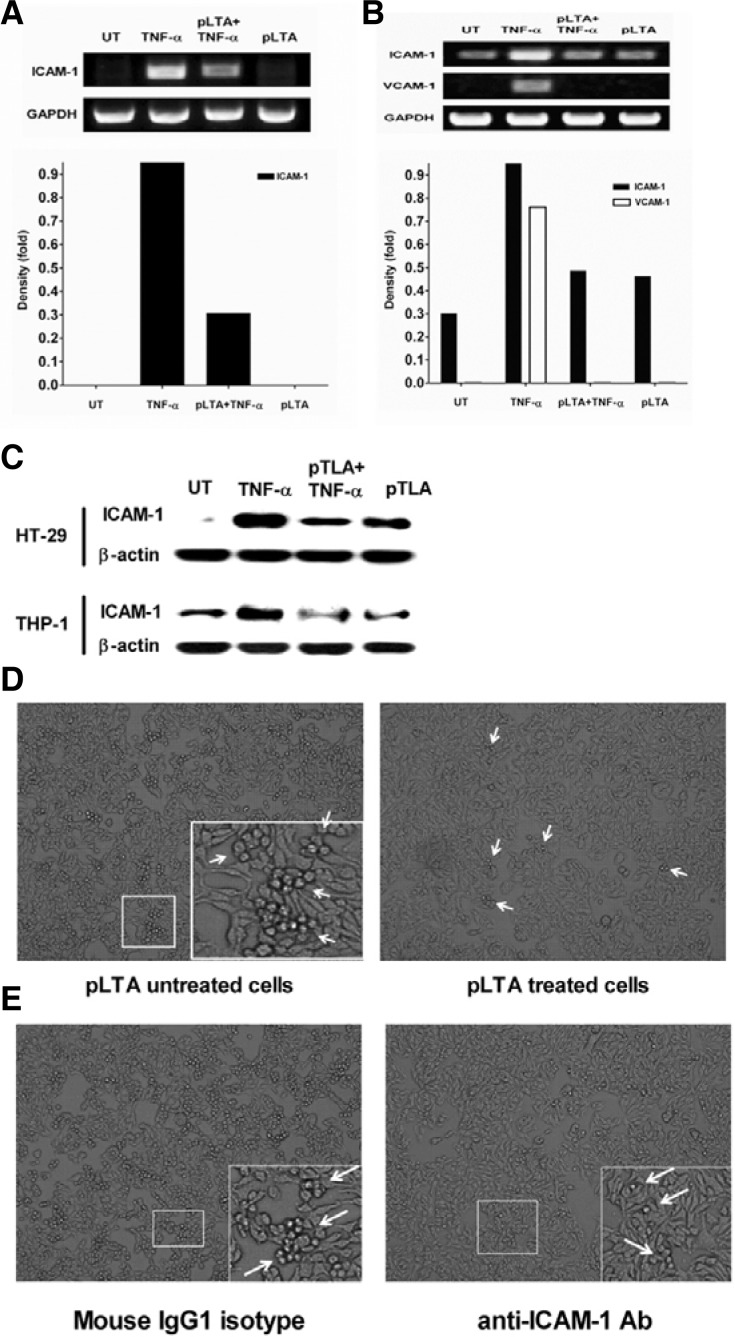
Adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) are implicated in cancer progression (O’Hanlon et al., 2002; Rosette et al., 2005). In this study, the effect of pLTA on the TNF-α-induced expression of adhesion molecules was examined. In HT-29 cells, ICAM-1 mRNA expression was significantly increased by rhTNF-α, while it slightly increased by pLTA (Fig. 4A, upper panel). Following incubation of HT-29 cells with rhTNF-α, I-CAM1 mRNA increased approximately 9-fold. However, VCAM-1 mRNA was not detected in the HT-29 cells. In contrast, ICAM-1 mRNA was significantly inhibited by pLTA (Fig. 4A, lower panel). In THP-1 cells, ICAM-1 and VCAM-1 mRNA were increased by rhTNF-α but not by pLTA. However, the rhTNF-α-mediated increases of these molecules were significantly inhibited by pLTA pre-treatment (Fig. 4B, upper panel). After treatment with rhTNF-α, ICAM-1 and VCAM-1 expression increased about 3- and 7.5-fold, respectively, compared to untreated cells. The expression of these molecules also decreased by pLTA (Fig. 4B, lower panel). Similar results were observed for protein levels. rhTNF-α increased ICAM-1 expression in THP-1 and HT-29 cells, and the expression of ICAM-1 was inhibited by pLTA in these cells (Fig. 4C). Since the adhesion of monocytes to IECs represents the expression of adhesion molecules and inflammation of both cells (Lee et al., 2010; Thapa et al., 2009), we examined whether pLTA inhibits the rhTNF-α-induced adhesion of monocytes to colon epithelial cells. As shown in Fig. 4D, high numbers of pLTA-untreated THP-1 cells adhered to HT-29 cells while minimal numbers of pLTA-treated THP-1 cells adhered to HT-29 cells, indicating that pLTA reduced the expression of the adhesion molecules which were involved in THP-1 cell adhesion to HT-29 cells. THP-1 cell adhesion to HT-29 cells was inhibited by ICAM-1 neutralization antibody (Fig. 4E). This result suggests that ICAM-1 mediated the interaction of both cells.
