Abstract
Abiotic and biotic stresses are the major factors that negatively impact plant growth. In response to abiotic environmental stresses such as drought, plants generate resistance responses through abscisic acid (ABA) signal transduction. In addition to the major role of ABA in abiotic stress signaling, ABA signaling was reported to downregulate biotic stress signaling. Conversely recent findings provide evidence that initial activation of plant immune signaling inhibits subsequent ABA signal transduction. Stimulation of effector-triggered disease response can interfere with ABA signal transduction via modulation of internal calcium-dependent signaling pathways. This review overviews the interactions of abiotic and biotic stress signal transduction and the mechanism through which stress surveillance system operates to generate the most efficient resistant traits against various stress condition.
Keywords: abscisic acid, Ca2+, guard cell, pathogen, R genes
INTRODUCTION
As sessile organisms facing diverse levels of stresses including abiotic environmental harsh condition and biotic pathogen attacks, plants are required to build up elaborate stress surveillance system and corresponding resistant mechanisms. Plant abiotic stress responses are largely controlled by phytohormone ABA through the regulation of its synthesis, transport, and onset of multi layers of signal transduction (Cutler et al., 2010; Kim et al., 2010). In addition to ABA’s major roles in abiotic stress resistant signal transduction, ABA has been shown to function during pathogen infection and the following plant immune response pathways (de Torres-Zabala et al., 2007; Fan et al., 2009). Although, in many cases, ABA affects negatively on the generation of immune responses there have been reports that ABA synthesis after pathogen recognition assists proliferation of the infected pathogen depending on the types of particular pathogen and host pairs (Ton et al., 2009).
This review focuses on an opposite way of regulation that is how biotic stress resistant responses can regulate abiotic stress signal transduction. Recent findings demonstrated that modulation of ABA signal transduction would occur via components previously known as major regulators of pathogen signaling. Especially control of abiotic responses by NB-LRR (Nucleotide Binding-Leucine Rich Repeat) R (Resistant) gene immune receptors and Ca2+ signaling as an integrator combining abiotic and biotic stress surveillance systems are particularly interesting and discussed more in detail.
INTERACTION OF ABA SIGNALING WITH BIOTIC STRESS RESPONSE PATHWAYS
In addition to the genetic interactions of ABA signaling with ethylene, gibberellins, and brassinosteroids signal transduction in embryonic and early postembryonic development (Beaudoin et al., 2000; Gazzarrini et al., 2004; Ghassemian et al., 2000; Zhang et al., 2009), novel roles of ABA signaling during pathogen infection and following defense responses against biotic stresses have been suggested (Asselbergh et al., 2008; Cao et al., 2011; Fujita et al., 2006; Ton et al., 2009). Jasmonic acid (JA) and salicylic acid (SA) are two major plant hormones regulating plant biotic stress signal transduction. Interactions between ABA and JA/SA signaling may coordinate to produce combined optimal resistant responses when plants face both abiotic and biotic stresses simultaneously (Fig. 1).
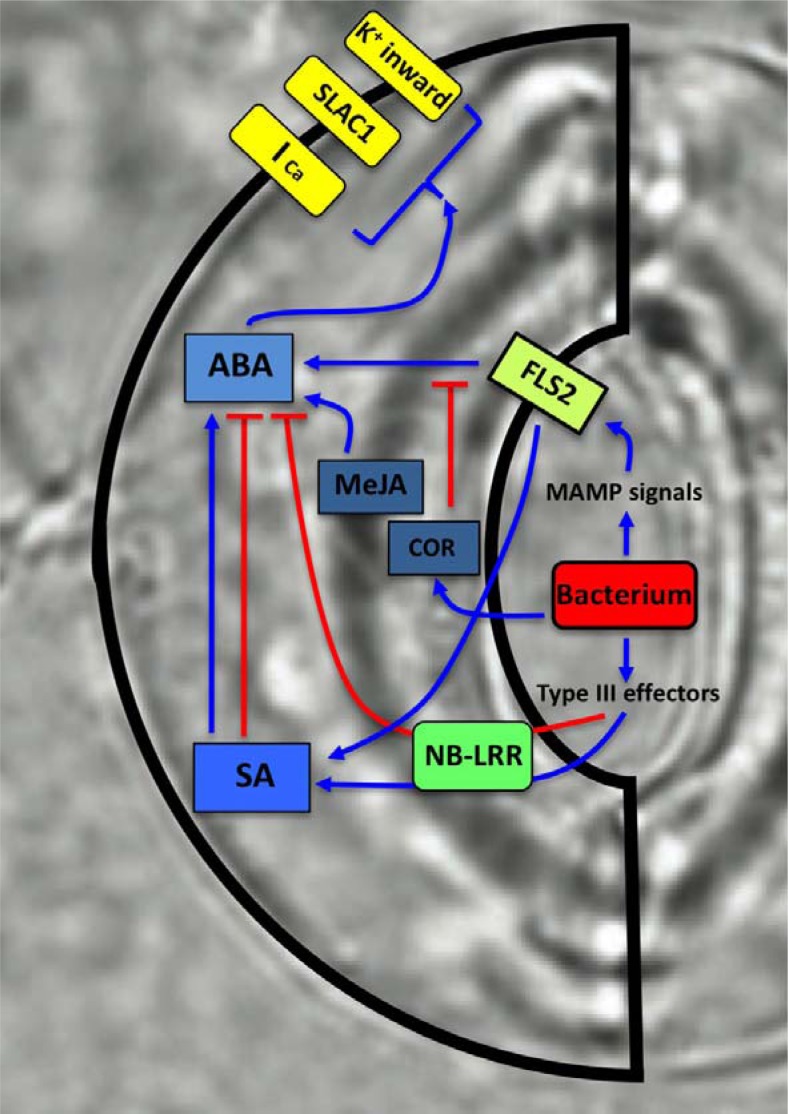
Fig. 1.
