Abstract
Resveratrol is a phytoalexin and polyphenol derived from grapes, berries, and peanuts. It has been shown to mediate death of a wide variety of cancer cells. Although resveratrol is considered an important potential chemotherapeutic agent, it is required at high doses to achieve a biologically or physiologically significant effect, which may be impractical for treating cancer. Thus, a more stable and potent derivative of resveratrol, with more effective tumoricidal activity, must be developed. A novel resveratrol analog, HS-1793, has recently been synthesized and was determined to exhibit a greater decrease in cancer cell viability than resveratrol. However, the underlying mechanism of HS-1793-induced cancer cell death remains unknown. We thus investigated the mechanism by which HS-1793 induces cell death and assessed whether this occurs through a mitochondrial-mediated mechanism. Using the MCF-7 breast cancer cell line, we determined that HS-1793 treatment significantly increased cell death at a relatively low dose compared with resveratrol. HS-1793 treatment more significantly decreased mitochondrial membrane potential, cellular ATP concentration, and cellular oxygen consumption rate than resveratrol treatment. At the molecular level, HS-1793 treatment down-regulated the expression of major mitochondrial biogenesis-regulating proteins, including mitochondrial transcriptional factor A (TFAM), Tu translation elongation factor (TUFM), and single-stranded DNA-binding protein. We conclude that HS-1793 acts by regulating the expression of TFAM and TUFM, leading to a block in normal mitochondrial function, which sensitizes cancer cells to cell death. We therefore propose that HS-1793 can be a useful chemosensitization agent, which together with other such agents can efficiently target cancer cells.
Keywords: HS-1793, mitochondrial biological function, oxygen consumption rate, resveratrol, TFAM
INTRODUCTION
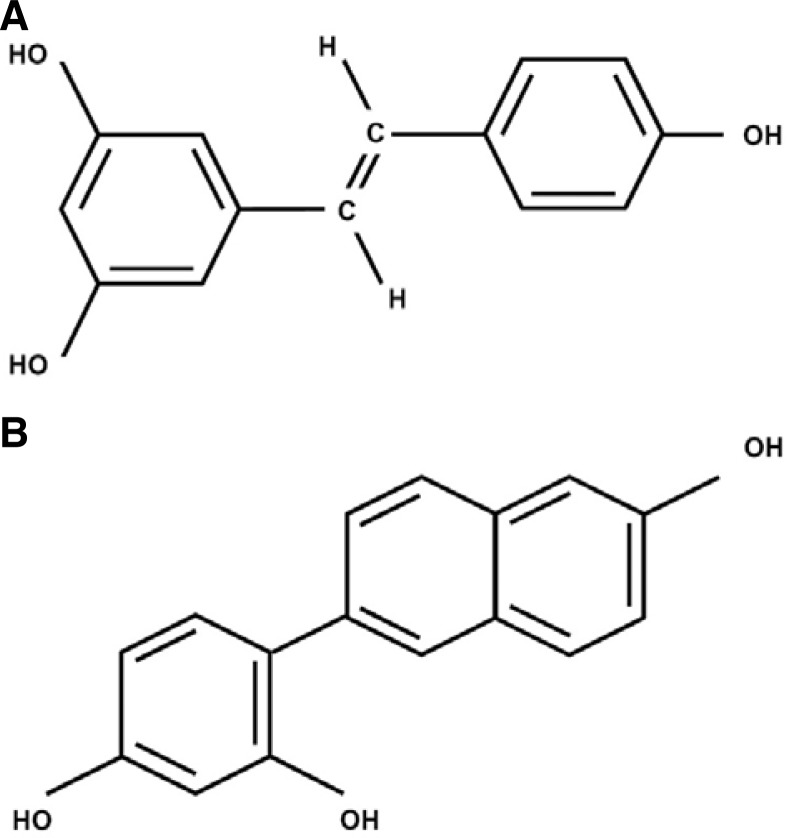
Resveratrol (3,5,4′-trihydroxystilbene, C14H12O3, Fig. 1A) is a phytoalexin present in numerous plant species. It has been implicated as a potential chemopreventive and chemotherapeutic drug in the treatment of human cancer (Bishayee, 2009). Many in vitro and animal model studies have shown that resveratrol treatment exhibits anticancer effects. For example, resveratrol treatment can reduce proliferation of several mammalian cancer cell lines (Bhat et al., 2001; Damianaki et al., 2000; Jang et al., 1997) and induce apoptosis in skin, colon, and breast cancer models in vivo (Alirol and Martinou, 2006; Bove et al., 2002; Fremont, 2000; Gusman et al., 2001; Hsieh et al., 1999). Furthermore, many studies have demonstrated that resveratrol can inhibit several events during carcinogenesis (e.g., tumor initiation, promotion and progression) (Bishayee 2009).
Fig. 1.
Chemical structure of resveratrol (A) and HS-1793 (B). (A) Resveratrol has two phenol rings (C14H12O3). (B) Synthetic resveratrol analog HS-1793 has three phenol rings.