Fig. 4.
pLTA inhibited THP-1 cell adhesion to HT-29 cells. HT-29 cells (A) or THP-1 cells (B) were treated with 100 μg/ml of pLTA for 20 h followed by 50 ng/ml of rhTNF-α for 6 h. rhTNF-α or pLTA alone were used as controls. After extracting total RNA, cDNA was synthesized and PCR was performed with specific primers for ICAM-1, VCAM-1, and GAPDH (upper panel). The I-CAM1 mRNA quantity was normalized with GAPDH after density analysis using Image J software (lower panel). (C) HT-29 and THP-1 cells were analyzed for ICAM-1 expression by Western blot. For adhesion assay, THP-1 and HT-29 cells were treated with 100 μg/ml pLTA for 20 h (D), 10 μg/ml anti-ICAM-1 or isotype control antibody for 1 h (E), and washed tree times with PBS to eliminate unbound pLTA or antibodies. Both cells were co-cultured with 50 ng/ml of rhTNF-α for 24 h and unattached THP-1 cells were removed using PBS. The attachment of pLTA to HT-29 cells was analyzed using a microscope. Arrows indicate colonized THP-1 cells on HT-29 cells. All experiments were repeated at least 3 times and a representative figure is displayed. UT indicates untreated cells.
LTA inhibited rhTNF-α-mediated NF-κB and MAPK activation
To analyze the inhibitory mechanism of pLTA on rhTNF-α-induced colon cell inflammation, a key signaling pathway initiated by TNF-α using inhibitors for signaling molecules was identified (Fig. 5A). In HT-29 cells, rhTNF-α-induced IL-8 expression was not affected by the PI3K, Akt, or JNK pathways. The inhibitor for IKK did not decrease rhTNF-α-mediated IL-8 production, while NF-κB and ERK inhibitors reduced IL expression. However, the p38 kinase inhibitor induced significant IL-8 inhibition. This result suggests that pLTA may inhibit the p38 signaling pathway as well as the NF-κB and ERK signaling pathways. To examine whether pLTA inhibits the NF-κB and MAPK pathways, key molecules were examined by western blotting after treatment with pLTA and rhTNF-α. As shown in Fig. 5B, rhTNF-α dramatically increased phospho-p38, phospho-ERK, and phosphor-JNK. However, pLTA pretreatment significantly inhibited the TNF-α-induced phosphorylation of MAPKs. The degradation of IκBα, an inhibitor of NF-κB, was increased by rhTNF-α and reduced by pLTA pretreatment. These results demonstrate that pLTA effectively inhibits TNF-α-induced NF-κB and MAPK signaling pathways.
Fig. 5.
