Abstract
Rice stripe virus (RSV) is a viral disease that seriously impacts rice production in East Asia, most notably in Korea, China, and Japan. Highly RSV-resistant transgenic japonica rice plants were generated using a dsRNAi construct designed to silence the entire sequence region of the RSV-CP gene. Transgenic rice plants were inoculated with a population of viruliferous insects, small brown planthoppers (SBPH), and their resistance was evaluated using ELISA and an infection rate assay. A correlation between the expression of the RSV-CP homologous small RNAs and the RSV resistance of the transgenic rice lines was discovered. These plants were also analyzed by comparing the expression pattern of invading viral genes, small RNA production and the stable transmission of the RSV resistance trait to the T3 generation. Furthermore, the agronomic trait was stably transmitted to the T4 generation of transgenic plants.
Keywords: movement protein (MP), Oryza sativa L., rice stripe virus (RSV), RNAi, RSV coat protein, silencing suppressor (NS3), small brown planthopper (SBPH)
INTRODUCTION
Rice stripe virus (RSV), one of the most economically significant pathogens of rice, causes repeated epidemics in China, Japan, and in particular, in Korea. RSV is transmitted to rice plants in a persistent, circulative-propagative manner via small brown planthoppers (SBPH) (Falk et al., 1998; Xiong et al., 2008a). RSV-infected rice plants exhibit chlorotic yellowish white stripes on the leaves and plant stunting. This results in a considerable yield loss (Hibino, 1996; Wei et al., 2009). In Korea, the first outbreak of RSV was recorded in 1965 and affected 40% of the rice hills (Toriyama, 2000). RSV re-emerged as a major rice pathogen in 2007, causing significant losses and raising serious concerns (Jonson et al., 2009a; 2009b).
A rice stripe resistance gene from the indica rice (Oryza sativa) cv. ‘Modan’ was introgressed into several Japonica rice cultivars (Hayano-Saito et al., 1998). The gene, Stvb-i, has provided stable resistance to rice stripe virus since the first introgression into japonica paddy rice cultivars approximately 30 years ago. However, the resistance breakdown can occur because of the emergence of new RSV strains, as well as an increase in the wintering and viruliferous rate of SBPH due to the global warming (Gu et al., 2005; Jonson et al., 2011). Therefore, it is vital that new resources of resistance are generated to meet future needs. Rice cultivars exhibiting stable resistance against new RSV strains must be developed by targeting conserved viral genes through biotechnology.
RNA interference (RNAi), a viral defense mechanism that is used by eukaryotic organisms to combat viruses, is especially important in plants. It is activated by double-stranded RNA (dsRNA) that is cleaved by cellular RNase III (Dicer) enzymes into small interfering RNAs (siRNA) of 21–24 bp (Deleris et al., 2006; Hamilton and Baulcombe, 1999; Waterhouse et al., 1998). One siRNA strand is incorporated into the RNA-induced silencing complex (RISC), which, in turn, targets and degrades RNA sequences complementary to the siRNA (reviewed by Voinnet, 2005). Therefore, in planta expression of untranslatable virus-derived sequences, such as double-stranded RNA (dsRNA), represents a method to target RNA silencing against the homologous infecting viruses (Di Nicola-Negri et al., 2005; Fuentes et al., 2006). Many attempts to engineer RNAi-mediated resistance to plant viruses have met with varying degrees of success, including immunity, delayed symptoms and an absence of resistance (Mansoor et al., 2006).
In the case of RSV, Hayakawa et al. (1992) attempted to generate RSV resistant rice plants using a RSV CP overexpression approach. This resulted in plants that were partially resistance to RSV, although no lines were agriculturally relevant. Although the use of CP sequences in single transgene constructs has been reported as successful against several viruses, there are no reports concerning the field testing of transgenic resistance against RSV (Dasgupa et al., 2003). Additionally, the use of the CMV RNA2-derived full sequence as a transgene resulted in extreme resistance to CMV, but a lower percentage of resistance was observed in transgenic lines harboring a construct containing a shorter viral RNA2 sequence. The resistance level conferred by a partial CP sequence approached 50% (Chen et al., 2004). To date, the use of full-length CP sequences for RNAi-mediated resistance to RSV has not been reported. In addition, Ma et al. (2011) demonstrated that transgenic rice containing a CP/SP chimeric gene displayed greater resistance than rice transformed with a partial CP gene containing the middle portion of the target gene. Therefore, a key element to acquiring efficient siRNA is optimal construct design, in accordance with the target gene (Chang et al., 2009).
The RSV genome contains four single-stranded RNA segments designated RNA 1 through 4, according to the molecular mass, and encodes seven total proteins (Toriyama and Watanabe, 1989). RNA 1 is negative sense and encodes a putative viral RNA-dependent RNA polymerase (RdRp; Toriyama et al., 1994). The three smaller RNA segments (RNAs 2, 3 and 4) are ambisense and contain two open reading frames (ORFs) (Takahashi et al., 1993; Zhu et al., 1991; 1992). One ORF is located in the 5′ end of the viral-sense RNA (vRNA), while the other is in the 5′ end of the viral complementary-sense RNA (vcRNA). vRNA 2 encodes NS2 of unknown function, and vcRNA 2 encodes pC2 glycoprotein. RNA 3 encodes NS3, a viral suppressor of RNA-silencing (Xiong et al., 2009), and pC3, a viral NCP. RNA 4 encodes SP, a major non-structural protein that accumulates in infected plants, and pC4, a viral movement protein (MP; Xiong et al., 2008b).
In this study, the rice stripe virus coat protein gene (RSV-CP) in the Suwon area, was isolated. Two vectors were constructed: an RNAi vector and an antisense vector containing the full-length cDNA, of high sequence homology to other RSV-CP genes (Lee et al., 2004). These vectors were used to generate transgenic rice plants displaying RSV resistance. Functional characterization of the pRNAi-RSV-CP-full or pAS-RSV-CP-full rice plant lines following infection by viruliferous small brown planthoppers was conducted. Additionally, invading viral gene expression differences were compared between phenotypically resistant pRNAi-RSV-CP-full transgenic rice and susceptible pAS-RSV-CP-full transgenic rice via semi-quantitative RT-PCR analysis. The T4 generation of the pRNAi-RSV-CP-full transgenic plants stably inherited the resistance trait and also displayed good field performance.
MATERIALS AND METHODS
Cloning of the RSV CP gene and sequence analysis
Viral RNAs from virus-infected leaves (cv, Ilpum-byeo) were extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. First strand cDNA was synthesized from 2 μg of total RNA at 42°C for 1 h using M-MLV Reverse Transcriptase (Stratagene). For full-length cDNA cloning, RSV CP-specific primers were designed based on the RSV CP sequence (GenBank Accession Number: X53563) which encodes the RSV CP gene (Zhu et al., 1991). The forward primer was 5′-CTAGTCATCTGCACCTTCTGCCTCATC-3′, and the reverse primer was 5′-AATGGGTACCAACAAGCCAGCCACTCT-3′. A DNA fragment (969 bp) containing the full coding region of the RSV CP gene was amplified using PCR with 0.05 μg of viral cDNA as a template. PCR conditions were as follows: initial denaturation for 4 min at 94°C; 35 cycles of 15 sec at 94°C, 30 s at 60°C, and 1 min at 72°C; and a final extension for 7 min at 72°C. The PCR products were separated by 0.8% agarose gel electrophoresis, recovered from the gel, and purified. The purified DNA fragments were cloned into the pGEM-T Easy Vector (Promega). After the DNA clones were sequenced, the sequence was compared with previous RSV CP gene sequences using the NCBI Blast search program.
