Abstract
Glycogen synthase kinase-3β (GSK-3β), which is a member of the serine/threonine kinase family, has been shown to be crucial for cellular survival, differentiation, and metabolism. Here, we present evidence that GSK-3β is associated with the karyopherin β2 (Kap β2) (102-kDa), which functions as a substrate for transportation into the nucleus. A potential PY-NLS motif (109IVRLRYFFY117) was observed, which is similar with the consensus PY NLS motif (R/K/H)X2–5PY in the GSK-3β catalytic domain. Using a pull down approach, we observed that GSK-3β physically interacts with Kap β2 both in vivo and in vitro. Secondly, GSK-3β and Kap β2 were shown to be co-localized by confocal microscopy. The localization of GSK-3β to the nuclear region was disrupted by putative Kap β2 binding site mutation. Furthermore, in transient transfection assays, the Kap β2 binding site mutant induced a substantial reduction in the in vivo serine/threonine phosphorylation of GSK-3β, where- as the GSK-3β wild type did not. Thus, our observations indicated that Kap β2 imports GSK-3β through its putative PY NLS motif from the cytoplasm to the nucleus and increases its kinase activity.
Keywords: GSK-3β, karyopherin β2, protein-protein interaction, PY NLS, subcellular localization
INTRODUCTION
Glycogen synthase kinase-3β (GSK-3β) is a member of the serine/threonine kinase family. GSK-3β was originally identified as an enzyme that phosphorylates gycogen synthase, the rate-limiting enzyme in glycogen biosynthesis (Doble and Woodgett, 2003; Hur and Zhou, 2010; Patel et al., 2004). Few enzymes exert as broad a regulatory influence on cellular function as GSK-3β. More than 40 proteins have been reported to be phosphorylated by GSK-3β, including over a dozen transcription factors (Kikuchi, 1999; Mishra, 2010). Thus, GSK-3β plays a role in many fundamental biological processes, including cell fate determination, metabolism, transcriptional control, and oncogenesis (Roberts et al., 2011; Topol et al., 2009; Yi et al., 2011; Zhai et al., 2011). GSK-3β also plays a central role in the Wnt signaling pathway (Doble and Woodgett, 2003; Fu et al., 2011; Kikuchi, 1999; Patel et al., 2004). Although most of these proteins have not yet met all of the criteria set out by Frame and Cohen necessary to prove that a protein is an in vivo substrate of GSK-3β, this large number of putative substrates illustrates the great potential of GSK-3β to affect many cellular functions (Cole et al., 2004; Frame et al., 2001). This suggests that the activity of GSK-3β must be carefully regulated by mechanisms that are individually tailored for each substrate to avoid indiscriminate phosphorylation by GSK-3β (Twomey and Mc-Carthy, 2006).
Although the mechanisms regulating GSK-3β are not fully understood, precise control appears to be achieved through a combination of phosphorylation, localization, and interactions with GSK-3β-binding proteins (Fu et al., 2011; Lustig and Behrens, 2003). GSK-3β is located predominantly in the cytosol, but is also in the nuclei and mitochondria (Hoshi et al., 1995; Meares and Jope, 2007; Sui et al., 2006). However, the mechanism by which GSK-3β localization is controlled remains unclear. Analysis of the amino acid sequence does not reveal the presence of any recognizable import or export sequences (Bijur and Jope, 2003; Meares and Jope, 2007). Thus, localization may be indirectly regulated through association with binding proteins. Others have suggested that GSK-3β binding protein regulates its subcellular localization by inhibiting nuclear export (Hongisto et al., 2008).
The majority of nucleocytoplasmic transport is mediated by the karyopherin β2 proteins (Kap β2 importins/exportins). There are 19 Kap βs in humans and 14 in yeast (Marfori et al., 2011; Xu et al., 2010). Ten of the yeast Kap βs import substrates from the cytoplasm to the nucleus. Kap βs recognize and bind substrates via nuclear localization signals (NLSs) for transport through the nuclear pore complex (Bonifaci et al., 1997). Once inside the nucleus, imported Kap βs bind the small GTPase RanGTP and release their substrates (Marfori et al., 2011; Xu et al., 2010).
Each import Kap β recognizes a different set of substrates with distinct NLSs. The best characterized NLS is the short, basic classic NLS (cNLS), which is recognized by the Kap α/Kap β1 heterodimer (yeast Kap60p/Kap95p). Monopartite cNLSs consist of a single cluster of basic residues with a consensus sequence of K(K/R)X(K/R), whereas bipartite NLSs have two clusters of basic residues separated by 10-12 amino acids (Bonifaci et al., 1997; Marfori et al., 2011; Suel et al., 2008; Xu et al., 2010). The cNLS is a relatively small well defined NLS that have concentrated binding energy. However, The PY-NLS recognized by Kap β2 (Kap104p in yeast) is a larger linear signal that is quite diverse in sequence, in contrast to the classic small monopartite cNLS (Bonifaci et al., 1997; Suel et al., 2008). Many structural and biochemical studies on Kap β2 revealed that the NLS of substrate proteins that contain an N-terminal hydrophobic or basic motif and a C-terminal (R/K/H)X2–5PY motif bind to Kap β2 (Lange et al., 2008).
Here we demonstrated that GSK-3β was a substrate of Kap β2 and identified its putative PY-NLS. Upon visual inspection of the GSK-3β amino acid sequence, which contains a Kap β2 binding RX2–5PY motif, we noticed that it also contains a potential Kap β2 binding motif (109IVRLRYFFY117) in its N terminal domain. Consequently, we set out to determine whether or not GSK-3β can interact with Kap β2. Our results also demonstrated that Kap β2 interacts with wild type GSK-3β through its potential PY NLS motif. In addition, we provide evidence that interaction with Kap β2 mediates the subcellular localization of GSK-3β, and also leads to the up-regulation of GSK-3β kinase activity. Thus, our observations might shed some light on the molecular mechanism underlying GSK-3β regulation, activation, and nuclear localization.
