Abstract
MicroRNAs (miRNAs) are regulatory small non-coding RNAs that can regulate gene expression by binding to gene elements, such as the gene promotor 5′UTR, mainly in the 3′UTR of mRNA. One miRNA targets many mRNAs, which can be regulated by many miRNAs, leading to a complex metabolic network. In our study, we found that the expression level of miR-590-5p is higher in the human hepatocellular carcinoma cell line HepG2 than in the normal hepatocellular cell line L02. Downregulation of miR-590-5p inhibited proliferation and invasion of hepatocellular carcinoma cells (HCCs). We also showed that expression of TGF-beta RII, which has been regarded as a regulator of tumor proliferation, invasion, and migration in hepatocellular carcinoma, is regulated by miRNA-590-5p. In addition, miR-590-5p downregulated the expression of TGF-beta RII by targeting the 3′UTR of mRNA. We also found that downregulation of miR-590-5p was associated with an elevation of TGF-beta RII and inhibition of proliferation and invasion in HepG2 cells. Furthermore, overexpression of miR-590-5p was associated with upregulation of TGF-beta RII and could promote proliferation and invasion in L02 cells. In conclusion, we determined that TGF-beta RII is a novel target of miRNA-590-5p. Thus, the role of TGF-beta RII in regulating proliferation and invasion of human HCCs is controlled by miR-590-5p. In other words, miR-590-5p promotes proliferation and invasion in human HCCs by directly targeting TGF-beta RII.
Keywords: HepG2, invasion, L02, miR-590-5p, proliferation, TGF-beta RII
INTRODUCTION
MicroRNAs (miRNAs) are regulatory endogenous, single stranded, small non-coding RNAs that regulate gene expression by binding to gene elements and control a wide range of physiological processes (Bartel, 2004; Esquela-Kerscher and Slack, 2006). miRNAs can cause mRNA cleavage or inhibit translation. However, a recent report showed that miR-369-3p can upregulate the expression of tumor necrosis factor alpha (Vasudevan et al., 2007). In addition, miRNAs have been shown to function as tumor suppressors or oncogenes (Bracken et al., 2009; Esquela-Kerscher et al., 2006; Huang et al., 2008; Kumar et al., 2007; Lee et al., 2007; Liu et al., 2009; Moriyama et al., 2009; Ryan et al., 2010).
Hepatocellular carcinoma is one of the most common cancers and the third leading cause of cancer death worldwide. Hepatocellular carcinoma has high fatality and mortality rates (Parkin et al., 2005). It has been confirmed that TGF-beta1 can combine with TGF-beta RII to regulate the EMT process to regulate migration and invasion in human carcinoma. TGF-beta1 can induce snail transcription factor in epithelial cell lines (Park et al., 2003; Peinado et al., 2003) and is downregulated in many cancer cells. In addition, overexpression of cyclinD1 in hepatocellular carcinoma leads to a mutation and decreased levels of TGF-beta RII, which can result in inhibition resistance due to proliferation made by TGF-beta1 (Jong et al., 2005). Moreover, overexpression of TGF-beta RII in cancer cells can inhibit the growth of cells (Deacu et al., 2004).
Several studies have demonstrated the role of TGF-beta RII in cancer cells, but little is known about the microRNAs that regulate it during cancer pathogenesis. In this study, we sought to identify a novel miRNA that can regulate proliferation and invasion of hepatocellular carcinoma cells (HCCs) and its target.
MATERIALS AND METHODS
Cell culture conditions
The cell lines used in this study included HepG2 and L02 purchased from Cell Bank Type Culture Collection of the Chinese Academy of Sciences (CBTCCCAS, China). The media used for the two cell lines was 1640 (Gibco), supplemented with 10% FBS (FBS; HyClone, USA), 100 U/ml penicillin sodium (Gibco), and 100 mg/ml streptomycin sulfate (Gibco). Cells were maintained at 37°C and 5% CO2 with media.
Transfection assays
For transfection, cells were grown to 80% confluency and transfected with the chemically synthesized miRNA-590-5p inhibitor (Ambion), TGF-beta RII siRNA (Santa Cruz, TGF-beta RII siRNA (h): sc-36657), pEGFP, or negative control vector by using Lipofectamine 2000 (Invitrogen, USA), according to the manufacturer’s recommendation.