RNAi Vector construction and rice transformation
To produce the RNAi construct for RSV-CP gene suppression, the 969-bp RSV-CP gene fragment (Accession no. GU230170.1) was cloned into the Gateway pENTR/D-TOPO cloning vector (Invitrogen). This vector carries two recombination sites (attL1 and attL2) for a LR Clonase reaction. Subsequently, the RSV-CP gene fragment was transferred to a pANDA destination vector by recombinase reaction repeats. For these reactions, the PCR-derived fragments were inserted into two regions flanked by two recombination sites (attB1 and attB2) in opposite directions, and the gus linker sequence was flanked by two inverted repeats (Miki and Shimamoto, 2004) (Fig. 1A). Ten colonies were randomly selected and used for tri-parental mating with Agrobacterium tumefaciens LBA4404 (plasmid-free) and E. coli HIT-DH5α containing the helper plasmid HB101. RNAi and antisense expression vectors containing the fully complementary RSV-CP gene transcribed into dsRNA were constructed and designated pRNAi-RSV-CP-full and pAS-RSV-CP-full, respectively. In these vectors, the RSV-CP gene was placed under the control of the maize Ubiquitin (Ubi) promoter for RNAi (Miki and Shimamoto, 2004) and the rice cytochrome C (OsCc1) promoter (Jang et al., 2002) for antisense (Fig. 1A). Rice calli [cv. Ilpumbyeo, highly susceptible cultivar (IP); and Daesanbyeo, susceptible cultivar (DS)] were transformed with RNAi, antisense and empty-vector constructs via Agrobacterium tumefaciens-mediated transformation as described by Kim et al. (2009). Twenty-four independent transgenic lines (Supplementary Table 1) were initially selected using 6 mg/L phosphinothricin followed by further selection with 0.3% basta herbicide spray.
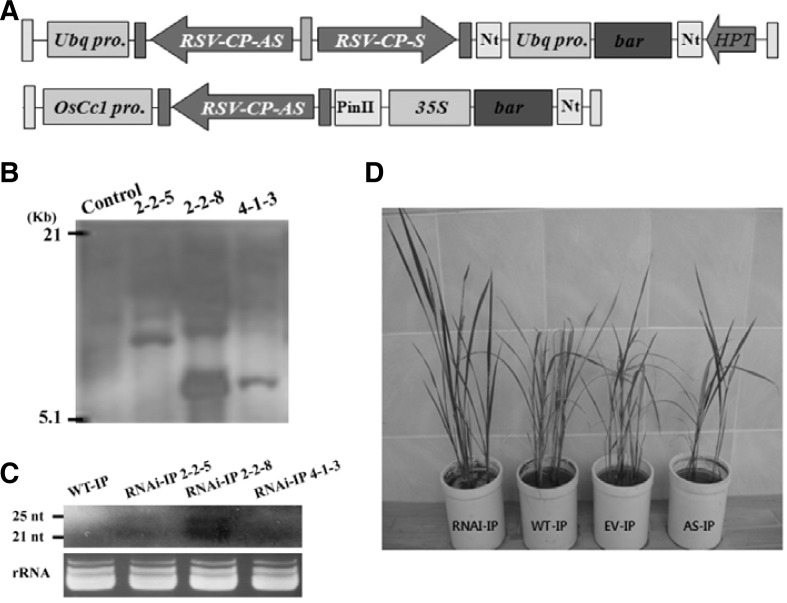
Fig. 1.
Generation and molecular analysis of transgenic rice plants with the fully complementary RNAi-RSV-CP-full construct in the highly susceptible cultivar, Ilpumbyeo (IP). (A) Schematic representation of the constructs for RSV-CP RNAi and antisense. Fully complementary RSV-CP gene derived from RNA 3 was cloned in antisense and sense orientations in pANDA-β to trigger RNAi. For RNAi vectors, namely pRNAi-RSV-CP-full, transcripts of the fully complementary RSV-CP were designed to be expressed constitutively under the control of the promoter (Ubq pro.) of the maize gene for ubiquitin. For antisense vectors, namely pAS-RSV-CP-full, transcripts of the RNAi-trigger region were designed to be expressed constitutively under the control of the promoter (OsCc1) of the rice gene for cytochrome C. (B) Southern blot analysis of T2 transgenic rice plants. Genomic DNA (20 μg) from wild-type and transgenic rice plants was digested with BamHI and hybridized with the DIG-labeled bar probe. Numbers indicate wild-type and transgenic lines. (C) Accumulation of transgene mRNA-derived siRNA in the leaves of the T2 transgenic rice plants transformed with an RNAi-RSV-CP-full construct exposed to viruliferous insects. (D) Representative phenotypes of RSV-inoculated T1 transgenic rice plants 8 weeks post inoculation (wpi). Plants in pots from left to right are transgenic rice plants harboring RNAi-, Antisense, and empty-vector control, respectively, exposed to viruliferous insects and non-transgenic wild-type (WT) inoculated with viruliferous insects.
Viral resistance assays of transgenic plants
RSV isolate KR was used for the virus resistance assays. Rice plants were inoculated with five nymphs (second or third instar) each and were kept in a cage containing 20 plants. After incubation at 27°C for 2–3 days under artificial light, the small brown planthoppers were killed with insecticide. Plants were transplanted to paddy soil in a green house (10 h at 27°C dark/14 h at 27°C light). The appearance of symptoms on developing leaves was evaluated 4–8 weeks after inoculation (Washio et al., 1968). Seedlings were evaluated and grouped into six classes (A, B, Bt, Cr, C and D) based on the severity of the RSV symptoms observed. Plants with poor growth and severe disease symptoms (A, B and Bt type) were considered as susceptible. Meanwhile, plants with a little poor growth or healthy and mild disease symptoms (Cr, C and D type) as resistance. The infection rate (the percentage of plants with susceptible symptoms) of tested lines was used for analyzing the resistance to RSV. Lines with infection rate values less than 29, from 30 to 39, from 40 to 69, and over 70 were classified as highly resistant, resistant, susceptible, and highly susceptible, respectively.
Southern blot analysis
Genomic DNA was extracted from rice (cv. Ilpumbyeo or Daesanbyeo) according to the cetyltrimethylammonium bromide (CTAB) method (Rogers and Bendich, 1985), and RNA was removed by the addition of 10 μg RNase A (Sigma-Aldrich Chemie GmbH). Approximately 20 μg of genomic DNA was digested with BamHI. The digested DNA fragments were fractionated on a 0.8% agarose gel in 0.5X TBE buffer. The supercoiled DNA ladder (Promega, USA) was included as a size marker. After electrophoresis, Southern blot analysis was conducted using a RSV-CP cDNA probe (330 bp) labeled with DIG-High Prime DNA Labeling. Signals were detected using the Detection Starter Kit II (Roche Diagnostics, Switzerland).
siRNA analysis of transgenic plants
siRNA analysis was performed according to Goto et al. (2003) with some modifications. Small RNA was extracted from 50 mg of plant leaves using the mirVana™ miRNA Isolation Kit (Ambion, #1560) according to the manufacturer’s instructions. One microgram of small RNA per sample was fractionated on a 17% polyacrylamide gel in 10X TBE buffer. Twelve nanograms of miRNA marker (NEB, #N0364) were included as a size marker. Probe labeling, hybridization and detection were performed according to the same protocol as the Southern blot analysis.
