Abstract
Translationally controlled tumor protein (TCTP), also termed P23 in human, belongs to a family of calcium- and tubulin-binding proteins, and it is generally regarded as a growth-regulating protein. Recently, Arabidopsis TCTP (AtTCTP) has been reported to function as an important growth regulator in plants. On the other hand, plant TCTP has been suggested to be involved in abiotic stress signaling such as aluminum, salt, and water deficit by a number of microarray or proteomic analyses. In this study, the biological functions of AtTCTP were investigated by using transgenic Arabidopsis plants overexpressing AtTCTP. Interestingly, AtTCTP overexpression enhanced drought tolerance in plants. The expression analysis showed that AtTCTP was expressed in guard cells as well as in actively growing tissues. Physiological studies of the overexpression lines showed increased ABA- and calcium-induced stomatal closure ratios and faster stomatal closing responses to ABA. Furthermore, in vitro protein-protein interaction analysis confirmed the interaction between AtTCTP and microtubules, and microtubule cosedimentation assays revealed that the microtubule binding of AtTCTP increased after calcium treatment. These results demonstrate that the overexpression of AtTCTP confers drought tolerance to plants by rapid ABA-mediated stomatal closure via the interaction with microtubules in which calcium binding enhances the interaction. Collectively, the present results suggest that the plant TCTP has molecular properties similar to animal TCTPs, such as tubulin- and calcium-binding, and that it functions in ABA-mediated stomatal movement, in addition to regulating the growth of plants.
Keywords: abscisic acid, drought tolerance, microtubule, stomatal closure, translationally controlled tumor protein
INTRODUCTION
Translationally controlled tumor protein (TCTP), also termed P21 in mouse (Chitpatima et al., 1988) and P23 in human (Gross et al., 1989), is a highly conserved and ubiquitously expressed protein in eukaryotic organisms (Bommer and Thiele, 2004). TCTP was initially identified in Ehrlich ascites tumor cells, as a translationally regulated protein. Subsequently, TCTP was found to be present in all eukaryotes including various normal cell types and its expression was reported to be regulated at the transcriptional level as well as the translational level (Schmidt et al., 2007; Thiele et al., 2000). Although the abundance and ubiquity of TCTPs suggest that they have important primary functions, their exact roles are still not fully understood. However, a wide range of biological functions have been reported for TCTPs, including cellular growth and proliferation (Cans et al., 2003; Chen et al., 2007; Hsu et al., 2007), cytokine-like activity as a histamine-releasing factor (MacDonald et al., 1995; Vonakis et al., 2008), anti-apoptotic activity (Li et al., 2001; Susini et al., 2008; Yang et al., 2005), stress signaling (Bonnet et al., 2000; Ermolayev et al., 2003; Sturzenbaum et al., 1998), and chaperone-like activity (Gnanasekar et al., 2009; Thaw et al., 2001).
TCTP is a calcium-binding protein (Feng et al., 2007; Kim et al., 2000; Sanchez et al., 1997) and has also been shown to be a tubulin-binding protein that associates with microtubules (Bazile et al., 2009; Gachet et al., 1999). In addition, the three-dimensional protein structure of TCTP reveals significant structural similarities to the Mss4/Dss4 family of proteins that bind to the GDP/GTP-free form of Rab proteins, which suggests a possible role for TCTP as a guanidine nucleotide exchange factor, or a guanidine nucleotide-free chaperone (Cans et al., 2003; Feng et al., 2007; Thaw et al., 2001). In mouse (Mus Musculus), homozygous knockout mutants of TCTP were embryogenic lethal, while heterozygous mutants displayed normal development (Chen et al., 2007). In addition, it has been reported that Drosophila TCTP (dTCTP) controls cell growth and proliferation by regulating Drosophila Rheb GTPase (Hsu et al., 2007). Despite this growing wealth of data on the molecular properties and biological functions of TCTPs, their precise role in cells still remains unclear. In particular, plant TCTPs have not been as well studied as animal TCTPs.
Recently, the functional roles of plant TCTPs have been reported in Arabidopsis thaliana. There are two TCTP genes in Arabidopsis thaliana genome, At3g16640 and At3g05540. Arabidopsis TCTPs have two distinct TCTP signatures that are highly conserved in all TCTPs, consistent with all known TCTP structures (Feng et al., 2007; Hinojosa-Moya et al., 2008; Thaw et al., 2001). Arabidopsis TCTPs also contains a basic domain (Q77-K119) for tubulin binding and a calcium-binding domain at the C-terminal region. Although there are two TCTPs in A. thaliana genome, it was reported that At3g05540 was a pseudogene, because no expression was detected (Berkowitz et al., 2008). Thus, there is one functional TCTP gene (At3g16640; AtTCTP) in Arabidopsis thaliana. Recently, it was reported that the AtTCTP functions as an important growth regulator in plants (Berkowitz et al., 2008; Brioudes et al., 2010). From the functional analysis of the Arabidopsis TCTP using a T-DNA insertion line, AtTCTP overexpression and RNAi lines, it was shown that the T-DNA insertion line (tctp-1; SAIL_28_C03) had a male gametophytic phenotype with impaired pollen tube growth and the RNAi lines exhibited slow vegetative growth phenotypes including reduced leaf expansion, reduced lateral root formation, and impaired root hair formation (Berkowitz et al., 2008). In contrast, the AtTCTP overexpression lines did not show any significant difference in phenotypes when compared with wild-type plant (Berkowitz et al., 2008). More recently, another AtTCTP knockout line (tctp-2; GABI_901E8) as well as tctp-1 was shown to be lethal, and this lethality was caused by retarded development of the embryos (Brioudes et al., 2010). In addition, it was also reported that the Arabidopsis TCTP plays a role in regulating mitotic growth by controlling cell cycle progression (Brioudes et al., 2010). These reports collectively suggest that plant TCTPs have an important role in controlling cell growth, similar to animal TCTPs. However, other studies suggest that the plant TCTP is involved in abiotic stress signaling such as aluminum, salt, and water deficit by transcriptomic or proteomic analyses (Ermolayev et al., 2003; Vincent et al., 2007). For examples, the expression level of plant TCTP increased in response to cold, salt, and heavy metal treatment (Ermolayev et al., 2003; Vincent et al., 2007), and decreased in response to pathogen infection (Ascencio-Ibanez et al., 2008; Fabro et al., 2008; Jones et al., 2006). Therefore, the biological function of plant TCTPs must be further investigated.
In the present study, we investigated the phenotypes of transgenic Arabidopsis plants overexpressing AtTCTP and found that the ectopic overexpression of AtTCTP enhanced drought tolerance in transgenic plants. Interestingly, AtTCTP was expressed relatively strong in guard cells and AtTCTP overexpression lines showed faster ABA-mediated stomatal closure than wild-type plants. We also found that AtTCTP interacted with microtubules and the interaction was increased by calcium treatment. Furthermore, ABA-induced microtubule deploymerization was shown to be accelerated in the overexpression lines. Therefore, our results suggest that the plant TCTP protein has microtubule- and calcium-binding properties, and might have a role in ABA-mediated stomatal closure as well as growth-regulating roles.