Although studies are ongoing to determine the mechanism of action of resveratrol, it is becoming clear that resveratrol interacts in multiple molecular cascades to promote apoptosis and reduce cell proliferation. For instance, resveratrol-induced apoptosis has repeatedly been reported to be accompanied by increased caspase activity (Ferry et al., 2002; Kim et al., 2004; Wolter et al., 2001). In addition, resveratrol-induced apoptosis was shown to be associated with Bax mitochondrial translocation (Mahyar-Roemer et al., 2002), inhibition of AKT activity (Brownson et al., 2002), up-regulation of the oncogene suppressor p53 (Narayanan et al., 2003), and down-regulation of cyclin D1 (Joe et al., 2002). In other studies, resveratrol has been shown to act via c-Jun NH2-terminal kinase (JNK), as resveratrol-induced p53-dependent transcriptional activity and apoptosis were blocked upon expression of a dominant-negative mutant of JNK or upon disruption of JNK1 or JNK2. In - addition to a proapoptotic role, resveratrol has been shown to exhibit antiproliferative effects in various cell types, which may be caused by a dose-dependent inhibition of ribonucleotide reductase activity (Fontecave et al., 1998). Similarly, resveratrol has been found to inhibit DNA polymerase (Sun et al., 1998) as well as ornithine decarboxylase, a key enzyme of polyamine biosynthesis that is enhanced in cancer (Schneider et al., 2000).
Although resveratrol has great potential as a chemotherapeutic and chemopreventive agent, one significant drawback is that resveratrol exhibits low cytotoxicity when compared with other chemotherapeutic agents; thus, a high concentration is needed to induce cancer cell death (Cecchinato et al., 2007; Clement et al., 1998). Recent studies have been undertaken to obtain synthetic analogs of resveratrol with more dynamic ranges in their biological effects (Szekeres et al., 2011). For example, resveratrol-based nitrovinylstilbenes (i.e., resveratrol analogs) have been shown to exhibit a cytotoxic effect on cancer cells - inducing cell cycle arrest and cell death - at lower concentrations than resveratrol (Reddy et al., 2011). Analogs RV32, RV01, and RV02 have been reported to inhibit ethanol-induced oxidative DNA damage in human peripheral lymphocytes (Yan et al., 2011). DHS (4-4′-dihydroxy-trans-stilbene) has been shown to exhibit more efficient anti-proliferative effects than resveratrol, by inhibiting DNA polymerase delta activity and DNA replication. Furthermore, DHS has been shown to more efficiently promote DNA damage in the presence of copper than resveratrol, and cancer cells have been reported to have higher copper levels than healthy cells. Thus, DHS may prove to efficiently kill cancer cells but not normal cells (Ebara et al., 2000; Savio et al., 2009; Zheng et al., 2006). Taken together, these studies suggest the utility of resveratrol analogs and their potential as effective chemotherapeutic agents.
In previous studies, we designed and synthesized a resveratrol analog, 4-(6-hydroxy-2-naphthyl)-1,3-benzenediol (HS-1793; C16H12O3, Fig. 1B), which elicits higher anti-tumor activity than resveratrol in various cancer cell lines (Jeong et al., 2009a; 2009b). In addition, HS-1793 overcame the resistance conferred by Bcl-2 in U937 leukemia cells. However, the molecular mechanism of the anticancer effect of HS-1793 is not fully defined. In the present study, we hypothesized that the potent anticancer effect of HS-1793 may be related to mitochondrial activity, because cell death by HS-1793 is induced in Bcl-2-mediated resistant cancer cells. To test this mechanism, we evaluated HS-1793-induced cell death in MCF-7 cells by assessing several parameters related to mitochondrial activity and the cell death pathway. We determined that HS-1793-treated MCF-7 cells showed disruption of mitochondrial function - mitochondrial membrane potential (ΔΨm), oxygen consumption, and ATP production. In addition, expression of both mitochondrial transcriptional factor A (TFAM) and Tu translation elongation factor (TUFM) decreased in HS-1793-treated MCF-7 cells. Therefore, our results reveal that HS-1793 promotes apoptosis in breast cancer cells and may be useful as a chemosensitization drug that induces breast cancer cell death in response to other anticancer drugs, such as tamoxifen, toremifene, and letrozole.
MATERIALS AND METHODS
Cell culture and treatments
Cells from the human breast adenocarcinoma cell line MCF-7 were obtained from the American Type Culture Collection (ATCC; USA). The culture medium used throughout these experiments was DMEM, containing 10% fetal bovine serum (FBS, PAA, Pasching, Austria) and 100 μg/ml penicillin-streptomycin. Resveratrol and HS-1793 were dissolved in ethanol at concentrations of 10 mM and 100 mM, and stored at −80°C until use. Cells were treated with resveratrol analogs, HS-1793 (0–90 μM) or resveratrol (0–150 μM). Cells were harvested 72 h after treatment and used for each experiment.
Reagents
Rabbit polyclonal TFAM and mouse monoclonal alpha tubulin (Santa Cruz Biotechnology, USA). Rabbit polyclonal anti-human caspase-3, caspase-9, and PARP (Cell Signaling Technology, USA). Mouse monoclonal anti-human TUFM (Sigma Aldrich, USA). HRP-conjugated donkey anti-rabbit and sheep antimouse IgG (Amersham Pharmacia Biotech, USA). For western blots, the enhanced chemiluminescent western blotting detection reagent (SuperSignal West Pico chemiluminescent substrate) was used (Pierce, USA). Rhod 2AM, TMRE, and Mito-SOX cell dyes were used (Molecular Probes, USA).
Flow cytometric analysis
Mitochondrial membrane potential (ΔΨm), mitochondrial Ca2+, and mitochondrial reactive oxygen species (ROS) levels were measured by FACS, using specific fluorescence probes: tetramethylrhodamine, ethyl ester, perchlorate (TMRE), Rhod 2AM (Invitrogen, USA) and MitoSOX, respectively. A total of 1 × 106 cells from each group were incubated in 200 nM TMRE or 5 μM MitoSOX for 20 min at 37°C in the dark. For measurement of mitochondrial Ca2+, a total of 1 × 106 cells from each group were incubated with 5 μM Rhod 2AM for 60 min on ice in the dark and were then used to measure TMRE, MitoSOX, and Rhod 2AM levels on a FACS caliber (BD, USA). The data were analyzed using WinMDI2.8 software for simultaneous estimation of each parameter.