Construction of luciferase reporter gene
The 3′UTR fragment of TGF-beta RII was amplified by PCR. The PCR was performed on the cDNA of HepG2 cells. The final product was inserted into the luciferase reporter vector pGL3cM (Promega) immediately downstream from the stop codon of luciferase at the XbaI site. The following primer set was used to amplify this fragment: TGF-beta RII, forward, 5′-GGCGAGCTCCTCTTCTGGGGCAGGCTGG-3′ with a sacI recognition site, and reverse, 5′-GGCTCTAGATTGTCAAATG CTAATGCTGTC-3′ with an XbaI recognition site. Mutations were generated by replacing the miRNA seed sequence, and the mutant sequences were inserted into the luciferase reporter vector (pGL3cM).
Ectopic expression vectors of TGF-beta RII
The fragment of TGF-beta RII ORF was amplified by PCR from the cDNA of L02 cells and inserted into the pEGFP vector. We deleted the Kozak sequence to prevent GFP expression of the pEGFP-N1 vector. Thus, we could remove the influence of the GFP fusion protein in regulating cancer cell proliferation, invasion, etc. The following primer set was used to amplify this fragment: TGF-beta RII, forward, 5′-GGCGTCGAGTGGGTC GGGGGCTGCTCCG-3′ with a small recognition site, and reverse, 5′-GGCGGATCCCTATTTGGTAGTGTTCAGCG-3′ with a BamHI recognition site.
Luciferase assay
HeLa cells (5 × 104) purchased from the Cell Bank Type Culture Collection of the Chinese Academy of Sciences (CBTCCCAS, China) were transfected with 300 ng of the UTR reporter, 10 ng of the transfection control Renilla vector, and 50 pmol of chemically synthesized miRNA-590-5p mimics or inhibitor (Ambion) with 2 μl of Lipofectamine 2000. Lysates were harvested 24 h after transfection. The reporter activity was measured by the Dual Luciferase Assay (Promega).
Transwell assay
Invasion assays were done using a modified transwell chamber system as described previously (Hu and Ivashkiv, 2004). Cells (2 × 105) were seeded on matrigel-coated (500 ng/ml) membrane inserts with a pore size of 8 mm (BD Bioscience) in the presence of DMEM supplemented with 10% FBS. The same media (600 μl) was placed in the lower wells. The cells on the upper side were scraped off with a rubber policeman, and the cells that had migrated into the lower compartment were fixed (4% paraformaldehyde in phosphate-buffered saline, PBS), stained with hochest33342, and counted from five random high power fields at 200× magnification in each well.
MTT assay
Cells (1.0 × 104 cells/ml) were cultured in 96-well plates for varying periods of time and exposed to fresh media every other day. During the last 4 h of each day of culture, the cells were treated with methyl thiazolyl tetrazolium (MTT, 50 μg per well, Sigma). The generated formazan was dissolved in DMSO, and the absorbance was measured at 570 nm to determine cell viability.
Real-time quantitative PCR
Total RNA was isolated using the Trizol reagent (Sigma) and was reverse transcribed using an mRNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCR was carried out using SYBR Premix Ex Taq™ (Code DRR041A, Takara). The qRT-PCR primers were as follows: GAPDH, forward, AAGG TGAAGGTCGGAGTCAAC, and reverse, GGGTCATTGAT GGCAACAATA; TGF-beta RII, forward, AAGATGACCGCT CTGACATCA, and reverse, CTTATAGACCTCAGCAAAGC GAC. The reactions were placed in a 96-well plate (ABI) using an Mx3000P system (Stratagene). The amount of target gene expression (2−ΔΔCt) was normalized using endogenous U6 as a reference.
Real-time quantitative PCR for miRNA
Quantitative RT-PCR was carried out using SYBR Premix Ex Taq™ (Code DRR041A, Takara). Total RNA isolated using the Trizol reagent (Sigma) was subsequently reverse transcribed to cDNA using the stem-loop reverse transcription primer for miRNA detection (Ruibo). The microRNA Reverse Transcription Kit (Applied Biosystems) was used to obtain the cDNA. U6 small nuclear RNA was used as an internal control for miRNA. The reactions were placed in a 96-well plate (ABI) using an Mx3000P system (Stratagene). The amount of target gene expression (2−ΔΔCt) was normalized using endogenous U6 as a reference.
Western blotting analysis
Cells were lysed with 1× lysis buffer, which was diluted from 5× lysis buffer [2.5 ml of 0.5 M Tris-HCl (pH 6.8), 0.39 g of DTT, 0.5 g of SDS, 0.025 g of bromophenol blue, and 2.5 ml of glycerine]. Equal amounts of protein were loaded onto 10% SDS-PAGE for electrophoresis and then transferred onto a nitrocellulose membrane. The membrane was then incubated with primary antibodies for 24 h at 4°C against TGF-betaRII and gapdh (Cell Signaling Technology). After incubation with secondary antibody, the signal was visualized by enhanced chemiluminescence.