MATERIALS AND METHODS
Plant materials and growth conditions
For the generation of transgenic lines overexpressing AtTCTP, the expression vector construct was transformed into Arabidopsis by the Agrobacterium-mediated transformation method (Clough and Bent, 1998). We also generated transgenic plants carrying the AtTCTPpro:GUS construct to investigate expression patterns of AtTCTP in plants. Transgenic lines segregating ∼3:1 for antibiotic or herbicide resistance in the T2 generation were selected, and the T3 or T4 homozygous generation was used for photographs, protein extraction, and phenotypic analyses. Several independent AtTCTP overexpression lines were obtained and most of them showed similar AtTCTP expression levels, so we used the overexpression lines #3 and #4 (i.e., OX3 and OX4) as the representative plants overexpressing AtTCTP. Arabidopsis plants in soil were grown routinely in a culture room (22°C with a 16 h photoperiod). For seedling assays, seeds were surface-sterilized, incubated at 4°C for three days in the dark, and placed on 0.8% phytoagar (w/v) media containing half-strength MS salts and vitamins with 2% sucrose, unless stated otherwise. The plates were then transferred to a growth chamber (22°C with a 16 h photoperiod).
Plant transformation constructs
For the AtTCTP overexpression lines, the cDNA of AtTCTP (At3g16640) was amplified from the Arabidopsis cDNA library by Pfu polymerase (Stratagene) using the following primers, 5′-CTGGATCCATGTTGGTGTACCAAGATC-3′ (forward) and 5′-CTGAGCTCTCAGCACTTGACCTCCTTC-3′ (reverse), and cloned into the pBI121 binary vector (Clontech) with BamHI and SacI under the control of the 35S promoter. For the AtTCTPpro: GUS construct, the AtTCTP promoter region of ∼2.0 kb (−1990 to −1) including 113 bp of 5′-UTR was amplified with the following primers, 5′-CGGATCCTTCTTCCATGGTGCATGC-3′ (forward) and 5′-GCCCCGGGTCGCTTATTGATTGTTTTCTCTCTCC-3′ (backward), and cloned into pBI101 with BamHI and SmaI. The integrity of all the constructs was confirmed by DNA sequencing.
Drought tolerance and water loss assays
For the drought tolerance assay, about 20 plants were sowed on soil and 10 plants that showed the same growth stage in each pot were kept and incubated further. All pots were fully irrigated before the start of the drought test, and then drought-stressed by terminating irrigation. The drought tolerance of each line was scored 18 days after termination of irrigation, and the plants were then re-irrigated. Drought tolerance was determined three days after re-irrigation, and pictures were also taken. The entire test was repeated at least three times with consistent results. For the water loss assay, leaves of similar developmental stages (third to fifth true rosette leaves) were detached from three-week-old soil-grown plants and water loss rates were measured by weighing freshly harvested leaves, placed abaxial side up on open Petri dishes on the laboratory bench, at designated time intervals, as described previously (Kang et al., 2002). The proportion of fresh weight losses was calculated based of the initial weight of the plant.
Histochemical GUS assay
With transgenic plants of 35Spro:GUS and AtTCTPpro:GUS constructs, the expression patterns of AtTCTP were investigated by histochemical GUS staining. The histochemical assays for GUS activity in the transgenic lines of 4-day-old seedlings and 3 week-old plants were performed as described previously (Jefferson et al., 1987). Briefly, the plants were incubated in GUS staining buffer (80 mM sodium phosphate, pH 7.0 buffer containing 0.4 mM potassium ferricyanide, 0.4 mM potassium ferrocyanide, 8 mM sodium-EDTA, 0.05% triton X-100, 0.8 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-gluc; USB) and 20% methanol) for 8 h and washed with absolute ethanol to remove chlorophylls with gentle agitation. For guard cell staining, epidermal strips were harvested from 3 week-old leaves and stained with the same method. GUS expression patterns were observed under Olympus BX51 microscopy equipped with Olympus UPIanF1 40X/0.75 as an objective lens and WH10X/22 as an ocular lens.
Measurements of stomatal apertures and stomatal closure responses
Measurements of stomatal apertures were performed by analyzing unstained epidermal strips from three-week-old leaves with a light microscope under bright-field illumination (Goh et al., 1999). Epidermal strips were prepared from the undersides of rosette leaves from mature plants. The detached epidermal strips were immediately pooled in sterile distilled water. All epidermis was then incubated in 10 mM Mes/KOH, 50 mM KCl, pH 6.1 at 22°C. Stomatal index was calculated by following formula: Stomatal index (%) = stomatal density/(stomatal density + epidermal cell density) × 100. For the light-induced stomatal opening, epidermis was incubated for 2 h in the dark before light application, and then white light (100 μmol·m−2·s−1) was applied for 1 or 3 h. Stomatal apertures were photographed under 10 × 20 magnification within 3 min and then compared with an eyepiece graticule that was calibrated with a 100 × 10 μm slide micrometer scale. To investigate the stomatal closure responses to ABA or calcium treatment, epidermal strips were incubated with pre-incubation buffer containing 10 mM Mes/KOH (pH 6.1), 30 mM KCl, and 0.1 mM CaCl2 for 4 h under white light. Stomatal closure percentages from 200 guard cells in each plant were scored 4 h after the addition of 50 μM ABA or 2 mM CaCl2 to the pre-opened stomata of Col-0 and overexpression lines under white light or at the indicated times with the ABA treatment. Epidermal strips were photographed under 10 × 100 magnification after ABA treatment. The incubation buffer containing the same volume of solvents for dissolving each chemical was used as a control. ABA [(+/−)-cis, trans-abscisic acid, Sigma] was dissolved in DMSO (Sigma) to make a 100 mM stock solution and then stored at −20°C. The experiments for the measurements of stomatal apertures and stomatal closure percentages were performed more than three times to exclude any biased data and most assays were conducted blind.
In vitro protein-protein interaction assay
For the preparation of recombinant proteins, AtTCTP was cloned into the pGEX 4T-1 vector with BamHI and SmaI using the primers, 5′-CGGGATCCATGTTGGTGTACCAAGATCTTCTC-3′ (forward) and 5′-GGCCCGGGTCAGCACTTGACCTCCTT-3′ (reverse). GST and GST-fused AtTCTP proteins were then purified by glutathione affinity chromatography, according to the manufacturer’s instructions (Amersham Biosciences). For GST pull-down assays, GST-AtTCTP or GST protein was bound to glutathione Sepharose™ 4B beads. The 5 μg of purified porcine tubulin (Cytoskeleton Inc.) was incubated at 37°C or 4°C in tubulin polymerization buffer (80 mM PIPES, pH 6.9, 1 mM MgCl2, 1 mM EGTA, 5% glycerol, 1 mM GTP) for 2 h. After the incubation, porcine tubulins were applied to 2.5 μg of GST-AtTCTP or GST protein and incubated for 1 h at 37°C or 4°C. Samples were then washed with a buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.1% NP-40), and precipitates were separated on SDS-PAGE gels, and immunodetection was performed with anti-β-tubulin and anti-GST antibodies (Santa Cruz Biotechnology). The amounts of tubulins used in this experiment were also shown (i.e., Tubulin control).
Microtubule cosedimentation assay
Total proteins were extracted from Col-0 wild type plants with an extraction buffer (50 mM PIPES (pH 7.0), 2 mM MgCl2, 1% casein, 20 μM leupeptin, 20 μM pepstatin, 1 mM PMSF, 4 mM DTT, 1% NP-40) and clarified to remove large protein aggregates by centrifugation at 18,000 × g for 30 min. For the microtubule cosedimentation assay, 250 μg of total protein was incubated at 37°C for the indicated time with 0.5 mM GTP, which induces microtubule polymerization. After incubation and centrifugation of samples at 18,000 × g for 30 min, supernatants were transferred to new tubes and precipitates were resuspended in the extraction buffer. Microtubules and AtTCTP recovered from the microtubule cosedimentation assays were detected by Western blot analyses with anti-β-tubulin (Santa Cruz Biotechnology) and anti-AtTCTP antibodies using an ECL™ Western blotting analysis system (GE Healthcare). Purified recombinant GST-AtTCTP protein was used to elicit polyclonal antisera production in rabbits.