RT-PCR analysis of transgenic plants
For semi-quantitative RT-PCR, total RNAs were isolated using the RNeasy Plant Mini Kit according to the manufacturer’s instructions (QIAGEN, Germany). The first strands of cDNA were synthesized using the AffinityScript™ Multiple Temperature Reverse Transcriptase Kit (Stratagene, USA). cDNA was amplified using 1.5 units of Ex Taq DNA polymerase (Takara, Japan), 100 μM of each dNTP, and 10 pmol of each gene-specific primer in a T1 thermal cycler (Biometra, Germany). Amplification was performed with 28 or 40 cycles of: 94°C for 30 s, 54–67°C for 30 s, and 72°C for 30 s. The primers used in this study are shown in Table 1.
Table 1.
Primer list for RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| NS2 | 5′-TCGGATCCATGGCATTACTCCTTTTCAATG-3′ | 5′-GCGTCGACTCACATTAGAATAGGACACTCA-3′ |
| NS3 | 5′-TCGGATCCATGAACGTGTTCACATCGTC-3′ | 5′-CAGTCGACCTACAGCACAGCTGGAGAG-3′ |
| CP | 5′-GAGGATCCATGGGTACCAACAAGCCAG-3′ | 5′-TCGTCGACCTAGTCATCTGCACCTTCTG-3′ |
| SP | 5′-TGGGATCCATGCAAGACGTACAAAGGAC-3′ | 5′-CTGTCGACCTATGTTTTATGAAGAAGAGGT-3′ |
| RdRp | 5′-TGAGAATGGGAGAGTTGTAAATCA-3′ | 5′-GGCTAAGCTACTCTTGAACACCTC-3′ |
| MP | 5′-GCGGATCCATGGCTTTGTCTCGACTTTT-3′ | 5′-ACGTCGACCTACATGATGACAGAAACTTC-3′ |
| 18S | 5′-AGCAAGCCATCGCTCTGGATACAT-3′ | 5′-TATAAGCAACATCCGCCGATCCCT-3′ |
Underlining indicates a restriction enzyme sites.
Double antibody sandwich (DAS)-ELISA
ELISA was conducted using the DAS-ELISA PathoScreen kit for RSV with an alkaline phosphatase label (Japan Plant Protection Association, Japan). Four weeks after inoculation, the newly developed leaves were harvested. One hundred micrograms of leaves were ground in 1.0 ml of extraction buffer [2 g bovine serum albumin, 20 g polyvinyl pyrrolidone, and 0.2 g sodium azide in 1000 ml of phosphate-buffered saline (PBS)-Tween20, pH 7.4]. Negative controls consisted of mock-inoculated (non-viruliferous insects only) rice plants. The RSV positive control was provided by the manufacturer of the ELISA kit (Japan Plant Protection Association, Japan). For ELISA, a 100-μl sample of the sap was incubated overnight in ELISA wells pre-coated with RSV polyclonal antibody at 4°C. Next, 100 μl of RSV polyclonal antibody conjugated to alkaline phosphatase enzyme conjugate (1/500 dilution with PBS-Tween20) was added to each well, followed by a 4-h incubation period at 37°C. The substrate solution was prepared by dissolving a p-nitrophenyl phosphate tablet in buffer (0.1 g magnesium chloride, 0.2 g sodium azide, and 97 ml diethanolamine in 1,000 ml of distilled water, pH 9.8) at a concentration of 1 mg/ml. One hundred microliters of the substrate solution were loaded into each well. After incubation for 30 min to 1 h at 37°C, the color intensity of each well was assessed at 405 nm using a BIORAD 550 Microplate reader (USA).
Field cultivation and trait evaluation
To evaluate the yield components of the transgenic plants under field conditions, five lines of RNAi-RSV-CP-full and one line of RSV-CP-AS transgenic T3 and T4 lines (Supplementary Table 1), together with the original cultivars Ilpumbyeo and Daesanbyeo, were planted in a field at the GMO Experimental Field of National Institute of Crop Science, Suwon, Korea. Hills containing one plant each were placed at a distance of 30 × 15 cm using conventional transplanting methods during the summer of 2009 and 2010. Ten plants in the center of each plot were chosen for the evaluation of six traits: culm length (CL) was measured in centimeters from the soil surface to the neck of the tallest tiller; panicle length (PL) was measured in centimeters from the panicle neck to the panicle tip; number of panicles (NP) was calculated as the number of panicles per plant; number of spikelets per panicle (NSP) was counted as the number of spikelets of the biggest panicle of the plant; filling rate (FR) was calculated as the number of fertile spikelets per panicle divided by the total number of spikelets per panicle and expressed as a percentage; and thousand-grain weight (1,000 GW) was measured in grams as the weight of 1,000 fully ripened (14% moisture) grains per line.
RESULTS AND DISCUSSION
Molecular analysis of transgenic plants of the highly susceptible cultivar, Ilpumbyeo
To determine the integration and copy number of the integrated T-DNA in transgenic lines harboring the pRNAi-RSV-CP-full, Southern blot analysis was conducted using genomic DNA isolated from the leaves of a wild-type plant and three selected transgenic lines (2-2-5, 2-2-8, and 4-1-3). Genomic DNA was digested with BamHI and hybridized with the DIG-labeled bar probe. Southern blot analysis revealed the presence of the target band in all of the transgenic plants, while no hybridized band was detected in the wild-type. This result implies that the T-DNA is successfully integrated into the genome of the transgenic lines, as well as stably transmitted to the next generation of transgenic plants (Fig. 1B). To investigate whether the transgene mRNA-derived siRNA was successfully produced in the leaves of the transgenic lines, small RNA northern blot analysis (Fig. 1C) was performed using a wild-type plant and three selected transgenic rice lines (2-2-5, 2-2-8, 4-1-3). A RSV-CP gene-specific DIG-labelled PCR product was used as a probe. Small RNA accumulated to a high level in all of the transgenic plants, but not in the wild-type plant. This result confirms the accumulation of siRNA in the transgenic rice plants (Fig. 1C).
RSV resistance analysis of the T1 transgenic plants
All T1 generation plants were sprayed with Basta for the selection of plants containing RNAi- or antisense vectors. For each transgenic line, 20 positive plants were selected and inoculated with RSV isolates via viruliferous vector insects. Wild-types (cv. IP, highly susceptible cultivar) were included as controls and monitored for the appearance of symptoms. After 4 weeks of inoculation, all of the pRNAi-RSV-CP-full transgenic plant lines originating from IP exhibited a resistant phenotype. The susceptible plants, harboring pAS-RSV-CP-full or the empty vector, exhibited the same symptoms as the wild-type susceptible plants under the experimental conditions (Fig. 1D).