MATERIALS AND METHODS
Antibodies
Monoclonal and polyclonal antibodies against, Actin, Kap β2, GSK-3β, phospho 9Ser GSK-3β and phospho 216Tyr GSK-3β were purchased from Santa Cruz Biotech Inc (Santa Cruz Biotech. USA) or Cell Signaling (Boston Ma, USA).
Cell culture and transfections
HEK293 cells were cultured in DMEM medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1000U penicillin-streptomycin (GIBCO BRL). Transfection was conducted with Lipofectamine and Plus Reagent (Invitrogen) in accordance with the manufacturer's instructions.
Plasmid constructs
Wild type human GSK 3β was purchased in HA- or GST-tagged mammalian expression vector (GeneCopoeia Co., USA). In order to generate the karyopherin beta 2-binding motif mutant, GSK-3β PY mutant construct, mutagenic primers (UP:5′-TGT AAC ATA GTC GCA TTG GCT TAT TTC TTC TAC-3′, DOWN:5′-GGA GTA GAA GAA ATA AGC CAA TGC GAC TAT GTT ACA-3′ and up:5′-ATA GTC CGA TTG CGT GCT TTC TTC GCC TCC AGT GGT-3′, DOWN: 5′-CTT CTC ACC ACT GGA GGC GAA GAA AGC ACG CAA TCG-3′) and a QuikChange Multi Mutagenesis Kit (Stratagene, West Cedar, USA) were utilized according to, the manufacturer’s instructions. GST-tagged recombinant proteins for GSK-3β, GSK-3β PY mutants were purified from Escherichia coli BL21 (DE3), after performing PCR. All constructions were confirmed by DNA sequencing.
Expression and purification of recombinant proteins
GST tagged GSK 3β WT was purchased from GeneCopia TM and its PY mutant was cloned with the same primer set used for generation of mammalian PYmutants. GST tagged protein GSK 3β (WT), PY NLS mutant (R111A, Y117A), or K292R mutant was expressed in Escherichia coli BL21 and purified with GST-agarose beads according to the manufacturer’s instruction (Amersham Biosciences Co). The purified proteins were used for the pull down assay with Kap β2.
Immunoprecipitation
Cells were routinely analyzed 48 h post-transfection. Cells were rinsed with ice-cold phosphate-buffered saline and resuspended in 1 ml of extraction buffer [10 mM Tris-HCl pH 7.4, 1 mM EDTA, 5 mM DTT, 100 mM NaCl, 1.0% Triton X-100, 60 mM n-octyl glucoside, 1 mM vanadate, 100 μM molybdate, 20 mM sodium fluoride and protease inhibitor cocktail (1 tablet per 10 ml extraction buffer)]. The pre-cleaned lysate was incubated for 1 h at 4°C with the appropriate antibody, and the resulting immune complexes were collected on Protein A-Sepharose beads (Pharmacia). Immune complexes were then captured by centrifugation, washed extensively in lysis buffer, and solubilized with 2 × sample buffer, prior to loading onto 10% SDS-PAGE gel.
GSK-3β pull down assay
Whole cell lysates of HEK293 cells was pre-cleaned with the glutathione agarose beads, and incubated with 1 μg of each glutathione agarose tagged recombinant GST-GSK 3β (WT), PY NLS mutant (R111A, Y117A), K292R mutant, at 4°C for 2 h on an end-over-end rotating shaker, in order to allow for the association of GSK-3β protein and Kap β2. The associated protein complexes were collected using the slurry of the glutathione agarose beads and washed extensively. After resuspension in 2× Laemmli sample buffer, samples were analyzed on 10% SDS-PAGE, The Western blot was performed with Kap β2 antibody.
Immunoblotting
The pull down or immunoprecipitated GSK-3β was resolved on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were then incubated in blocking buffer (5% dried skim milk in PBS and 0.05% Tween-20), and probed with specific antibodies, followed by horseradish peroxidase-conjugated secondary antibody. Immune complexes were detected with the chemiluminescence western blotting detection system (Pierce, USA).
Confocal microscopy
HEK293 cells were seeded overnight at 60% confluence onto culture slides coated with human fibronectin (Becton Dickinson, USA). The following day, cells were transfected HA-GSK 3β (WT), PY NLS mutant (R111A, Y117A), K292R mutant, and allowed to grow for an additional 48 h. The cells were washed several times with ice-cold PBS and fixed in 2% paraformaldehyde for 10 min. The fixed cells were permeabilized with 0.1% Triton X-100 for 10 minutes and blocked for 2 h in PBS containing 0.1% BSA-C (Aurion, The Netherlands) and 0.1% Tween. Following incubation with a polyclonal antibody against Kap β2, the cells were washed and stained further with a conjugated donkey anti-rabbit IgG prior to processing the slides for immunofluorescence. After an additional 20 min of incubation at 37°C, the cells were fixed, permeabilized, and decorated with either an anti- Kap β2 or HA antibody. As a secondary antibody, Alexa Fluor 568 or 488-conjugated donkey anti-rabbit or antimouse (Molecular Probes, Inc., USA) was used. Confocal microscopy analysis was performed LSM710 (Zeiss, Germany) at the Center for Experimental Research Facilities of Chungbuk National University (Kuo et al., 2011; Lee et al., 2011).
FACS analysis
HA-GSK 3β (WT), PY NLS mutant (R111A, Y117A), K292R mutant, or pcDNA vector was transfected and the rate of apoptosis measured by Annexin V-PE apoptosis detection kit I (BD Biosciences, USA), according to the manufacturer’s instructions. The cells were vortexed gently and incubated for 15 min at 25°C in the dark. 400 μl of binding buffer was added to each tube. Within 1 h, FACS was examined using FACS Calibur (BD Science) in The Core Facility of Chungbuk National University (Lee et al., 2011).