Statistical analysis
The results were evaluated by the Student’s t-test for nonpaired data. Statistical significance was defined as a value of p < 0.05* or p < 0.01**. Values are reported as mean ± s.d.
RESULTS
miR-590-5p is upregulated in human HCCs
We detected the expression level of miRNA-590-5p in several hepatocellular carcinoma cell lines and the normal hepatocellular cell line L02. We found that the levels of miRNA-590-5p in the hepatocellular carcinoma cell lines HepG2, HeP3B, and MHCC97L were higher than in the normal hepatocellular cell line L02; HepG2 cells had the highest levels (Fig. 1A). Therefore, we hypothesized that miRNA-590-5p might play an important role in regulating the development and formation of hepatocellular carcinoma. We also found that there was a reverse correlation between TGF-beta RII and miR-590-5p in hepatocellular cancer cells (Fig. 1B). So, we wanted to know whether miR-590-5p can regulate TGF-beta RII in hepatocellular cancer cells.
Fig. 1.
miR-590-5p is upregulated in human HCCs. (A) Detection of the relative expression of miR-590-5p in L02 and various hepatocellular carcinoma cell lines. The relative miR-590-5p level (mean ± SD) is shown. P values are from Student’s t-tests. All data are presented as means ± s.d. of three independent experiments. **p < 0.01. (B) Detection of the relative expression of TGF-beta RII in L02 and various hepatocellular carcinoma cell lines. The relative TGF-beta RII expression level (mean ± SD) is shown. P values are from Student’s t-tests. All data are presented as means ± s.d. of three independent experiments. **p < 0.01. The Western blot shows the TGF-beta RII protein level.
miR-590-5p downregulates TGF-beta RII by targeting the 3′UTR of TGF-beta RII
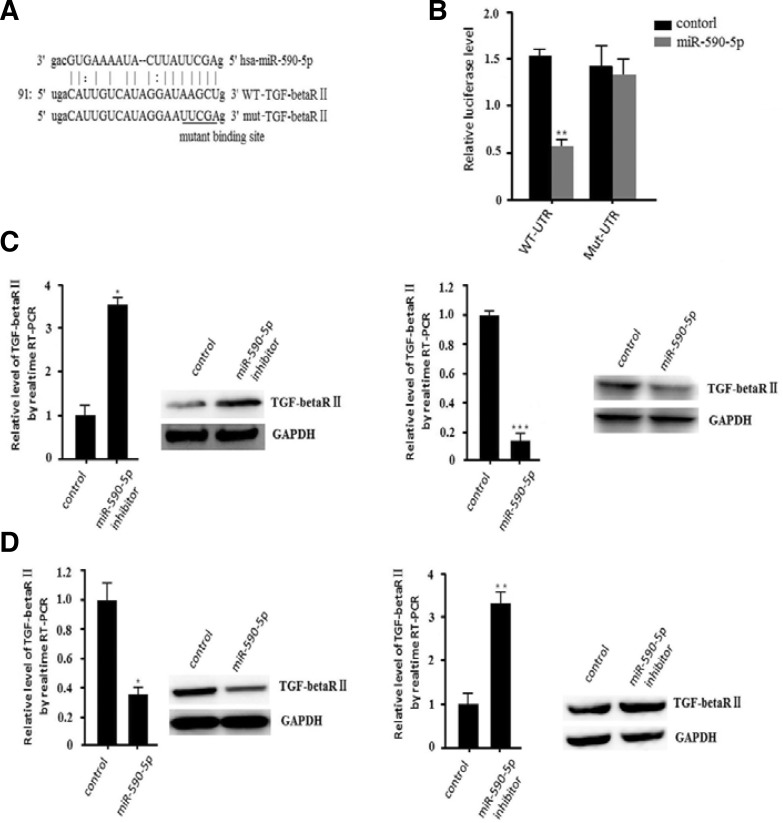
We used online prediction software (Miranda, target scan) to find the targets of miR-590-5p. We found that TGF-beta RII is one of the targets (Fig. 2A). We hypothesized that the negative correlation between miR-590-5p and TGF-beta RII revealed that TGF-beta RII may be regulated by miR-590-5p, which may play an important role in regulating hepatocellular cancer cell growth.