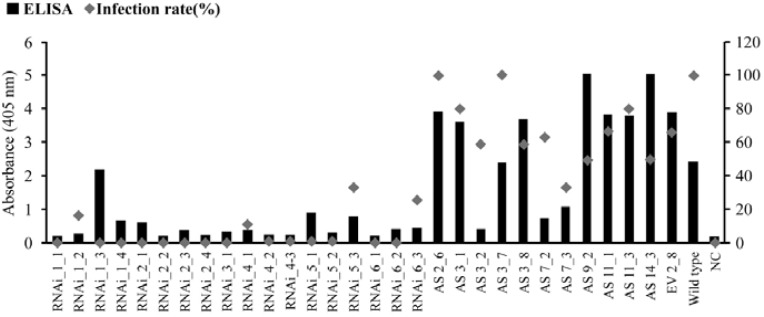
Six independent transgenic lines (Supplementary Table 1), originating from IP and containing the pRNAi-RSV-CP-full and pAS-RSV-CP-full constructs, were used for ELISA. The RSV infection rate of the pRNAi-RSV-CP-full and pAS-RSV-CP-full transgenic plants ranged from 0–38% and 37–100%, respectively (Fig. 2). Wild-type control plants had a 100% infection rate. At 4 weeks post inoculation (wpi), leaf extracts from individual plants were examined for the presence of RSV using a double-antibody sandwich (DAS) enzyme-linked immunosorbent assay (ELISA) with polyclonal antibodies. Although the pAS-RSV-CP-full transgenic plants showed high level of CP gene expression (data not shown), most of them, with the exception of three lines (pAS-RSV-CP-full 3-2, 7-2, 7-3), exhibited viral levels comparable to wild-type rice plants (Fig. 2). By contrast, RSV was not detected in the pRNAi-RSV-CP-full transgenic plants, with the exception of one line (pRNAi-RSV-CP-full 1–3). This result confirms the enhanced RSV resistance of the pRNAi-RSV-CP-full transgenic plants (Fig. 2). Thus, the extent of RSV resistance observed in the transgenic plants generated with pRNAi-RSV-CP-full was greater than the resistance of the pAS-RSV-CP-full transgenic plants.
Fig. 2.
Detection by ELISA (purple bar) with RSV polyclonal antibody, and the infection rate (navy diamond) in T1 transgenic and wild-type (WT) Ilpumbyeo rice plants 8 weeks post inoculation. Values refer to absorbance at 405 nm, measured after hydrolysis of substrate for 1 h. n = 2. NC, negative control.
Genetic stability of the transgenic RSV resistance
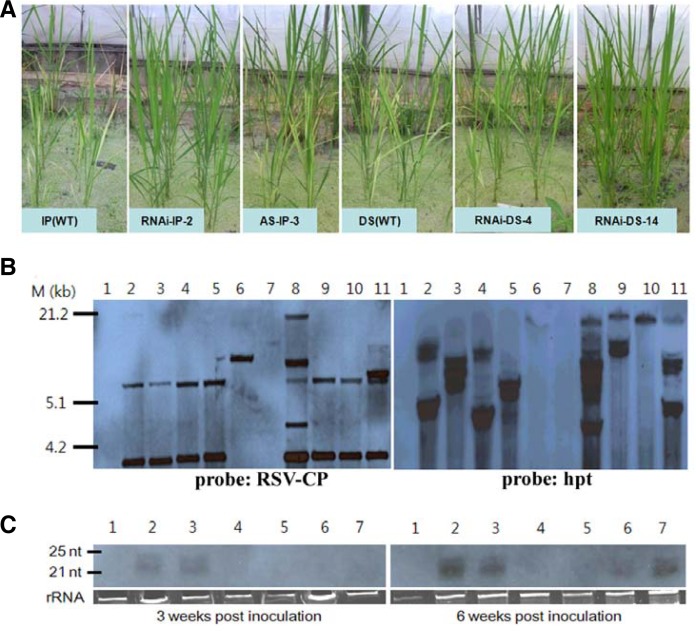
To observe the stable inheritance of the RSV resistance phenotype, T3 generation seedlings of pRNAi-RSV-CP-full and pAS-RSV-CP-full transgenic rice plants of highly susceptible and moderately susceptible wild-type rice (cv. IP and DS) were exposed to viruliferous insects. The representative phenotypes of the RSV-inoculated transgenic T3 plants are shown in Fig. 3A. Typical symptoms appeared in IP wild-type plants at 3 wpi. However, typical symptoms emerged at 6 wpi in pAS-RSV-CP-full transgenic plants and susceptible DS non-transgenic plants. Of the pRNAi-RSV-CP-full transgenic rice plants, all with the exception of one transgenic line (pRNAi-DS-4-1-1-1), showed immunity to RSV infection. These plants were characterized by an absence of symptoms for the duration of the test, regardless of whether the background was the highly susceptible cultivar IP lines or susceptible cultivar DS lines. This indicates that the RNAi-mediated resistance was stably inherited over generations, with the exception of pRNAi-DS-4-1-1-1, a transgenic line harboring multiples copies of the transgene (Fig. 3B).
Fig. 3.
RSV resistance of transgenic T3 rice plants with the RNAi-RSV-CP-full construct and of control plants in two cultivars (highly susceptible cultivar, Ilpumbyeo; susceptible cultivar, Daesanbyeo). (A) Representative phenotypes of RSV-inoculated transgenic T3 rice plants 6 weeks post inoculation (wpi). Plants in paddy greenhouse are non-transgenic wild-type (IP or DS) inoculated with viruliferous insects; and transgenic rice plants harboring the RNAi_RSV-CP fully complementary sequence or antisense RSV-CP full #AS-IP-3-2-1-1, respectively, exposed to viruliferous insects. IP(WT): Highly susceptible non-transgenic cultivar. RNAi-IP-2: Transgenic rice(IP) plants harboring RNAi_RSV-CP #IP-2-2-1-3; AS-IP-3: Transgenic rice(IP) plants harboring antisense RSV-CP #AS-IP-3-2-1-1; DS(WT): Susceptible non-transgenic cultivar; RNAi-DS-4 or 14: Transgenic rice(DS) plants harboring RNAi_RSV-CP #DS-4-1-1-1 or #DS-14-2-1-2. (B) Southern blot analysis of transgenic rice T3 plants. Genomic DNA (20 μg) from wild-type and transgenic rice plants was digested with BamHI and hybridized with the DIG-labeled bar or hpt probe. Numbers indicate wild-type and transgenic lines. Line 1, wild-type IP; line 2, RNAi-IP 2-2-1-3; line 3, RNAi-IP 2-4-2-1; line 4, RNAi-IP 9-1-4-1; line 5, RNAi-IP 9-2-1-3; line 6, Antisense IP 3-2-1-1; line 7, wild-type DS; line 8, RNAi-DS 4-1-1-1; line 9, RNAi-DS 5-2-4-1; line 10, RNAi-DS 9-1-1-2; line 11, RNAi-DS 14-2-1-2. (C) Detection of siRNAs in the leaves of RSV-inoculated transgenic T3 rice plants 3 or 6 wpi with an RNAi-RSV-CP-full construct. Numeric designations of transgenic lines are shown above the panels. Approximately 1 μg of total RNA was probed with digoxigenin-labeled transgene-specific RNA. The lower panel shows the detection of rRNA, included to confirm the loading of equal amounts of RNA in each lane. Numbers indicate wild-type and transgenic lines. Line 1, highly susceptible wild-type IP; line 2, RNAi-IP 2-2-1-3; line 3, RNAi-IP 9-1-4-1; line 4, Antisense IP 3-2-1-1; line 5, susceptible wild-type DS; line 6, RNAi-DS 4-1-1-1; line 7, RNAi-DS 14-2-1-2.