Western blot analysis
Cell lysates were centrifuged at 14,000 rpm for 15 min at 4°C. Protein concentrations of cell lysates were determined by Bradford protein assay (Bio-Rad), and 50 μg proteins were loaded per lane onto 7.5–15% SDS-polyacrylamide gels. The gels were transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, USA) and reacted with a specific antibody. Immunostaining with antibodies was performed using SuperSignal West Pico-enhanced chemiluminescence substrate and detected with LAS-3000PLUS (Fuji Photo Film Company, Japan).
Cellular ATP concentration analysis
ATP concentrations in the HS-1793- (30–90 μM) or resveratrol-treated (90–150 μM) cells were analyzed by ATP bioluminescent somatic cell assay kit (FLASC, Sigma-Aldrich, USA). Briefly, 100 μl ATP assay mix working solution, 100 μl somatic cell ATP-releasing reagent, and 50 μl ultrapure water were added and vortexed in each of the assay vials. After treatment with either HS-1793 or resveratrol, cells were harvested. Then, 1 × 105 cells were added to each assay vial. Luminescence was detected by SpectraMax M2 (Molecular Devices, USA). ATP concentration was calculated using a standard curve with known concentrations (0, 1.25, 2.5, 5, and 10 μM) of ATP standards. Finally, ATP concentration was expressed as micromole ATP/1 × 105 cells.
Oxygen consumption measurements
XF24 analysis was performed as previously described (Qian and Van Houten, 2010). Briefly, the XF24 cell culture plates (Part No. 100777-004, Seahorse Bioscience, USA) are shaped like typical 24-well plates, with rows A–D and columns 1–6. The seeding surface of each well is the same size as on a typical 96-well plate. Typical seeding density was 10,000 cells per well, which were seeded into 200 μl cell suspension per well, leaving temperature-correction wells empty (A1, B4, C3, and D6). Next, the plate was incubated (37°C, 5% CO2) for 16 h. Cells were treated with resveratrol (0–150 μM) or HS-1793 (0–90 μM) for 48 h. Before XF24 analysis, the media was changed to 750 μl XF Assay Medium-modified DMEM (0 mM glucose, Part No. 100965-000, Seahorse Bioscience, USA) and then incubated at 37°C without CO2 for 1 h. To prepare a sensor cartridge for calibration, 1.0 ml Seahorse Bioscience XF24 Calibrant (pH 7.4) (Part No. 100840-000, Seahorse Bioscience, USA) was added to each well of the 24-well plate. Then, the sensor cartridge was placed on top of the plate and incubated at 37°C without CO2 overnight. The oxygen consumption rate (OCR) was measure by the XF24 analyzer and XF24 software. After measuring the OCR, the XF24 assay results were normalized to cell number. Cell number for each well was counted using a hemocytometer. Live cells were also counted and the XF assay results were normalized to cell number using XF assay software.
Real-time PCR
For real-time PCR of TFAM (forward 5′-ccgaggtggttttcatctgt-3′ and reverse 5′-tccgccctataagcatcttg-3′) and TUFM (forward 5′-tagcaagaacatccgcactg-3′ and reverse 5′-gtcccaagtcagggagaaca-3′) mRNA, total RNA was extracted from cultured cells using the RNeasy plus Mini kit (QIAGEN, USA) following the manufacturer’s instructions. Reverse transcription (RT) PCR was performed with the Superscript III reverse transcriptase kit (Invitrogen, USA) using 2 μg fractionated cellular RNA as a template, which was purified as described above. Real-time PCR was carried out using SYBR Premix Ex Taq (Takara, Japan). Reactions were prepared following the manufacturer’s protocol. All reactions were carried out in triplicate (Bio-Rad, USA). Specific primers were used to detect the presence of each mRNA. Standard thermal cycling conditions included a hot start of 2 min at 50°C and 10 min at 95°C. The DNA was amplified through 50 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C for both the TFAM and TUFM genes. Data analysis was carried out using Bio-Rad software (iCycler iQTM) and Microsoft Excel. Expression values are presented relative to the measurements for beta-tubulin values in the corresponding samples.
Quantitative PCR for mitochondrial DNA
To evaluate the effects of HS-1793 or resveratrol on mitochondrial biogenesis, we tested mitochondrial DNA content ratios (Clay Montier et al., 2009). Total DNA including chromosomal DNA and mitochondrial DNA was extracted from HS-1793- or resveratrol-treated cells using the Gentra Puregene kit (QIAGEN) following the manufacturer’s instructions. Real-time PCR was carried out using SYBR Premix Ex Taq (Takara, Japan). Reactions were prepared following the manufacturer’s protocol. All reactions were carried out in triplicate (Bio-Rad). Standard thermal cycling conditions included a hot start of 2 min at 50°C and 10 min at 95°C. We designed Primer F 5′-attacccactcacgggagct-3′ (mtDNA 21–40) and Primer R 5′-atagtagtagggtcgtggtg-3′ (mtDNA 5141–5160). The DNA (mtDNA 101–5210) was amplified through 60 cycles of 15 s at 95°C, 30 s at 58°C, and 300 s at 72°C. Data analysis was carried out using Bio-Rad software (iCycler iQTM) and Microsoft Excel.
Statistical analysis
Four independent in vitro experiments were performed. Statistical results were expressed as the mean ± standard deviation of the triplicate results obtained from triplicates of each independent experiment. Statistical significance of differences was determined by the paired Kruskal-Wallis nonparametric test. A P value less than 0.05 were considered significant.