Fig. 2.
miR-590-5p downregulates TGF-beta RII expression by targeting the 3′UTR of TGF-beta RII.
To investigate whether TGF-beta RII can be directly targeted by miR-590-5p, we engineered luciferase reporters that have either the wild-type 3′UTRs of this gene or mutant UTRs with a six base pair (bp) replacement of the miRNA seed sequence (Fig. 2A). Reporter constructs such as these are widely used to provide experimental evidence that miRNAs can directly repress translation initiation (Veerla et al., 2004). Control miRNA mimics cannot affect the reporter activities. The mimics of miR-590-5p significantly reduced the luciferase activities of the wild-type TGF-beta RII 3′UTR more than 60% compared to the negative control. In contrast, the mutant binding site reporters were not repressed by miR-590-5p (Fig. 2B). This result showed that miR-590-5p directly targets TGF-beta RII 3′UTRs. We further examined whether miR-590-5p can repress endogenous TGF-beta RII in HepG2 cells. We downregulated miR-590-5p by transfecting the miR-590-5p inhibitor, which can inhibit the function of endogenous miR-590-5p. Quantitative real-time RT-PCR revealed that the mRNA level of TGF-beta RII was higher in the miR-590-5p inhibitor-transfected population than in the population transfected with inhibitor control (Fig. 2C). Next, we transfected miR-590-5p mimics into the cells and found that TGF-beta RII was downregulated. Western blot analysis also confirmed these results (Fig. 2C). In contrast, after transfecting miR-590-5p mimics, we found that both TGF-beta RII mRNA and protein levels were downregulated in the L02 cell line (Fig. 2C). We also found that miR-590-5p inhibitor could upregulate the expression of TGF-beta RII. These results indicate that TGF-beta RII was directly targeted by miR-590-5p in human HCCs. Thus, miRNA-590-5p may be a strong candidate to promote cancer proliferation and invasion.
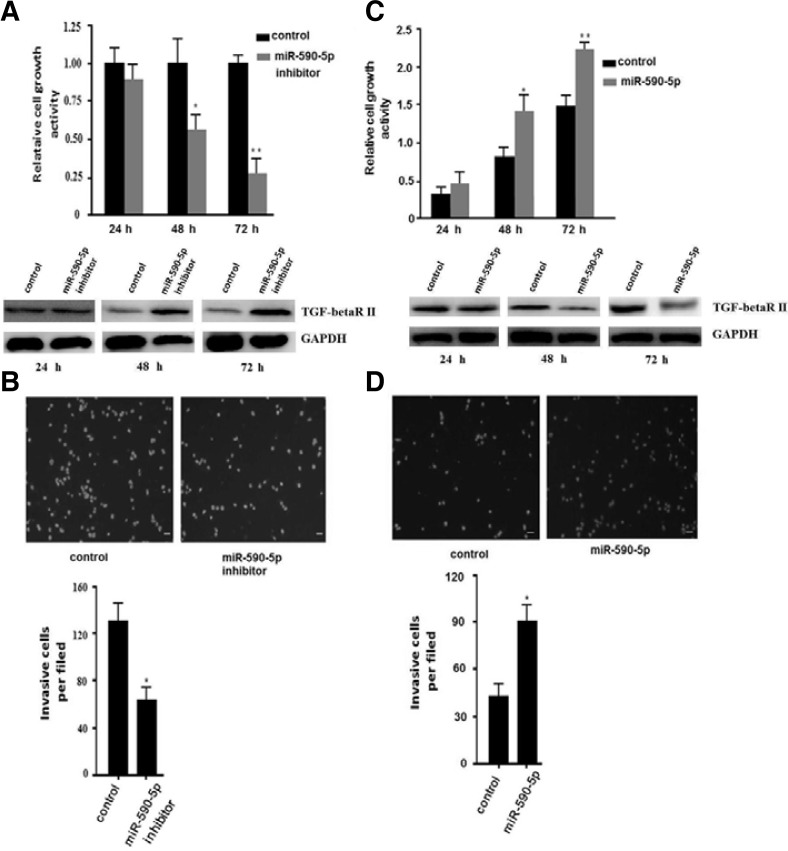
miR-590-5p promotes proliferation and invasion in hepatocellular carcinoma
It is well known that TGF-beta RII is associated with cell cycle regulation. Therefore, we wanted to determine whether miR-590-5p could regulate proliferation of HepG2 cells. We transfected miR-590-5p inhibitor in HepG2 cells for the proliferation assay. The MTT assay demonstrated that miR-590-5p inhibitor could reduce proliferation of HepG2 cells, especially 48 or 72 h after transfection (Fig. 3A). Invasion is also one of the important characteristics of hepatocellular carcinoma, even in the early stage. So, we examined the invasion of HepG2 cells transfected with miR-590-5p inhibitor or negative control. To examine invasion, the cells were seeded at a density of 2 × 105 cells/ml. In order to remove the influence of miRNA in proliferation, we incubated the cells at 37°C for 16 h and counted the number of cells. The miR-590-5p inhibitor could significantly promote invasion of HepG2 cells. The invasion of cells transfected with miR-590-5p inhibitor decreased about 50% compared to the negative control (Fig. 3B). Then, we performed the study in L02 cells. We transfected miR-590-5p mimics or mimic control in L02 cells for the proliferation assy. We found that miR-590-5p could promote proliferation of L02 cells (Fig. 3C). In the invasion assay, we determined that miR-590-5p could also promote invasion of L02 cells (Fig. 3D). These results showed that miR-590-5p can significantly promote proliferation and invasion in human HCCs.
