Abstract
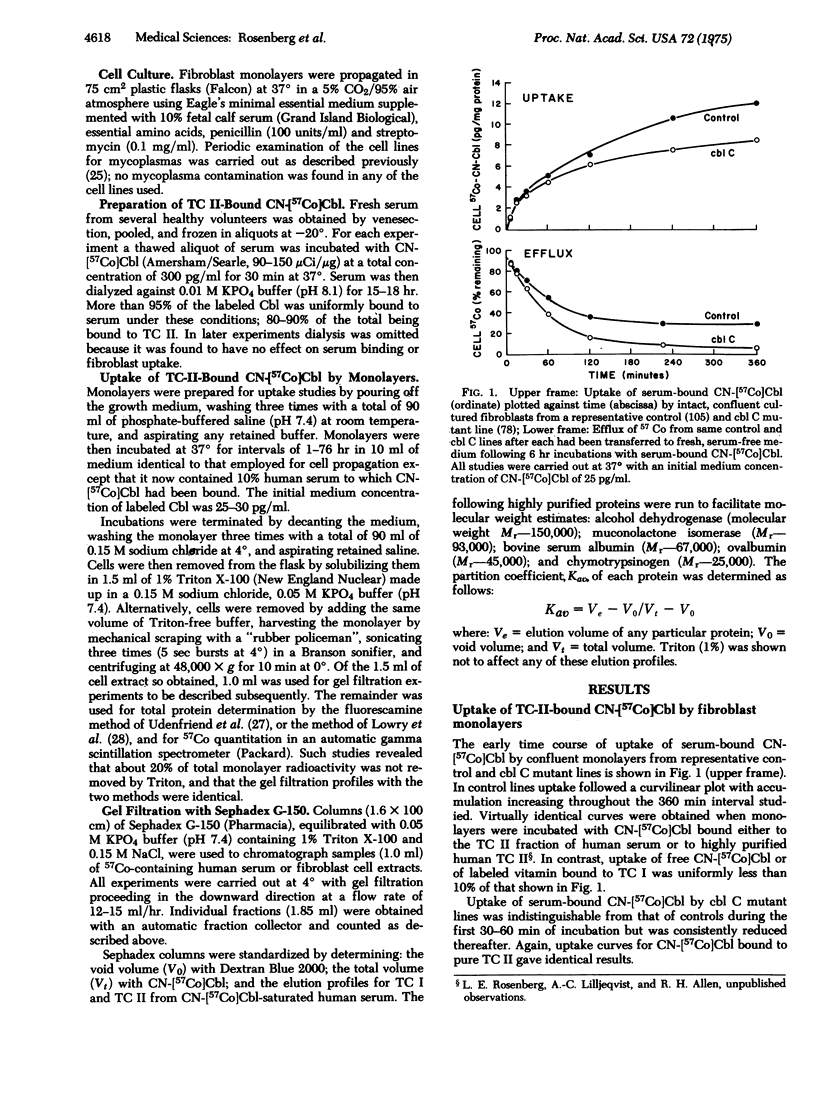
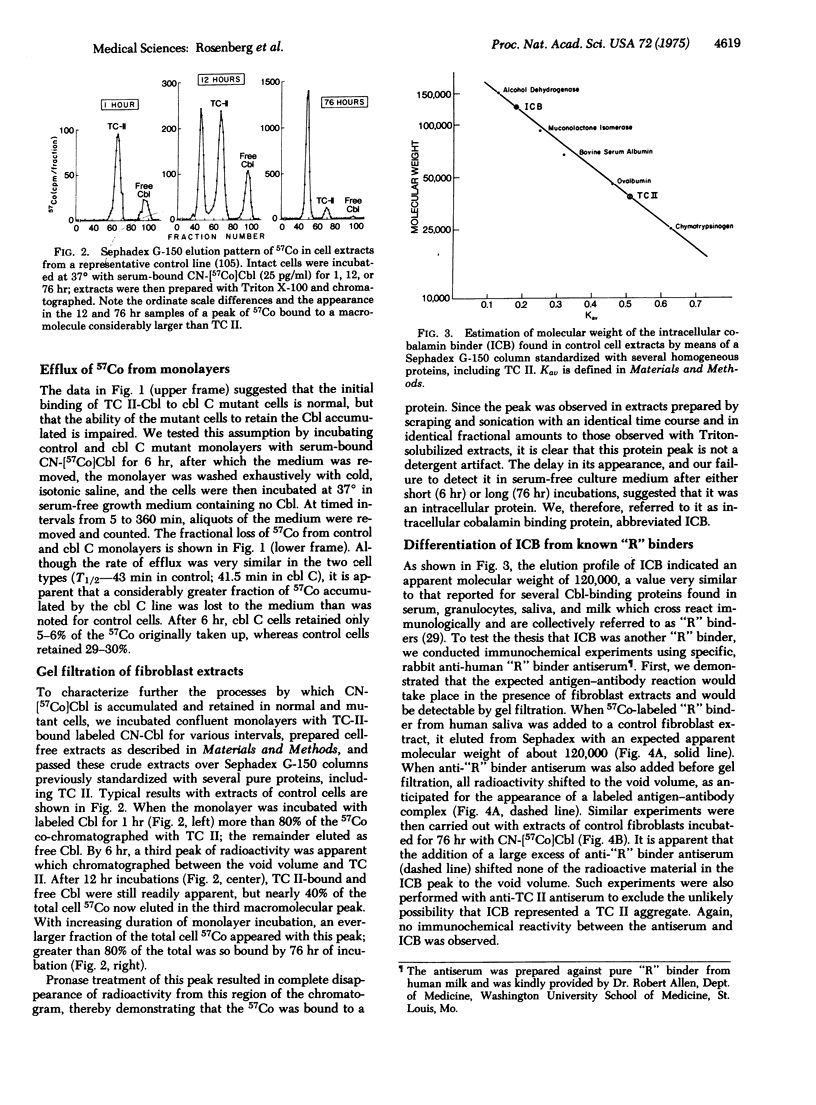
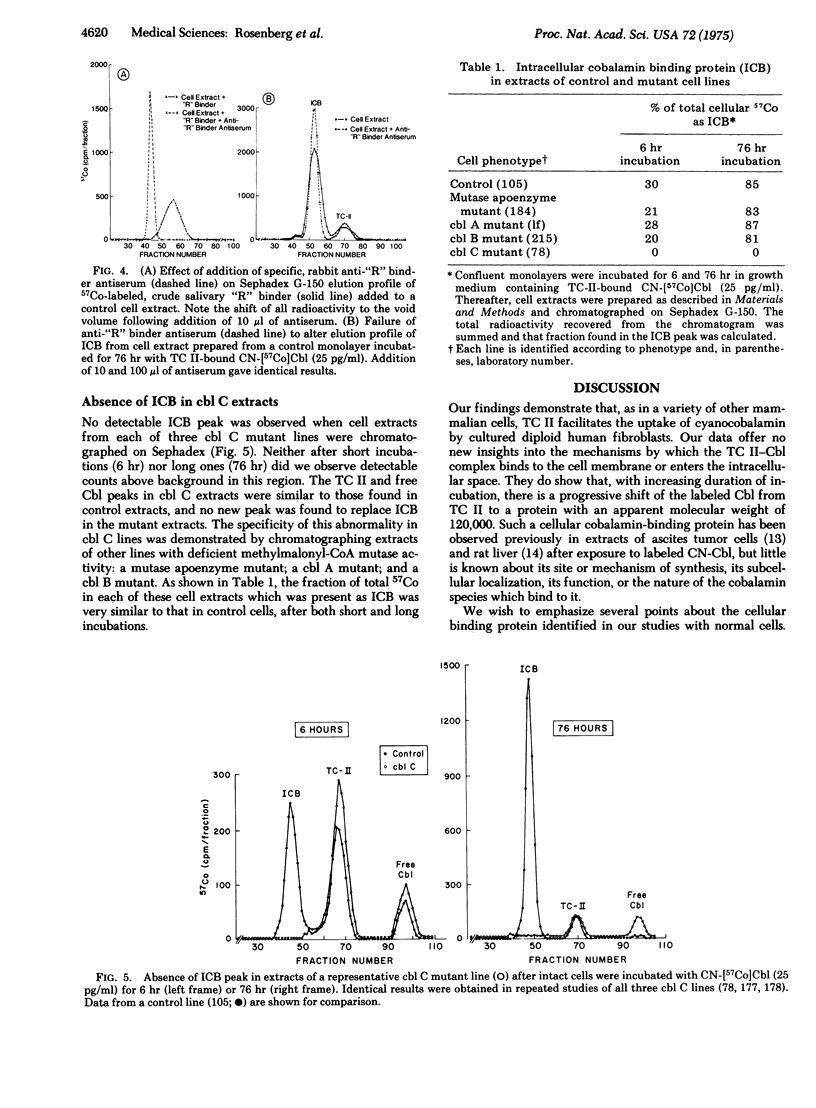
Three distinct classes of human mutations (cbl A, cbl B, and cbl C) cause defective synthesis of cobalamin (Cbl; vitamin B12) coenzymes. Cultured fibroblasts from that unique class (cbl C) deficient in the synthesis of both Cbl coenzymes, 5′-deoxyadenosylcobalamin (AdoCbl) and methylcobalamin (MeCbl), were used to explore the underlying defect. We compared the uptake of transcobalamin II(TC II)-bound cyano[57Co]cobalamin (CN-Cbl) by cbl cells with that of other control and mutant cell lines. Although the cbl C cells initially took up CN-[57Co]Cbl normally, they were unable to retain it. To characterize this “leak” further, cell extracts were prepared following incubation and chromatographed on Sephadex G-150. After incubations of 1-2 hr, most of the CN-[57Co]Cbl accumulated by control cells was still bound to TC II; the remainder was free. Thereafter, an ever-increasing fraction of the labeled Cbl eluted with an intracellular cobalamin-binding protein (ICB); more than 80% of the total was so bound after 76 hr incubations. ICB had an apparent molecular weight similar to that of several Cbl “R” binders (about 120,000), but was distinguished from them by its failure to react with specific anti-“R”binder antiserum. Significantly, no ICB was detected in extracts of three different cbl C lines even aftr prolonged incubations, whereas its appearance in cbl A, cbl B, and mutase apoenzyme mutants was normal. We propose: that ICB is required for retention of cobalamins by cells; and that cbl C cells “leak” cobalamins and show defective synthesis of Cbl coenzymes because they lack this intracellular binder.