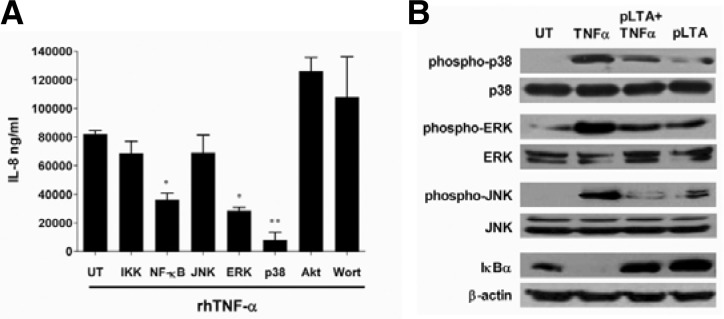
pLTA inhibited rhTNF-α-mediated p38 pathway. (A) HT-29 cells were pretreated with various signaling inhibitors for 30 min and then retreated with rhTNF-α for 24 h. Culture supernatants were collected and IL-8 expression was analyzed using ELISA. UT indicates untreated cells. The data are given as means ± standard deviations for triplicate samples in a single experiment. *, p < 0.05; **, p < 0.01; compared to untreated cells (UT). (B) HT-29 cells were treated with 100 μg/ml of pLTA for 20 h followed by 50 ng/ml of rhTNF-α for 1 h. Treatments of rhTNF-α or pLTA alone were used as controls. The variation of signaling molecules was analyzed by Western blotting. UT indicates untreated cells.
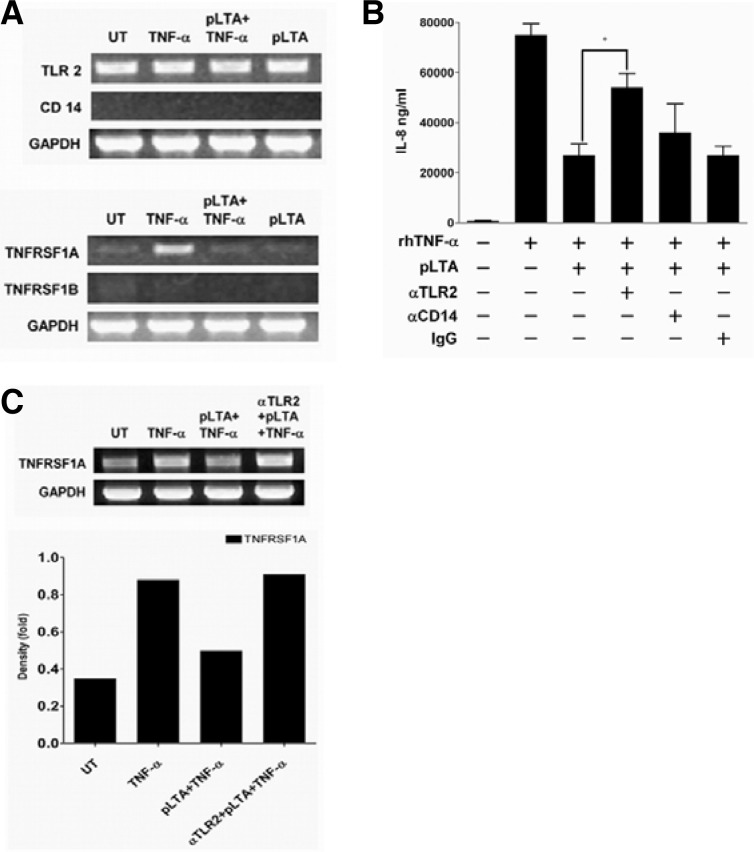
The role of receptors in the inhibition of IL-8 by LTA
LTA is recognized by TLR2 and CD14. TNF-α induces signaling pathways through the TNF receptor superfamilies (TNFRSF) 1A and 1B, which are responsible for cell survival and cell death, respectively (Narayanan and Patial, 2010; Triantafilou et al., 2004). In this study, we examined the role of receptors on the regulation of IL-8 expression. In HT-29 cells, CD14 mRNA was not amplified with CD14-specific primers, and there was no significant change in TLR2 mRNA (Fig. 6A, upper panel). CD14 mRNA in THP-1 cells was significantly increased by LPS and aLTA but not by pLTA and rhTNF-α (data not shown). This indicates that pLTA and TNF-α do not induce CD14 mRNA production in HT-29 cells. TNFRSF1A production was significantly increased by rhTNF-α treatment while TNFRSF1B mRNA was not detected in the HT-29 cells (Fig. 6A, lower panel). However, the TNFRSF1A was almost completely inhibited by pLTA pretreatment. In an experiment using anti-TLR2 and -CD14 antibodies for neutralization, anti-TLR2 antibody reversed the inhibition of rhTNF-α-mediated IL-8 expression by pLTA, while anti-CD14 and control IgG did not (Fig. 6B). This experiment indicates that TLR2 plays an important role in the inhibition of rhTNF-α-induced IL-8 expression by pLTA. However, rhTNF-α-induced IL-8 expression was not completely recovered in HT-29 cells treated with anti-TLR2 neutralization antibody, indicating the existence of another signaling pathway that is responsible for IL-8 reduction. One possible mechanism could be a reduction of TNFRSF1A, which affects rhTNF-α-mediated IL-8 expression. Therefore, the next experiment examined the effect of the pLTA-TLR2 signaling pathway on TNFRSF1A expression. As expected, TNFRSF1A mRNA was significantly inhibited by pLTA pretreatment (Fig. 6C, upper panel) while TLR2 neutralization using specific anti-TLR2 antibody did not inhibit pLTA-induced TNFRSF1A production. The intensity bar indicates that a 50% decrease in rhTNF-α-induced TNFRSF1A expression by pLTA was recovered in anti-TLR2 antibody treated cells (Fig. 6C, lower panel). These data indicates that pLTA inhibits TNF receptor expression as well as IL-8 expression through TLR2.