Fig. 3.
miR-590-5p regulates the proliferation and invasion of human HCCs. (A) Inhibition of miR-590-5p could inhibit the proliferation of HepG2 cells. An MTT assay was used to determine relative cellular proliferation at 24, 48, and 72 h. After transfection with miR-590-5p, the 48 h and 72 h data showed a statistically significant difference. All data are presented as means ± s.d. of three independent experiments. The change of protein level was detected by Western blot and is shown at the bottom. (B) Inhibition of miR-590-5p in HepG2 cells significantly inhibited cell invasion. HepG2 cells transfected with miR-590-5p inhibitor or negative inhibitor control. Representative images are shown at the top, and the quantification of three independent experiments is shown at the bottom. Representative photomicrographs of the membrane-associated cells assayed using hochest33342 staining (200×) are shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05, **p < 0.01. The change of protein level was detected by Western blot and is shown at the bottom. (A) L02 cells were transfected with miR-590-5p mimics or negative control. The MTT assay was used to determine relative cellular proliferation at 24, 48, and 72 h. All data are presented as means ± s.d. of three independent experiments. *p < 0.05, **p < 0.01. (B) HepG2 cells transfected with miR-590-5p mimics or negative control. Representative images are shown at the top, and the quantification of three independent experiments is shown at the bottom. Representative photomicrographs of the membrane-associated cells assayed using hochest-33342 staining (200×) are shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05.
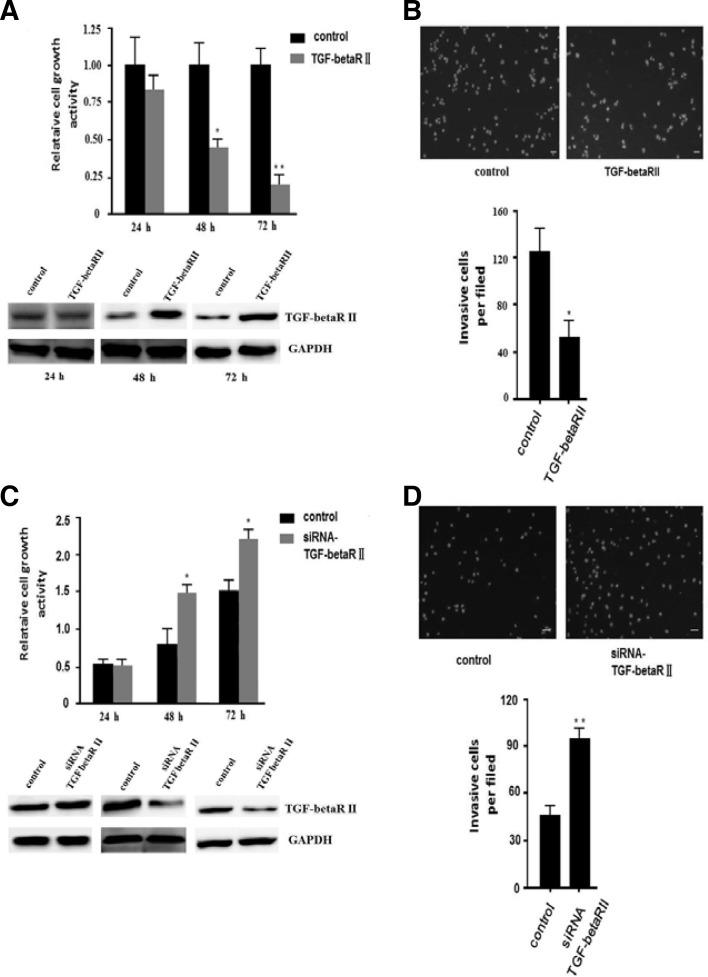
TGF-beta RII can suppress proliferation and invasion in hepatocellular carcinoma
To determine the impact of TGF-beta RII on proliferation of HepG2 cells, we transfected the pEGFP-TGF-beta RII ectopic expression vector into HepG2 cells. The overexpression of TGF-beta RII was confirmed by Western blot at indicated times (Fig. 4A). Then, we examined the impact of TGF-beta RII on the proliferation of HepG2 cells by an MTT assay. We found that TGF-beta RII significantly reduced HepG2 cell proliferation (Fig. 4A). Next, we performed a transwell invasion assay. The invasion of ectopic cells expressing TGF-beta RII significantly decreased about 50% compared to the negative control (Fig. 4B). We also transfected TGF-beta RII siRNA or negative control into L02 cells to knockdown TGF-beta RII. We found that downregulation of TGF-beta RII can promote both proliferation (Fig. 4C) and invasion (Fig. 4D) in L02 cells. Thus, TGF-beta RII can play an important role in reducing cancer proliferation and invasion.