Summary of guard cell signaling displaying cross-talks of ABA and biotic stress responses. Depending on the types of pathogen-plant host pairs SA signaling can affect either negatively or positively guard cell ABA signal transduction. Negative regulation of ABA signaling by NB-LRR can be independent of SA signaling. Whereas coronatine from pathogen was shown to inhibit ABA responses, MeJA treatment was reported to induce stomatal closing. See the text for more detailed discussion.
Both wound and pathogen Botrytis cinerea treatment induce biosynthesis of ABA, JA, and SA (Pan et al., 2008) suggesting synergistic or antagonistic interactions among these induced hormone signaling to generate defense responses. MeJA (methyl jasmonate) treatment induces stomatal closing through a CORONATINE INSENSITIVE1 (COI1)- and JASMO-NATE RESISTANT1 (JAR1)-dependent signaling pathway (Munemasa et al., 2007; Suhita et al., 2004). JA-triggered activation of S-type anion channel and ICa-channel activities is probably under the control of the same second messengers elicited by ABA because MeJA does not induce stomatal closures in ABA insensitive2-1 (abi2-1) as well as in coi1 (Fig. 1). In regulation of merging signals for ABA- and JA-triggered stomatal closures, guard cell abundant myrosinase THIOGLUCOSIDE GLUCO-HYDROLASE1 (TGG1) may have a role. tgg1 showed defects in ABA-inhibition of inward K+-channel activity and stomatal opening (Zhao et al., 2008) and ttg1 ttg2 produced reduced responses in the ABA- and JA-induced stomatal closures (Islam et al., 2009) suggesting a role of glucosinolate metabolism in the guard cell ABA signaling.
However an antagonistic effect of ABA in JA signaling was also presented based on the observation that exogenous ABA treatment repressed the expression of JA-dependent defense genes (Anderson et al., 2004). Additionally, mutations in the positive ABA signaling bHLH (basic Helix-Loop-Helix) transcription factor AtMYC2 and the ABA biosynthesis gene ABA DEFICIENT2 (ABA2) produced increased resistance to pathogen (Anderson et al., 2004). As a response to the flg22 perception by FLAGELLIN SENSITIVE2 (FLS2), plants close the possible bacterial entrance sites, guard cells by inducing stomatal closures (Melotto et al., 2006) and inhibiting stomatal openings (Zhang et al., 2008). The MAMP (microbe-associated molecular pattern)-triggered stomatal response requires components of ABA signal transduction OPEN STOMATA1/SnRK2.6 (OST1) and G-protein subunit GPA1 for stomatal closures and openings, respectively (Fig. 1). Stomatal closures by flg22 can be repressed by the compound coronatine secreted by PstDC 3000 and also by mutations in ABA and SA biosynthesis genes (Melotto et al., 2006; Zeng et al., 2010) suggesting interactions between ABA and SA/JA signaling in guard cells.
SA is a central biotic stress hormone inducing systemic resistance to bacterial and fungus pathogens. Whereas pathogen infection induces biosynthesis of ABA and SA, an antagonistic effect of ABA on both SA biosynthesis and signaling via control of transcription was observed (de Torres Zabala et al., 2009; Fan et al., 2009; Mosher et al., 2010; Yasuda et al., 2008) (Fig. 1). Although the detailed mechanism through which ABA-SA antagonism balances the downstream responses remain under investigation, ABA-RESPONSIVE1 (ABR1) expression by Xanthomonas campestris infection was shown to control antagonistic biosynthesis of ABA and SA and produce the disease resistance (Choi and Hwang, 2011).