Fig. 6.
pLTA regulated rhTNF-α-mediated receptor expression. (A) HT-29 cells were treated with 100 μg/ml of pLTA for 20 h followed by 50 ng/ml of rhTNF-α for 6 h. Treatment with rhTNF-α or pLTA alone was used as control. After extracting total RNA, cDNA was synthesized and PCR was performed with specific primers for TLR2, CD14, TNFRSF1A, TNFRSF1b, and GAPDH (upper and lower panel). (B) HT-29 cells were pretreated with anti-TLR2, -CD14, or control IgG for 30 min and then restimulated with 50 ng/ml of rhTNF-α for 24 h. IL-8 expression was analyzed from culture supernatants by ELISA. Data are given as means ± standard deviations for triplicate samples in a single experiment. *, p < 0.05. (C) HT-29 cells were treated with 100 μg/ml of pLTA for 20 h following anti-TLR2 antibody treatment for 30 min and then restimulated with 50 ng/ml of rhTNF-α for 6 h. After total RNA extraction and cDNA synthesis, the TNFRSF1A mRNA level was amplified by PCR using specific TNFRSF1A primers (upper panel). The TNFRSF1A mRNA quantity was normalized with GAPDH after density analysis using Image J software (lower panel). At least 3 different experiments were conducted. UT indicates untreated cells.
DISCUSSION
In a previous study, we have shown that pLTA inhibited LPS- or aLTA-induced TNF-α production in THP-1 cells (Kim et al., 2008a; 2008b). In addition, pLTA has low immune activation effects while LPS and aLTA induce high levels of TNF-α production. Interestingly, pLTA does not increase the activation of NF-κB and MAP Kinases. However, signaling activation by LPS and aLTA is significantly inhibited by pLTA pretreatment. These results suggest that pLTA has a fine-tuning effect on pathogen-mediated inflammation in THP-1 cells. Monocytes are a major source of inflammatory cytokines and play an important role in the innate and adaptive immune systems of vertebrates. When bacteria invade epithelium from the intestinal lumen through M-cells, monocytes recognize invading microorganisms through TLR and enhance the production of pro-inflammatory cytokines. These cytokines activate innate immune cells, such as neutrophils and natural killer cells, and acquired immune responses (Rescigno, 2011; Sheikh and Plevy, 2010).
Unlike monocytes, IECs did not respond to bacterial ligands and IFN- γ (Fig. 1). Many bacterial species can contribute to the tumorigenesis of IEC. Escherichia coli, which causes inflammatory bowel disease (IBD) and colorectal cancer (CRC), contributes to the colonization of IEC and the production of IL-8, IFN- γ, and TNF-α. It also mediates the adhesion of carcinoembryonic antigen-related molecule 6. Although bacterial cell wall components, such as LPS and LTA, contribute to TLR-mediated inflammation (Terzic et al., 2010), these ligands may not directly alter IEC inflammation (Fig. 1). The TLR-mediated signaling pathway via bacterial ligands may be limited in IEC because of impaired expression of TLR accessory molecules. Compared to THP-1 cells, IECs are lacking in CD14 and TLR expression in response to bacterial stimulation (Fig. 6A), which may cause impairment of bacterial cell wall ligand-mediated inflammation in epithelial cells.