Fig. 4.
TGF-beta RII can suppress cell proliferation and invasion in HepG2 cells. (A) TGF-beta RII could suppress HepG2 cell proliferation. The cell growth activity of HepG2 cells at the indicated times was determined by an MTT assay. Overexpressed TGF-beta RII in HepG2 cells was detected by Western blot (shown at the bottom). The relative cell growth activity is shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05, **p < 0.01. (B) Ectopic expression of TGF-beta RII decreased HepG2 cell invasion. Representative images are shown at the top, and the quantification of three independent experiments is shown at the bottom. Representative photomicrographs of the membrane-associated cells assayed using hochest33342 staining (200×) are shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05. (C) Knockdown of TGF-beta RII promoted the cell growth activity of L02 cells. The knockdown effect of siRNA TGF-beta RII in L02 cells was detected by Western blot (shown at the bottom). The relative cell growth activity is shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05. (D) Knockdown of TGF-beta RII promoted L02 cell invasion. Representative images are shown at the top, and the quantification of three independent experiments is shown at the bottom. Representative photomicrographs of the membrane-associated cells assayed using hochest 33342 staining (200×) are shown. All data are presented as means ± s.d. of three independent experiments. **p < 0.01.
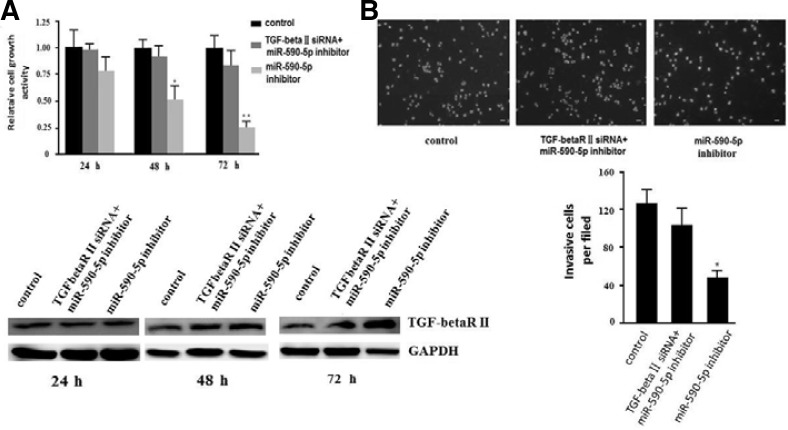
miR-590-5p can regulate proliferation and invasion of the malignant hepatocellular cancer cell line HepG2 by targeting TGF-beta RII
To prove our hypothesis that miR-590-5p can regulate proliferation and invasion by targeting TGF-beta RII in malignant hepatocellular cancer, we performed a rescue experiment in HepG2 cells. We transfected HepG2 cells with miR-590-5p inhibitor or negative control. Eight hours later, the overexpression of miRNA-590-5p could be detected (data not shown). Then, we transfected TGF-beta RII siRNA or negative control into HepG2 cells transfected with miR-590-5p inhibitor or negative inhibitor control, respectively. Western blot showed that TGF-beta RII siRNA downregulated the level of TGF-beta RII protein, which was increased by miR-590-5p inhibitor (Fig. 5A). Knockdown of TGF-beta RII in HepG2 cells with miR-590-5p inhibitor showed an approximately 50% increase in proliferation compared to HepG2 cells with miR-590-5p inhibitor transfected with siRNA control 48 h later, nearly the same as HepG2 cells transfected with negative inhibitor control and siRNA control (Fig. 5A). We also found that HepG2 cells with miR-590-5p inhibitor-transfected TGF-beta RII siRNA showed an approximately 50% increase in invasion ability compared to HepG2 cells with miR-590-5p inhibitor transfected with siRNA negative control, nearly as the same as HepG2 cells with negative inhibitor control and siRNA control (Fig. 5B). These results indicated that miRNA-590-5p can directly downregulate the expression of TGF-beta RII to promote proliferation and invasion of malignant hepatocellular cancer.