In addition, microtubule cosedimentation assays were carried out with the addition of recombinant GST-AtTCTP protein to the total protein extract. The 5 μg of purified GST-AtTCTP was added to 250 μg of the total protein extract, and microtubule polymerizations were performed for the microtubule cosedimentation assays. Microtubule-bound GST-AtTCTP was also quantified by using Quant-iT™ Protein Assay Kit (Invitrogen). Following microtubule cosedimentation, the precipitates were obtained at the time indicated, and total protein concentrations of the precipitates were determined. Using the protein concentration of plant extract as a control, the protein concentrations of samples from GST-AtTCTP were analyzed within the incubation time. Microtubule-bound GST-AtTCTP was calculated by subtracting the protein concentration of precipitates without GST-AtTCTP from the concentration of precipitates with GST-AtTCTP at each time point. Each quantification assay was repeated at least three times and the averages were used for the present data. To examine the effects of calcium on the interaction of AtTCTP with MTs, we also performed the cosedimentation assays in the presence of 5 mM CaCl2 or 5 mM EGTA for the times indicated.
Analysis of microtubule array and depolymerization in guard cells
GFP-MBD construct [GFP fused to microtubule binding domain (MBD) of microtubule associated protein 4 (MAP4)] was provided by Dr. Jozef Šamaj (University of Bon) and cloned into pCAMBIA 3300 vector with HindIII for transformation of Col-0 and OX3 plants. To investigate microtubule array and depolymerization in guard cells, epidermal strips were harvested from three-week-old leaves and incubated with pre-incubation buffer for 2 h under white light, and then transferred to 10 μM ABA containing pre-incubation buffer under white light. The incubated epidermal strips were sampled at the indicated times, and then mounted for the observation of confocal images with a Laser Scanning Confocal Microscope (Leica TCS SP5 AOBS/Tandem) at Korea Basic Science Institute, Gwangju center. The confocal acquired z-stacks were also reconstructed into 3D image projections using the IMARIS software (Bitplane AG) and converted the confocal images into surface mode images. This software allowed the construction of an isosurface for each fluorescent channel (GFP channel; microtubule arrays) and the investigation of microtubule arrays more precisely (Pierce et al., 2010).
RESULTS
Overexpression of Arabidopsis TCTP enhances drought tolerance
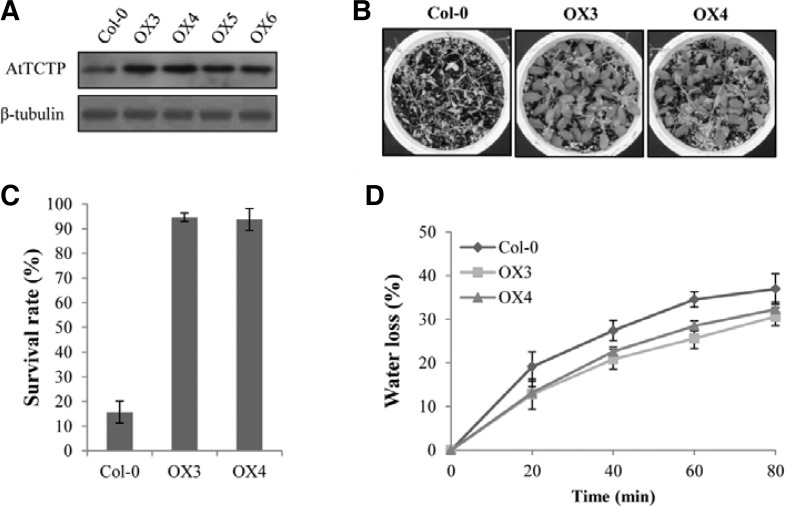
To investigate the effects of AtTCTP overexpression in plants, transgenic lines overexpressing AtTCTP (hereafter, OX lines) were generated in a Col-0 background. Western blot analysis confirmed that the AtTCTP proteins were expressed higher in the OX lines than Col-0 plant (Fig. 1A). When we compared the phenotypes of the OX lines with Col-0 at the stages of three-week-old and bolted plants, no significant differences were observed (Supplementary Fig. S1). This is consistent with the previous report that AtTCTP overexpression plants showed no detectable phenotypic differences compared to wild-type (Berkowitz et al., 2008). However, during the investigation of the plants’ responses to dehydration stress, we repeatedly found that the AtTCTP overexpression lines showed greatly enhanced tolerance to drought (Fig. 1B). Eighteen days after termination of irrigation, the OX lines showed higher tolerance to dehydration stress compared with Col-0 plants. Approximately 95% of the overexpression plants (OX3 and OX4) survived under the same conditions for which only ∼16% of wild-type plants survived (Fig. 1C). In addition, when the water loss rates from detached leaves of Col-0 and overexpression plants were analyzed, the OX plants showed slower water loss rates than Col-0 (Fig. 1D). Since the stomatal indexes of Col-0 (27.3 ± 2.1%) and the overexpression lines (28.1 ± 1.5% for OX3 and 27.7 ± 1.2% for OX4) were not significantly different, our results suggest that AtTCTP overexpression plants were more tolerant to drought due to reduced water loss rates.
Fig. 1.
Drought stress responses of AtTCTP overexpression (OX) lines. (A) Western blot analysis of AtTCTP proteins in OX lines. Crude extracts from ten-day-old seedlings of Col-0 and OX lines were used for the western blot analysis with the AtTCTP-specific antibody. The β-tubulin was shown as a loading control. (B) Drought tolerance assays of the OX lines. The plants were re-irrigated 18 days after drought treatment, and the pictures were taken three days after re-hydration. (C) Average survival rates of the OX lines compared with Col-0. Each analysis with 30 plants was repeated three times. Error bar = SD (n = 3). (D) Water loss rates with detached leaves from the Col-0 and OX lines. Leaves at similar developmental stages were excised and weighed at the indicated after detachment. The proportion of fresh weight losses was calculated on the basis of the initial weight of the leaves. Each data point represents the mean with SD (n = 20).