Moreover findings a regulatory function of race-specific immune receptor NB-LRR proteins in drought/humidity responses support further complex interactions between ABA and SA signaling components. The CC (coiled-coil)-NB-LRR mutant activated disease resistance1 (adr1) was originally isolated as a disease resistant mutant. Overexpression of ADR1 conferred a specific drought resistant phenotype via ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) and ABA INSENSI-TIVE1 (ABI1) pathways (Chini et al., 2004). The Toll and interleukin-1 receptor homolog (TIR)-NB-LRR-WRKY mutant sensitive low humidity1 (slh1) is hypersensitive to low humidity by induction of hyperactive disease responses (Noutoshi et al., 2005). Constitutive lesion phenotypes of the TIR-NB-LRR mutant ssi4 (suppressor of salicylic acid insensitivity of npr1-5) is also suppressed by high humidity treatment perhaps via down-regulation of the mitogen activated protein (MAP) kinases MPK3/6 (Zhou et al., 2004). Involvement of MAPK pathways during interactions between abiotic and biotic stress signaling is additionally supported by the identification of a novel allele in KEEP ON GOING (KEG) as a suppressor of enhanced disease resistance and ABA hypersensitive phenotypes of enhanced disease resistance1 (edr1) (Wawrzynska et al., 2008). EDR1 encodes a putative MAP Kinase Kinase Kinase. Humidity control of disease responses and ABA-insensitivity produced by induction of disease signaling genes was also observed in the constitutive expression of PR genes22 (cpr22) mutant, of which mutation causes to express a mutant chimeric cyclic nucleotidegated ion channel11/12 (CNGC11/12) protein (Mosher et al., 2010). Since CNGCs are known to function as ligand-gated channels maintaining cation homeostasis and Ca2+ transport (Kudla et al., 2010), it is plausible to hypothesize that Ca2+ regulation is important for cross-talks of abiotic and biotic stress signaling.
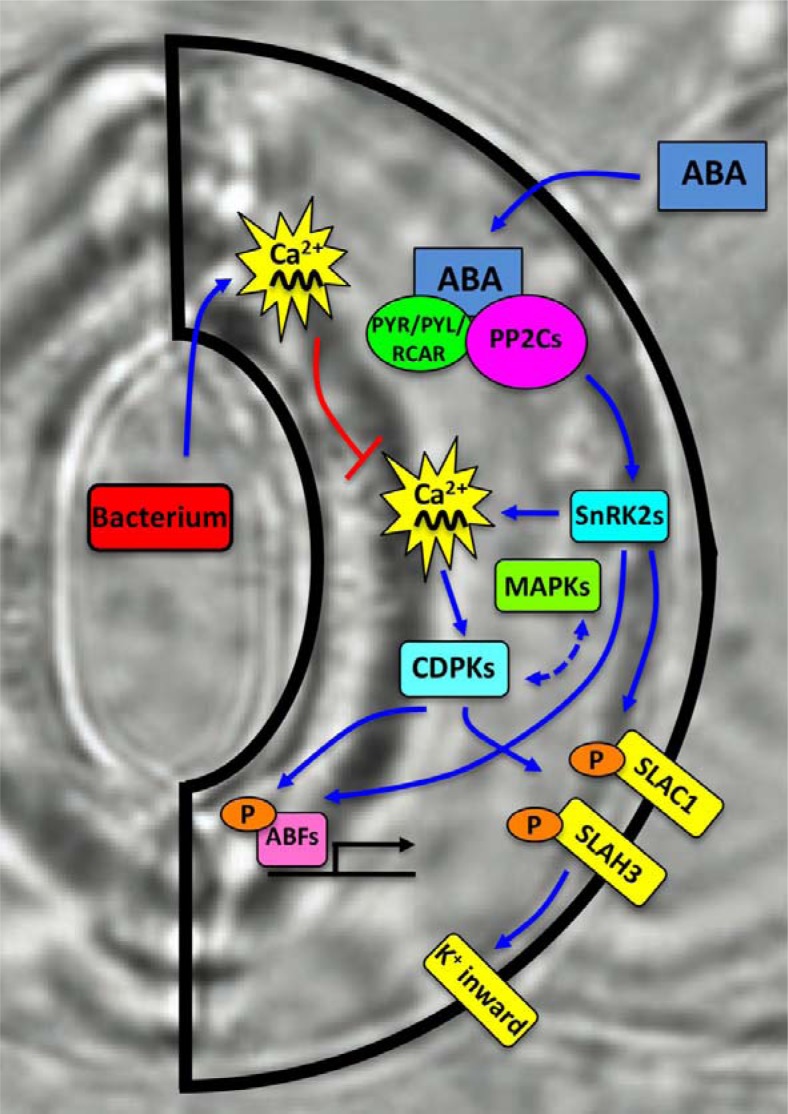
Another evidence revealing NB-LRR receptors as an important regulator of ABA signal transduction was presented based on the finding of novel small compound DFPM and the following chemical genetics approach (Kim et al., 2011) (Fig. 1). DFPM was isolated from a screen of small compound library as an ABA signaling inhibitor. Gene expression and genetic analyses of DFPM indicated that ABA signaling interference by DFPM was caused by activation of NB-LRR and subsequent signaling pathways. As induction of disease response pathways by DFPM efficiently interfered with guard cell ABA signal transduction, plant infection by Pseudomonas syringae affected negatively ABA-induction of gene expression as well as ABA-induced stomatal closures (Kim et al., 2011). Notably, analyses of pathogen signaling mutants showed that major early regulators of effector-triggered immunity pathways, including EDS1, PHYTOALEXIN DEFICIENT4 (PAD4), REQUIRED FOR Mla12 RESISTANCE (RAR1), and SUPPRESSOR OF G2 ALLELE OF SKP1B (SGT1B), are required for the DFPM- as well as for biological Pseudomonas-inhibition of ABA signal transduction. SA signaling and the EDS16 and NONEXPRESSER OF PR GENES1 (NPR1) loci were not necessary for this interference of ABA signaling, demonstrating a