Abstract
The asymmetry of environmental stimuli and the execution of developmental programs at the organism level require a corresponding polarity at the cellular level, in both unicellular and multicellular organisms. In plants, cell polarity is important in major developmental processes such as cell division, cell enlargement, cell morphogenesis, embryo-genesis, axis formation, organ development, and defense. One of the most important factors controlling cell polarity is the asymmetric distribution of polarity determinants. In particular, phosphorylation is implicated in the polar distribution of the determinant protein factors, a mechanism conserved in both prokaryotes and eukaryotes. In plants, formation of local gradients of auxin, the morphogenic hormone, is critical for plant developmental processes exhibiting polarity. The auxin efflux carriers PIN-FORMEDs (PINs) localize asymmetrically in the plasma membrane and cause the formation of local auxin gradients throughout the plant. The asymmetry of PIN distribution in the plasma membrane is determined by phosphorylation-mediated polar trafficking of PIN proteins. This review discusses recent studies on the role of phosphorylation in polar PIN trafficking.
Keywords: auxin, auxin transport, PIN-FORMED (PIN), phosphorylation, protein trafficking
INTRODUCTION
Cell polarity refers to the asymmetric distribution of cellular components such as organelles, molecules, and ions along a particular axis (Cove, 2000). Cell polarity occurs across kingdoms, from unicellular prokaryotes to multicellular eukaryotes, and plays a pivotal role in development (Nelson, 2003). Uneven distributions of cellular components can be caused either by environmental stimuli or endogenous cues. Cell polarity is brought about by the highly coordinated actions of many different cellular mechanisms. In prokaryotes and eukaryotes, polar protein localization and trafficking plays a decisive role in the generation and maintenance of cell polarity.
In plants, recent studies of several asymmetrically localized plasma membrane (PM) proteins have shed light on the mechanisms of cell polarity and the roles of these proteins in plant development. For example, the PIN-FORMED (PIN) proteins are polar-localized transmembrane auxin efflux carriers that play a rate-limiting role in polar auxin transport and are implicated in diverse plant developmental processes. Different PIN proteins display distinct apical, basal or lateral polarity in the PM, depending on the PIN and the tissue type. PINs thereby determine the direction of auxin flow throughout the plant body (Wiśniewska et al., 2006). Auxin influx carriers such as AUXIN-RESISTANT1 (AUX1) and LIKE AUX1 (LAX) show polar localization in certain cell types (Dettmer and Friml, 2011). Some nutrient transporters, such as boron transporters, are also polarly localized. The plant indole-3-buryric acid transporter, PIS1/ABCG37 exhibits polar localization on the outer side of the epidermal cells similar to the pathogen-defense related transporter PEN3/PDR8/ABCG36 (Ito and Gray, 2006; Langowski et al., 2010; Stein et al., 2006).
The mechanisms underlying polar localization of plant membrane transporters have been actively studied for the last decade. In this review we discuss one such mechanism, the role of phosphorylation in modulating polar intracellular trafficking of the auxin transporters, mainly focusing on PIN proteins.
POLAR AUXIN TRANSPORT AND PINs
The plant hormone auxin plays fundamental roles in growth, development, cell division, cell expansion, primary axis formation, root meristem maintenance, lateral root formation, root hair growth, vasculature differentiation, flowering, and tropic responses of shoots and roots (Davies, 2004). Auxin is synthesized primarily in the shoot apex region, including in young growing leaves, and is transported through the stem and root to the root tip; this directional process is called polar auxin transport (PAT). PAT leads to spatial auxin maxima and minima, and local auxin gradients that are required for fundamental plant developmental processes such as organogenesis and cell differentiation, enlargement, and division.
PAT occurs by a chemiosmotic mechanism (Raven, 1975; Rubery and Sheldrake, 1974). Indole-3-acetic acid (IAA, the natural auxin, with a pKa of ∼4.75) is partially protonated (IAAH) at relatively low apoplastic pH (∼5.5) and can enter the cell by diffusion from any direction. By contrast, the de-protonated IAA (IAA−) in the apoplast must be imported into the cell by the auxin influx carriers (AUX1 and LAXs). Once inside the cell, in neutral cytosolic pH, most IAAH is ionized to IAA−, which is then exported by efflux carriers such as PINs and members of the P-glycoprotein/ATP-binding cassette protein subfamily B (PGP/ABCB), indicating that auxin efflux carriers can act as a determining factor in PAT (Cho et al., 2007a; 2007b; Kleine-Vehn and Friml, 2008).
PIN proteins, named after the pin-shaped inflorescence of the Arabidopsis pin1 mutant, regulate diverse auxin-mediated developmental processes (Kleine-Vehn and Friml, 2008). PIN proteins are polar-localized in plant cells in accordance with directional auxin flow. For instance, in the root tip, auxin moves through the stele downward to the meristem and columella, in which the auxin maximum is formed for maintenance of root stem cell activity. Auxin next turns upward to the differentiation zone through the epidermis where some flows back to the root tip via the cortex (Blilou et al., 2005). Consistent with this pattern of auxin flow, PIN1, PIN3, and PIN7 are localized at the basal (toward root tip) side of stele cells (Blilou et al., 2005; Friml et al., 2002a; 2002b), and PIN4 is localized at the basal side in root stem cells (Friml et al., 2002a). PIN3 is localized at the inner-lateral side of pericycle cells (Friml et al., 2002b), likely redirecting auxin back into the stele by lateral translocation of auxin. In the columella cells, PIN3 and PIN7 exhibit apolar localization, enabling the lateral allocation of auxin to the epidermis (Friml et al., 2002b; Kleine-Vehn et al., 2010). PIN2 is localized at the apical side (toward the shoot) in epidermal cells and at the basal side in cortex cells (Luschnig et al., 1998; Müller et al., 1998), allowing auxin flow upward from the root tip and downward again through the cortex.