Arabidopsis TCTP is expressed in guard cells as well as in actively-proliferating tissues
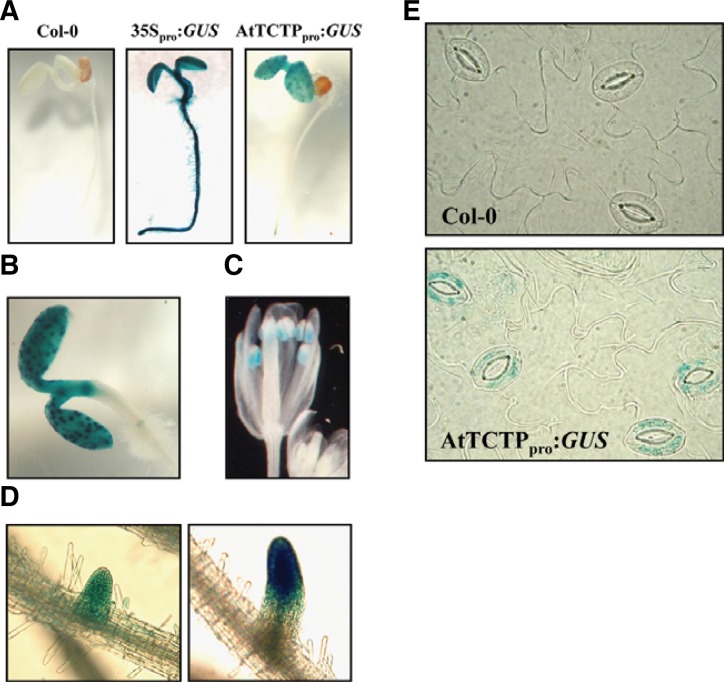
To gain insights into AtTCTP functions in plants, tissue-specific expression of AtTCTP was first examined by northern blot analysis with 10-d-old seedlings (Supplementary Fig. S2A). AtTCTP was highly expressed in all the tested tissues including cotyledon, true leaf with hypocotyl and root, although its expression was relatively a little less in roots. We also generated transgenic plants carrying approximately 2.0 kb of AtTCTP promoter fused to a GUS reporter gene (AtTCTPpro:GUS) to investigate the spatial expression patterns of this gene. Histochemical analyses revealed that AtTCTP was expressed in actively-proliferating tissues, such as shoot apical meristem, root tips, lateral root initiation regions, and anthers in flower, while little expression was observed in tissues in which cell division was complete (Fig. 2). This is similar to the previous results with a AtTCTP-GFP construct carrying about 1 kb upstream region and the full genomic AtTCTP including intron sequences, which showed a high and ubiquitous expression of AtTCTP in actively dividing and differentiating cells (Berkowitz et al., 2008). These expression patterns were also consistent with animal TCTPs, which are abundantly expressed in mitotically active and growing tissues but show low expression in post-mitotic tissues (Bommer and Thiele, 2004; Thiele et al., 2000). Interestingly, during the investigation, we also found that AtTCTP was strongly expressed as spots in leaves. Later, the spots were confirmed to be guard cells (Figs. 2B and 2E). Therefore, our results indicate that the plant TCTP is expressed in guard cells, as well as in mitotically active tissues. This suggests a possible role for plant TCTPs in guard cell function.
Fig. 2.
Expression analysis of AtTCTP in seedlings and adult plants. (A) GUS expression patterns of four-day-old seedlings under white light conditions. The region approximately 2.0 kb upstream (−1990 to −1) of AtTCTP was used as the AtTCTP promoter and was fused to the GUS gene (AtTCTPpro:GUS) for plant transformation. A Col-0 wild-type plant and a transgenic plant carrying the 35S promoter (35Spro:GUS) were used as a negative and a positive control, respectively. (B–D) AtTCTP expression in cotyledon (B), flower (C), and lateral root (D). (E) Guard cell expression of AtTCTP. Epidermal strips of Col-0 and AtTCTPpro:GUS plants were harvested and stained.
Since it has been reported that plant TCTP shows increased expressional levels in response to water and osmotic stress (Ermolayev et al., 2003; Vincent et al., 2007), we performed the expression analysis of AtTCTP in the presence of ABA using the transgenic plants carrying AtTCTPpro:GUS construct. The results showed that GUS expression was detected in whole roots of transgenic seedlings with AtTCTPpro:GUS in the presence of ABA, whereas that was detected only in root apical meristem in the absence of ABA (Supplementary Fig. S2). These results indicate that the expression of AtTCTP might be regulated by ABA, suggesting a possible role of AtTCTP in response to ABA.
AtTCTP is involved in the regulation of ABA-mediated stomatal closure
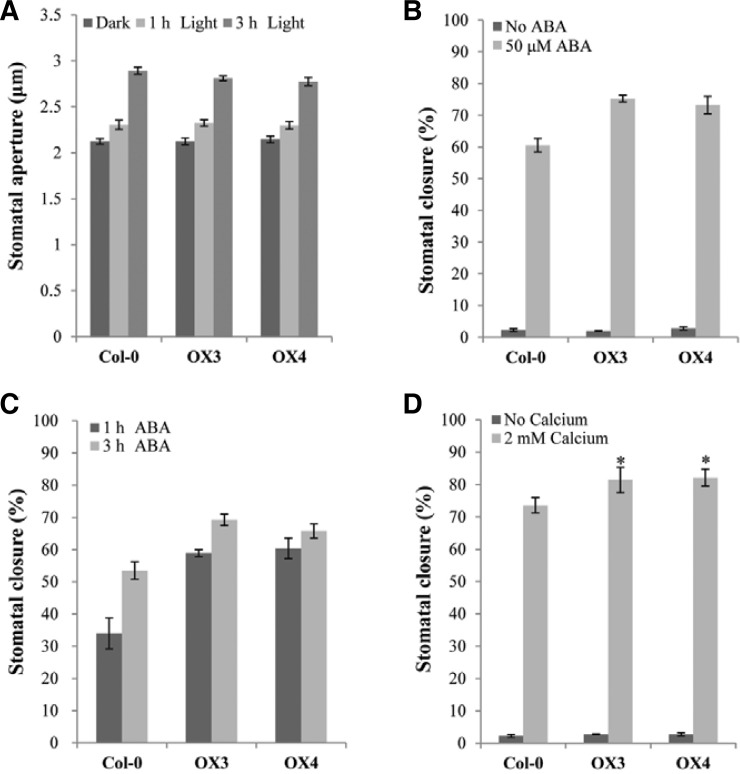
Since our results showed a potential function for AtTCTP in drought tolerance with reduced water loss rates and relatively strong expression of AtTCTP in guard cells, we hypothesized that AtTCTP play a biological role in stomatal movement. It is well known that the apertures of stomatal pores are regulated by ABA, which increases the cytosolic calcium level in guard cells and results in stomatal closure (Blatt, 2000). Exogenous calcium is also known to induce stomatal closure by increasing cytosolic calcium levels (Allen et al., 1999; McAinsh et al., 1995). In this study, we first measured stomatal apertures in leaf epidermal strips of the overexpression and wild-type plants with light treatments. When the leaf epidermal strips were transferred from dark to light conditions, the overexpression plants, as well as wild-type plants, showed normal stomatal opening with increased apertures (Fig. 3A). When stomatal closure was induced by ABA treatment, the OX plants as well as wild-type plants showed closed stomata with reduced apertures, but the OX plants showed greater ABA-mediated stomatal closure ratios (i.e. greater reduction ratios in apertures) than wild-type plants (Fig. 3B; aperture data in Supplementary Fig. S3A). To examine ABA-hypersensitive stomatal closure of the OX plants more closely, we also investigated ABA-mediated stomatal closure ratios in a time-dependent manner (Fig. 3C; aperture data in Supplementary Fig. S3B). The results of this analysis showed that more than 60% of guard cells in AtTCTP overexpression plants were closed 1 h after ABA treatment, while less than 40% of the guard cells were closed in Col-0 plants, confirming that the overexpression plants underwent faster ABA-mediated stomatal closure than wild-type plants. Since exogenous calcium is also known to induce stomatal closure, we tested calcium-induced stomatal closure and obtained the results of increased stomatal closure ratios in the OX plants with the calcium treatment (Supplementary Fig. 3D). In addition, we investigated fusicoccin-induced stomatal opening. The fungal toxin fusicoccin is known as an activator of plasma membrane H+-ATPase, which leads to irreversible stomatal opening (Chen et al., 2007; Johansson et al., 1993). Our results showed that the OX plants opened stomata normally in response to fusicoccin treatment (Supplementary Fig. S4), suggesting that the OX plants had no defect in H+-pumping of the plasma membrane. Therefore, our results suggest that AtTCTP is involved in ABA- and calcium-mediated stomatal closure, but not in light or H+-pumping induced stomatal opening.
Fig. 3.