difference to the converse ABA interference of biotic stress signaling. DFPM interference of ABA signaling occurs at the events downstream of intracellular Ca2+ and Ca2+-activation of anion channels, where-as upstream ABA signaling events involving PYR/PYL/RCAR receptors and SnRK2 kinase activation were not affected by DFPM treatment (Kim et al., 2011) (Fig. 2). Therefore regulation of Ca2+-dependent downstream signaling steps probably at the bottleneck position may adjust stress responses after simultaneous or serial perception of abiotic and biotic stimuli.
Fig. 2.
A guard cell model illustrating ABA signaling from ABA perception to downstream ion channel regulation and gene expression. This model focuses on the role of internal Ca2+ signals and cross-regulation of protein kinases including SnRK2s, CDPKs, and MAPKs. Ca2+-activation of protein kinases elicits phosphorylation of downstream targets resulting ion channel activation or induction of gene expression. Ca2+ signals triggered by pathogen infection affect ABA signal transduction either through cross regulation of common downstream factors or by consuming Ca2+ messengers of which homeostasis to be maintained for priming of guard cell ABA responses.
CALCIUM SIGNAL REGULATION AT THE POINT OF ABA AND DISEASE SIGNALING INTERACTIONS
By changing amplitude, duration, and frequency of Ca2+ transients accordingly to environmental and developmental stimuli, plant cells interpret the source of input signals and then produce proper responses (Dodd et al., 2010; Sanders et al., 2002). Considering various responses controlled by Ca2+ signals, plant genome is expected to encode diverse Ca2+ sensors and mechanisms to transmit the general secondary signals to specific target components. The model plant Arabidopsis thaliana contains at least 250 proteins containing EF-hand motifs that can bind Ca2+ ions (Day et al., 2002). Calcium-dependent protein kinases (CDPKs or gene name CPKs) receive calcium stimuli using EF-hand motifs within the calmodulin-like domain at the C-terminal. Ca2+-bindings to CDPKs activate the serine-threonine kinase domain at the N-terminal and phosphorylate downstream targets to relay the signal. At low levels of calcium, the middle autoinhibitory domain, which is intervening between kinase and calmodulin-like domains, suppresses the kinase activity of CDPKs. With previous reports on the roles of CDPKs in abiotic signal transduction as well as during biotic disease stress responses (Ludwig et al., 2004), CDPK is proposed as a candidate regulator to function through Ca2+ signals at the point of ABA and disease signaling interactions (Kim et al., 2011). The Arabidopsis genome has 34 CDPK genes. With different affinities to Ca2+ ions and with diverse subcellular and tissue-specific localizations, particular groups of CDPKs may define a specific signaling pathway from upstream Ca2+ signals to downstream targets. Alternatively, CDPKs may play a role as a converging point of multiple signal transductions by combining different sources of Ca2+ signals from various stimuli into a regulation of common downstream factors. Otherwise CDPKs could function as nexus regulators where both Ca2+ signal convergence and specification occur.