Protein stability experiments
HEK293 cells (2.5 × 105 cells per well) in 10 cm plates were transfected with 1.0 μg of expression vector with HA-GSK 3β (WT), PY NLS mutant (R111A, Y117A), or K292R mutant plasmid. The medium was replaced with medium containing 200 μg/ml cycloheximide 36 h after transfection (0-h time point). Cell lysates were harvested at 0, 8, 16, 24 h and analyzed by immunoprecipitation and Western blotting using anti-HA antibodies, and assayed in five time repeats. The relative optical density (OD) was measured by image analysis of the dried SDS-PAGE gel with the Fuji Image Quant software (Fujifilm, Japan), according to the manufacturer’s instructions.
RESULTS
GSK-3β interacts with Karyopherin β2 through its putative PY-NLS
Related Kap β2 binding motifs are found in most Kap β2-associated proteins (Marfori et al., 2011; Xu et al., 2010). Diverse PY-NLS sequences are consistent with their weak consensus motifs composed of a loose N-terminal hydrophobic or basic motif and a C-terminal RX2–5PY motif (Lange et al., 2008; Suel et al., 2008). The composition of N-terminal motifs divides PY-NLSs into hydrophobic and basic subclasses (hPY- and bPY-NLSs). PY-NLSs contain four consecutive predominantly hydrophobic residues (consensus Φ1-G/A/S-Φ3-Φ4, where Φ is a hydrophobic residue), while the equivalent region in bPYNLSs is a stretch of 4–20 amino acids that are enriched in basic residues (Bonifaci et al., 1997; Lange et al., 2008; Marfori et al., 2011; Suel et al., 2008; Xu et al., 2010).
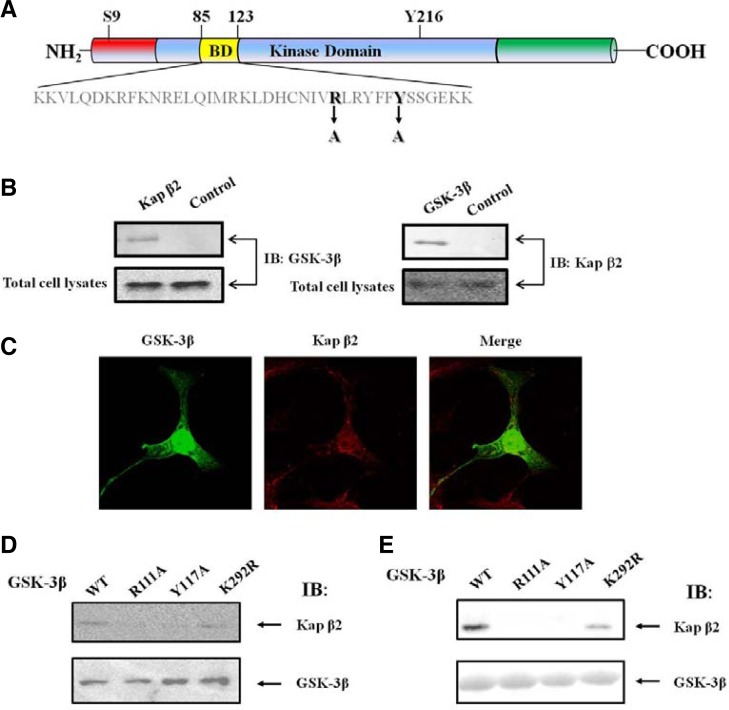
Based on this information, we found that GSK-3β contains the potential Kap β2-binding motifs (109IVRLRYFFY117) within its binding domain (Fig. 1A). Thus, the presence of a putative conserved Kap β2 binding motif in GSK-3β clearly suggests that GSK-3β can bind to Kap β2. Meares and Jope reported that they identify an NLS motif in GSK3β that is necessary for its nuclear import and is sufficient to drive the nuclear import of yellow fluorescent protein (Meares and Jope, 2007). They suggested that an NLS motif in GSK-3β which is localized in the binding domain (BD) (Fig. 1) does not include R111 or Y117 sequence as the key amino acid sequences for its putative NLS function. Instead, two basic sequences (85KK86 and 103RK105) were emphasized as its NLS. Because the BD does not contain a classic NLS, the nuclear localization of GSK-3β seems to be not mediated by importin α (Mo et al., 2000; Takeda et al., 2000). We assumed that these two basic sequences are the part of PY-NLS. Furthermore, we did not detect the binding of importin α with GSK-3β in HEK293 cell, even though two basic sequences (85KK86 and 103RK105) of GSK-3β seems to be a good candidate site for the binding with importin α (data not shown).
Fig. 1.
The putative PY NLS in GSK-3β and interaction between exogenous GSK-3β and Kap β2. (A) GSK-3β contains the putative-conserved Kap β2 binding motif (109IVRLRYFFY117) within its binding domain (yellow). Two point mutants were prepared in order to define the binding region. GSK-3β mutants in the Kap β2 putative binding motifs, were also prepared, and the mutated sequences were indicated as Y117A (109IVRLRYFFY 117 changes to 109IVRLRYFFA 117) or R111A (109IVRLRYFFY 117 changes to 109IVALRYFFY 117). For these mutants control, we used the unrelated GSK-3β K292R mutant. (B) Following immunoprecipitation (IP) using an anti-Kap β2 antibody, an immunoblot (IB) was performed using an antibody against GSK-3β (left). The immunoprecipitated GSK-3β complexes were applied to the immunoblot, using an anti-Kap β2 antibody (right). For the negative control, normal mouse serum was used for immunoprecipitation. (C) Confocal fluorescence micrographs showing the endogenous GSK-3β and Kap β2 in HEK293 cells. Kap β2 was visualized by immunofluorescence in fixed and permeablized cells using polyclonal antibodies to human Kap β2 or GSK-3β and Alexa Fluor 568 conjugated donkey anti-rabbit IgG or Alexa Fluor 488 conjugated mouse anti-rabbit IgG. The yellow pattern resulting from the merging of red and green colors indicates the co-localization of both proteins at a specific region of the nuclear membrane and nuclear. (D) HEK293 cells were transiently transfected with expression vectors, HA-GSK-3β WT, R111A, Y117A. Following immunoprecipitation (IP) using an anti-HA antibody, either Kap β2 (upper lane) or GSK-3β (down lane) was detected with the immunoblot (IB) using an antibody against Kap β2 or GSK-3β. (E) In vitro pull down assay with the fusion protein of GSK-3β (WT, R111A, Y117A, K292R). Whole cell lysates of HEK293 cells was incubated with 1 μg of each glutathione agarose tagged recombinant GSK-3β (WT, R111A, Y117A, K292R). The immunoblot was performed to detect Kap β2 with its antibody (upper lane). The recombinant GSK-3β (WT, R111A, Y117A, K292R) protein amount were monitored with the coomasaie blue staining (bottom lane).