THE STRUCTURE OF PIN PROTEINS
Different PIN family members have different structures and localizations. The Arabidopsis PIN protein family consists of eight members, among which six PINs (PIN1-4, 6 and 7) have a long central hydrophilic loop (∼300 amino acids) connecting the five transmembrane helices on each end. The other two PINs (PIN5 and 8) have a very short (∼50 amino acids or less) hydrophilic loop (Fig. 1) (Ganguly et al., 2010; Mravec et al., 2009). Most of the ‘long-looped’ PINs (PIN1-4 and 7) localize in the PM, but the ‘short-looped’ PINs (PIN5 and 8) localize either predominantly in the internal compartment (endoplasmic reticulum) (e.g. PIN5) or both in the internal compartment and PM (e.g. PIN8) (Ganguly et al., 2010; Mravec et al., 2009). There is no direct evidence available on the role of the transmembrane domain (TM) in auxin transport, but several lines of evidence support the idea that the PIN hydrophilic loop acts in intracellular trafficking of PIN proteins. The PIN hydrophilic loop of the long-looped PINs contains several conserved phosphorylation motifs and sites that are targeted by some kinases (Fig. 1) to modulate PIN trafficking and its subcellular polarity. The absence of a similar long hydrophilic loop in the ‘short-looped’ PINs may explain their different subcellular localizations.
Fig. 1.

Putative PIN structures. (A) The PIN1 structure representing Arabidopsis long-looped PINs (PIN1∼4, 6 and 7). (B) The PIN5 structure representing Arabidopsis short-looped PINs (PIN5 and PIN8). Both the long- and short-looped PINs are predicted to contain five transmembrane helices (∼150 amino acids) spanning the plasma membrane in both the N-and C-terminal ends, which are connected by a hydrophilic loop (HL) on the predicted cytosolic side. In the long-looped PINs, the HL is over 300 residues long (328 for PIN1) whereas in the short-looped PINs, the HL is ∼50 residues long (42 for PIN5). The long-looped PIN-HLs carry several phosphorylation sites that are required for polar trafficking of the PINs. Shown here are the three TPRXS sites (circles) whose serine residues are targeted by the PID kinase, and Ser-337:340 (triangles), which are targeted by yet unknown kinases. The short-looped PINs lack these conserved phosphorylation motifs.
PINs UNDERGO ENDOCYTIC RECYCLING
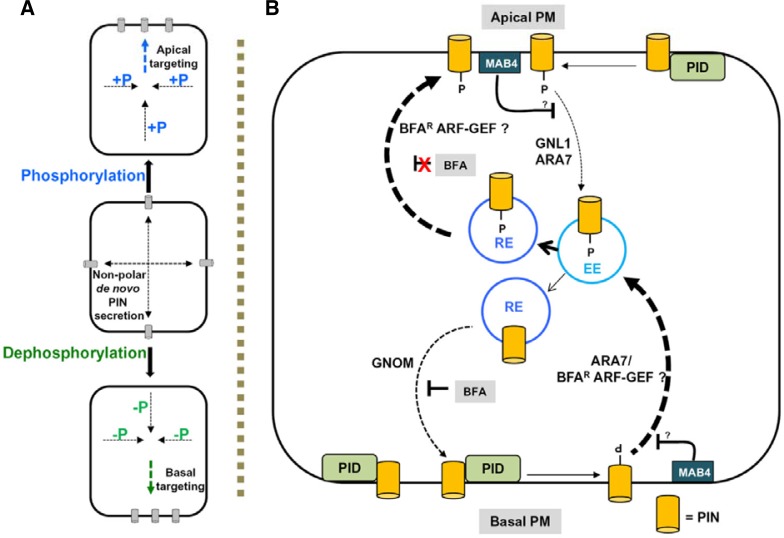
Polar PIN localization controls the direction of auxin flow between cells and thereby directs auxin gradients and distribution throughout the plant. PIN proteins undergo continuous cycles of endocytosis and exocytosis, which act in both determination and maintenance of PIN polarity (Kleine-Vehn and Friml, 2008). This dynamic process of PIN recycling is constitutive and rapid, but is also well coordinated and tightly regulated. Geldner et al. (2001) first showed recycling of PIN proteins through endocytosis and exocytosis. The fungal toxin brefeldin A (BFA) blocks the trafficking or exocytosis of PIN proteins from the endosomes to the PM, resulting in PIN accumulation into so-called “BFA compartments” within the cell (Geldner et al., 2001; 2003). BFA interferes with various trafficking pathways within the cell and specifically inhibits ADP-ribosylation factor-GDP/GTP exchange factor (ARF-GEF)-mediated vesicle trafficking (Donaldson and Jackson, 2000). PIN trafficking to the base of a cell (basal targeting) is regulated by the BFA-sensitive GNOM protein, an ARF-GEF (Fig. 2B) (Steinmann et al., 1999). In the embryo and the postembryonic root of the gnom mutant, basal PIN1 localization was lost but the apical localization of other PINs and AUX1 remained unaffected (Kleine-Vehn et al., 2006; 2008a; Steinamann et al., 1999). Similarly the BFA-mediated inhibition of apical PIN targeting was much weaker than basal PIN targeting, suggesting that the apical localization may be mediated by one (or more) BFA-resistant ARF-GEF (Kleine-Vehn et al., 2008). However, other PINs localize by different mechanisms; for example, in addition to GNOM, a BFA-resistant ARF-GEF protein GNL1 (GNOM-like 1) was found to be primarily involved in PIN2 trafficking (Geldner et al., 2003; Richter et al., 2007; Teh and Moore, 2007). In gnl1 mutant roots, the internalization of PIN2 from the PM was inhibited after BFA treatment. This indicates that GNL1 may act in coordination with a BFA-sensitive ARF-GEF to regulate PIN trafficking (Fig. 2B) (Teh and Moore, 2007).
Fig. 2.