To confirm the maintenance of RNAi in the T3 generation, the accumulation of transgene-specific siRNAs was analyzed using total RNA from pRNAi-RSV-CP-full and pAS-RSV-CP-full transgenic T3 rice plants. As shown in Fig. 3C, small RNA molecules of 21–24 nt accumulated in all RNAi-RSV-CP-full transgenic T3 plants. In the pAS-RSV-CP-full transgenic line and wild-type rice plants, transgene-specific siRNAs were not detected. From these results, the level of siRNA accumulation correlates with the levels of RSV resistance. High levels of transgene-specific siRNAs, a hallmark of RNA silencing (Hamilton and Baulcombe, 1999), accumulated in the leaves of transgenic resistant lines in a manner consistent with the phenotypic symptoms.
dsRNAi-mediated viral resistance is more effective than antisense approaches. Lennefors et al. (2008) demonstrated that a dsRNA transcript from a BNYVV replicase gene-based construct could be considered a “strong silencing inducer.” This is in contrast to ssRNA produced from a CP transgene, considered a “weak silencing inducer” (Andika et al., 2005; 2006). The mechanism of initial dsRNA recognition via the RNA silencing process is not yet known. However, the transgene of pRNAi-RSV-CP-full readily produces a dsRNA that is recognizable. By contrast, production of dsRNA from an antisense RNA transcript requires further cellular steps. These may include complementary-strand synthesis by a cellular RNA-dependent RNA polymerase and/or interaction with the complementary viral RNA strands. Interestingly, in the pRNAi-RSV-CP-full transgenic T3 plants originating from the highly susceptible IP cultivar, RSV-CP specific siRNAs were readily detected at 3 wpi. However, small RNA molecules of 21–24nt were detected at 6 wpi in the pRNAi-RSV-CP-full transgenic T3 plants originating from the susceptible DS cultivar. This finding is novel. Transgenic rice plants originating from highly susceptible and susceptible cultivars were not compared according to the weeks post inoculation by viruliferous insects. The siRNAs detected in this study are believed to be small RNA molecules produced by degradation of the infecting virus. The small RNA accumulation increased according to the period after inoculation, as well as the original cultivar’s susceptibility to RSV.
RT-PCR analysis of invading viral gene expression
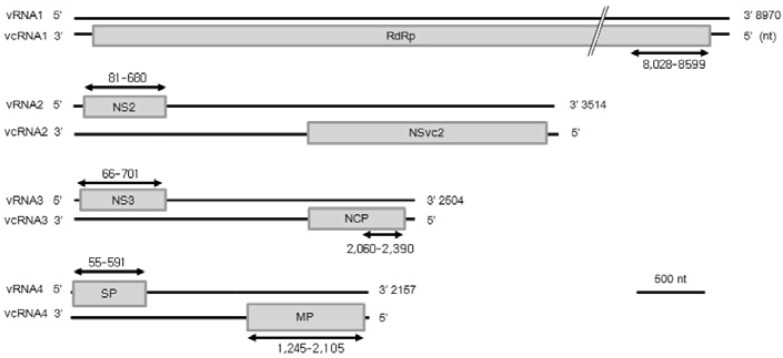
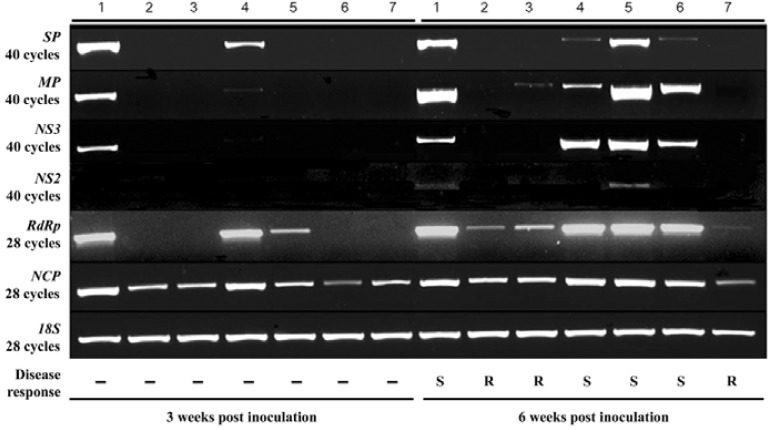
RT-PCR was used to analyze the potential correlation between the extent of resistance to RSV infection and the expression pattern of RSV genes. RSV gene-specific primer sets (see Table 1 for primer sequences) were used to analyze expression in the two wild-type cultivars, highly susceptible IP and susceptible DS, and for the T3 generation transgenic lines harboring pRNAi-RSV-CP-full or pAS-RSV-CP-full construct. The RSV genome positions of six target gene-specific primers are shown in Fig. 4. Expression differences of six viral genes were notable between the highly susceptible wild-type IP, susceptible wild-type DS, pRNAi-RSV-CP-full (cv. IP, DS) and pAS-RSV-CP-full (cv. IP) transgenic lines according to the weeks post inoculation (Fig. 5).
Fig. 4.
Genetic map of RNAs 1 through 4 of the RSV genome. The lines represent RNA segments and the boxes denote genes encoded by the viral-sense RNAs (vRNAs) and viral complementary sense RNAs (vcRNAs). RdRp, RNA-dependent RNA polymerase; NS3, viral suppressor of RNA-silencing; NCP, nucleocapsid; SP, disease-specific protein; MP, movement protein. Each of the six arrows indicates the position of RSV gene specific primer sets used for the study of infected viral gene expression.
Fig. 5.
Reverse-transcription polymerase chain reaction (RT-PCR) and small RNA detection of Rice stripe virus (RSV) in RSV-inoculated transgenic T3 rice plants three and 6 weeks post inoculation (wpi). RT-PCR or semi-quantitative RT-PCR detection of RSV genes (using specific primer sets shown in Table 1) in the leaves of RSV-inoculated non-transgenic plants (IP or DS) and transgenic rice plants transformed with the RNAi-RSV-CP-full or Antisense-RSV-CP construct. SP, disease-specific protein; MP, movement protein; NS3, viral suppressor of RNA-silencing (40 cycles); RdRp, RNA-dependent RNA polymerase; CP, capsid protein (28 cycles), respectively. 18S served as a control. Disease responses are represented below bottom panel (R, resistant; S, susceptible; -, not evaluated). Numbers indicate wild-type and transgenic lines. Line 1, highly susceptible wild-type IP; line 2, RNAi-IP 2-2-1-3; line 3, RNAi-IP 9-1-4-1; line 4, Antisense IP 3-2-1-1; line 5, susceptible wild-type DS; line 6, RNAi-DS 4-1-1-1; line 7, RNAi-DS 14-2-1-2.
The transcription of RSV genes was influenced by host immunity. No viral gene expression, with the exception of the transgene CP, was detected in any of the pRNAi-RSV-CP-full transgenic lines at 3 wpi. By contrast, all of the viral genes except NS2, whose function is unknown, were transcribed in the IP wild-type at the same stage. Interestingly, while the transcription level of MP and NS2 was increased at 6 wpi in the IP wild-type plants, the transcription of the RSV genes was dramatically altered between 3 and 6 wpi in the pAS-RSV-CP-full transgenic line. At 3 wpi, the transcription of two genes, the RNA-dependent RNA polymerase (RdRP) and disease specific protein (SP), was observed in the pAS-RSV-CP-full (cv. IP) transgenic line. On the other hand, while SP expression was relatively lower than that of IP wild-type during the early infection stage, the transcription of NS3 and MP increased at 6 wpi in the antisense transgenic line. In a previous study, the timing of SP expression was earlier than CP (Wu et al., 2006). Furthermore, the expression of SP was limited to young leaf tissues early in the infection cycle (Liang et al., 2005). Thus, these results indicate that SP, a disease-specific protein which accumulates in infected plants, may be easily suppressed by antisense mediated RNA silencing. This is in contrast to the limited success obtained by the suppression of NS3 and MP. For instance, not all RNAi constructs that target viral RNAs are equally effective in preventing RSV infection. Shimizu et al. (2010) reported that nearly 90% of the dsRNAi-trigger p4 (SP) transgenic plants were susceptible to RSV infection. Upon considering these results, it is suggested that down-regulation of SP expression appears to be insufficient for the suppression of viral gene expression related to RSV proliferation. However, it is enough to induce the expression of NS3, a gene silencing suppressor.