RESULTS
Resveratrol analog, HS-1793, exhibits a greater antitumor effect than resveratrol
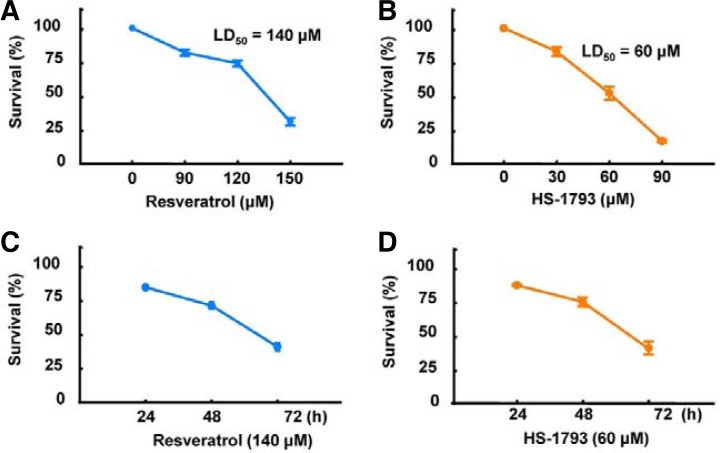
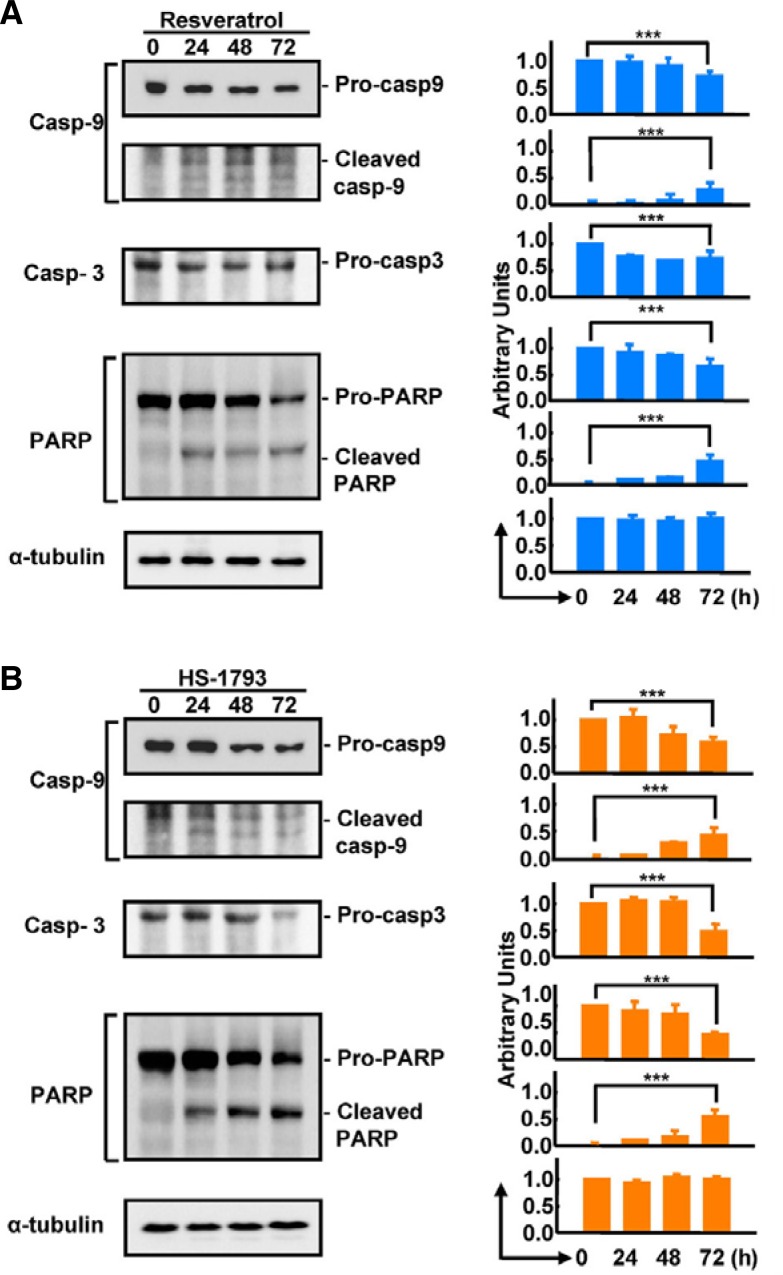
To compare the anticancer effects of HS-1793 and resveratrol, we treated breast cancer cells (MCF-7 cells) with resveratrol (Fig. 2A) or HS-1793 (Fig. 2B) in both dose- and time-dependent manners. HS-1793 treatment significantly decreased the survival rate of MCF-7 cells compared with resveratrol treatment (P < 0.005). Determination of the half-maximal lethal dose (LD50) revealed that the LD50 of HS-1793 (60 μM) was significantly lower than resveratrol (140 μM). Using the same concentrations throughout the study, the cell survival rate was determined to be significantly reduced by both 140 μM resveratrol (Fig. 2C) and 60 μM HS-1793 treatment (Fig. 2D) after 48 h. To validate whether necrosis or apoptosis was induced by HS-1793 or resveratrol, we assessed activation of caspases-3 and -9, which are key executioners of apoptosis, during HS-1793 and resveratrol treatment. Exposure of MCF-7 cells to resveratrol or HS-1793 strongly stimulated caspase activity (Figs. 3A and 3B). In particular, pro-caspase-3 and PARP were significantly decreased and cleaved PARP was inversely increased by HS-1793 treatment. These results show that HS-1793 treatment significantly increases apoptotic cell death by only one-half dose of resveratrol.