Phosphorylation-mediated polar PIN trafficking. (A) PIN proteins are synthesized and secreted to the PM first in a non-polar manner; they subsequently become polar upon phosphorylation and recycling. Phosphorylated PINs show predominantly apical polarity whereas dephosphorylated PINs show predominant basal localization. (B) Model describing the mechanism of phosphorylation and endocytosis-mediated basal-to-apical PIN polarity switch. After undergoing PID-mediated phosphorylation in the PM, PINs are predominantly recruited to the apical targeting pathway through subsequent early (EE) and recycling (RE) endosomes in a BFA resistant (BFAR) manner, whereas the dephosphorylated PINs are predominantly recruited, in a similar manner, to a GNOM-depen-dent BFA sensitive basal targeting pathway. ARA7 and GNL1 mediate PIN endocytosis from the PM. Conversely, the polarly localized MAB4 inhibits PIN endocytosis by an unknown mechanism.
Newly synthesized PIN1 proteins are first secreted to the PM in a non-polar manner, after which the PINs undergo endocytic recycling to generate proper polarity (Fig. 2A) (Dhonukse et al., 2008). The Rab5-GTPase ARA7 (RabF2b) and its regulator VPS9A (vacuolar protein sorting) are responsible for the polarization of apolarly secreted PIN proteins in the Arabidopsis embryo and root (Dhonukse et al., 2008). Recent evidence suggests that polarization of PIN proteins is mediated by both ARF-GEF and Rab5-GTPase. GNOM acts in determination of basal PIN polarity, but RAB5-GTPases seem to be the common regulator of both apical and basal PIN trafficking (Dhonukse et al., 2008; 2010). The current model of PIN polarization suggests that ARA7 mediates the internalization of PIN proteins from the PM into the endosomes, and these endosomes are targeted either to the basal or the apical PM by GNOM or other ARF-GEF proteins (Fig. 2B). However, ARA7 resides in several endomembrane compartments such as early endosomes (Ueda et al., 2004), multivesicular bodies (MVBs; Haas et al., 2007), and vacuolar membrane (Ebine et al., 2011), but has never been reported to be present in the PM. Therefore, the actual mechanism by which ARA7 inhibits PIN internalization from the PM remains unknown. In this regard, more evidence is needed to understand the synchronization between the ARF-GEFs and Rab-GTPases and how they collectively regulate PIN polarity.
PIN POLARITY IS DETERMINED BY ITS PHOSPHORYLATION STATUS
Constitutive recycling through different trafficking pathways determines the polar localization of PIN proteins in the PM. Phosphorylation has been implicated in this polar trafficking of PIN proteins (Kleine-Vehn and Friml, 2008). When expressed in a single cell type (root epidermis) PIN1, PIN2, PIN3 and PIN4 show distinct and different polar localizations. PIN1 is basal and PIN2 is apical in the root epidermal cells, suggesting that the polarity-determining cue may reside within the PIN protein itself (Wiśniewska et al., 2006). This hypothesis is strongly supported by the observation that a GFP insertion disrupting the PIN1 hydrophilic loop triggers a basal-to-apical shift of PIN1 polarity in root epidermal cells (Wiśniewska et al., 2006). Several PINs have been shown to move between different sides of the PM under different cellular and environmental conditions. This phenomenon has been termed transcytosis (Kleine-Vehn et al., 2008). Inhibition of the GNOM-mediated basal PIN targeting machinery by prolonged BFA treatment leads to a basal-to-apical switch in PIN polarity (Kleine-Vehn et al., 2008; Rahman et al., 2010). Basally localized PIN1 in the stele undergoes transcytosis after prolonged (∼12 h) BFA treatment and shows predominantly apical PM localization (Kleine-Vehn et al., 2008). Similar transcytosis is observed when PIN2, which is basal in the young cortex cells, moves to the apical PM after prolonged (∼48 h) BFA treatment (Rahman et al., 2010).
The earliest evidences for the role of phosphorylation in auxin transport came from studies showing that the kinase inhibitors staurosporine and K25A inhibited auxin efflux from tobacco (Nicotiana tabacum) suspension culture cells (Delbarre et al., 1998). Later, loss-of-function mutant studies of a serine-threonine protein kinase PINOID (PID) belonging to the AGC kinase family (named after the protein kinase A, G, and C families) also indicated a role of phosphorylation in polar auxin transport (Benjamins et al., 2001; Christensen et al., 2000). Moreover, PID was also found to facilitate auxin efflux from Arabidopsis root hair cells and from tobacco BY-2 cells (Lee and Cho, 2006). Later studies demonstrated that PID is involved in determination of PIN polarity. Overexpression of PID led to a basal-to-apical switch of PIN1, PIN2 and PIN4 proteins in Arabidopsis root tissues (Friml et al., 2004). PID was shown to phosphorylate the central hydrophilic loop of PIN proteins in vitro; also, the protein phosphatase 2A (PP2A) antagonizes this PID-mediated PIN phosphorylation (Michniewicz et al., 2007). Consistent with these observations, pid loss-of-function mutants showed preferential basal targeting of PINs (Friml et al., 2004), whereas pp2a loss-of-function led to preferential apical PIN targeting in embryos and roots (Michniewicz et al., 2007). These results led to the hypothesis that phosphorylated PINs are recruited predominantly to the apical targeting pathway and the phospho-defective PINs are recruited predominantly to the basal targeting pathway (Michniewicz et al., 2007).