Stomatal movement of AtTCTP overexpression lines. (A) Light-induced stomatal opening. White light (100 μmol·m−2·s−1) was applied to dark-incubated epidermal strips for light-induced stomatal opening. Stomatal apertures of ten randomly selected stomata from Col-0 and OX lines were measured under the microscope. Each assay was repeated three times. Error bar = SE (n = 3). (B) ABA-induced stomatal closure. Stomatal closure ratios were scored 4 h after the addition of 50 μM ABA. (C) ABA-induced stomatal closure ratios in a time-dependent manner. Stomatal closure ratios were scored at the indicated times after ABA treatment. (D) Calcium-induced stomatal closure. Stomatal closure ratios were scored 4 h after the addition of 2 mM CaCl2. For (B) to (D), stomata had been pre-opened by incubating for 4 h in white light. 200 randomly selected stomata were used for the assay and each assay was repeated three times. Error bar = SD (n = 3). Asterisks show significant differences with a P < 0.01 (t-test) vs. Col-0.
The interaction between AtTCTP and microtubules is accelerated by calcium
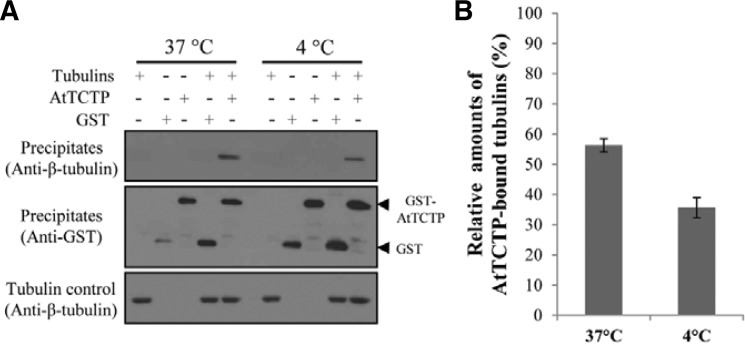
TCTPs are known as microtubule- and calcium-binding proteins (Feng et al., 2007; Gachet et al., 1999). Microtubules (MTs) are known to play an important role in stomatal movement, and stomatal closure is accompanied by microtubule depolymerization (Galatis and Apostolakos, 2004). Therefore, we performed in vitro protein-protein interaction assays to elucidate the interaction of AtTCTP with microtubules. These assays were performed by GST pull-down experiments using purified GST-AtTCTP and porcine tubulins at 37°C or 4°C. It has been known that tubulins exist mainly as heterodimers of α- and β-tubulins at 4°C, while they form microtubules at 37°C (Desai and Mitchison, 1997; Shelanski et al., 1973). The results of these experiments showed that AtTCTP interacted with both heterodimers and MTs in which AtTCTP interacted more strongly with MTs than heterodimers (Fig. 4).
Fig. 4.
Interaction of AtTCTP with microtubules. (A) In vitro protein-protein interaction between AtTCTP and tubulins. Purified GST-AtTCTP and porcine tubulin proteins were used for the pull-down assays. Precipitates were separated on SDS-PAGE gels, and immunodetection was performed with anti-β-tubulin and anti-GST antibodies. The amount of tubulin added in each assay was also shown as a control (i.e., Tubulin control). (B) Relative amounts of AtTCTP-bound tubulin at 37°C or 4°C compared to the amount of tubulin added in the reactions (i.e. [Tubulin in precipitates]/[Tubulin in Tubulin control]). Each assay was repeated three times. Error bar = SD (n = 3).
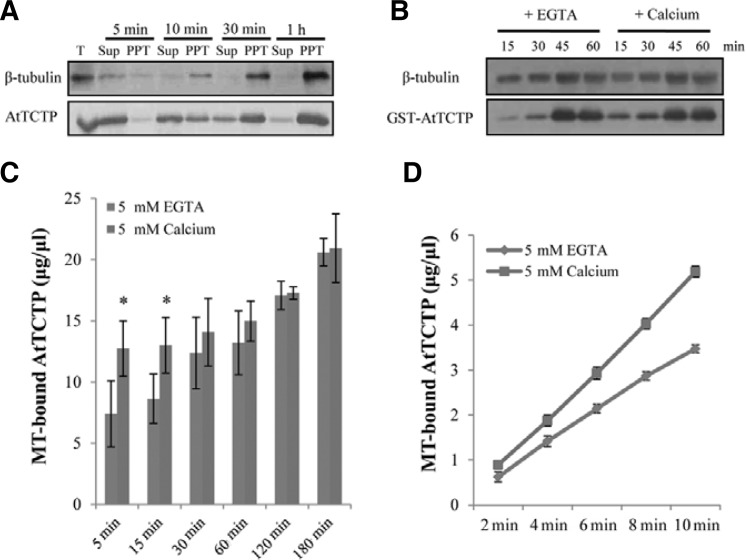
We also performed microtubule cosedimentation assays to quantitatively detect MT-bound AtTCTP. In these experiments, we induced polymerization of MTs by incubating plant total protein extracts with GTP at 37°C and the synthesized MTs were harvested at the indicated time intervals. We then used western blot analyses to detect MTs and AtTCTP using β-tubulin and AtTCTP specific antibodies. Under these experimental conditions, synthesized MTs were detected in the precipitates of the total protein extracts from wild-type plants after 5 min of incubation and the amounts increased with incubation time (Fig. 5A). At the beginning of microtubule polymerization, AtTCTP was mostly found in the supernatants, but was detected in the precipitates after 5 min of incubation with MTs. The levels of both MTs and AtTCTP decreased in the supernatants but increased in the precipitates with respect to incubation time.
Fig. 5.
Calcium effects on the interaction of AtTCTP with microtubules. (A) Microtubule cosedimentation assay from the wild-type plant Col-0. Microtubules were polymerized in the presence of 0.5 mM GTP at 37°C and harvested at the indicated times. AtTCTP and microtubules in the supernatant (Sup) and precipitate (PPT) of the reactions were detected by western blot analysis. One fifth of the supernatant samples relative to the precipitate were used for these assays. T, total crude extract. (B) AtTCTP interaction with MTs in the presence of EGTA or calcium. Microtubules were polymerized in the presence of 0.5 mM GTP at 37°C for 30 min and microtubule cosedimentation assays were performed with Col-0 total crude protein extracts after the addition of 5 mM EGTA or 5 mM calcium. Samples were harvested at the indicated times. (C, D) Quantitative analysis of the interaction between AtTCTP and MTs in the presence of 5 mM EGTA or 5 mM calcium. Each analysis was re peated three times. Error bar = SD (n = 3). Asterisks show significant differences with a P < 0.05 (t-test) vs. MT-bound AtTCTP in the presence of EGTA.
TCTPs are also known as calcium-binding proteins and the AtTCTP overexpression plants showed enhanced calcium-induced stomatal closure, indicating the possible involvement of calcium in AtTCTP function. Furthermore, calcium is known as a factor in microtubule depolymerization (O’Brien et al., 1997). Thus, in order to examine the effect of calcium on the microtubule binding of AtTCTP, we conducted microtubule cosedimentation assays with total protein extracts supplemented with recombinant GST-AtTCTP proteins in the presence of calcium or EGTA (Fig. 5B). The results of these experiments showed that the interaction between AtTCTP and MTs increased in the presence of calcium, when compared with the interaction in the presence of EGTA. In our experimental conditions, there was no significant difference in MT amounts in the presence of calcium and EGTA within 60 min. In contrast, after 15 min of incubation, MT-bound GST-AtTCTP was clearly detected in the presence of calcium but only low levels were detected in the presence of EGTA. Quantitative analysis of MT-bound AtTCTP also showed increased binding of AtTCTP with MTs in the presence of calcium, especially at the early time point (Fig. 5C). These results indicated that the interaction between AtTCTP and MTs could be accelerated in the presence of calcium. To further investigate the effects of calcium on the interaction, we performed the quantitative analysis over a narrow range of time and confirmed the accelerated binding of AtTCTP in the presence of calcium (Fig. 5D). Collectively, our results demonstrate the interaction between Arabidopsis TCTP and microtubules, and suggest that cytosolic calcium positively regulates the interaction of AtTCTP with MTs.