Since GSK-3β seems to contain the putative Kap β2 binding motif (see Fig. 1A), we set out to determine whether the endogenous Kap β2 formed a protein complex with GSK-3β in HEK293 cells. As shown in Fig. 1B, the GSK-3β immunoprecipitate contained Kap β2 (right). Antibodies directed against Kap β2 were also able to successfully capture GSK-3β from the same lysates, corroborating the hypothesis that the two proteins were indeed physically associated (Fig. 1B left). Furthermore, we attempted to determine whether GSK-3β exists together with Kap β2 in the cell by confocal microscopy (Fig. 1C). The results of this analysis indicated that the endogenous GSK- 3β (green) and Kap β2 (red) were, indeed, merged (yellow) in the nuclear. Thus these findings strongly suggest that endogenous GSK-3β interacts with Kap β2 in the HEK293 cell.
In order to determine further whether the putative PY NLS in GSK-3β interacts with Kap β2, we constructed HA-GSK-3β PY NLS point mutants (R111A, Y117A; Fig. 1A), which included related Kap β2 binding motifs, and K292R (included related Kap β2 binding motifs). After transfected in HEK293 cell, each HA-GSK-3β protein was purified with HA antibody. As expected Kap β2 containing the potential candidate Kap β2-binding motifs (Fig. 1A), wild-type GSK-3β brought down Kap β2 from HEK293 cell lysates in high quantities, while the GSK-3β PY mutant (R111A and Y117A) did not result in appreciable pulldown of Kap β2 (Fig. 1D). However, GSK-3β K292R mutant which exclude the putative PY NLS sequence did co-immunoprecipitate with Kap β2 from HEK293 cell lysates in high quantities, as the wild-type GSK-3β (Fig. 1D). The GSK-3β K292R mutant was utilized as a control for its PY NLS(Eun Jeoung et al., 2008).
To confirm further that the motif (109IVRLRYFFY117) in BD functions as PY NLS of GSK-3β, we performed Kap β2 pull down assay with GST GSK-3β WT, R111A, Y117A, and K292R fusion proteins expressed in E. coli. We observed that wild-type and K292R GSK-3β fusion proteins brought down Kap β2 from HEK293 cell lysates in high quantities, while the GSK-3β PY mutant (R111A and Y117A) fusion proteins did not (Fig. 1E). This result was consistent with the co-immunoprcipitation result in Fig. 1D. Thus, together these results (Figs. 1A–1E) demonstrated unequivocally that GSK-3β binds to Kap β2 with its putative PY NLS motif.
The interaction between the exogenous GSK-3β and Kap β2 is required for its nuclear localization in the HEK293 cell
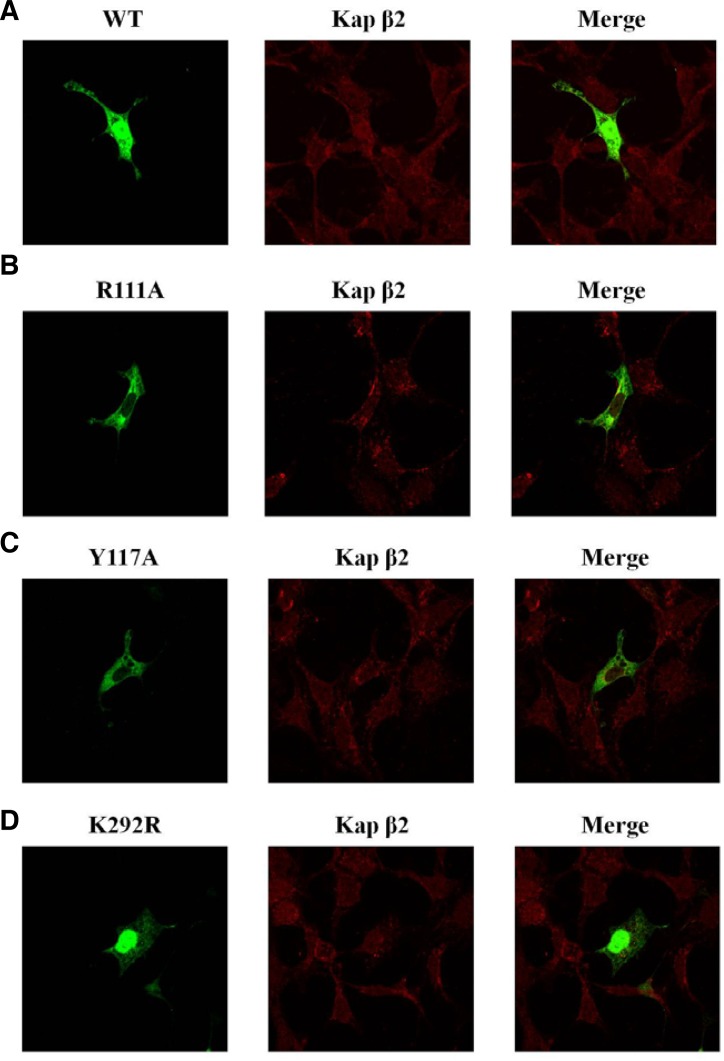
In order to better understand the effects of the interactions between GSK-3β and Kap β2, confocal microscopic analysis was performed (Figs. 2A–2D). In a finding consistent with the endogenous GSK-3β results shown in Fig. 1C, we observed that exogenous HA-GSK-3β WT (green) and Kap β2 (red) were merged together (yellow) in the nuclear (Fig. 2A). The exogenous HA-GSK-3β PY NLS mutants (R111A, Y117A green), however, was not merged or co-localized with Kap β2 (red) in the nuclear (Figs. 2B and 2C), which was probably due to the mutations in the Kap β2 binding sites. They were slightly merged (yellow) with Kap β2 (red) in the nuclear rim (Figs. 2B and 2C). Further, GSK-3β K292R mutant which excluded the putative PY NLS sequence was merged well and co-localized in the nuclear strongly with Kap β2 (Fig. 2D), similar with GSK-3β WT (Fig. 2A).