Fig. 2.
Effects of HS-1793 and resveratrol on the survival rate of MCF-7 cells. Resveratrol (90 to 150 μM) (A) or HS-1793 (30 to 90 μM) (B) were added to MCF-7 cells for 72 h, and cell survival rates were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Half-maximal lethal dose (LD50) of HS-1793 was remarkably lower than resveratrol (60 μM vs. 140 μM, respectively). The LD50 of resveratrol (C) or HS-1793 (D) in treated cells was determined at 24, 48, and 72 h in MCF-7 cells. The time-dependent cytotoxic effect of LD50 was not significantly different between HS-1793 and resveratrol in MCF-7 cells.
Fig. 3.
Effects of HS-1793 and resveratrol on caspase activity in MCF-7 cells. Western blot analysis showed that caspase-9, caspase-3, and PARP activities were increased by LD50 treatment of resveratrol (A) and HS-1793 (B) (***P < 0.005) in a time-dependent manner.
Resveratrol analog, HS-1793, impairs mitochondrial function
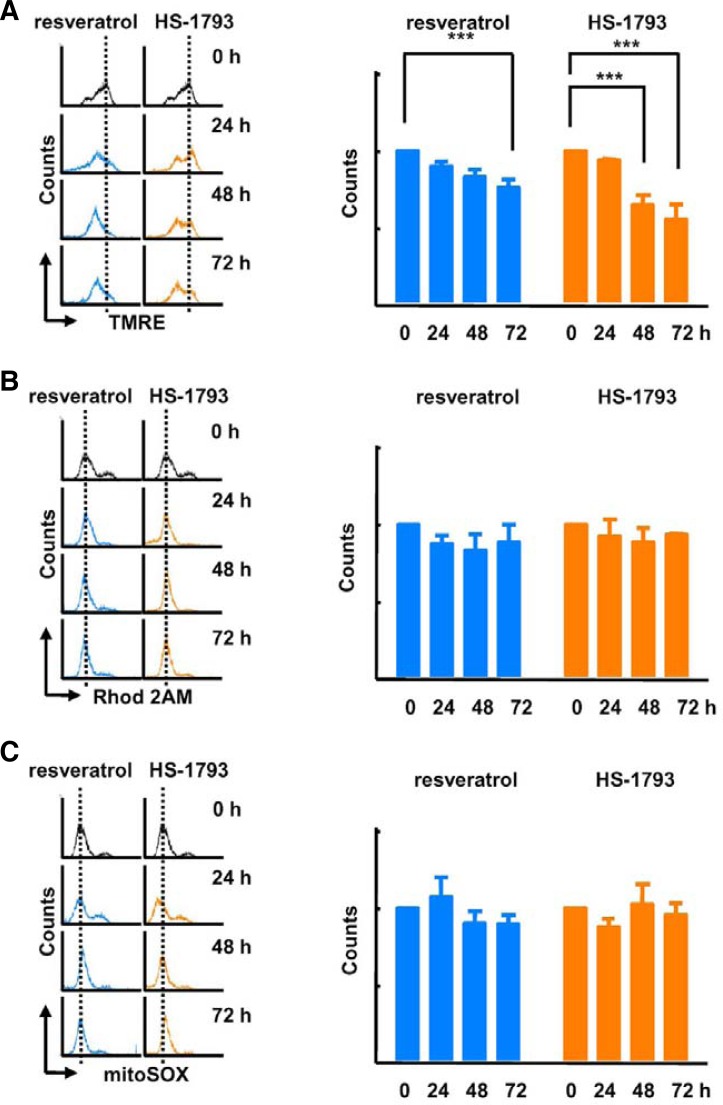
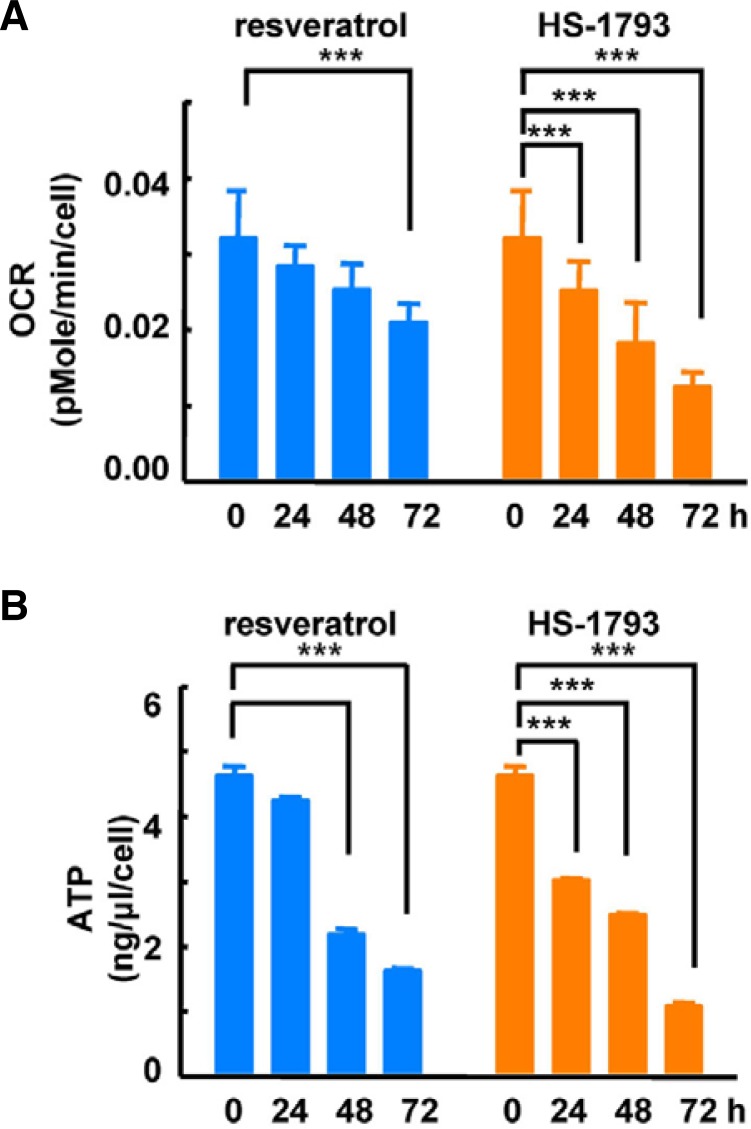
To investigate the effects of HS-1793 treatment on mitochondrial function, we compared mitochondrial membrane potential after resveratrol and HS-1793 treatments. We observed a significant depolarization of the membrane 48 h after HS-1793 treatment (Fig. 4A). Additionally, although resveratrol treatment reduced mitochondrial membrane potential, the effect was lower than after HS-1793 treatment. Next, we analyzed whether these treatments influenced mitochondrial calcium and ROS levels. Neither HS-1793 nor resveratrol treatment significantly changed the levels of mitochondrial calcium (Fig. 4B) and ROS (Fig. 4C). Following this experiment, we evaluated mitochondrial oxidative phosphorylation activity by determining the OCR and cellular ATP concentration. HS-1793 treatment exerted a stronger influence on oxygen consumption and ATP synthesis than resveratrol treatment. The OCR and cellular ATP levels were significantly reduced by HS-1793 treatment after 24 h (Fig. 5). At 24 h, HS-1793 more strongly inhibited mitochondrial oxidative phosphorylation compared with resveratrol. Taken together, these results indicate that HS-1793 effectively suppresses mitochondrial energy metabolism in MCF-7 cells, independently of Ca2+ and ROS levels.
Fig. 4.