CPK3 and CPK6 are the major CDPKs expressed in guard cells and regulate ABA induction of S-type anion channel and Ca2+-permeable channels (Mori et al., 2006). Single cell type-based guard cell microarray analyses identified that CPK3 and CPK6 expressions were mainly localized in guard cells. Disruption of these two genes produced defects in ABA- and Ca2+-activation of S-type anion channel. Additionally, ROS (reactive oxygen species)-activation of ICa-channel was also reduced in the cpk3 cpk6 mutant suggesting CPK3 and CPK6 might control downstream of ROS and Ca2+ signaling steps in ABA signal transduction (Mori et al., 2006) (Fig. 2). Furthermore, with the pronounced phenotypes of the double mutant cpk3 cpk6 compared to the individual single mutants, functional overlaps of CPK3 and CPK6 activity in guard cell ABA signal transduction were expected (Mori et al., 2006).
In contrast to the CPK3 and CPK6’s function as positive regulators, another CDPK, CPK10 was reported as a negative regulator of ABA and Ca2+-dependent stomatal closing responses in guard cells. The mutant cpk10 was more sensitive to ABA and drought treatment, whereas the overexpression of CPK10 displayed insensitive responses to both treatments (Zou et al., 2010), suggesting a complex network of CDPK’s activity determines downstream of ABA signaling pathways.
While genetic evidence pointed out CPK3, CPK6, and CPK10 as important regulators of S-type anion channel currents, cell biological and biochemical evidence showed that CPK21 and CPK23 directly interact with SLAC1 S-type anion channel. Phosphorylation of SLAC1 may be required to activate anion channel currents (Geiger et al., 2010). Interestingly, SLAC1 activation by CPK21 was Ca2+-dependent but CPK23 activated SLAC1 in a Ca2+-insensitive manner indicating a bifurcating point of Ca2+ signaling pathways resides here. Another anion channel SLAH3 (SLAC1 homolog3) was shown to mediate nitrate anion conductance in guard cells (Geiger et al., 2011). Patch clamp analyses of the mutant slah3-1 showed a defect in generation of nitrate-induced anion currents. CPK21 was shown to interact with SLAH3 directly and activate SLAH3 by phosphorylation (Geiger et al., 2011). Moreover, in response to osmotic stress, CPK21 is activated for production of resistant responses against osmotic stress. The mutant cpk21 was more resistant to osmotic stress condition and exhibited transcriptional activation of stress-related marker genes even without stresses (Franz et al., 2011).
CPK4, CPK11, and CPK32 were shown to be involved in regulation of general ABA signal transduction in tissues other than guard cells. Consistent to their roles in mediating ABA signaling in many different types of tissues, CPK32 was reported to interact directly with ABA responsive bZIP transcription factor4 (ABF4) and upregulate ABA-induced gene expression (Choi et al., 2005). Similarly CPK4 and CPK11 were shown to biochemically target ABF1 and ABF4 (Zhu et al., 2007). The lack of phosphorylation of ABF1 and ABF4, reduced ABA-induction of gene expression including ABI4, ABI5, RAB18, and KIN1, and more susceptible phenotypes to drought stress in cpk4-1 cpk11-2 (Zhu et al., 2007) well correlate with the proposed functions of CDPKs as an important positive regulator of ABA signal transduction. It is noteworthy to compare the above observation with recent results showing cpk4-2 cpk11-2 showed a defect in Ca2+ oscillation-induced stomatal closures, whereas no effects on the ABA-induced stomatal closures (Hubbard et al., 2011). This discrepancy may be because different mutant alleles of cpk4 were used for each genetic analysis. It is also intriguing that ABA-activated OST1/SnRK2.6 can phosphorylate ABF3, which is then predicted to stabilize ABF3 (Sirichandra et al., 2010). However, the presence of multiple protein kinase bands nearby CPK4 in in-gel-kinase assays (Zhu et al., 2007) indicate that a protein kinase(s) other than CPK4 or SnRK2s could also be involved in parallel to consummate the fine-tuning of ABA and biotic signal transduction.