AGC1 KINASES DIRECTLY PHOSPHORYLATE THE PIN HYDROPHILIC LOOP
Recently, it was reported that PID directly phosphorylates the serine residue of the conserved TPRXS motifs (three in PIN1-4, 7 and two in PIN6) in the PIN hydrophilic loop (Dhonukshe et al., 2010; Huang et al., 2010). Two other members of the AGC1 (or AGCVIIIa, Bögre et al., 2003) kinase subfamily of proteins (WAG1 and WAG2) also phosphorylate the same TPRXS motif (Dhonukshe et al., 2010). Phospho-mimetic mutations (Ser to Glu) of the three serine residues led to predominantly apical PIN localization; conversely, the phospho-defective mutations (Ser to Ala) led to predominantly basal PIN localization (Dhonukshe et al., 2010; Huang et al., 2010). Similarly, the apically localized PIN2 protein showed predominantly basal localization in the pid/wag1/wag2 triple mutant, (Dhonukshe et al., 2010). Moreover, phosphorylated PINs were targeted to the apical PM in a BFA resistant manner (Dhonukse et al., 2010; Kleine-Vehn et al., 2009), which is again consistent with the BFA resistant apical PIN localization mechanism (see above). These data collectively suggest that the phosphorylation status and polarity of PINs depend on the cell type where they are expressed, and constitutive phosphorylation or dephosphorylation of PINs disrupts cell type-specific polar PIN localization, thereby altering polar auxin transport. In summary, PINs, after being secreted to the PM in a non-polar fashion, are phosphorylated in the PM by the AGC1 kinase family members. These phosphorylated PIN proteins then have reduced affinity for the GNOM-dependent basal recruiting pathway and are recruited preferentially to the apical GNOM-independent trafficking pathway (Figs. 2A and 2B). Thus, AGC1 kinases and GNOM antagonistically regulate PIN polarity. This process of PIN polarization is different from that of animal models where polar sorting of cargo takes place in so-called sorting endosomes (Farr et al., 2009) but not in the PM. In plants, AGC1 kinases, being predominantly localized at the PM, may not affect the polar targeting of PIN proteins that are newly synthesized or present in the various endosomal compartments, but only phosphorylate PINs in the PM (Dhonukse et al., 2008).
Several lines of recent evidence suggest that PID also acts in lateral polarity of PIN3 in the hypocotyl endodermis (Ding et al., 2011). Upon phototropic stimulation, PIN3 gradually disappears from the outer lateral PM side of the illuminated endodermis cell, but the inner lateral PM side of the illuminated cell and both lateral PM sides of the shaded cell maintain normal levels of PIN3 signals (Ding et al., 2011). This light-mediated polarization of PIN3 in the illuminated endodermal cells is likely to cause diversion of auxin flow towards the shaded side of the hypocotyl, causing auxin-mediated cell elongation and therefore hypocotyl bending towards the direction of the light (Ding et al., 2011). The PID kinase was implicated in this light-mediated polarization of PIN3 in the hypocotyl. Overexpression of PID strongly inhibited the light-mediated inner lateral polarization of PIN3 in the illuminated endodermis, thereby making the hypocotyl defective in phototropism (Ding et al., 2011). Similar PID-mediated inhibition of PIN3 polarization was observed in the hypocotyl endodermal cells after gravity stimulus (Rakusova et al., 2011). Interestingly, although loss of phototropin 1 (the blue light receptor belonging to the AGC2 subgroup) impaired PIN3 polarization in the hypocotyl endodermis, it did not directly phosphorylate the PIN3 hydrophilic loop in vitro (Ding et al., 2011). Zourelidou et al. (2009) reported that D6PK (D6 protein kinase), another AGC1 subgroup protein kinase, might be implicated in auxin transport, most likely by modulating PIN activity. D6PK was able to phosphorylate the hydrophilic loop of several PINs in vitro and in Arabidopsis protoplasts. The kinase was polarly co-localized with PINs, and its mutation caused defects in stem auxin transport. However, because the loss or overexpression of D6PK did not affect PIN polarity, it still remains to be determined whether D6PK-mediated PIN phosphorylation is directly involved in modulation of PIN polarity.
So far, only three AGC1 kinases have been shown to directly target the PIN hydrophilic loop to affect PIN polarity. However, some experimental results indicate that other kinases, in addition to those three AGC1s, are involved in PIN phosphorylation and polar trafficking. Zhang et al. (2010) identified two PID-independent phosphorylation sites in the PIN1 hydrophilic loop (Ser 337 and 340) that also affect polar PIN localization. The corresponding phospho-mimetic version (Asp 337 and 340) of the PIN1 protein exhibited predominantly apical polarity in the root epidermal cells.
These lines of evidence suggest that only certain phosphorylation sites in the PIN hydrophilic loop are likely to be functional for polar trafficking of PINs, and that the AGC kinases are the major but not the only players that modulate PIN polarity. Moreover, the PIN1 phospho-mimetic version (i.e., Asp 337 and 340), which showed apical polarity in the root epidermis, did not show apical localization in the root vasculature, suggesting that different tissues may require different phosphorylation codes for specific trafficking mechanisms. These results raise the question of why there are so many phosphorylation sites and why so many kinases are involved in phosphorylation of these sites to modulate PIN polarity.
SIGNALING TO AGC1 KINASES
The 3-phosphoinositide-dependent protein kinase-1 (PDK1) positively regulates PID activity in vitro through phosphorylation (Zegzouti et al., 2006a). PDK1 interacts with several AGCVIII kinase family members by binding to their hydrophobic C-terminal PDK1-interacting fragment (PIF) domain (Zegzouti et al., 2006b). Hence, PDK1 may act as a master regulator of some AGCVIII kinases (Zhang and McCormick, 2008). PID does have a PIF domain, but WAG1 and WAG2 do not possess a PIF domain (Zegzouti et al., 2006b), implying that these WAGs may not be direct substrates of PDK1. Moreover, PID, which is an auto-activating kinase (autophosporylation), may not need PDK1 during normal development (Sauer et al., 2006). Although PDK1 increases both trans-phosphorylation and autophosphorylation of several AGCVIII kinases in vitro (Anthony et al., 2006; Devarenne et al., 2006), its role in polar PIN localization remains to be deciphered.