The effects of AtTCTP overexpression on microtubule destabilization
It has been reported that exogenously added ABA disrupted cortical MTs in guard cells, and that this was accompanied by stomatal closure (Jiang et al., 1996). Cortical MTs in fully opened guard cells are transversely oriented from the ventral wall to the dorsal wall. When the stomatal aperture was decreased, these MTs became twisted and patched, and broke down into diffuse fragments when the stomata were closed (Lahav, 2004; Marcus et al., 2001; Yu et al., 2001). Since AtTCTP interacts with MTs and the interaction is enhanced by calcium, the drought tolerance of the AtTCTP overexpression plants might be due to MT destabilization by AtTCTP. Thus, we investigated MT depolymerization by observing MT arrays during stomatal closure.
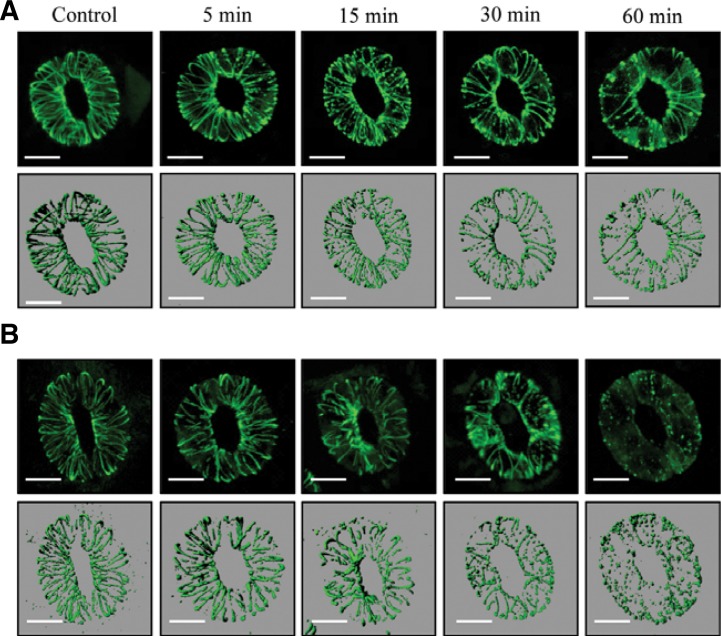
To investigate MT arrays in guard cells upon ABA treatment, we obtained GFP-MBD construct (GFP fused to microtubule binding domain (MBD) of microtubule associated protein 4 (MAP4)) and generated transgenic Col-0 and AtTCTP overexpression (OX3) plants with the GFP-MBD construct (hereafter, GFP-MBD/Col-0 and GFP-MBD/OX3). MT arrays upon ABA treatment were then analyzed in a time-dependent manner using a laser scanning confocal microscope. In GFP-MBD/Col-0 plant, we could observe that the fibrous microtubules (type I) were destroyed into fragmented forms (type II) and then punctuate or speckle forms (type III) along with the ABA treatment (Supplementary Fig. S5). In our experimental condition, microtubule arrays of GFP-MBD/Col-0 plant were not changed for 1 h, when ABA was not added (Supplementary Fig. S5A). On the other hand, ABA-induced MT depolymerization (i.e., fragmented MT arrays) was clearly detected within 60 min, although the stomatal apertures were not significantly changed in this condition during one hour (Supplementary Figs. S5B and S5C). Our results showed that the ABA-induced MT depolymerization was accelerated in the GFP-MBD/OX3 plant compared to the GFP-MBD/Col-0 plant (Fig. 6). In the GFP-MBD/OX3 plant, ABA-induced MT depolymerization began as quickly as 5 min, and most MT arrays disappeared within 60 min, whereas that in the GFP-MBD/Col-0 plant began about 15 min and some MT arrays still remained at 60 min. Therefore, these results suggest that AtTCTP plays a role in the ABA-mediated stomatal closure possibly by regulating the microtubule depolymerization.
Fig. 6.
ABA-induced microtubule depolymerization of Col-0 and OX3 plants transformed with GFP-MBD construct. Microtubule arrays of GFP-MBD/Col-0 (A) and GFP-MBD/OX3 (B) transgenic plants were observed after 10 μM ABA treatments in a time-dependent manner using a laser scanning confocal microscope. Bar = 10 μm.
DISCUSSION
Arabidopsis TCTP is involved in ABA-mediated responses
Since the AtTCTP knockout plants showed lethal phenotypes and it has been shown that the Arabidopsis TCTP has a role in regulating mitotic growth by controlling cell cycle progression (Brioudes et al., 2010), the AtTCTP overexpression plants are expected to show some phenotypes related to the plant growth. However, previous studies showed no detectable phenotypic differences between wild-type and AtTCTP overexpression plants (Berkowitz et al., 2008), or a little accelerated growth in which the growth of the overexpression plants was about 2 day ahead at 48 day post-germination (Brioudes et al., 2010). In the present study, we could not find significant differences in growing phenotypes between wild-type and overexpression plants (Supplementary Fig. S1). It is likely that the effect of AtTCTP overexpression with 35S promoter is not so strong in the transgenic plant to exhibit differences in growing phenotypes, because the TCTP is already highly and ubiquitously expressed protein. Thus, we investigated other phenotypes than growth. Based on the other previous studies that suggested the involvement of plant TCTP in abiotic stress signaling such as water deficit (Vincent et al., 2007), we investigated drought stress responses and found significantly enhanced drought tolerance in the AtTCTP overexpression plants (Fig. 1). Further studies revealed that the AtTCTP overexpression lines showed hypersensitive phenotype to ABA-induced stomatal closure (Fig. 3). These results suggest that the OX plants responded rapidly to dehydration stress and closed their stomata quickly, which resulted in the plants being tolerant to drought. This correlation was also confirmed by analyzing water loss rates from detached leaves of Col-0 and overexpression plants (Fig. 1D). In the aspect that TCTPs have usually been described as growth-related proteins and originally ABA has been identified as growth-inhibiting compounds, the involvement of plant TCTPs in the ABA response such as stomatal movement may be well co-related as a plant-specific function of TCTPs.
In the present study, we showed that the expression of AtTCTP was possibly regulated by ABA (Supplementary Fig. 2). When we analyzed cis-elements in the AtTCTP promoter using PlantPAN program (plantpan.mbc.nctu.edu.tw), three putative ABA-responsive elements (core motif; -ACGT-) were found in −1065, −1006, and −789 bp upstream regions of start codon. Therefore, our results suggest that the expression of AtTCTP might be regulated by ABA through these ABA-responsive elements. The expression analysis of AtTCTP also confirmed its expression in guard cells as well as in actively cell-proliferating tissues (Fig. 2). Although AtTCTP expression in actively dividing tissues is expected from previous studies that animal TCTPs are abundantly expressed in mitotically active and growing tissues (Bommer and Thiele, 2004; Thiele et al., 2000), the expression of AtTCTP in guard cells is distinct from animal TCTPs because guard cells are plant-specific tissues that modulate stomatal apertures in response to diverse environmental conditions including drought stress (Hetherington and Woodward, 2003). The expression of AtTCTP in guard cells and the possible regulation by ABA is well co-related to the functional role of AtTCTP in stomatal movement.