Elevated Ca2+ influx and transients followed by pathogen recognition comprises a major part of disease signal transduction (Du et al., 2009). One of the major responses by infection-induced cytosolic Ca2+ influx is the generation of hypersensitive response (HR) and ROS production. Moreover pharmacological experiments with Calmodulin (CaM) antagonist and the calmodulin-like24-4 (cml24-4) mutant demonstrated that Ca2+ influx mediates generation of Nitric oxide (NO) signals through activation of CaM or CML (Ma et al., 2008). For the decoding of pathogen-induced Ca2+ signature, AtSR1 also known as Ca2+/calmodulin-binding transcription factor3 (CAMTA3) acts as a negative regulator of SA signaling-mediated HRs by binding to the promoter of the EDS1 gene (Du et al., 2009). In addition, CDPKs could also play a role as positive regulators in disease responses by transferring Ca2+ signals to downstream target elements. A series of genetic and cell biological evidence proposed that CPK4 and CPK11 along with CPK5 and CPK6 might be activated by a MAMP (microbe-associated molecular patterns) signal flg22 (Boudsocq et al., 2010). Activation of CDPKs by flg22, which was dependent on the flg22 receptor FLS2, produced innate immunity against bacterial attacks. Interestingly, cpk5 cpk6 cpk11 loss-of-function mutants and C-terminally truncated autoactivated form of CPK5 and CPK11 demonstrated that CDPKs and MAPKs signaling pathways are independently regulated after the flg22 activation. Moreover none of the ABA-regulated genes were recovered as co-regulated genes with the CPK11-regulated genes. These data suggest that stimulus-specific signaling pathways separately exist as well as cross-talks of signaling networks impose stress responses. How the activation of same CPKs by different sources of stimuli can distinguish the proper downstream targets remains to be investigated. One possibility is that different stimuli generate different characteristics of Ca2+ signatures and that encodes the specificity of CPK activation and downstream target selection. Alternatively, pathway-specific regulators in the process are stimulated independent of Ca2+ signals and that contributes to selective activation of relevant CDPKs in specific signal transduction.
Additional function of CDPKs reported is for regulation of MeJA-induced stomatal closing. The mutant studies using cpk3-1, cpk6-1, cpk4-1, and cpk11-2 indicated that only CPK6 among them specifically controls MeJA-induced stomatal movement regulation (Munemasa et al., 2011). The fact that MeJA-triggered production of ROS and NO was not affected in cpk6-1 while Ca2+-permeable cation channel and S-type anion channel were not activated by MeJA in cpk6-1 suggested again that the position of Ca2+ signaling step is at downstream of ROS production and upstream of ion channel regulation.
It has been presented that activation of CDPKs as well as MAPK pathways comprise of critical steps of plant immune responses (Boudsocq et al., 2010; Wurzinger et al., 2011). Cross-talks between these two kinase pathways participate in generating diverse resistant mechanisms. Moreover MAPK pathways have been reported to be a part of abiotic stress or ABA signal transduction. Besides a role in disease signaling, the MAP kinase kinase, MKK2 was reported to function as a negative regulator of cold and salt stress signal transduction (Teige et al., 2004). In contrast, guard cell preferentially expressed MPK9 and MPK12 act as positive regulators of ABA signaling at downstream of ROS and Ca2+ signals and upstream of S-type anion channel regulation (Jammes et al., 2009). The mutant mpk9-2 mpk12-1 provided genetic evidence displaying disruption of ABA and Ca2+-stimulated stomatal closures. Furthermore mpk9-2 mpk12-1 exhibited more susceptibility to Pseudomonas infections (Jammes et al., 2011) suggesting a cross-talk between ABA and disease response signaling operates through MAPK pathways. It will be informative to test a hypothesis whether MAPK pathways can be affected by CPDK activation or vice versa (Fig. 2).
CONCLUSIONS
Recent results demonstrate that plant biotic stress signaling rapidly interferes with ABA signal transduction through modulation of downstream Ca2+ signaling. Cytosolic Ca2+-transient activation of ABA signal transduction can be primed by prior exposure to Ca2+ concentrations or to ABA treatment. For example, pre-exposure to high Ca2+ concentration is required to produce activation of cytosolic Ca2+-induced S-type anion channels (Allen et al., 2002). ABA pre-treatment to guard cells augments the cytosolic Ca2+-induced S-type anion channel activation (Chen et al., 2010; Siegel et al., 2009). This calcium sensitivity priming hypothesis can offer an explanation how different sources of cytosolic Ca2+ transients can antagonistically control ABA and disease signal transduction. When pathogen-induced cytosolic Ca2+ influx activates disease response pathways, consumption of intracellular Ca2+ bursts may deprime calcium sensitivity mechanisms for ABA signal transduction (Fig. 2). In an opposite way, ABA or abiotic stress-triggered signal transduction passes through intracellular Ca2+ messengers and this affects negatively or in some specific cases positively priming the mode of disease signal transduction. Further investigation will shed light on how Ca2+ signals offer cross-talks when plants face various stresses. Calcium-binding proteins especially including CDPKs could mediate network interactions and thereby maintain stress surveillance system in plants. These findings will be instrumental for dissection of abiotic and biotic stress signaling interactions and characterization of the underlying molecular mechanisms adjusting plant adaptive responses against combined abiotic and biotic stress exposures.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant by the Korean Government (Ministry of Education, Science, and Technology) (2011-0011760 and 2011-0031386).