A Bric-a-Brac (BTB) scaffold protein, MACCHI-BOU4/ENHANCER OF PINOID/NAKED PINS IN YUC MUTANTS 1 (MAB4/ENP/NPY1), acts together with PID in PAT and organ formation (Cheng et al., 2007; Furutani et al., 2007). Recent findings showed that the Arabidopsis MAB4 protein and four of its close homologs, MAB4/ENP/NPY1-LIKE1 (MEL1), MEL2, MEL3 and MEL4, form a subfamily, regulate root gravitropism, and are functionally redundant (Furutani et al., 2011). The MAB4 subfamily proteins are polarly localized similarly to PIN proteins and regulate PIN internalization from the PM. PIN proteins were retained at the PM where the MAB4 proteins were present, whereas PINs were internalized from the PM where the MAB4 proteins were absent. Cumulatively, these data suggest that apolar AGC1 kinases might promote PIN internalization only from the PM where MAB4 proteins are absent (Fig. 2B) (Furutani et al., 2011). However, the molecular mechanism by which MAB4 regulates PIN internalization by inhibiting PID action remains unknown. These studies collectively indicate that diverse regulators operate upstream of PID to regulate PIN polarity and PAT.
POLAR PROTEIN TRAFFICKING OF BORON TRANSPORTERS
Boron transporters BOR1 and BOR4 also show asymmetric localization in the plasma membrane. Boron, an essential nutrient for plants, is toxic in excessive concentrations, and can be transported through apoplastic flow. However, the casparian band on the endodermal cell walls acts as a barrier to apoplastic ion flow between the outer root surface side and the inner xylem side; therefore, boron uptake from the root surface to the xylem requires at least two transmembrane transport components (Takano et al., 2008). One is a member of the major intrinsic protein (MIP) family, NIP5;1 (nodulin 26-like intrinsic protein), for boron import, which was identified by screening for genes upregulated in boron deficient Arabidopsis roots (Takano et al., 2006). The other is the boron exporter BOR1, identified by analysis of the Arabidopsis bor1-1 mutant, which has a high sensitivity to boron deficiency (Takano et al., 2002). BOR1 localizes at the inner lateral side of the PM in the root columella, lateral root cap, epidermis, endodermis, and pericycle; moreover, under boron deficient conditions bor1-1 mutants show retarded growth and reduced boron accumulation mainly in shoot tissues, suggesting that BOR1 is responsible for loading of boron into the xylem (Takano et al., 2002; 2010).
It has been proposed that under boron-deficient conditions BOR1 is continuously internalized from the PM into BFA-sensitive and Rab-GTPase Ara7-positive endosomes for recycling and upon exposure to high concentrations of boron, BOR1 is sorted into MVB for degradation; this sorting provides a rapid and efficient mechanism to regulate BOR1 activity (Fuji et al., 2009; Takano et al., 2005; 2008). Three tyrosine residues in the largest cytosolic loop of BOR1 are required for its polar and vacuolar trafficking (Takano et al., 2010), although the underlying mechanism remains to be elucidated. BOR4, one of the six BOR1 paralogs, is also a boron exporter. In contrast to BOR1, BOR4 is not degraded in response to high boron supply, and is localized to the outer lateral side of the PM in the root epidermis; thus BOR4 is likely to be important for boron export from the root to the soil (Fuji et al., 2009; Miwa et al., 2007). So far there is no indication that BOR4 undergoes endocytosis and/or recycling. Łangowski and coworkers (2010) characterized the polar localization of BOR4 in detail. Unlike apical- or basal-localized PINs, BOR4 polarity is already established during the secretion of the newly synthesized protein and is independent of known molecular components for apical or basal targeting such as GNOM, AXR4, PINOID, and PP2A. This suggests a unique polar targeting mechanism for the outer lateral polarity of the boron transporters.
POLAR PROTEIN TRAFFICKING IN PROKARYOTES AND EUKARYOTES
Polar protein trafficking occurs ubiquitously in unicellular and multicellular organisms. Certain bacterial cells possess a highly organized and dynamic internal architecture (Gitai, 2005). Lacking cell compartmentalization, bacteria are polarized at the molecular level and, interestingly, many bacterial proteins also localize to a particular site or ‘poles’ of the cell (Ebersbach and Jacobs-Wagner, 2007; Gitai, 2005; Rudner and Losick, 2010). Most bacteria have two poles, the new pole originating from the most recent division and the old pole from the previous division. Bacterial protein polarization occurs through multiple mechanisms (Ebersbach and Jacobs-Wagner, 2007). For example, the bacterial protein ActA (a surface protein) polarizes by a unique mechanism in Listeria monocytogenes (Kocks et al., 1993). ActA is secreted to certain discrete sites of the bacterial cell and associates with the peptidoglycan layer in the bacterial cell wall. During cell elongation the helically patterned peptidoglycan growth pushes the older peptidoglycan layer and the associated ActA (Rafelski et al., 2006). The ActA protein thus remains trapped by the older pepidoglycan layer and exhibits polar localization also in the daughter cells. A somewhat similar mechanism of maintaining polar PIN localization has been recently identified in plants. Plants use connections between the PM and certain cellulose based components in the cell wall to trap the movement of polar PINs (PIN1 and PIN2). The PM-cell wall association probably restricts the lateral diffusion of the PINs and thus helps maintain polar PIN localization (Feraru et al., 2010).