On the other hand, the transcription pattern of infected RSV in the DS wild-type cultivar and the pRNAi-RSV-CP-full transgenic lines (original cultivar, DS) was significantly different to that of IP wild-type plants. In the RSV-infected DS wild-type plants, low level of RdRP expression was shown at 3 wpi (Fig. 5). At 6 wpi, the transcription of all six genes was increased. These results indicate that the accumulation of RSV transcripts in the host plant depends upon the susceptibility to RSV. This means that the DS wild-type cultivar may be more tolerant to RSV infection than the IP cultivar. In addition, transcription of RdRP, NS3, and MP increased in the RSV-infected DS pRNAi-RSV-CP-full transgenic plants containing multiple copies of the transgene and exhibiting a susceptible phenotype. By contrast, no RSV genes except for the CP transgene were transcribed in the DS pRNAi-RSV-CP-full transgenic plants showing highly resistant phenotypes. These results suggest that the suppression of RdRP, NS3 and MP is essential for the stable inheritance of RNAi-mediated RSV resistance by targeting the fully complementary RSV-CP gene. In particular, typical symptoms appeared in the highly susceptible IP wild-type at 3 wpi. At 6 wpi, typical symptoms appeared in pAS-RSV-CP-full transgenic IP rice plants and pRNAi-RSV-CP-full transgenic DS rice plants harboring three transgene copies and showing the susceptible phenotype (Fig. 5). These results imply that the transcription of two genes (NS3 and MP) was critical for the appearance of typical RSV symptoms. Furthermore, the expression level of these two genes varied between the antisense susceptible line and the RNAi-susceptible line (Fig. 5, lanes 4 and 6, respectively). While the transcription level of NS3 was greater than that of MP in lane 4, the expression level of MP was greater than that of NS3 in lane 6. These results suggest that although the same phenotypic symptoms are present, the infection mechanism of RSV may be diverse as evidenced by the initial phase of infection (Atabekov, 1975). It seems that the induced expression of NS3 was sufficient as a counter-defense by RSV in antisense susceptible lines. Meanwhile, relatively lower NS3 expression levels appeared in the RNAi-susceptible line, and relatively lower amounts of small RNAs accumulated (Fig. 3B). This may also suggest that the RNAi-susceptible transgenic line is more tolerant than the antisense susceptible line.
The expression level of SP was lower than the levels of the other genes in lane 4 and 6, suggesting that lower SP gene expression may induce the host defense mechanism (Jari and Valkonen, 2003). This may sequentially activate the expression of the viral gene silencing suppressor NS3 and the movement protein MP. Shimizu and co-workers (2010) demonstrated that high level RSV resistance was shown in pRdRp-, pNCP-, and pMP-specific RNAi transgenic rice plants. Low levels of RSV resistance were observed in pNS3- and pNS2-specific RNAi lines. This may indicate that genes related to viral replication are key determinants for the appearance of typical symptoms in the host. Meanwhile, viruses evolve more rapidly than resistant hosts. This is because the virus overcomes cultivar-specific resistance genes by evolving suppressors and thus, countering silencing-mediated defense (Choi et al., 2004).
The RSV-CP gene sequence homology was very high, where-as the sequence similarity of the SP gene was lowest among RSV isolates (Ma et al., 2011). Among the 100 accessions listed in NCBI, the RSV-CP genes were 93–100% identical at the nucleotide level and 96–100% identical the amino acid level (data not shown). This suggests that RSV-CP may be an effective target gene for broad spectrum resistance for the prevention of resistance breakdown due to virus mutation. For instance, Kumar et al. (2006) used a sequence conserved among a variety of flaviviruses as an effective target fragment. This fragment efficiently silenced both Japanese encephalitis virus and West Nile virus.
While previous studies have reported the used of RSV CP and SP sequences in single transgene constructs (Ma et al., 2011; Shimizu et al., 2010), the RNAi effectiveness was quite different. For instance, RNAi transgenic rice plants harboring pSP-specific sequences did not exhibit enhanced RSV resistance (Shimizu et al., 2010). However, CP/SP double targeting RNAi lines showed stronger RSV resistance than CP or SP single targeting RNAi lines (Ma et al., 2011). These discrepancies may be due to the size and chromosomal position of transgene. Ma et al. (2011) used targeting fragments that corresponded to the middle of the full-length CP and SP coding regions, respectively. Determining the targets involved in viral sequence specificity, configuration, genome position and size of the transgene is important for efficient RNA silencing (Hutvagner et al., 2000).
In this study, the production of transgenic rice plants harboring the full sequence of the RSV-CP single gene and originating from two cultivars of varying susceptibilities did result in dsRNAi-mediated transgenic resistance against RSV. The dsRNA length determined the quantity of siRNA molecules that were produced after DICER cleavage (Berstein et al., 2001), with a greater length providing higher diversification and more efficient RNA degradation. Variations in sequence size and position may explain the different efficiencies in conferring resistance. Chen et al. (2004) reported that when using the long inverted repeat constructs, a remarkable 75% resistance was shown in the R0 lines, while transformants harboring the short inverted repeats transgene exhibited a considerable yet lower percentage of resistance (30%).
Agronomic traits of field-grown pRANi-RSV-CP-full or pAS-RSV-CP-full T4 generation transgenic plants
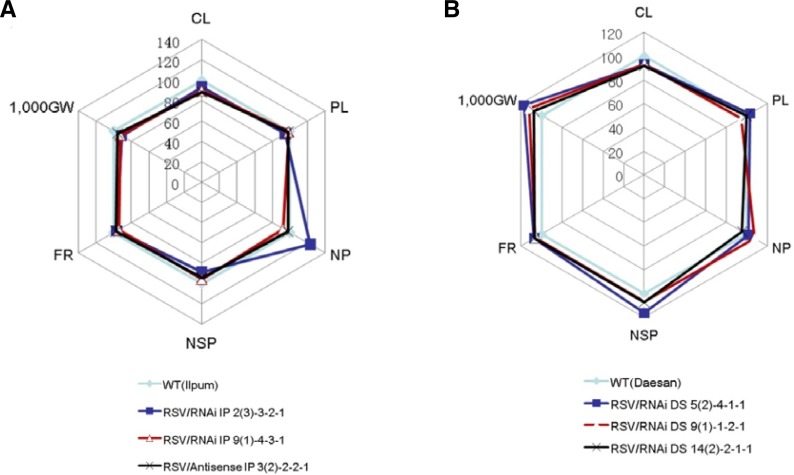
To investigate whether the pRNAi-RSV-CP-full and pAS-RSV-CP-full transgenes influenced the agronomic traits of the transgenic plants under field conditions, three independent T4 homozygous lines (Supplementary Table 1) of the pRNAi-RSV-CP-full and pAS-RSV-CP-full plants, together with their respective wild-type controls, were transplanted in a paddy field and grown to harvest. The yield parameters of these plants revealed that the grain yield component of the pRNAi-RSV-CP-full plants (cv. DS) was similar, perhaps even better, than that of the wild-type control under field conditions (Fig. 6B). However, trait differences related to grain yield were apparent between the transgenic lines (cv. IP) and the wild-type (Fig. 6A). These appear to be due to somaclonal variation occurring during the transformation process, an artifact of the longer IP culture period compared to that of DS (Phillips et al., 1994). Meanwhile, the initial generation level of difference between the transgenic lines and the wild-type cultivars in agronomic traits was decreased by generation iteration (data not shown). This implies that the genes critical for rice plant growth were unaffected by the transgene.