Keywords: methylmalonicacidemia, homocystinuria, vitamin B12, coenzyme synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER B. A., PARANCHYCH W. Selective uptake of specifically bound cobalt-58 vitamin B12 by human and mouse tumour cells. Nature. 1961 Jul 22;191:393–395. doi: 10.1038/191393a0. [DOI] [PubMed] [Google Scholar]
- Dillon M. J., England J. M., Gompertz D., Goodey P. A., Grant D. B., Hussein H. A., Linnell J. C., Matthews D. M., Mudd S. H., Newns G. H. Mental retardation, megaloblastic anaemia, methylmalonic aciduria and abnormal homocysteine metabolism due to an error in vitamin B12 metabolism. Clin Sci Mol Med. 1974 Jul;47(1):43–61. doi: 10.1042/cs0470043. [DOI] [PubMed] [Google Scholar]
- Finkler A. E., Hall C. A. Nature of the relationship between vitamin B12 binding and cell uptake. Arch Biochem Biophys. 1967 Apr;120(1):79–85. doi: 10.1016/0003-9861(67)90600-5. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., Moe P. G., Hammond K. B., Mudd S. H., Uhlendorf B. W. Homocystinuria with methylmalonic aciduria: two cases in a sibship. Biochem Med. 1970 Dec;4(5):500–515. doi: 10.1016/0006-2944(70)90080-3. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Mahoney M. J., Ruddle F. H., Rosenberg L. E. Genetic complementation in heterokaryons of human fibroblasts defective in cobalamin metabolism. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3181–3185. doi: 10.1073/pnas.72.8.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräsbeck R. Intrinsic factor and the other vitamin B12 transport proteins. Prog Hematol. 1969;6:233–260. [PubMed] [Google Scholar]
- HALL C. A., FINKLER A. E. THE DYNAMICS OF TRANSCOBALAMIN II. A VITAMIN B12 BINDING SUBSTANCE IN PLASMA. J Lab Clin Med. 1965 Mar;65:459–468. [PubMed] [Google Scholar]