Phosphorylation-mediated polar protein localization is pivotal for cell division of certain bacterial species. The dimorphic aquatic bacterium Caulobacter crescentus undergoes asymmetric cell division to generate a sessile ‘stalked cell’ and a motile ‘swarmer cell’ (Matroule et al., 2004). In the predivision Caulobacter cells, the single-domain response regulator DivK exhibits a dynamic bipolar localization and rapidly shuttles between the two poles. This rapid polar movement of DivK between the swarmer and the stalked pole is guided by its phosphorylation status. The DivJ kinase phosphorylates DivK at the stalked pole and the phosphorylated DivK (DivK∼P) then moves to the swarmer pole. Also, the PleC phosphatase dephosphorylates DivK∼P at the swarmer pole, triggering the movement of DivK to the stalked pole. However, this rapid ‘pole-to-pole’ movement of DivK only continues as long as the cytoplasm of both cells (old and new) is connected. Once the cell has divided, the new cell predominantly retains dephosphorylated DivK and the old cell predominantly retains phosphorylated DivK (Fig. 3) (Ebersbach and Jacobs-Wagner, 2007; Matroule et al., 2004). In addition to the movement of the DivK protein, the coordinated action of several other regulatory proteins (including kinases and phosphatases) is required for a well-organized asymmetric division of the Caulobacter cell. The polar movement of DivK depending on its phosphorylation status bears a striking resemblance to PIN polarization in plant cells, which is mediated by the PID kinase and PP2A phosphatase (Fig. 3).
Fig. 3.
Phosphorylation-mediated polar protein trafficking in plants, bacteria, and mammals. The phosphorylation mediated protein polarity switch operates in plants (PINs in Arabidopsis thaliana), bacteria (DivK in Caulobacter crescentus) and mammals (PARs in mouse) despite wide differences in kinases and their substrates. (A) In plants, PID-mediated phosphorylation of PIN retargets it to the apical PM. (B) Similarly in bacteria, phosphorylation of DivK by DivJ, which localizes in the ‘stalked cell pole’, translocates DivK to the ‘swarmer cell pole’. In the ‘swarmer cell pole’, DivK is subsequently dephosphorylated by the PleC phosphatase, which again redirects DivK back to the ‘stalked cell pole’. (C) In mammals, phosphorylation also serves as the major regulating factor of PAR protein polarity. In mouse epithelial cells, the basolateral PAR1 protein strictly maintains the apical PAR3 polarity by phosphorylating and subsequently redirecting it back to its original apical membrane from the basolateral sides. Conversely, aPKC-PAR6-mediated phosphorylation of PAR1 redirects PAR1 back to the basolateral sides of the epithelial cells.
In animals such as insects, nematodes, and mammals, a group of PAR (partition defective) proteins establish epithelial cell polarity and bilateral symmetry (Goldstein and Macara, 2007; Nance and Zallen, 2011). In mammals, three major polarity complexes regulate the apical and basolateral polarity of epithelial cells, the PAR (CDC42-PAR3-PAR6-aPKC), Crumb (Crb-PALS-PATJ) and Scribble (Scrib-Dlg-Lgl) complexes. PAR and Crumb promote apical polarity and Scribble promotes basolateral polarity (Goldstein and Macara, 2007). Movement of Scribble towards the apical domain is antagonized by the aPKC (a Ser/Thr kinase) protein of the PAR complex. aPKC phosphorylates LGL and thereby promotes its dissociation from the cell cortex, thus maintaining the apical polarity of the PAR complex (Bryant and Mostov, 2008; Plant et al., 2003). Interestingly, the PAR genes encode a variety of scaffold and signaling proteins, and the PAR proteins are themselves polarly localized. Among the six PARs, PAR1 (a Ser/Thr kinase) and PAR3 (a PDZ domain protein) show distinct basolateral and apicobasal membrane polarity in fly epithelial cells, and their phosphorylation by several regulatory proteins inhibits their attachment to the membrane. However, PAR1 kinase itself can phosphorylate PAR3 in order to maintain its own basolateral localization and also stabilize microtubule organization to maintain cell polarity (Fig. 3) (Goldstein and Macara, 2007).
Regulation of PIN polarity by phosphorylation shows substantial similarity to PAR protein localization in epithelial cells (Fig. 3). However, in case of PIN proteins, phosphorylation and endocytic recycling, rather than the cytoskeletal organizational cues, are the key factors that maintain polarity (Geldner, 2008). The distinct polar localization of individual PINs and the presence of multiple phosphorylation sites in the PIN cytosolic loop indicate more complex regulation of polar protein trafficking in plants. By contrast, PAR proteins are under tight regulation by diverse scaffold proteins, kinases, small GTPases, cytoskeletal proteins and other signaling molecules, most of which are either absent or yet to be identified in plants. The identification and characterization of such regulatory proteins for PIN phosphorylation and polar trafficking will shed more light on the mechanism by which PINs target specifically to a certain membrane side. It is possible that different phosphorylation sites in the PIN hydrophilic loop are targeted by different kinases to confer different localization cues to certain PIN species in a cell type-specific manner. Future studies to identify and decipher the PIN phosphorylation code for specific upstream kinases and downstream interacting partners will illuminate the core mechanism of phosphorylation-mediated modulation of PIN trafficking.
In bacterial and mammalian systems, several proteins interacting with those ‘polar’ proteins have been identified (Nance and Zallen, 2011; Tsokos et al., 2010). In plants, PID homologs are the only known PIN interactors. Future studies to identify PIN interacting proteins based on PIN phosphorylation status should provide a much better understanding of polar protein trafficking in plants.
Acknowledgments
This work was supported by grants from the Mid-career Researcher Program [2011-0017242, National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology (MEST)] and the Next-Generation BioGreen 21 programs (TAGC PJ00820701 and SSAC PJ00814102) of Rural Development Administration. AG was partly supported by the graduate student fellowship from Korea Research Foundation.