Fig. 6.
Agronomic traits of RNAi-RSV-CP and Antisense-RSV-CP rice plants grown under field conditions. Spider plots of the agronomic traits of three independent homozygous T4 lines of RNAi-RSV-CP-full plants and the corresponding WT were drawn using Microsoft Excel. Highly susceptible wild type IP and RNAi IP 2(3)-3-2-1, 9(1)-4-3-1 and antisense IP 3(2)-2-2-1 transgenic lines are shown in (A). Susceptible wild type DS and RNAi DS 5(2)-4-1-1, 9(1)-1-2-1 and 14(2)-2-1-1 transgenic lines are represented in (B). Each data point represents the percentage of the mean values (n = 20). The mean measurements from the WT controls were assigned a 100% reference value. CL, culm length; PL, panicle length; NP, number of panicles per hill; NSP, number of spikelets per panicle; FR, filling rate; 1,000 GW, 1000 grain weight.
To date, comparisons of viral expression patterns between wild-type and transgenic rice plants harboring pRNAi- or pAS-constructs showing susceptible phenotypes have not been reported. This is a result of little attention being placed on susceptible transgenic plants. It is thus important to evaluate the expression pattern of RSV genes in transgenic plants harboring RSV-CP-full to verify the interaction between the virus and the plant for effective inheritable RNAi-mediated RNA-silencing.
Overall, the results presented here demonstrate that the application of an RNAi construct targeting the fully complementary RSV-CP gene appears to be a highly efficient method for the production of stable RSV resistant rice plants. Furthermore, these plants do not exhibit undesirable growth phenotypes at T4 generation level. In particular, efficient suppression of viral genes corresponding to the silencing suppressor and movement, as well as that of viral replication related genes, is essential for the stable inheritance of RNAi-mediated transgenic resistance against RSV. Additionally, the RSV-resistant transgenic rice plants produced in this study possess an advantage in bio-safety assessments, as they show good field performance without the accumulation of foreign proteins.
Supplementary Material
Acknowledgments
This work was supported by grants from the R&D project (No.PJ006750) of the National Institute of Crop Science and the Next-Generation BioGreen 21 Program (The National Center for GM Crops No.PJ008070), of the Rural Development Administration, Republic of Korea.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Andika I.B., Kondo H., Tamada T. Evidence that RNA silencing-mediated resistance to Beet necrotic yellow vein virus is less effective in roots than in leaves. Mol. Plant Microbe Interact. 2005;18:194–204. doi: 10.1094/MPMI-18-0194. [DOI] [PubMed] [Google Scholar]
- Andika I.B., Kondo H., Rahim M.D., Tamada T. Lower levels of transgene silencing in roots is associated with reduced DNA methylation levels at non-symmetrical sites but not at symmetrical sites. Plant Mol. Biol. 2006;60:423–435. doi: 10.1007/s11103-005-4429-7. [DOI] [PubMed] [Google Scholar]
- Atabekov J.G. Host specificity of plant viruses. Ann. Rev. Phytopathol. 1975;13:127–145. [Google Scholar]
- Bernstein E., Caudy A.A., Hammond S.M., Hannon G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Chang C.I., Kang H.S., Ban C., Kim S., Lee D.K. Dual-target gene silencing by using, synthetic siRNA duplexes without triggering antiviral responses. Mol. Cells. 2009;27:689–695. doi: 10.1007/s10059-009-0093-0. [DOI] [PubMed] [Google Scholar]
- Chen Y.K., Dick L., Rob G., Marcel P. High frequency induction of RNA-mediated resistance against Cucumber mosaic virus using inverted repeat constructs. Mol. Breed. 2004;14:215–226. [Google Scholar]
- Choi C.W., Qu F., Ren T., Morris T.J. Relationship between plant viral encoded suppressor to post-transcriptional gene silencing and elicitor to R gene-specific host resistance. Plant Pathol. J. 2004;20:22–29. [Google Scholar]
- Dasgupta I., Malathi V.G., Mukherjee S.K. Genetic engineering for virus resistance. Curr. Sci. 2003;84:341–354. [Google Scholar]
- Deleris A., Gallego-Bartolome J., Bao J., Kasschau K.D., Carrington J.C., Voinnet O. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science. 2006;313:68–71. doi: 10.1126/science.1128214. [DOI] [PubMed] [Google Scholar]
- Di Nicola-Negri E., Brunetti A., Tavazza M., Ilardi V. Hairpin RNA-mediated silencing of Plum pox virus P1 and HC-Pro genes for efficient and predictable resistance to the virus. Transgenic Res. 2005;14:989–994. doi: 10.1007/s11248-005-1773-y. [DOI] [PubMed] [Google Scholar]
- Falk B.W., Tsai J.H. Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 1998;36:139–163. doi: 10.1146/annurev.phyto.36.1.139. [DOI] [PubMed] [Google Scholar]
- Fuentes A., Ramos P.L., Fiallo E., Callard D., Sanchez Y., Peral R., Rodriguez R., Pujol M. Intron-hairpin RNA derived from replication associated protein C1 gene confers immunity to Tomato yellow leaf curl virus infection in transgenic tomato plants. Transgenic Res. 2006;15:291–304. doi: 10.1007/s11248-005-5238-0. [DOI] [PubMed] [Google Scholar]
- Goto K., Kanazawa A., Kusaba M., Masutaaj C., Baulcombe D.C. A simple and rapid method to detect plant siRNAs using Nonradioactive probes. Plant Mol. Biol. Rep. 2003;21:51–58. [Google Scholar]
- Gu K.L., Wang Z.L., Yang G., Ma L., Xu J.J. The consequence of outbreak of the small brown planthopper (Laodelphax striatellus Fallén) and rice stripe virus and their control measures. J. Anhui Agric. Sci. 2005;33:44. [Google Scholar]
- Hamilton A.J., Baulcombe D.C. A species of small antisense RNA in post-transcriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Zhu Y., Itoh K., Kimura Y., Izawa T., Shimamoto K., Toriyama S. Genetically engineered rice resistant to rice stripe virus, an insect-transmitted virus. Proc. Natl. Acad. Sci. USA. 1992;89:9865–9869. doi: 10.1073/pnas.89.20.9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano-Saito Y., Tsuji T., Fujii K., Saito K., Iwasaki M., Saito A. Localization of the rice stripe disease resistance gene, Stvb-i by graphical genotyping and linkage analyses with molecular markers. Theor. Appl. Genet. 1998;101:59–63. [Google Scholar]
- Hibino H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 1996;34:249–274. doi: 10.1146/annurev.phyto.34.1.249. [DOI] [PubMed] [Google Scholar]