Fig. 2.
The subcellular localization of exogenous GSK-3β PY mutants. Confocal fluorescence micrographs of HA-GSK-3β WT, R111A, Y117A, or K292R in HEK293 cells. Kap β2 was visualized by immunofluorescence in fixed and permeablized cells using a polyclonal antibody against human a Kap β2 and Alexa Fluor 568 conjugated donkey anti-rabbit IgG. The yellow pattern resulting from the merging of red and green colors indicates colocalization of both proteins at a specific region of the plasma membrane or cytoplasm, similar to the results obtained for endogenous GSK-3β shown in Fig. 1C. All constructs were shown as green color and performed to determine whether it merged with Kap β2. The transfected HA- GSK-3β wt (detected in both the cytoplasm and the nucleus) was merged (yellow) with GSK- 3β nuclear speckles around nuclear pore (A). The transfected HA-GSK-3β PY mutant (R111A, Y117A) was not detected in nuclear speckles around nuclear pore, and was not merged with Kap β2 in the nuclear (B and C). To control the specificity of GSK-3β PY mutant (R111A, Y117A) subcellular localization, that of GSK-3β K292R mutant was also visualized in (D).
Together, these confocal data strongly suggested that Kap β2 interacts with GSK-3β through its putative consensus motifs (109IVRLRYFFY117) in BD of GSK-3β, and the protein-protein interaction between GSK-3β and Kap β2 is a requirement for its localization to the nuclear.
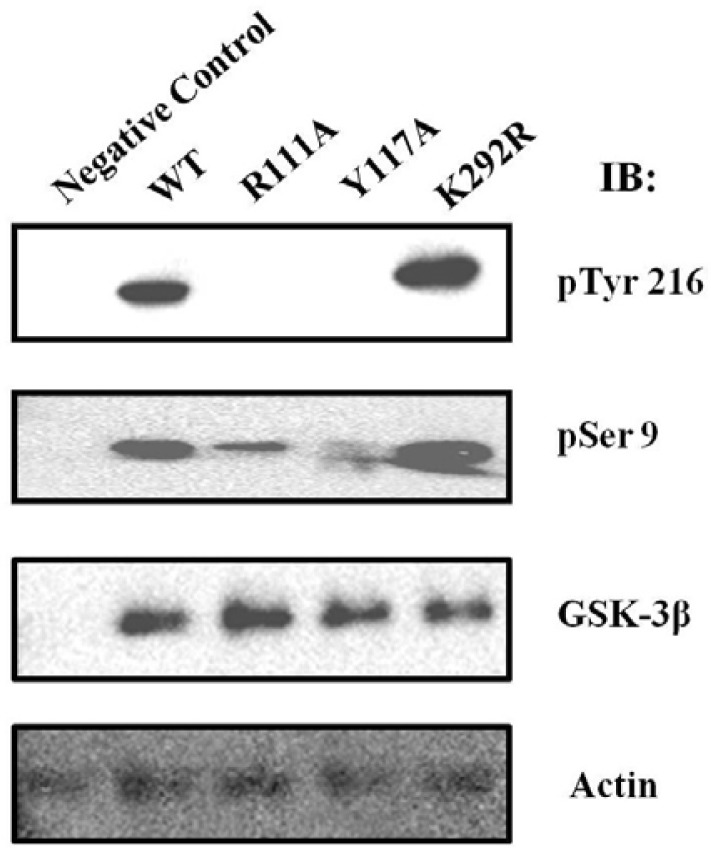
The subcellular localization GSK-3β influenced on its 216Tyr or 9Ser residue phosphorylation
As shown in Figs. 1 and 2, the putative PY NLS motif of GSK-3β contributes to the physical interaction with Kap β2 and its nuclear localization. In order to characterize the functional consequences of this protein-protein interaction and subcellular localization, we transiently expressed HA-GSK-3β WT, PY mutant (R111A or Y117A), or its control mutant K292R in the HEK293 cell, and compared their phosphorylation status with the phosphor specific antibody against pTyr 216 or pSer 9 of GSK-3β. We also used an anti-GSK-3β antibody to monitor GSK-3β expression (Fig. 3). As shown in Fig. 3, we observed that the tyrosine 216 residue of GSK-3β PY mutant (R111A or Y117A) was not phosphorylated, whereas that of GSK-3β WT, or K292R mutant was well phosphorylated (Fig. 3, upper lane). The Ser 9 residue of GSK-3β PY mutant (R111A or Y117A) was slightly phosphorylated, whereas that of GSK-3β WT, or K292R mutant was well phosphorylated (Fig. 3, middle lane). The GSK-3β expression level was not altered dramatically (Fig. 3, bottom lane). Thus, the nuclear localized GSK-3β seems to be related with the phosphoryltion on its Ser 9 and Tyr 214.
Fig. 3.