REFERENCES
- Anthony R.G., Khan S., Costa J., Pais M.S., Bögre L. The Arabidopsis protein kinase PTI1-2 is activated by convergent phosphatidic acid and oxidative stress signaling pathways downstream of PDK1 and OXI1. J. Biol. Chem. 2006;281:37536–37546. doi: 10.1074/jbc.M607341200. [DOI] [PubMed] [Google Scholar]
- Benjamins R., Quint A., Weijers D., Hooykaas P., Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128:4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Bögre L., Ökrész L, Henriques R, Anthony R.G. Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 2003;8:424–431. doi: 10.1016/S1360-1385(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Bryant D.M., Mostov K.E. From cells to organs building polarized tissue. Nat. Rev. Mol. Cell. Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Qin G., Dai X., Zhao Y. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2007;104:18825–18829. doi: 10.1073/pnas.0708506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Lee O.R., Ganguly A., Cho H.-T. Auxin-signaling short and long. J. Plant Biol. 2007a;50:79–89. [Google Scholar]
- Cho M., Lee S.H., Cho H-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007b;19:3930–3943. doi: 10.1105/tpc.107.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S.K., Dagenais N., Chory J., Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Cove D.J. The generation and modification of cell polarity. J. Exp. Bot. 2000;51:831–838. [PubMed] [Google Scholar]
- Davies P.J. The plant hormones: their nature, occurrence and function. In: Davies P.J., editor. In Plant Hormones: Biosynthesis, Signal Transduction, Action! 3rd ed. Dordrecht, Netherlands: Kluwer Academic Publishers; 2005. pp. 1–15. [Google Scholar]
- Dettmer J., Friml J. Cell polarity in plants: when two do the same, it is not the same. Curr. Opin. Plant Biol. 2011;23:686–696. doi: 10.1016/j.ceb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Devarenne T.P., Ekengren S.K., Pedley K.F., Martin G.B. Adi3 is a Pdk1-interacting AGC kinase that negatively regulates plant cell death. EMBO J. 2006;25:255–265. doi: 10.1038/sj.emboj.7600910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., Tanaka H., Goh T., Ebine K., Mahonen A.P., Prasad K., Blilou I., Geldner N., Xu J., Uemura T., et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456:962–966. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dhonukshe P., Huang F., Galvan-Ampudia C.S., Mähönen A.P., Kleine-Vehn J., Xu J., Quint A., Prasad K., Friml J., Scheres B., et al. Plasma membrane bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development. 2010;137:3245–3255. doi: 10.1242/dev.052456. [DOI] [PubMed] [Google Scholar]
- Ding Z., Galván-Ampudia C.S., Demarsy E., Łangowski Ł, Kleine-Vehn J, Fan Y, Morita M.T., Tasaka M., Fankhauser C., Offringa R., et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 2011;13:447–452. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- Ebersbach G., Jacobs-Wagner C. Exploration into the spatial and temporal mechanisms of bacterial polarity. Trend Microbiol. 2008;3:101–108. doi: 10.1016/j.tim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Ebine K., Fujimoto M., Okatani Y., Nishiyama T., Goh T., Ito E., Dainobu T., Nishitani A., Uemura T., Sato M.H., et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011;13:853–860. doi: 10.1038/ncb2270. [DOI] [PubMed] [Google Scholar]
- Farr G.A., Hull M., Mellman I., Caplan M.J. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J. Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E., Feraru M.I., Kleine-Vehn J., Martinière A., Mouille G., Vanneste S., Vernhettes S., Runions J., Friml J. PIN polarity maintenance by the cell wall in Arabidopsis. Curr. Biol. 2011;21:338–343. doi: 10.1016/j.cub.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Friml J., Benková E., Blilou I., Wisniewska J., Hamann T., Ljung K., Woody S., Sandberg G., Scheres B., Jürgens G., et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002a;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002b;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Friml J., Yang X., Michniewicz M., Weijers D., Quint A., Tietz O., Benjamins R., Ouwerkerk P.B., Ljung K., Sandberg G., et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fuji K., Miwa K., Fujiwara T. The interacellular transport of transporters membrane trafficking of mineral transporters. Curr. Opin. Plant Biol. 2009;12:699–704. doi: 10.1016/j.pbi.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Furutani M., Kajiwara T., Kato T., Treml B.S., Stockum C., Torres-Ruiz R.A., Tasaka M. The gene MACCHI-BOU 4/ENHANCER OF PINOID encodes an NPH3-like protein and reveals similarities between organogenesis and phototropism at the molecular level. Development. 2007;134:3849–3859. doi: 10.1242/dev.009654. [DOI] [PubMed] [Google Scholar]
- Furutani M., Sakamoto N., Yoshida S., Kajiwara T., Robert H.S., Friml J., Tasaka M. Polar-localized NPH3-like proteins regulate polarity and endocytosis of PIN-FORMED auxin efflux carriers. Development. 2011;138:2069–2078. doi: 10.1242/dev.057745. [DOI] [PubMed] [Google Scholar]
- Ganguly A., Lee S.H., Cho M., Lee O.R., Ryu H., Cho H.-T. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010;153:1046–1061. doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N. Cell polarity in plants a PARspective on PINs. Curr. Opin. Plant Biol. 2008;12:42–48. doi: 10.1016/j.pbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jurgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Gitai Z. The new bacterial cell biology: moving parts and subcellular architecture. Cell. 2005;120:577–586. doi: 10.1016/j.cell.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Macara I.G. The PAR proteins: Fundamental players in animal cell polarization. Dev. Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas T.J., Sliwinski M.K., Martínez D.E., Preuss M., Ebine K., Ueda T., Nielsen E., Odorizzi G., Otegui M.S. The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell. 2007;19:1295–1312. doi: 10.1105/tpc.106.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Zago M.K., Abas L., Van M.A., Galvan-Ampudia C.S., Offringa R. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell. 2010;22:1129–1142. doi: 10.1105/tpc.109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Gray W.M. A gain-of-function mutation in the Arabidopsis pleiotropic drug resistance transporter PDR9 confers resistance to auxinic herbicides. Plant Physiol. 2006;142:63–74. doi: 10.1104/pp.106.084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Dhonukshe P., Swarup R., Bennett M., Friml J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell. 2006;18:3171–3181. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Dhonukshe P., Sauer M., Brewer P.B., Wisniewska J., Paciorek T., Benkova E., Friml J. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Huang F., Naramoto S., Zhang J., Michniewicz M., Offringa R., Friml J. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell. 2009;21:3839–3849. doi: 10.1105/tpc.109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Ding Z., Jones A.R., Tasaka M., Morita M.T., Friml J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA. 2010;107:22344–22349. doi: 10.1073/pnas.1013145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Hellio R., Gounon P., Ohayon H., Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J. Cell Sci. 1993;105:699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- Langowski L., Ruzicka K., Naramoto S., Kleine-Vehn J., Friml J. Trafficking to the outer polar domain defines the root-soil interface. Curr. Biol. 2010;20:904–908. doi: 10.1016/j.cub.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Cho H.-T. PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2006;18:1604–1616. doi: 10.1105/tpc.105.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C., Gaxiola R.A., Grisafi P., Fink G.R. EIR1, a root specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matroule J.Y., Lam H., Burnette D.T., Jacobs-Wagner C. Cytokinesis monitoring during development; rapid pole-to-pole shuttling of a signaling protein by localized kinase and phosphatase in Caulobacter. Cell. 2004;118:579–590. doi: 10.1016/j.cell.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Michniewicz M., Zago M.K., Abas L., Weijers D., Schweighofer A., Meskiene I., Heisler M.G., Ohno C., Zhang J., Huang F., et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Miwa K., Takano J., Omori H., Seki M., Shinozaki K., Fujiwara T. Plants tolerant of high boron levels. Science. 2007;318:1417. doi: 10.1126/science.1146634. [DOI] [PubMed] [Google Scholar]
- Mravec J., Skupa P., Bailly A., Hoyerova K., Krecek P., Bielach A., Petrasek J., Zhang J., Gaykova V., Stierhof Y.D., et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- Müller A., Guan C., Galweiler L., Tanzler P., Huijser P., Marchant A., Parry G., Bennett M., Wisman E., Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J., Zallen J.A. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.W. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant P.J., Fawcett J.P., Lin D.C., Holdorf A.D., Binns K., Kulkarni S., Pawson T. A polarity complex of mPar-6 and atypical PKC binds phosphorylates and regulates mammalian LGL. Nat. Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Rafelski S.M., Theriot J.A. Mechanism of polarization of Listeria monocytogenes surface protein ActA. Mol. Microbiol. 2006;59:1262–1279. doi: 10.1111/j.1365-2958.2006.05025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Takahashi M., Shibasaki K., Wu S., Inaba T., Tsurumi S., Baskin T.I. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells. Plant Cell. 2010;22:1762–1776. doi: 10.1105/tpc.110.075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusová H., Gallego-Bartolomé J., Vanstraelen M., Robert H.S., Alabadí D., Blázquez M. A., Benková E., Friml J. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J. 2011;67:817–826. doi: 10.1111/j.1365-313X.2011.04636.x. [DOI] [PubMed] [Google Scholar]
- Raven J.A. Transport of indolacetic acid in plant cells in relation to pH and electrical potential gradients and its significance for polar IAA transport. New Phytol. 1975;74:163–172. [Google Scholar]
- Rubery P.H., Sheldrake A.R. Carrier-mediated auxin transport. Planta. 1974;188:101–121. doi: 10.1007/BF00388387. [DOI] [PubMed] [Google Scholar]
- Rudner D.J., Losick R. Protein Subcellular Localization in Bacteria. Cold Spring Harb. Perspect Biol. 2010;2:a000307. doi: 10.1101/cshperspect.a000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Dittgen J., Sanchez-Rodriguez C., Hou B.H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Galweiler L., Palme K., Jurgens G. Co-ordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- Takano J., Noguchi K., Yasumori M., Kobayashi M., Gajdos Z., Miwa K., Hayashi H., Yoneyama T., Fujiwara T. Arabidopsis boron transporter for xylem loading. Nature. 2002;420:337–340. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]
- Takano J., Miwa K., Yuan L., Von Wiren N., Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Wada M., Ludewig U., Schaaf G., Wirén N., Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Miwa K., Fujiwara T. Boron transport mechanisms collaboration of channels and transporters. Trends Plant Sci. 2008;13:451–457. doi: 10.1016/j.tplants.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA. 2010;107:5220–5225. doi: 10.1073/pnas.0910744107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh O.K., Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–496. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- Tsokos C.G., Perchuk B.S., Laub M.T. A dynamic complex of signaling proteins uses polar localization to regulate cell-fate asymmetry in Caulobacter crescentus. Dev. Cell. 2011;20:329–341. doi: 10.1016/j.devcel.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Uemura T., Sato M.H., Nakano A. Functional differentiation of endosomes in Arabidopsis cells. Plant J. 2004;40:783–789. doi: 10.1111/j.1365-313X.2004.02249.x. [DOI] [PubMed] [Google Scholar]
- Wiśniewska J., Xu J., Seifertová D., Brewer P.B., Růžička K., Blilou I., Rouquié D., Benková E., Scheres B., Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- Zegzouti H., Anthony R.G., Jahchan N., Bögre L., Christensen S.K. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006a;103:6404–6409. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H., Li W., Lorenz T.C., Xie M., Payne C.T., Smith K., Glenny S., Payne G.S., Christensen S.K. Structural and functional insights into the regulation of Arabidopsis AGC VIIIa kinases. J. Biol. Chem. 2006b;281:35520–30. doi: 10.1074/jbc.M605167200. [DOI] [PubMed] [Google Scholar]
- Zhang Y., McCormick S. AGCVIII kinases at the crossroads of cellular signaling. Trends Plant Sci. 2008;14:689–695. doi: 10.1016/j.tplants.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang J., Nodzyński T., Pĕnčík A., Rolčík J., Friml J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc. Natl. Acad. Sci. USA. 2010;107:918–922. doi: 10.1073/pnas.0909460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M., Müller I., Willige B.C., Nill C., Jikumaru Y., Li H., Schwechheimer C. The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development. 2009;136:627–636. doi: 10.1242/dev.028365. [DOI] [PubMed] [Google Scholar]