HS-1793 induced depolarization of mitochondrial membrane potential. Flow cytometry data indicated that (A) TMRE (mitochondrial membrane potential) significantly decreased by 60 μM HS-1793 or 140 μM resveratrol treatment at 48 and 72 h. The intensities of (B) Rhod 2AM (mitochondrial calcium) and (C) MitoSOX (mitochondrial ROS) were not altered by 60 μM HS-1793 or 140 μM resveratrol treatment (***P < 0.005).
Fig. 5.
HS-1793 reduced mitochondrial oxidative phosphorylation. Resveratrol (140 μM) or 60 μM HS-1793 were added to MCF-7 cells for 72 h and then washed out of each well. To measure the OCR, we used an XF24 analyzer. Treatment with HS-1793 (60 μM) or resveratrol (140 μM) significantly reduced (A) OCR and (B) ATP production in a time-dependent manner at 24, 48, and 72 h (***P < 0.005).
HS-1793 treatment down-regulates mitochondrial biogenesis regulatory proteins
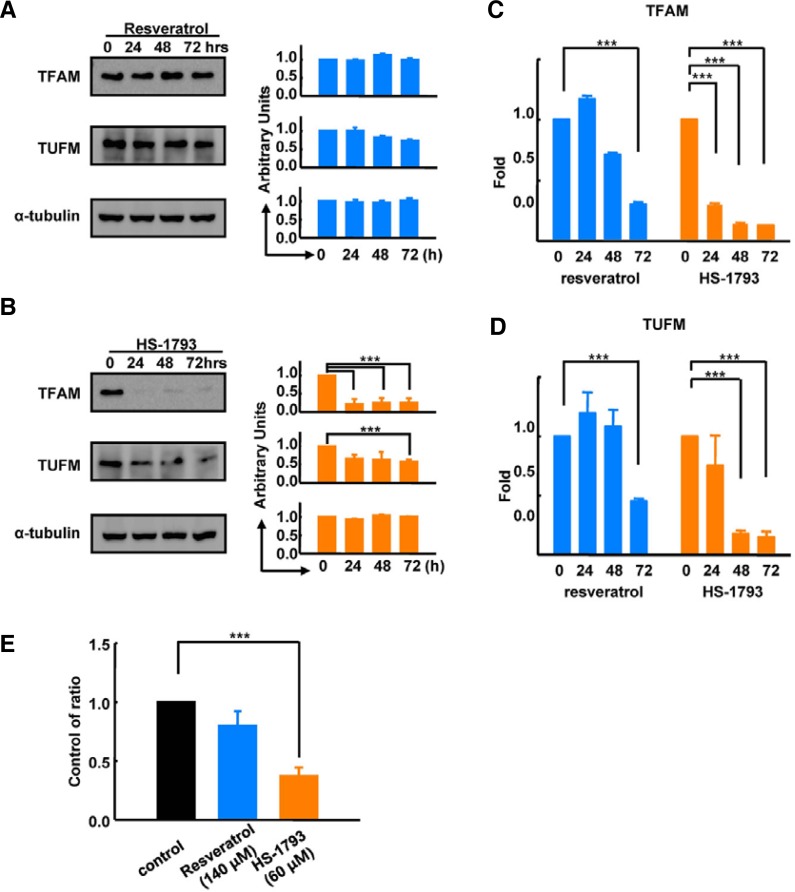
To determine the molecular mechanism of HS-1793-induced mitochondrial dysfunction, we analyzed protein expression of three mitochondria biogenesis regulatory proteins, including TFAM, TUFM, and single-stranded DNA-binding protein (SSBP). Resveratrol treatment had no effect on TFAM, TUFM, or SSBP protein levels (Fig. 6A). However, HS-1793 treatment significantly down-regulated TFAM expression to approximately 20% of control levels after 24 h of treatment (Fig. 6B). Likewise, TUFM expression was down-regulated to 50% of control levels 48 h after HS-1793 treatment. To test whether these decreases in TFAM and TUFM were regulated by the transcription of coding genes, we analyzed the mRNA levels of TFAM and TUFM. Real-time PCR data showed that HS-1793 treatment significantly decreased the amount of TFAM (Fig. 6C) and TUFM (Fig. 6D) transcripts. We analyzed alterations of mitochondrial DNA content in control, resveratrol-, and HS-1793-treated MCF-7 cells to validate the final consequence of the down-regulation of both TFAM and TUFM. Consistent with the protein and gene levels, DNA content and mitochondrial biogenesis were significantly decreased in HS-1793-treated MCF-7 cells compared with control and resveratrol treatment (Fig. 6E). Taken together, these results suggest that HS-1793 inhibits mitochondrial energy metabolism by repressing transcription of mitochondrial biogenesis regulatory genes, such as TFAM and TUFM. However, the exact mechanism of how HS-1793 regulates transcription of these genes remains to be investigated.