Fig. 5.
Knockdown of TGF-beta RII could reverse the effect of inhibition of miR-590-5p in HepG2 cells. (A) TGF-bRII siRNA could knockdown the upregulation of TGF-b RII by miRNA-590-5p inhibitor to rescue the proliferation of HepG2 cells. Control means cells transfected with miRNA inhibitor control and siRNA control. The protein expression of TGF-beta RII is shown at the bottom. All data are presented as means ± s.d. of three independent experiments. *p < 0.05, **p < 0.01. (B) Knockdown of TGF-beta RII could reverse the decrease of invasion caused by miR-590-5p inhibitor as determined by a transwell assay in HepG2 cells. Representative images are shown at the top, and the quantification is shown at the bottom. Representative photomicrographs of the membrane-associated cells assayed using hochest33342 staining (200×) are shown. All data are presented as means ± s.d. of three independent experiments. *p < 0.05, **p < 0.01.
DISCUSSION
Several studies have shown that TGF-beta RII plays an important role in cell growth and cancer formation and development (Bedossa et al., 2004; Fukushima et al., 2003). TGF-beta RII has been regarded as a tumor suppressor, which can result in cancer due to its silence or mutation (Fukushima et al., 2003). Silenced or mutated TGF-beta RII cannot combine with TGF-beta1 that can inhibit the proliferation of cancer cells, which make cells proliferate without inhibition and have less apoptosis. The inhibition of TGF-beta RII, regarded as a tumor suppressor, has been found in many hepatocellular carcinomas. Proliferation, migration, and invasion are major characteristics of human cancer. Cancer cells evolve to evade apoptosis so that they can escape from the surveillance system and survive in the harsh tumor growth environment, such as low nutrition and hypoxia and migrate or invade to other tissues for propagation (Hanahan and Weinberg, 2000). We have shown that overexpression of TGF-beta RII can inhibit proliferation and invasion in HepG2 cells, which has been reported previously. However, the misexpressed miRNAs that regulate TGF-beta RII have not been reported in hepatocellular carcinoma.
The miRNAs discovered recently represent an important class of endogenous regulator genes by binding to nucleotide elements. It has been confirmed that the misexpression of specific miRNAs is detected in many cancer types, including HCCs (Lu et al., 2005; Parkin, 2001; Volinia et al., 2006). Despite the fact that abnormal transcription of miRNAs is related to many human cancers, relatively little is known regarding the molecular mechanisms of how they regulate biological pathways. Bioinformatics-based prediction software can identify miRNAs and their targets, providing an easier way to study the relationship between miRNAs and their targets.
Making sense of the regulation of gene expression by miRNAs can shed light on the endogenous regulation mechanism in hepatocellular carcinoma, helping us to make miRNA a safe therapeutic approach to treat diseases instead of chemotherapy drugs or radiotherapy. In this study, we found that miRNA-590-5p was differentially expressed in the human hepatocellular carcinoma cell line HepG2 and the normal hepatocellular cell line L02. So, we hypothesized that miRNA-590-5p may be a potential tumor regulator, even an oncogene. Using miRNA target prediction software and experiments, we found that TGF-beta RII is directly regulated by miR-590-5p in the human hepatocellular carcinoma cell line HepG2. We also found that TGF-beta RII was differentially expressed in HepG2 and L02 cells. Overexpression of miR-590-5p accounted for the suppression of TGF-beta RII. In addition, inhibition of miR-590-5p could upregulate TGF-beta RII expression. Further study is needed to determine the different expression levels of miR-590-5p between different hepatocellular carcinoma cell lines, cancer tissues, and normal tissues to find out whether miR-590-5p is a marker that indicates the grade of HCC. Focusing on well-known genes that play an important role in HCC might yield a greater understanding of HCC formation and development. New regulatory miRNAs may be used as diagnostic markers of HCC and safe therapeutic approaches to treat human cancer.