REFERENCES
- Allen G.J., Murata Y., Chu S.P., Nafisi M., Schroeder J.I. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell. 2002;14:1649–1662. doi: 10.1105/tpc.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.P., Badruzsaufari E., Schenk P.M., Manners J.M., Desmond O.J., Ehlert C., Maclean D.J., Ebert P.R., Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B., De Vleesschauwer D., Hofte M. Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant Microbe Interact. 2008;21:709–719. doi: 10.1094/MPMI-21-6-0709. [DOI] [PubMed] [Google Scholar]
- Beaudoin N., Serizet C., Gosti F., Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M., Willmann M.R., McCormack M., Lee H., Shan L., He P., Bush J., Cheng S.H., Sheen J. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F.Y., Yoshioka K., Desveaux D. The roles of ABA in plant-pathogen interactions. J. Plant Res. 2011;124:489–499. doi: 10.1007/s10265-011-0409-y. [DOI] [PubMed] [Google Scholar]
- Chen Z.H., Hills A., Lim C.K., Blatt M.R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 2010;61:816–825. doi: 10.1111/j.1365-313X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- Chini A., Grant J.J., Seki M., Shinozaki K., Loake G.J. Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004;38:810–822. doi: 10.1111/j.1365-313X.2004.02086.x. [DOI] [PubMed] [Google Scholar]
- Choi D.S., Hwang B.K. Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell. 2011;23:823–842. doi: 10.1105/tpc.110.082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.I., Park H.J., Park J.H., Kim S., Im M.Y., Seo H.H., Kim Y.W., Hwang I., Kim S.Y. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression and modulates its activity. Plant Physiol. 2005;139:1750–1761. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. Abscisic acid emergence of a core signaling network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Day I.S., Reddy V.S., Shad Ali G., Reddy A.S. Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-10-research0056. RESEARCH0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres-Zabala M., Truman W., Bennett M.H., Lafforgue G., Mansfield J.W., Rodriguez Egea P., Bogre L., Grant M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007;26:1434–1443. doi: 10.1038/sj.emboj.7601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres Zabala M., Bennett M.H., Truman W.H., Grant M.R. Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 2009;59:375–386. doi: 10.1111/j.1365-313X.2009.03875.x. [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Kudla J., Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Du L., Ali G.S., Simons K.A., Hou J., Yang T., Reddy A.S., Poovaiah B.W. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457:1154–1158. doi: 10.1038/nature07612. [DOI] [PubMed] [Google Scholar]
- Fan J., Hill L., Crooks C., Doerner P., Lamb C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009;150:1750–1761. doi: 10.1104/pp.109.137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz S., Ehlert B., Liese A., Kurth J., Cazale A.C., Romeis T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant. 2011;4:83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- Fujita M., Fujita Y., Noutoshi Y., Takahashi F., Narusaka Y., Yamaguchi-Shinozaki K., Shinozaki K. Crosstalk between abiotic and biotic stress responses a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gazzarrini S., Tsuchiya Y., Lumba S., Okamoto M., McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev. Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Maierhofer T., Al-Rasheid K.A., Scherzer S., Mumm P., Liese A., Ache P., Wellmann C., Marten I., Grill E., et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 2011;4:ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- Ghassemian M., Nambara E., Cutler S., Kawaide H., Kamiya Y., McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard K.E., Siegel R.S., Valerio G., Brandt B., Schroeder J.I. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann. Bot. 2011;109:5–17. doi: 10.1093/aob/mcr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.M., Tani C., Watanabe-Sugimoto M., Uraji M., Jahan M.S., Masuda C., Nakamura Y., Mori I.C., Murata Y. Myrosinases, TGG1 and TGG2 redundantly function in ABA and MeJA signaling in Arabidopsis guard cells. Plant Cell Physiol. 2009;50:1171–1175. doi: 10.1093/pcp/pcp066. [DOI] [PubMed] [Google Scholar]
- Jammes F., Song C., Shin D., Munemasa S., Takeda K., Gu D., Cho D., Lee S., Giordo R., Sritubtim S., et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F., Yang X., Xiao S., Kwak J.M. Two Arabidopsis guard cell-preferential MAPK genes, MPK9 and MPK12, function in biotic stress response. Plant Signal. Behav. 2011;6 doi: 10.4161/psb.6.11.17933. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Bohmer M., Hu H., Nishimura N., Schroeder J.I. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Hauser F., Ha T., Xue S., Bohmer M., Nishimura N., Munemasa S., Hubbard K., Peine N., Lee B.H., et al. Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr. Biol. 2011;21:990–997. doi: 10.1016/j.cub.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A.A., Romeis T., Jones J.D. CDPK-mediated signalling pathways: specificity and cross-talk. J. Exp. Bot. 2004;55:181–188. doi: 10.1093/jxb/erh008. [DOI] [PubMed] [Google Scholar]