- Hutvagner G., Mlynarova L., Nap J. Detailed characterization of the post-transcriptional gene-silencing-related small RNA in a GUS gene-silenced tobacco. RNA. 2000;6:1445–1454. doi: 10.1017/s1355838200001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.C., Choi W.B., Lee K.H., Song S.I., Nahm B.H., Kim J.K. High-level and ubiquitous expression of the rice cytochrome c gene (OsCc 1) and its promoter activity in transgenic plants provides a useful promoter for transgenesis of monocots. Plant Physiol. 2002;129:1473–1481. doi: 10.1104/pp.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jari P., Valkonen T. Plant resistance to Infection with viruses. eLS on line library. 2003. [DOI]
- Jonson G.-M., Choi H.-S., Kim J.-S., Choi I.-R., Kim K.-H. Sequence and phylogenetic analyses of the RNA1 and RNA2 segments of Korean Rice stripe virus isolates and comparison with those of China and Japan. Arch. Virol. 2009a;154:1705–1708. doi: 10.1007/s00705-009-0493-7. [DOI] [PubMed] [Google Scholar]
- Jonson G.-M., Choi H.-S., Kim J.-S., Choi I.-R., Kim K.-H. Complete genome sequence of the RNAs 3 and 4 segments of rice stripe virus isolates in Korea and their phylogenetic relationships with Japan and China isolates. Plant Pathol. J. 2009b;25:142–150. [Google Scholar]
- Jonson G.-M., Lian S., Choi H.S., Lee G.-S., Kim C.S., Kim K.H. Genetic reassortment of Rice stripe virus RNA segments detected by RT-PCR restriction enzyme analysis-based method. Plant Pathol. J. 2011;27:148–155. [Google Scholar]
- Kim Y.H., Park H.M., Choi M.S., Yun H.T., Choi I.S., Shin D.B., Kim C.K., Lee J.Y. The effects of co-cultivation medium and culture conditions on rice transformation efficiency. Korean J. Breed. Sci. 2009;41:252–260. [Google Scholar]
- Kumar P., Lee S.K., Shankar P., Manjunath N. A single siRNA suppresses fatal encephalitis induced by two different fl aviviruses. PLoS Med. 2006;3:505–514. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.C., Hong Y.K., Kawk D.Y., Park S.T., Choi J.M., Lee K.W. Cloning and sequencing of the Korean isolate of rice stripe virus. Plant Pathol. J. 2004;20:313–315. [Google Scholar]
- Lennefors B.L., Yndgaard F., van Roggen P., Savenkov E.I., Valkonen J.P.T. Efficient dsRNA-mediated transgenic resistance to Beet necrotic yellow vein virus in sugar beets is not affected by other soilborne and aphid-transmitted viruses. Transgenic Res. 2008;17:219–228. doi: 10.1007/s11248-007-9092-0. [DOI] [PubMed] [Google Scholar]
- Liang D., Qu Z., Ma X., Hull R. Detection and localization of rice stripe virus gene products in vivo. Virus Genes. 2005;31:211–221. doi: 10.1007/s11262-005-1798-6. [DOI] [PubMed] [Google Scholar]
- Ma J., Song Y., Wu B., Jiang M., Li K., Zhu C., Wen F. Production of transgenic rice new germplasm with strong resistance against two isolations of rice stripe virus by RNA interference. Transgenic Res. 2011 doi: 10.1007/s11248-011-9502-1. [DOI] [PubMed] [Google Scholar]
- Mansoor S., Amin I., Hussain M., Zafar Y., Briddon R.W. Engineering novel traits in plants through RNA-interference. Trends Plant Sci. 2006;11:559–565. doi: 10.1016/j.tplants.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Miki D., Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004;45:490–495. doi: 10.1093/pcp/pch048. [DOI] [PubMed] [Google Scholar]
- Phillips R.L., Kaeppler S.M., Olhoft P. Genetic instability of plant tissue cultures: breakdown of normal controls. Proc. Natl. Acad. Sci. USA. 1994;91:5222–5226. doi: 10.1073/pnas.91.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.O., Bendich A.J. Extraction of DNA from milligram amounts of fresh herbarium and mummified plant tissues. Plant Mol. Biol. 1985;5:69–76. doi: 10.1007/BF00020088. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nakazono-Nagaoka E., Uehara-Ichiki T., Sasaya T., Omura T. Targeting specific genes for RNA interference is crucial to the development of strong resistance to rice stripe virus. Plant Biotechnol. J. 2010;9:503–512. doi: 10.1111/j.1467-7652.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Toriyama S., Hamamatsu C., Ishihama A. Nucleotide sequence and possible ambisense coding strategy of rice stripe virus RNA segment 2. J. Gen. Virol. 1993;74:769–773. doi: 10.1099/0022-1317-74-4-769. [DOI] [PubMed] [Google Scholar]
- Toriyama S. Rice stripe virus. CMI/AAB Description of Plant Viruses. 2000. No. 375.
- Toriyama S., Watanabe Y. Characterization of singleand double stranded RNAs in particles of rice stripe virus. J. Gen. Virol. 1989;70:505–511. [Google Scholar]
- Toriyama S., Takahashi M., Sano Y., Shimizu T., Ishihama A. Nucleotide sequence of RNA1, the largest genomic segment of rice stripe virus the prototype of the tenuiviruses. J. Gen. Virol. 1994;75:3569–3579. doi: 10.1099/0022-1317-75-12-3569. [DOI] [PubMed] [Google Scholar]
- Voinnet O. Induction and suppression of RNA silencing: Insights from viral infections. Nat. Rev. Genet. 2005;6:206–221. doi: 10.1038/nrg1555. [DOI] [PubMed] [Google Scholar]
- Washio O., Ezuka A., Toriyama K., Sakurai Y. Testing method for genetics of and breeding for resistance to rice stripe disease. Bull. Chugoku Agric. Exp. Sta. Ser. A. 1968;16:39–197. [Google Scholar]
- Waterhouse P.M., Graham M.W., Wang M.B. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA. 1998;95:13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T.Y., Yang J.G., Liao F.R., Gao F.L., Lu L.M., Zhang X.T., Li F., Wu Z.J., Lin Q.Y., Xie L.H., et al. Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 2009;90:1025–1034. doi: 10.1099/vir.0.006858-0. [DOI] [PubMed] [Google Scholar]
- Wu S.J., Zhong H., Zuo H., Gu M.H., Liang G.H. Research progress in molecular biology of rice stripe virus and gene engineering of virus resistance. Acta Agric. Jiangxi. 2006;18:72–77. [Google Scholar]
- Xiong R.Y., Cheng Z.B., Wu J.X., Zhou Y.J., Zhou T., Zhou X.P. First report of an outbreak of Rice stripe virus on wheat in China. Plant Pathol. 2008a;57:397. [Google Scholar]
- Xiong R., Wu J., Zhou Y., Zhou X. Identification of movement protein of the Tenuivirus rice stripe virus. J. Virol. 2008b;82:12304–12311. doi: 10.1128/JVI.01696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Wu J., Zhou Y., Zhou X. Characterization and subcellular localization of an RNA silencing suppressor encoded by rice stripe tenuivirus. Virology. 2009;387:29–40. doi: 10.1016/j.virol.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hayakawa T., Toriyama S., Takahashi M. Complete nucleotide sequence of RNA 3 of rice stripe virus an ambisense coding strategy. J. Gen. Virol. 1991;72:763–767. doi: 10.1099/0022-1317-72-4-763. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hayakawa T., Toriyama S. Complete nucleotide sequence of RNA4 of rice stripe virus isolate T and comparison with another isolate and with maize stripe virus. J. Gen. Virol. 1992;73:1309–1312. doi: 10.1099/0022-1317-73-5-1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.