Fig. 6.
HS-1793 down-regulated mitochondrial biogenesis regulatory proteins. (A) Western blot data showed that 140 μM resveratrol treatment did not change the levels of mitochondrial biogenesis regulatory proteins, including TFAM and TUFM, in MCF-7 cells. (B) HS-1793 (60 μM) treatment significantly down-regulated the protein expression levels of both TFAM and TUFM. Real-time PCR data indicated that TFAM (C) and TUFM (D) were also significantly decreased by HS-1793 treatment. (E) Resveratrol (140 μM) or 60 μM of HS-1793 were added to MCF-7 cells for 72 h and the mitochondrial DNA ratio was measured using real-time PCR. HS-1793 treatment decreased the mitochondrial DNA ratio by 50% (***P < 0.005).
DISCUSSION
Although resveratrol is a promising anticancer agent, resveratrol analog HS-1793 may be a more effective agent to treat cancer, as HS-1793 exhibits higher stability and enhanced antiproliferative and proapoptotic effects than resveratrol. In the present study, we investigated the effect of HS-1793 treatment on mitochondria-mediated cell death in MCF-7 cells.
We have shown that HS-1793 induces cell death by inhibiting transcription of mitochondrial biogenesis regulatory genes, TFAM and TUFM, in MCF-7 cells. When HS-1793 was added to MCF-7 cells, TFAM and TUFM expression was decreased and mitochondrial biogenesis and oxidative phosphorylation were perturbed. As a result, HS-1793-treated cells underwent mitochondrial-mediated apoptosis in mitochondrial calcium- and ROS-independent manners. Generally, in response to apoptotic triggers such as accumulation of ROS and Ca2+, ΔΨm is immediately dissipated by the opening of the membrane permeability transition pore and osmotic swelling of the mitochondrial matrix (Bae et al., 2011). This eventually leads to OMP (outer membrane permeability). Upon OMP, cytotoxic proteins within the intermembrane space, such as cytochrome c and Smac, are released into the cytosol and initiate mitochondrial-mediated cell death (Baines et al., 2007; Bouchier-Hayes et al., 2008; Nakagawa et al., 2005).
Our data suggest that mitochondrial proteins TFAM and TUFM are decreased by HS-1793 treatment, which in turn, induces mitochondrial dysfunction and cell death. These nuclear-encoding mitochondrial proteins are localized to mitochondria and play roles in mitochondrial DNA replication and translation. TFAM is an essential and important component for maintenance of mitochondrial biological function, as well as for maintenance of proper mitochondrial copy number, mitochondrial morphology (Ekstrand et al., 2004; Kang et al., 2007; Larsson et al., 1998), and respiratory chain function (Ekstrand et al., 2004; 2007; Falkenberg et al., 2002; Ho et al., 2010; Larsson et al., 1998). TFAM regulates expression of 13 protein components of mitochondrial oxidative phosphorylation complexes for electron transport and ATP synthesis (Shadel and Clayton, 1997). In addition, recent studies have reported a role of TFAM in oncogenesis. TFAM, which is increased in thyroid oncocytoma (Savagner et al., 2003), and mitochondrial DNA (mtDNA) depletion cause apoptosis in osteosarcoma cells (Dey and Moraes, 2000). Moreover, loss of the TFAM gene reduces tumorigenesis in an oncogenic Kras-driven mouse model of lung cancer (Weinberg et al., 2010). Given the significance of TFAM in mitochondrial biogenesis and tumorigenesis, these data support the idea that the inhibition of TFAM by HS-1793 is the cause of the observed antiproliferative and proapoptotic effects. However, the exact target of HS-1793 remains to be determined.