Both positive regulators, such as proinflammatory cytokines TNF-α, IL-1, and IL-6, and negative regulators, such as transforming growth factor-β (TGF-β), IL-10, and single immunoglobulin IL-1R-related molecule (SIGIRR) induce colon cancer development (Danese and Mantovani, 2010). The transcription factors NF-κB and MAPKs are particularly important in colon cancer development (Danese and Mantovani, 2010; Huang et al., 2010). Activation of NF-κB contributes to the activation of innate immunity, inflammation, cell proliferation, and survival. NF-κB also controls the expression of inflammatory cytokines, adhesion molecules, inducible nitric oxide synthase, and a number of anti-apoptotic genes (Mantovani et al., 2008; Naugler and Karin, 2008). In our studies, IECs did not induce IL-8 production in response to IFN- γ (Fig. 1A). We also did not detect expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IFN- γ from HT-29 cells (data not shown). Although IFN- γ did not affect HT-29 cell inflammation, TNF-α mediated NF-κB/MAPK activation and increased the expression of IL-8, NO, and ICAM-1. In particular, p38, ERK, and NF-κB inhibitors significantly inhibited IL-8 production (Fig. 5A), indicating that these signaling pathways are primarily used for TNF-α-mediated inflammation in HT-29 cells.
Inflammation causes inflammatory diseases in IECs. Therefore, the inhibition of inflammation is critical for intestinal homeostasis. In this study, we showed that TNF-α-mediated production of inflammatory molecules such as IL-8, iNOS, NO, and adhesion molecules was inhibited by pLTA pretreatment. LTA is a cell wall component of Gram-positive bacteria that has numerous biological functions including neutrophil recruitment, impairment of osteoclastogenesis, and induction of inflammation in the mammary gland and lung. It also causes septic shock and multiorgan failure (Bougarn et al., 2010; De Kimpe et al., 1995; Knapp et al., 2008; Rainard et al., 2008; Yang et al., 2009). LTA plays important roles in both innate and adaptive immunity (Chan et al., 2007; Kim et al., 2007b; Seo et al., 2008). Although precise mechanical differences have not yet been reported, LTAs have differential immunostimulatory effects on host cells according to origin. For example, LTA isolated from S. aureus and B. subtilis induces significant NO production from RAW264.7 cells, while LTA isolated from L. plantarum induces only mild NO production (Ryu et al., 2009). The structural differences of LTAs may possibly account for the differences in cellular responses to aLTA and pLTA. Previously, we reported that the two LTAs have different glycolipid anchors (Jang et al., 2011). The biological roles of LTA glycolipid anchors have drawn great attention, since they are known to be key molecules that trigger innate immunity in hosts. The immunostimulatory activity of aLTA was remarkably diminished by removal of the fatty acid acyl group of LTA (Ohshima et al., 1988). We also demonstrated that the acyl group of pLTA was responsible for inhibiting LPS-induced TNF-a production, not the D-alanine substituent in the phosphoglycerol moiety of pLTA (Kim et al., 2008a). Based on the glycolipid analysis conducted in a previous study, the glycolipid moiety of pLTA contains unsaturated fatty acids and a third acyl chain on the conserved trihexosyldiacyl ceramide structure. The glycolipid from aLTA consists only of saturated fatty acids attached to the dihexosyl-diacyl glycerol structure. These structural differences may recruit different receptor complexes on the cell surface. LTA is a macro-molecule composed of phosphate chain, sugar units, and glycolipid anchors. Due to their complexity, some LTAs are structurally uncharacterized. These LTAs may induce serious immune responses related to arthritis, nephritis, uveitis, encephalo-myelitis, meningeal inflammation, periodontal lesions, septic shock, and multiorgan failure. Although a previous study (Kim et al., 2008a) used pLTA for an in vivo septic shock mouse model, the application of LTAs to a human disease model is needed to learn more about their safety.
In conclusion, inflammatory cytokines such as TNF-α cause a number of inflammatory diseases in intestinal epithelial cells. In this study, we showed that pLTA inhibits TNF-α-induced inflammatory responses in HT-29 cells through inhibition of the NF-κB and MAPK signaling pathways. Our results suggest that LTA could be an effective component for maintaining intestinal homeostasis.
Acknowledgments
A National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Education, Science and Technology) (No. 2010-0012091) supported this work.