Comparison the phosphorylation at 9Ser and 216Tyr residue of GSK-3β with its PY mutant. HA-GSK-3β WT or its mutant (R111A, Y117A, or K292R) was transfected and immunoprecitated with HA antibody, as described in “Material and Methods”. The phosphorylation of GSK-3β was detected with IB using an anti-phospho 216Tyr (upper lane) or 9Ser (middle lane) GSK-3β antibody. The untansfectied HEK293 cells was immunoprecitated with HA antibody as the negative control. The amount of GSK-3β protein in the experiment was monitored by GSK-3β antibody (under lane).
It was reported that GSK-3β self-phosphorylates on the 216Tyr residue in its activation loop, in a fashion similar to the activation mechanisms of other AGC family protein kinases (Cole et al., 2004; Frame et al., 2001). Thus GSK-3β WT, or K292R mutant seems to be more active than GSK-3β PY mutant (R111A or Y117A). It has been reported that the Ser 9 residue of GSK-3β is phosphorylated and inhibited its activity by Akt or SGK (Moule et al., 1997; Tanioka et al., 2011). However, it is not clear why the more activated form of GSK-3β by the phosphorylation on its Tyr 216 is more phosphorylated on its Ser 9. We assumed that the self phosphorylation on Tyr 216 induces its structure which its 9 Ser can be easily accessible by Akt or SGK.
Furthermore, we assumed that the nuclear localization of GSK-3β seems to be related with its kinase activity, because WT, or K292R mutant (mainly nuclear localization) are more phosphorylated on its Tyr 216 than that of GSK-3β PY mutant (R111A or Y117A) (Figs. 2 and 3). This result also suggests that the association of GSK-3β with Kap β2 (Figs. 1D and 1E) is related to its 216Tyr residue self-phosphorylation (Fig. 3, upper lane). However, we cannot rule out potential changes in the kinase activity of GSK-3β PY NLS mutant by the site-directed mutagenesis.
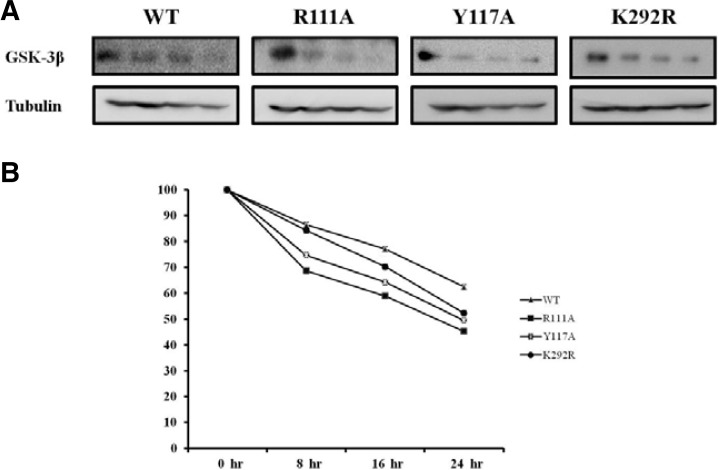
The nuclear localization of GSK-3β enhances on its protein stability
To evaluate the effect of Kap β2 binding on GSK-3β protein stability, we performed pulse-chase experiments as described in the Materials and Methods section. Each HA-GSK-3β WT, R113A, Y117A, K292R mutant expression vector was transfected into HEK293 cells and immunoprecipitated using HA monoclonal Ab following cycloheximide treatment (Fig. 4A). The exogenous GSK-3β proteins were chased for the indicated time periods (0, 8, 16, 24 h), and then immunoprecipitated using a polyclonal anti-HA antibody and subjected to SDS-PAGE, followed by western blot with an anti-Kap β2 antibody. To control for the protein amount, the tubulin level was monitored in each sample by western blotting (Fig. 4A). The quantification of the pulse-chase experiment, as determined by image analysis of the dried SDS-PAGE gel using the Fuji Image Quant software, is shown in Fig. 4B. GSK-3β K292R mutant, which was transported into the nuclear region and very active, was used as the control. The tubulin amount of each sample was also visualized with its specific antibody in the Western blot (bottom).
Fig. 4.
The protein stability of GSK-3β and its PY mutant. HEK293 cells (2.5 × 105 cells per well) in 100 mm plates were transfected with 8.0 μg of expression vector with HA-GSK 3β wt or its PY mutant plasmid. The medium was replaced with medium containing 200 μg/ml cycloheximide 36 h after transfection (0-hr time point). Cell lysates were harvested at 0, 8, 16, and 24 h then analyzed by immunoprecipitation and Western blotting using anti-HA antibodies, and assayed in five time repeats. The relative optical density (OD) was measured by image analysis of the dried SDS-PAGE gel with the Fuji Image Quant software (Fujifilm, Japan), according to the manufacturer’s instructions.
As shown in Fig. 4, the protein stability of the GSK-3β PY NLS mutant (R113A, Y117A) was 70% that of GSK-3β WT or K292R mutant, suggesting that the nuclear localization of GSK- 3β (Fig. 2) improved its protein stability (Fig. 4) and activity (Fig. 3). We assumed that the cytoplasmic GSK-3β PY NLS mutant is more easily available to the protein degradation apparatus than the nuclear GSK-3β WT or K292R mutant.
The nuclear localization of GSK-3β effects on the cell viability
We also measured cell viability using FACS analysis to determine whether the subcellular localization of GSK-3β influenced the cell viability (Ku et al., 2011; Tanioka et al., 2011). As shown in Table 1, our FACS results indicate that the GSK-3β PY NLS mutants (R113A, Y117A) increased the cell survival rate significantly, compared to the HA-GSK-3β WT or K292R mutant which interacts with Kap β. As shown in Table 1, GSK-3β PY mutant (R113A or Y117) appeared to be 2.5 time less effective on HEK293 cell apoptosis than the HA-GSK-3β WT or K292R mutant did. Thus, it seems to be that nuclear localization of GSK-3β is also required for its effects on cell viability. However, because GSK-3β PY mutant (R113A or Y117) was less self-phosphorylated on Tyr 216 (Fig. 3), it seems to be that the effect of GSK-3β on the cell viability is related its kinase activity.