AtTCTP plays a role in ABA-induced stomatal closure
Stomatal opening in the AtTCTP overexpression lines was normally induced by treating dark-incubated epidermises with light, whereas the OX plants showed ABA-hypersensitive stomatal closure (Fig. 3). The OX plants showed increased ABA-induced stomatal closure ratios (Fig. 3B), faster closing responses to ABA (Fig. 3C), and drought-tolerant phenotypes (Fig. 1). These results indicate that AtTCTP is involved in ABA-mediated stomatal closure but not in light-induced stomatal opening. In addition, there was no difference in stomatal movement by fusicoccin treatment (Supplementary Fig. S4). Therefore, AtTCTP might play a role in stomatal closure in the downstream of ABA.
TCTPs have a tubulin-binding property (Gachet et al., 1999), and microtubules are known to play an important role in regulating stomatal movement (Marcus et al., 2001; Yu et al., 2001). MTs are rod-like cytoplasmic polymers assembled from dimers of α- and β-tubulins and are found in all eukaryotic cells. MTs are polarized with the α-tubulin at the minus end and the β-tubulin at the plus end of the structure (Heald and Nogales, 2002; Howard and Hyman, 2003). In vivo, MTs show dynamic instability based on alternating phases of polymerization and catastrophic depolymerization. This enables MTs to re-arrange quickly into arrays for various functions including stomatal movement. Therefore, we investigated the interaction of AtTCTP with MTs or tubulin heterodimers by GST pull-down experiment. Our results showed that AtTCTP interacted with MTs more strongly than tubulin heterodimers (Fig. 4). This suggests a possibility that AtTCTP might have a role in MT polymerization or depolymerization.
Since TCTPs also have a calcium-binding property (Feng et al., 2007; Kim et al., 2000) and it is known that ABA-mediated stomatal closure is accompanied by an increase in cytosolic calcium concentration (McAinsh et al., 1995), we also tested the effect of calcium on the interaction of AtTCTP with MTs. If cytosolic calcium was increased, MTs should be depolymerized with stomata closure. In this scenario, calcium would have a positive effect on the interaction of AtTCTP with MTs. The microtubule cosedimentation results confirmed an increase in the interaction of AtTCTP with MTs in the presence of calcium, especially during the early stages of the interaction (Fig. 5). Since MTs are highly dynamic and quickly re-arranged into arrays via polymerization and catastrophic depolymerization, a rapid interaction of AtTCTP with MTs would have an influence on MT depolymerization, which could explain why the overexpression plants showed quick stomatal closure. Collectively, these results show that AtTCTP interacts with MTs and suggest that the interaction might be regulated by calcium binding. However, the mechanism by which the interaction of AtTCTP with MTs results in MT depolymerization needs to be answered in the future. Recently, TCTPs have been reported to function as guanidine nucleotide exchange factors for the translation elongation factor eEF1A, and for Rheb, a Ras superfamily GTPase (Cans et al., 2003; Hsu et al., 2007). Therefore, AtTCTP may influence the GTPase activity of β-tubulin that is known to be important for MT depolymerization.
MT organization during stomatal closing and opening has been previously demonstrated (Marcus et al., 2001; Yu et al., 2001). Microinjecting living guard cells of Vicia faba with fluorescent tubulin showed that cortical MTs are transversely oriented from the ventral wall to the dorsal wall in fully opened guard cells, and are broken down into diffuse fragments by stomatal closure (Yu et al., 2001). Therefore, we investigated the MT arrays to show MT depolymerization in response to ABA. When MT arrays and its depolymerization patterns were investigated with Col-0 and OX3 plants carrying the GFP-MBD construct, the GFP-MBD/OX3 plant showed faster ABA-induced MT depolymerization than GFP-MBD/Col-0 (Fig. 6). These results suggest that AtTCTP might function in the regulation of MT stability during ABA-mediated stomatal closure. Based on the present results, we may propose a possible mechanism for the regulation of ABA-mediated stomatal closure by AtTCTP: Under dehydration conditions such as drought, ABA is synthesized from root and transported to guard cells, which increases the cytosolic calcium concentration. The elevated cytosolic calcium in the guard cells binds to AtTCTP, which stimulate the interaction of AtTCTP with microtubules. Finally, the interaction results in microtubule depolymerization by yet unknown mechanism, which induce the stomatal closure. However, further studies are necessary to elucidate the exact mechanism for AtTCTP function in plant stress signaling.
TCTPs belong to an evolutionally highly conserved and abundantly expressed family of eukaryotic proteins that has been implicated in many cellular functions related to cell growth. Especially, a number of microarray and proteomic studies consistently show its induction in plants by water and osmotic stress. The present study provides an initial insight on the involvement of plant TCTPs in stress signaling. Particularly, our results in the present study suggest that the plant TCTP functions in ABA-mediated stomatal movement, in addition to regulating the growth of plants.
Supplementary Material
Acknowledgments
We thank Dr. Jozef Šamaj for the GFP-MBD construct. This work was supported in part by Next-Generation BioGreen 21 Program (SSAC, grant no. PJ008044), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant no. 2010-0012628).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Allen G.J., Kwak J.M., Chu S.P., Llopis J., Tsien R.Y., Harper J.F., Schroeder J.I. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Ascencio-Ibanez J.T., Sozzani R., Lee T.J., Chu T.M., Wolfinger R.D., Cella R., Hanley-Bowdoin L. Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2008;148:436–454. doi: 10.1104/pp.108.121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile F., Pascal A., Arnal I., Le Clainche C., Chesnel F., Kubiak J.Z. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis. 2009;30:555–565. doi: 10.1093/carcin/bgp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz O., Jost R., Pollmann S., Masle J. Characterization of TCTP, the translationally controlled tumor protein, from Arabidopsis thaliana. Plant Cell. 2008;20:3430–3447. doi: 10.1105/tpc.108.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt M.R. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- Bommer U.A., Thiele B.J. The translationally controlled tumour protein (TCTP) Int. J. Biochem. Cell Biol. 2004;36:379–385. doi: 10.1016/s1357-2725(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Bonnet C., Perret E., Dumont X., Picard A., Caput D., Lenaers G. Identification and transcription control of fission yeast genes repressed by an ammonium starvation growth arrest. Yeast. 2000;16:23–33. doi: 10.1002/(SICI)1097-0061(20000115)16:1<23::AID-YEA503>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Brioudes F., Thierry A.M., Chambrier P., Mollereau B., Bendahmane M. Translationally controlled tumor protein is a conserved mitotic growth integrator in animals and plants. Proc. Natl. Acad. Sci. USA. 2010;107:16384–16389. doi: 10.1073/pnas.1007926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cans C., Passer B.J., Shalak V., Nancy-Portebois V., Crible V., Amzallag N., Allanic D., Tufino R., Argentini M., Moras D., et al. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc. Natl. Acad. Sci. USA. 2003;100:13892–13897. doi: 10.1073/pnas.2335950100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Wu P.S., Chou C.H., Yan Y.T., Liu H., Weng S.Y., Yang-Yen H.F. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific manner. Mol. Biol. Cell. 2007;18:2525–2532. doi: 10.1091/mbc.E07-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitpatima S.T., Makrides S., Bandyopadhyay R., Brawerman G. Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988;16:2350. doi: 10.1093/nar/16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Ermolayev V., Weschke W., Manteuffel R. Comparison of Al-induced gene expression in sensitive and tolerant soybean cultivars. J. Exp. Bot. 2003;54:2745–2756. doi: 10.1093/jxb/erg302. [DOI] [PubMed] [Google Scholar]