- Hall C. A. Transport of vitamin B 12 in man. Br J Haematol. 1969 May;16(5):429–433. doi: 10.1111/j.1365-2141.1969.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Hom B. L. Plasma turnover of 57cobalt-vitamin B12 bound to transcobalamin I and II. Scand J Haematol. 1967;4(5):321–332. doi: 10.1111/j.1600-0609.1967.tb01633.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahoney M. J., Hart A. C., Steen V. D., Rosenberg L. E. Methylmalonicacidemia: biochemical heterogeneity in defects of 5'-deoxyadenosylcobalamin synthesis. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2799–2803. doi: 10.1073/pnas.72.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E., Mudd S. H., Uhlendorf B. W. Defective metabolism of vitamin B 12 in fibroblasts from children with methylmalonicaciduria. Biochem Biophys Res Commun. 1971 Jul 16;44(2):375–381. doi: 10.1016/0006-291x(71)90610-3. [DOI] [PubMed] [Google Scholar]
- Morrow G., 3rd, Barness L. A., Cardinale G. J., Abeles R. H., Flaks J. G. Congenital methylmalonic acidemia: enzymatic evidence for two forms of the disease. Proc Natl Acad Sci U S A. 1969 May;63(1):191–197. doi: 10.1073/pnas.63.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Levy H. L., Abeles R. H., Jennedy J. P., Jr A derangement in B 12 metabolism leading to homocystinemia, cystathioninemia and methylmalonic aciduria. Biochem Biophys Res Commun. 1969 Apr 10;35(1):121–126. doi: 10.1016/0006-291x(69)90491-4. [DOI] [PubMed] [Google Scholar]
- Newmark P. A. The mechanism of vitamin B 12 by the kidney of the rat in vivo. Biochim Biophys Acta. 1972 Jan 28;261(1):85–93. doi: 10.1016/0304-4165(72)90317-0. [DOI] [PubMed] [Google Scholar]
- Newmark P., Newman G. E., O'Brien J. R. Vitamin B12 in the rat kidney. Evidence for an association with lysosomes. Arch Biochem Biophys. 1970 Nov;141(1):121–130. doi: 10.1016/0003-9861(70)90114-1. [DOI] [PubMed] [Google Scholar]