REFERENCES
- Bougarn S., Cunha P., Harmache A., Fromageau A., Gilbert F.B., Rainard P. Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells. Clin. Vaccine Immunol. 2010;17:1797–1809. doi: 10.1128/CVI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning D.D., Diehl W.C., Hsu M.H., Schraufstatter I.U., Ye R.D. Autocrine regulation of interleukin-8 production in human monocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1129–L1136. doi: 10.1152/ajplung.2000.279.6.L1129. [DOI] [PubMed] [Google Scholar]
- Buchholz K.R., Stephens R.S. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect. Immun. 2007;75:5924–5929. doi: 10.1128/IAI.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz K.R., Stephens R.S. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect. Immun. 2008;76:3150–3155. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M.A. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Chan K.G., Mayer M., Davis E.M., Halperin S.A., Lin T.J., Lee S.F. Role of D-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity. Infect. Immun. 2007;75:3033–3042. doi: 10.1128/IAI.01549-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese S., mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- De Kimpe S.J., Kengatharan M., Thiemermann C., Vane J.R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen E., Morath S., Flo T., Schromm A., Hartung T., Thiemermann C., Espevik T., Golenbock D., Foster D., Solberg R., et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 2002;8:149–156. [PubMed] [Google Scholar]
- Ellingsen E.A., Morath S., Flo T.H., Schromm A.B., Hartung T., Thiemermann C., Espevik T., Golenbock D.T., Foster S.J., Solberg R., et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 2002;8:BR149–156. [PubMed] [Google Scholar]
- Erdman S.E., Rao V.P., Poutahidis T., Rogers A.B., Taylor C.L., Jackson E.A., Ge Z., Lee C.W., Schauer D.B., Wogan G.N., et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc. Natl. Acad. Sci. USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A.F., Bian Q., Jiang J.K., Thomas C.J., Taylor A., Pereira P., Shang F. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol. Biol. Cell. 2009;16:3690–3699. doi: 10.1091/mbc.E08-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2002;2:171–179. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Huang P., Han J., Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218–226. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K.S., Baik J.E., Han S.H., Chung D.K., Kim B.G. Multi-spectrometric analyses of lipoteichoic acids isolated from Lactobacillus plantarum. Biochem Biophys. Res. Commun. 2011;407:823–830. doi: 10.1016/j.bbrc.2011.03.107. [DOI] [PubMed] [Google Scholar]
- Kim H.G., Gim M.G., Kim J.Y., Hwang H.J., Ham M.S., Lee J.M., Hartung T., Park J.W., Han S.H., Chung D.K. Lipoteichoic acid from Lactobacillus plantarum elicits both the production of Interleukin-23p19 and suppression of pathogen-mediatedInterleukin-10 in THP-1 cells. FEMS Immunol. Med. Microbiol. 2007a;49:205–214. doi: 10.1111/j.1574-695X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Yang J.S., Woo S.S., Kim S.K., Yun C.H., Kim K.K., Han S.H. Lipoteichoic acid and muramyl dipeptide synergistically induce maturation of human dendritic cells and concurrent expression of proinflammatory cytokines. J. Leukoc. Biol. 2007b;81:983–989. doi: 10.1189/jlb.0906588. [DOI] [PubMed] [Google Scholar]
- Kim H.G., Kim N.R., Gim M.G., Lee J.M., Lee S.Y., Ko M.Y., Kim J.Y., Han S. H., Chung D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008a;180:2553–2561. doi: 10.4049/jimmunol.180.4.2553. [DOI] [PubMed] [Google Scholar]
- Kim H.G., Lee S.Y., Kim N.R., Ko M.Y., Lee J.M., Yi T.H., Chung S.K., Chung D.K. Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production. J. Microbiol. Biotechnol. 2008b;18:1191–1196. [PubMed] [Google Scholar]
- Knapp S., von Aulock S., Leendertse M., Haslinger I., Draing C., Golenbock D.T., van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J. Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- Kolios G., Brown Z., Robson R.L., Roberson D.A.F., Westwick J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br. J. Pharmacol. 1995;116:2866–2872. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolios G., Robertson D.A.F., Jordan N.J., Minty A., Caput D., Ferrara P., Westwick J. Interleukin-8 production by the human colon epithelial cell line HT-29: modulation by interleukin-13. Br. J. Pharmacol. 1996;119:351–359. doi: 10.1111/j.1476-5381.1996.tb15993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Park S.Y., Thapa D., Choi M.K., Chung I.M., Park Y.J., Yong C.S., Choi H.G., Kim J.A. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-α production and its signaling. Exp. Mol. Med. 2010;42:143–154. doi: 10.3858/emm.2010.42.2.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mattsson E., Verhage L., Rollof J., Fleer A., Verhoef J., van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1β and interleukin-6. FEMS Immunol. Med. Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Meron-Sudai S., Matityahou A., Keisari Y., Cox K.H., Hasty D.L., Ofek I. Lipoteichoic acid synergizes with glycosphingolipids to potently stimulate secretion of interleukin-6 from human blood cells. Clin. Vaccine Immunol. 2008;15:309–315. doi: 10.1128/CVI.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi W.S., Dean M., Seuanez H.N., Mukaida N., Matsushima K., O’Brien S.J. Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily. Hum. Genet. 1990;84:185–187. doi: 10.1007/BF00208938. [DOI] [PubMed] [Google Scholar]
- Narayanan P., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler W.E., Karin M. NF-kappaB and canceridentifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008;28:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus F.C., Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon D.M., Fitzsimons H., Lynch J., Tormey S., Malone C., Given H.F. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur. J. Cancer. 2002;38:2252–2257. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Beuth J., Yassin A., Ko H.L., Pulverer G. Stimulation of human monocyte chemiluminescence by staphylococcal lipoteichoic acid. Med. Microbiol. Immunol. 1988;177:115–121. doi: 10.1007/BF00232891. [DOI] [PubMed] [Google Scholar]
- Otte J.M., Cario E., Podolsky D.K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Rainard P., Fromageau A., Cunha P., Gilbert F.B. Staphylococcus aureus lipoteichoic acid triggers inflammation in the lactating bovine mammary gland. Vet. Res. 2008;39:52. doi: 10.1051/vetres:2008034. [DOI] [PubMed] [Google Scholar]
- Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rosette C., Roth R.B., Oeth P., Braun A., Kammerer S., Ekblom J., Denissenko M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- Ryu Y.H., Baik J.E., Yang J.S., Kang S.S., Im J., Yun C.H., Kim D.W., Lee K., Chung D.K., Ju H.R., et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int. Immunopharmacol. 2009;9:127–133. doi: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Seo H.S., Michalek S.M., Nahm M.H. Lipoteichoic acid is important in innate immune responses to gram-positive bacteria. Infect. Immun. 2008;76:206–213. doi: 10.1128/IAI.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S.Z., Plevy S.E. The role of the macrophage in sentinel responses in intestinal immunity. Curr. Opin. Gastroenterol. 2010;26:578–582. doi: 10.1097/MOG.0b013e32833d4b71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh A.P., Sharma B., Owen L.B., Singh R.K. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6:111–116. doi: 10.2217/fon.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y.M., Ahn S.M., Jang M.S., Moon Y.S., Kim S.H., Cho K.K., Han S.H., Yun C.H. Immunomodulatory effect of resistin in human dendritic cells stimulated with lipoteichoic acid from Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2008;376:599–604. doi: 10.1016/j.bbrc.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Standiford T.J., Arenberg D.A., Danforth J.M., Kunkel S.L., Van Otteren G.M., Strieter R.M. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes a cellular and molecular analysis. Infect. Immun. 1994;62:119–125. doi: 10.1128/iai.62.1.119-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic J., Grivennikov S., Karin E., Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Thapa D., Lee J.S., Park M.A., Cho M.Y., Park Y.J., Choi H.G., Jeong T.C., Kim J.A. Inhibitory effects of clotrimazole on TNF-alpha-induced adhesion molecule expression and angiogenesis. Arch. Pharm. Res. 2009;32:593–603. doi: 10.1007/s12272-009-1416-6. [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Manukyan M., Mackie A., Morath S., Hartung T., Heine H., Triantafilou K. Lipoteichoic acid and tolllike receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J. Biol. Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- Waugh D.J.J., Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Yang J., Ryu Y.H., Yun C.H., Han S.H. Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J. Leukoc. Biol. 2009;86:823–831. doi: 10.1189/jlb.0309206. [DOI] [PubMed] [Google Scholar]