Table 1.
The comparison of cell survival ratio of GSK-3β wt with its PY mutants
| GSK-3 β | Rate of apoptosis (%) by FACS |
|---|---|
| WT | 25 +/− 3 |
| R111A | 10 +/− 4 |
| Y117A | 11 +/− 5 |
| K292R | 23 +/− 4 |
| (Vector only) | 7 +/− 2 |
|
| |
| Mean value of 5 repeats | |
HA-GSK-3β WT, its PY mutant (R111A, Y117A), K292R mutant or pcDNA 3.0 was transfected and the rate of apoptosis measured by FACS. HA-GSK-3β PY mutant (R111A, Y117A), which was not dominantly localized into the nuclear was less effective on the cell apoptosis, compared to GSK-3β WT constructs. The apoptotic effect of GSK-3β PY mutant (R111A or Y117A) was reduced to 2.5 times that of GSK-3β WT.
In summary, our data indicated that GSK-3β binds to Kap β2 (as its new partner protein) through its binding domain (85-120aa), which contains the putative PY NLS motif (109IVRLRYFFY117) for Kap β2, and that the interaction with Kap β2 regulates both GSK-3β activity and its functions through its nuclear localization control.
DISCUSSION
The role of GSK-3β in signal transduction has been most clearly characterized in the context of PI3K-Akt kinase signaling, in which it functions as a hub protein kinase (Doble and Woodgett, 2003; Hur and Zhou, 2010; Kikuchi, 1999; Mishra, 2010; Patel et al., 2004).
In this study, we demonstrated that Kap β2 can function as one of GSK-3β nuclear transporters, and the putative GSK-3β PY-NLS is localized in its BD with the site-directed mutagenesis analysis (Fig. 1).
Although our data suggests that the interaction of GSK-3β and Kap β2 through PY NLS binding enhances GSK-3β kinase activity and controls its subcellular localization, our findings also raise several questions regarding the interaction of GSK-3β and Kap β2 (Bonifaci et al., 1997; Lange et al., 2008; Suel et al., 2008; Xu et al., 2010). It remains unknown as to whether the self-phosphorylation or the kinase activity of GSK-3β was actually necessary for its functional interaction with Kap β2 in vivo. Further experiments are clearly warranted in order to gain a greater understanding of the biological implications regarding the high degree of conservation of a well-defined Kap β2 binding motif in GSK-3β. Moreover, the fashion and mechanisms by which the interactions between GSK-3β and Kap β2 are controlled still need to be evaluated under physiological conditions. In addition, it remains to be determined whether GSK-3β PY NLS mutation itself affects kinase and self-phosphorylation activity, regardless of protein-protein interactions with Kap β2. It is also necessary to ascertain whether the post translational modification of Kap β2 is required for the activation and/or regulation of GSK-3β or for the interaction between GSK-3β and Kap β2.
In present time, we do not know whether the PY NLS mutation of GSK-3β directly affected self phosphorylation, which is required for its kinase activity. To address this question, however, we constructed GSK-3β Y216A mutant, which could not be self-phosphorylated, but it was found in the nucleus with the confocal analysis (data not shown), Thus, this observation suggested that the nuclear localization of GSK-3β seems to be independent of self-phosphorylation on its 216 Tyr. Therefore our result (Fig. 2) which the blockade of nuclear localization of GSK-3β PY NLS mutant (R111A or Y117) is due to the inhibition of Kap β2 binding on its BD by the point mutation, but not the self-phosphorylation on its 216 Tyr or its kinase activity.
The identification of adaptor/substrate proteins and signaling properties of GSK-3β provide some indications of possible therapeutic strategies for the inhibition of GSK-3β activity (Cole et al., 2004; Frame et al., 2001; Lustig and Behrens, 2003; Meares and Jope, 2007; Sui et al., 2006; Twomey and McCarthy, 2006). Further characterization of the biological ramifications of the interaction between GSK-3β and Kapβ in terms of cell survival, differentiation, and Akt kinase signaling is also needed. The activated GSK-3β mediates the phosphorylation of a variety of intracellular substrates (Frame et al., 2001; Hoshi et al., 1995; Twomey and McCarthy, 2006). However, many other authentic substrate proteins of GSK-3β remain to be identified. Thus, even though the role of GSK-3β kinase activity is largely that of a hub kinase in PI3K-Akt kinase signaling, the identification of other GSK-3β substrate proteins may facilitate the characterization of the biological functions of GSK-3β in the cell.
In conclusion, in this study, we demonstrated that Kap β2 binds to GSK-3β through its PY motif (109IVRLRYFFY117) in its catalytic domain. Although the functional significance of this interaction remains poorly understood, the positive regulation of GSK-3β activity by Kap β2 may well represent a relevant consequence of the different signaling pathways in which GSK-3β is involved. The results on both the GSK-3β self-phosphorylation activity and the kinase activity of GSK-3β revealed that these activities are inhibited upon engagement with Kap β2 (Fig. 3). Thus, Kap β2 acts as an agonistic to GSK-3β signal transduction. However, although the data in this study suggest that the Kap β2 may function as a positive regulator of GSK-3β signaling, the precise mechanisms underlying the subcellular localization of GSK-3β requires further characterization in order to gain better insight into the overall function of the Kap β2 signal transduction pathway.
In the future, this identification of a GSK3β PY NLS provides new strategies to decipher and manipulate its cellular actions in gene expression and apoptosis and its involvement in human diseases.