- Ma W., Smigel A., Tsai Y.C., Braam J., Berkowitz G.A. Innate immunity signaling: cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008;148:818–828. doi: 10.1104/pp.108.125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Mori I.C., Murata Y., Yang Y., Munemasa S., Wang Y.F., Andreoli S., Tiriac H., Alonso J.M., Harper J.F., Ecker J.R., et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)- permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher S., Moeder W., Nishimura N., Jikumaru Y., Joo S.H., Urquhart W., Klessig D.F., Kim S.K., Nambara E., Yoshioka K. The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiol. 2010;152:1901–1913. doi: 10.1104/pp.109.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S., Oda K., Watanabe-Sugimoto M., Nakamura Y., Shimoishi Y., Murata Y. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 2007;143:1398–1407. doi: 10.1104/pp.106.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S., Hossain M.A., Nakamura Y., Mori I.C., Murata Y. The Arabidopsis calcium-dependent protein kinase, CPK6 functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutoshi Y., Ito T., Seki M., Nakashita H., Yoshida S., Marco Y., Shirasu K., Shinozaki K. A single amino acid insertion in the WRKY domain of the Arabidopsis TIR-NBS-LRR-WRKY-type disease resistance protein SLH1 (sensitive to low humidity 1) causes activation of defense responses and hypersensitive cell death. Plant J. 2005;43:873–888. doi: 10.1111/j.1365-313X.2005.02500.x. [DOI] [PubMed] [Google Scholar]
- Pan X., Welti R., Wang X. Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry. 2008;69:1773–1781. doi: 10.1016/j.phytochem.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Sanders D., Pelloux J., Brownlee C., Harper J.F. Calcium at the crossroads of signaling. Plant Cell. 2002;14(Suppl):S401–417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.S., Xue S., Murata Y., Yang Y., Nishimura N., Wang A., Schroeder J.I. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. Plant J. 2009;59:207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Davanture M., Turk B.E., Zivy M., Valot B., Leung J., Merlot S. The Arabidopsis ABA-activated kinase OST1 phosphorylates the bZIP transcription factor ABF3 and creates a 14-3-3 binding site involved in its turnover. PLoS One. 2010;5:e13935. doi: 10.1371/journal.pone.0013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D., Raghavendra A.S., Kwak J.M., Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M., Scheikl E., Eulgem T., Doczi R., Ichimura K., Shinozaki K., Dangl J.L., Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Ton J., Flors V., Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14:310–317. doi: 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wawrzynska A., Christiansen K.M., Lan Y., Rodibaugh N.L., Innes R.W. Powdery mildew resistance conferred by loss of the ENHANCED DISEASE RESISTANCE1 protein kinase is suppressed by a missense mutation in KEEP ON GOING a regulator of abscisic acid signaling. Plant Physiol. 2008;148:1510–1522. doi: 10.1104/pp.108.127605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzinger B., Mair A., Pfister B., Teige M. Cross-talk of calcium-dependent protein kinase and MAP kinase signaling. Plant Signal. Behav. 2011;6:8–12. doi: 10.4161/psb.6.1.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M., Ishikawa A., Jikumaru Y., Seki M., Umezawa T., Asami T., Maruyama-Nakashita A., Kudo T., Shinozaki K., Yoshida S., et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20:1678–1692. doi: 10.1105/tpc.107.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Melotto M., He S.Y. Plant stomata: a check-point of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010;21:599–603. doi: 10.1016/j.copbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., He S.Y., Assmann S.M. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56:984–996. doi: 10.1111/j.1365-313X.2008.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cai Z., Wang X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 2009;106:4543–4548. doi: 10.1073/pnas.0900349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang W., Stanley B.A., Assmann S.M. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Menke F.L., Yoshioka K., Moder W., Shirano Y., Klessig D.F. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 2004;39:920–932. doi: 10.1111/j.1365-313X.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Zhu S.Y., Yu X.C., Wang X.J., Zhao R., Li Y., Fan R.C., Shang Y., Du S.Y., Wang X.F., Wu F.Q., et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J.J., Wei F.J., Wang C., Wu J.J., Ratnasekera D., Liu W.X., Wu W.H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010;154:1232–1243. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]