- Fabro G., Di Rienzo J.A., Voigt C.A., Savchenko T., Dehesh K., Somerville S., Alvarez M.E. Genome-wide expression profiling Arabidopsis at the stage of Golovinomyces cichoracearum haustorium formation. Plant Physiol. 2008;146:1421–1439. doi: 10.1104/pp.107.111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Liu D., Yao H., Wang J. Solution structure and mapping of a very weak calcium-binding site of human translationally controlled tumor protein by NMR. Arch. Biochem. Biophys. 2007;467:48–57. doi: 10.1016/j.abb.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Gachet Y., Tournier S., Lee M., Lazaris-Karatzas A., Poulton T., Bommer U.A. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J. Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- Galatis B., Apostolakos P. The role of the cytoskeletn in the morphogenesis and function of stomatal complexes. New Phytol. 2004;161:613–639. doi: 10.1046/j.1469-8137.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M., Dakshinamoorthy G., Ramaswamy K. Translationally controlled tumor protein is a novel heat shock protein with chaperone-like activity. Biochem. Biophys. Res. Commun. 2009;386:333–337. doi: 10.1016/j.bbrc.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C.-H., Schreiber U., Hedrich R. New approach of monitoring changes in chlorophyll a fluorescence of single guard cells and protoplasts in response to physiological stimuli. Plant Cell Environ. 1999;22:1057–1070. [Google Scholar]
- Gross B., Gaestel M., Bohm H., Bielka H. cDNA sequence coding for a translationally controlled human tumor protein. Nucleic Acids Res. 1989;17:8367. doi: 10.1093/nar/17.20.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Nogales E. Microtubule dynamics. J. Cell Sci. 2002;115:3–4. doi: 10.1242/jcs.115.1.3. [DOI] [PubMed] [Google Scholar]
- Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Moya J., Xoconostle-Cazares B., Piedra-Ibarra E., Mendez-Tenorio A., Lucas W.J., Ruiz-Medrano R. Phylogenetic and structural analysis of translationally controlled tumor proteins. J. Mol. Evol. 2008;66:472–483. doi: 10.1007/s00239-008-9099-z. [DOI] [PubMed] [Google Scholar]
- Howard J., Hyman A.A. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422:753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- Hsu Y.C., Chern J.J., Cai Y., Liu M., Choi K.W. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.J., Nakajima N., Kondo N. Disruption of microtubules by abscisic acid in guard cells of Vicia faba L. Plant Cell Physiol. 1996;37:697–701. [Google Scholar]
- Johansson F., Sommarin M., Larsson C. Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal Inhibitory domain. Plant Cell. 1993;5:321–327. doi: 10.1105/tpc.5.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M., Thomas V., Bennett M.H., Mansfield J., Grant M. Modifications to the Arabidopsis defense proteome occur prior to significant transcriptional change in response to inoculation with Pseudomonas syringae. Plant Physiol. 2006;142:1603–1620. doi: 10.1104/pp.106.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.Y., Choi H.I., Im M.Y., Kim S.Y. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002;14:343–357. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Jung Y., Lee K., Kim C. Identification of the calcium binding sites in translationally controlled tumor protein. Arch. Pharm. Res. 2000;23:633–636. doi: 10.1007/BF02975253. [DOI] [PubMed] [Google Scholar]
- Lahav M., Abu-Abied M., Belausov E., Schwartz A., Sadot E. Microtubules of guard cells are light sensitive. Plant Cell Physiol. 2004;45:573–582. doi: 10.1093/pcp/pch067. [DOI] [PubMed] [Google Scholar]
- Li F., Zhang D., Fujise K. Characterization of fortilin, a novel antiapoptotic protein. J. Biol. Chem. 2001;276:47542–47549. doi: 10.1074/jbc.M108954200. [DOI] [PubMed] [Google Scholar]
- MacDonald S.M., Rafnar T., Langdon J., Lichtenstein L.M. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- Marcus A.I., Moore R.C., Cyr R.J. The role of microtubules in guard cell function. Plant Physiol. 2001;125:387–395. doi: 10.1104/pp.125.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh M.R., Webb A., Taylor J.E., Hetherington A.M. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E.T., Salmon E.D., Erickson H.P. How calcium causes microtubule depolymerization. Cell Motil. Cytoskeleton. 1997;36:125–135. doi: 10.1002/(SICI)1097-0169(1997)36:2<125::AID-CM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Pierce D.R., Hayar A., Williams D.K., Light K.E. Developmental alterations in olivary climbing fiber distribution following postnatal ethanol exposure in the rat. Neuroscience. 2010;169:1438–1448. doi: 10.1016/j.neuroscience.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.C., Schaller D., Ravier F., Golaz O., Jaccoud S., Belet M., Wilkins M.R., James R., Deshusses J., Hochstrasser D. Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis. 1997;18:150–155. doi: 10.1002/elps.1150180127. [DOI] [PubMed] [Google Scholar]
- Schmidt I., Fahling M., Nafz B., Skalweit A., Thiele B.J. Induction of translationally controlled tumor protein (TCTP) by transcriptional and post-transcriptional mechanisms. FEBS J. 2007;274:5416–5424. doi: 10.1111/j.1742-4658.2007.06069.x. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C.R. Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzenbaum S.R., Kille P., Morgan A.J. Identification of heavy metal induced changes in the expression patterns of the translationally controlled tumour protein (TCTP) in the earthworm Lumbricus rubellus1. Biochim. Biophys. Acta. 1998;1398:294–304. doi: 10.1016/s0167-4781(98)00077-3. [DOI] [PubMed] [Google Scholar]
- Susini L., Besse S., Duflaut D., Lespagnol A., Beekman C., Fiucci G., Atkinson A.R., Busso D., Poussin P., Marine J.C., et al. TCTP protects from apoptotic cell death by antagonizing bax function. Cell Death Differ. 2008;15:1211–1220. doi: 10.1038/cdd.2008.18. [DOI] [PubMed] [Google Scholar]
- Thaw P., Baxter N.J., Hounslow A.M., Price C., Waltho J.P., Craven C.J. Structure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperones. Nat. Struct. Biol. 2001;8:701–704. doi: 10.1038/90415. [DOI] [PubMed] [Google Scholar]
- Thiele H., Berger M., Skalweit A., Thiele B.J. Expression of the gene and processed pseudogenes encoding the human and rabbit translationally controlled tumour protein (TCTP) Eur. J. Biochem. 2000;267:5473–5481. doi: 10.1046/j.1432-1327.2000.01609.x. [DOI] [PubMed] [Google Scholar]
- Vincent D., Ergul A., Bohlman M.C., Tattersall E.A., Tillett R.L., Wheatley M.D., Woolsey R., Quilici D.R., Joets J., Schlauch K., et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007;58:1873–1892. doi: 10.1093/jxb/erm012. [DOI] [PubMed] [Google Scholar]
- Vonakis B.M., Macglashan D.W., Jr, Vilarino N., Langdon J.M., Scott R.S., MacDonald S.M. Distinct characteristics of signal transduction events by histamine-releasing factor/translationally controlled tumor protein (HRF/TCTP)-induced priming and activation of human basophils. Blood. 2008;111:1789–1796. doi: 10.1182/blood-2007-07-104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yang F., Xiong Z., Yan Y., Wang X., Nishino M., Mirkovic D., Nguyen J., Wang H., Yang X.F. An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene. 2005;24:4778–4788. doi: 10.1038/sj.onc.1208666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Huang R.F., Wang X.C., Yuan M. Microtubule dynamics are involved in stomatal movement of Vicia faba L. Protoplasma. 2001;216:113–118. doi: 10.1007/BF02680138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.