Supplementary Material
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grants (2009-0076024 and 2009-0069007) funded by the Korea government (Ministry of Education, Science and Technology) to S S Kang. We also appreciated The Core Facility of Chungbuk National University.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Bijur G.N., Jope R.S. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14:2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- Bonifaci N., Moroianu J., Radu A., Blobel G. Karyopherin beta2 mediates nuclear import of a mRNA binding protein. Proc. Natl. Acad. Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A., Frame S., Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble B.W., Woodgett J.R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun Jeoung L., Sung Hee H., Jaesun C., Sung Hwa S., Kwang Hum Y., Min Kyoung K., Tae Yoon P., Sang Sun K. Regulation of glycogen synthase kinase 3beta functions by modification of the small ubiquitin-like modifier. Open Biochem. J. 2008;2:67–76. doi: 10.2174/1874091X00802010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S., Cohen P., Biondi R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zheng S., An N., Athanasopoulos T., Popplewell L., Liang A., Li K., Hu C., Zhu Y. beta-catenin as a potential key target for tumor suppression. Int. J Cancer. 2011;129:1541–1551. doi: 10.1002/ijc.26102. [DOI] [PubMed] [Google Scholar]
- Hongisto V., Vainio J.C., Thompson R., Courtney M.J., Coffey E.T. The Wnt pool of glycogen synthase kinase 3beta is critical for trophic-deprivation-induced neuronal death. Mol. Cell. Biol. 2008;28:1515–1527. doi: 10.1128/MCB.02227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M., Sato M., Kondo S., Takashima A., Noguchi K., Takahashi M., Ishiguro K., Imahori K. Different localization of tau protein kinase I/glycogen synthase kinase-3 beta from glycogen synthase kinase-3 alpha in cerebellum mitochondria. J. Biochem. 1995;118:683–685. doi: 10.1093/oxfordjournals.jbchem.a124965. [DOI] [PubMed] [Google Scholar]
- Hur E.M., Zhou F.Q. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell Signal. 1999;11:777–788. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Ku B.M., Lee Y.K., Jeong J.Y., Ryu J., Choi J., Kim J.S., Cho Y.W., Roh G.S., Kim H.J., Cho G.J., et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3beta pathways in U87MG human glioma cells. Mol Cells. 2011;31:275–279. doi: 10.1007/s10059-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.W., Wang W.H., Liu S.T. Mapping signals that are important for nuclear and nucleolar localization in MCRS2. Mol Cells. 2011;31:547–552. doi: 10.1007/s10059-011-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A., Mills R.E., Devine S.E., Corbett A.H. A PYNLS nuclear targeting signal is required for nuclear localization and function of the Saccharomyces cerevisiae mRNA-binding protein Hrp1. J. Biol. Chem. 2008;283:12926–12934. doi: 10.1074/jbc.M800898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.J., Shin S.H., Hyun S., Chun J., Kang S.S. Mutation of a putative S-nitrosylation site of TRPV4 protein facilitates the channel activates. Animal Cells Syst (Seoul) 2011;15:95–106. doi: 10.1080/19768354.2011.555183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B., Behrens J. The Wnt signaling pathway and its role in tumor development. J. Cancer Res. Clin. Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- Marfori M., Mynott A., Ellis J.J., Mehdi A.M., Saunders N.F., Curmi P.M., Forwood J.K., Boden M., Kobe B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys Acta. 2011;1813:1562–1577. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Meares G.P., Jope R.S. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3: functional effects in apoptosis. J. Biol. Chem. 2007;282:16989–17001. doi: 10.1074/jbc.M700610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y.Y., Wang C., Beck W.T. A novel nuclear localization signal in human DNA topoisomerase I. J. Biol. Chem. 2000;275:41107–41113. doi: 10.1074/jbc.M003135200. [DOI] [PubMed] [Google Scholar]
- Moule S.K., Welsh G.I., Edgell N.J., Foulstone E.J., Proud C.G., Denton R.M. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J. Biol. Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- Patel S., Doble B., Woodgett J.R. Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem. Soc. Trans. 2004;32:803–808. doi: 10.1042/BST0320803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.M., Pronobis M.I., Poulton J.S., Waldmann J.D., Stephenson E.M., Hanna S., Peifer M. Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol Cell. 2011;22:1845–1863. doi: 10.1091/mbc.E10-11-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suel K.E., Gu H., Chook Y.M. Modular organization and combinatorial energetics of proline-tyrosine nuclear localization signals. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z., Kovacs A.D., Maggirwar S.B. Recruitment of active glycogen synthase kinase-3 into neuronal lipid rafts. Biochem. Biophys. Res. Commun. 2006;345:1643–1648. doi: 10.1016/j.bbrc.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Takeda K., Haque M., Nagoshi E., Takemoto M., Shimamoto T., Yoneda Y., Yamanishi K. Characterization of human herpesvirus 7 U27 gene product and identification of its nuclear localization signal. Virology. 2000;272:394–401. doi: 10.1006/viro.2000.0364. [DOI] [PubMed] [Google Scholar]
- Tanioka T., Tamura Y., Fukaya M., Shinozaki S., Mao J., Kim M., Shimizu N., Kitamura T., Kaneki M. Inducible nitricoxide synthase and nitric oxide donor decrease insulin receptor substrate-2 protein expression by promoting proteasome-dependent degradation in pancreatic beta-cells: involvement of glycogen synthase kinase-3beta. J. Biol. Chem. 2011;286:29388–29396. doi: 10.1074/jbc.M110.192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L., Chen W., Song H., Day T.F., Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J. Biol. Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey C., McCarthy J.V. Presenilin-1 is an unprimed glycogen synthase kinase-3beta substrate. FEBS Lett. 2006;580:4015–4020. doi: 10.1016/j.febslet.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Xu D., Farmer A., Chook Y.M. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Curr. Opin. Struct. Biol. 2010;20:782–790. doi: 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F., Pereira L., Hoffman J.A., Shy B.R., Yuen C.M., Liu D.R., Merrill B.J. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat. Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P., Sciarretta S., Galeotti J., Volpe M., Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ. Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.