- Peirce K., Abe T., Cooper B. A. Incorporation and metabolic conversion of cyanocobalamin by Ehrlich ascites carcinoma cells in vitro and in vivo. Biochim Biophys Acta. 1975 Feb 13;381(2):348–358. doi: 10.1016/0304-4165(75)90240-8. [DOI] [PubMed] [Google Scholar]
- Pletsch Q. A., Coffey J. W. Properties of the proteins that bind vitamin B 12 in subcellular fractions of rat liver. Arch Biochem Biophys. 1972 Jul;151(1):157–167. doi: 10.1016/0003-9861(72)90484-5. [DOI] [PubMed] [Google Scholar]
- Rappazzo M. E., Hall C. A. Transport function of transcobalamin II. J Clin Invest. 1972 Jul;51(7):1915–1918. doi: 10.1172/JCI106995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retief F. P., Vandenplas L., Visser H. Vitamin B12 binding proteins in liver disease. Br J Haematol. 1969 Mar;16(3):231–240. doi: 10.1111/j.1365-2141.1969.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A. C., Hsia Y. E., Rosenbloom F. M. Vitamin B12 dependent methylmalonicaciduria: defective B12 metabolism in cultured fibroblasts. Biochem Biophys Res Commun. 1969 Nov 6;37(4):607–614. doi: 10.1016/0006-291x(69)90853-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A., Hsia Y. E. Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency. Science. 1968 Nov 15;162(3855):805–807. doi: 10.1126/science.162.3855.805. [DOI] [PubMed] [Google Scholar]
- Ryel E. M., Meyer L. M., Gams R. A. Uptake and subcellular distribution of vitamin B12 in mouse L1210 leukemic lymphoblasts. Blood. 1974 Sep;44(3):427–433. [PubMed] [Google Scholar]
- Tan C. H., Hansen H. J. Studies on the site of synthesis of transcobalamin-II. Proc Soc Exp Biol Med. 1968 Mar;127(3):740–744. doi: 10.3181/00379727-127-32789. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]