In conclusion, this study found that the role of miR-590-5p in HCCs is to promote proliferation and invasion. TGF-beta RII is the direct and functional target of miR-590-5p. Furthermore, we also discovered that suppression of miR-590-5p repressed cell proliferation and invasion in the hepatocellular carcinoma cell line HepG2. Understanding the function of miR-590-5p and its targets clarified a new regulation mechanism of miRNAs in human HCCs and may provide a new therapeutic strategy for hepatocellular carcinoma.
REFERENCES
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Peltier E., Terris B., Franco D., Poynard T. Transforming growth factor-beta 1 (TGF-beta1) and TGF-beta1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology. 1995;121:760–766. [PubMed] [Google Scholar]
- Bracken C.P., Gregory P.A., Khew-Goodall Y., Goodall G.J. The role of microRNAs in metastasis and epithelial-mesenchymal transition. Cell. Mol. Life Sci. 2009;10:1682–1699. doi: 10.1007/s00018-009-8750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacu E., Mori Y., Sato F., Yin J., Olaru A., Sterian A., Xu Y., Wang S., Schulmann K., Berki A., et al. Activin type II receptor restoration in ACVR2-deficient colon cancer cells induces transforming growth factor-beta response pathway genes. Caner Res. 2004;64:7690–7696. doi: 10.1158/0008-5472.CAN-04-2082. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Fukushima T., Mashiko M., Takita K., Otake T., Endo Y., Sekikawa K., Takenoshita S. Mutational analysis of TGF-beta typeII receptor, Smad2, smad3, smad4, smad6 and smad7 genes in colorectal cancer. J. Exp. Clin. Cancer Res. 2003;22:315–320. [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ivashkiv L.B. Costimulation of chemokine receptor signaling by matrix metalloproteinase-9 mediates enhanced migration of IFN-alpha dendritic cells. J. Immunol. 2006;176:6022–6033. doi: 10.4049/jimmunol.176.10.6022. [DOI] [PubMed] [Google Scholar]
- Huang Q., Gumireddy K., Schrier M., le Sage C., Nagel R., Nair S., Egan D.A., Li A., Huang G., Klein-Szanto A.J., et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- Jong H.S., Lee H.S., Kim T.Y., Im Y.H., Park J.W., Kim N.K., Bang Y.J. Attenuation of transforming growth factor beta-induced growth inhibition in human hepatocellular carcinoma cell lines by cyclin D1 overexpression. Biochem. Biophys. Res. Commun. 2002;292:383–389. doi: 10.1006/bbrc.2002.6666. [DOI] [PubMed] [Google Scholar]
- Kumar M.S., Lu J., Mercer K.L., Golub T,R., Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- Lee D.Y., Deng Z., Wang C.H., Yang B.B. Micro-RNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tang H., Lang Y., Liu M., Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Ohuchida K., Mizumoto K., Yu J., Sato N., Nabae T., Takahata S., Toma H., Nagai E., Tanaka M. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol. Cancer Ther. 2009;8:1067–1074. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- Park D.Y., Sol M.Y., Suh K.S., Shin E.C., Kim C.H. Expressions of transforming growth factor (TGF)-beta typeII receptor and their relation with apoptosis during chemical hepatocarcinogenesis in rats. Hepatol. Res. 2003;27:205–213. doi: 10.1016/s1386-6346(03)00264-x. [DOI] [PubMed] [Google Scholar]
- Parkin D.M. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. 2002 Global cancer statistics. CA. Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Peinado H., Quintanilla M., Cano A. Transfoming growth factor beta-1 induces snial transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003;278:21113–2112. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Ryan B.M., Robles A.I., Harris C.C. Genetic variation in microRNA networks: the implications for cancer research. Nat. Rev. Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S., Tong Y., Steitz J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Veerla S., Lindgren D., Kvist A., Frigyesi A., Staaf J., Persson H., Liedberg F., Chebil G., Gudjonsson S., Borg A., et al. MiRNA expression in urothelial carcinomas: important roles of miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and metastasis, and frequent homozygous losses of miR-31. Int. J. Cancer. 2009;124:2236–2242. doi: 10.1002/ijc.24183. [DOI] [PubMed] [Google Scholar]
- Volinia S., Calin G.A., Liu C.G., Ambs S., Cimmino A., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]