It is well known that PGC-1 alpha and Sirtuin-1 (Sirt1) exert positive effects on mitochondrial biogenesis under normal physiological conditions (Kaeberlein et al., 2005; Luo et al., 2001; Motta et al., 2004; Muth et al., 2001; Vaziri et al., 2001; Yeung et al., 2004). Sirt1 regulates the activity and expression of PGC-1 alpha (Nie et al., 2009). Interestingly, it has been shown that TFAM is regulated by nuclear respiratory factor 1 and PGC-1 alpha (Ventura-Clapier et al., 2004; Zhang et al., 2011). Thus, it is possible that resveratrol and HS-1793 both target these upstream regulators of TFAM. We examined these signaling pathways and found that treatment of MCF-7 cells with resveratrol and HS-1793 reduced both the activation of Sirt1 (i.e., phosphorylation) and its protein levels, and reduced the protein levels of downstream mediators, such as PGC-1 alpha and STAT3 (Supplementary Fig. S1). Thus, Sirt1 may be the main target of HS-1793.
A main result of HS-1793 treatment, however, is to dampen mitochondrial biogenesis, and in turn, promote cell death and attenuate proliferation of MCF-7 cells. Mitochondria appear to be important for this action to occur, as treatment of mitochondria-deficient (ρ0) HeLa cells with HS-1793, but not resveratrol, did not induce cell death to the same level as in normal HeLa cells (Supplementary Fig. S2). The mitochondria-specific effect of HS-1793 can be utilized potentially to target cancer cells but not normal cells. For example, mitochondria in cancer cells structurally and functionally differ from their normal counterparts (Gogvadze et al., 2008). Moreover, cancer cells exhibit an extensive metabolic reprogramming that renders them more susceptible to mitochondrial perturbations than non-immortalized cells (Bellance et al., 2009; Kroemer and Pouyssegur, 2008). Thus, this difference can be exploited by mitochondria-targeted compounds, such as HS-1793, creating a bias towards cancer cells but not normal cells. Further, HS-1793 can be used as a chemosensitization agent, whereby affecting mitochondrial dysfunction may enhance the chemotoxic effects of anticancer agents, such as tamoxifen.
In summary, our findings propose that HS-1793 acts as a modifier in mitochondrial activity, leading to the activation of the mitochondrial-mediated apoptosis pathway in MCF-7 cells. This underlying mechanism will provide a fundamental basis for creating a therapeutic modality in resveratrol analog-mediated cancer therapy. Future studies will focus on understanding the underlying mechanism that links TFAM to mitochondrial dysfunction and the effects of HS-1793 in cancer combination therapy.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health, Welfare and Family affairs, Republic of Korea (0920040) and the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2009-0093193 and 2010-0020224), Republic of Korea.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Alirol E., Martinou J.C. Mitochondria and cancer: is there a morphological connection? Oncogene. 2006;25:4706–4716. doi: 10.1038/sj.onc.1209600. [DOI] [PubMed] [Google Scholar]
- Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Sheiko T., Craigen W.J., Molkentin J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellance N., Lestienne P., Rossignol R. Mitochondria from bioenergetics to the metabolic regulation of carcinogenesis. Front Biosci. 2009;14:4015–4034. doi: 10.2741/3509. [DOI] [PubMed] [Google Scholar]
- Bhat K.P., Lantvit D., Christov K., Mehta R.G., Moon R.C., Pezzuto J.M. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- Bouchier-Hayes L., Munoz-Pinedo C., Connell S., Green D.R. Measuring apoptosis at the single cell level. Methods. 2008;44:222–228. doi: 10.1016/j.ymeth.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove K., Lincoln D.W., Tsan M.F. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2002;291:1001–1005. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- Brownson D.M., Azios N.G., Fuqua B.K., Dharmawardhane S.F., Mabry T.J. Flavonoid effects relevant to cancer. J. Nutr. 2002;132:3482S–3489S. doi: 10.1093/jn/132.11.3482S. [DOI] [PubMed] [Google Scholar]
- Cecchinato V., Chiaramonte R., Nizzardo M., Cristofaro B., Basile A., Sherbet G.V., Comi P. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem. Pharmacol. 2007;74:1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Clay Montier L.L., Deng J.J., Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J. Genet. Genomics. 2009;36:125–131. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement M.V., Hirpara J.L., Chawdhury S.H., Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- Damianaki A., Bakogeorgou E., Kampa M., Notas G., Hatzoglou A., Panagiotou S., Gemetzi C., Kouroumalis E., Martin P.M., Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell Biochem. 2000;78:429–441. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Dey R., Moraes C.T. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-x(L) in osteosarcoma cells. J. Biol. Chem. 2000;275:7087–7094. doi: 10.1074/jbc.275.10.7087. [DOI] [PubMed] [Google Scholar]
- Ekstrand M.I., Falkenberg M., Rantanen A., Park C.B., Gaspari M., Hultenby K., Rustin P., Gustafsson C.M., Larsson N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Ekstrand M.I., Terzioglu M., Galter D., Zhu S., Hofstetter C., Lindqvist E., Thams S., Bergstrand A., Hansson F.S., Trifunovic A., et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M., Gaspari M., Rantanen A., Trifunovic A., Larsson N.G., Gustafsson C.M. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- Ferry S., Matsuda M., Yoshida H., Hirata M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFkappaB-mediated cell survival pathway. Carcinogenesis. 2002;23:2031–2041. doi: 10.1093/carcin/23.12.2031. [DOI] [PubMed] [Google Scholar]
- Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O. Resveratrol a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998;421:277–279. doi: 10.1016/s0014-5793(97)01572-x. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gusman J., Malonne H., Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- Ho D.J., Calingasan N.Y., Wille E., Dumont M., Beal M.F. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp. Neurol. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Hsieh T.C., Burfeind P., Laud K., Backer J.M., Traganos F., Darzynkiewicz Z., Wu J.M. Cell cycle effects and control of gene expression by resveratrol in human breast carcinoma cell lines with different metastatic potentials. Int. J. Oncol. 1999;15:245–252. doi: 10.3892/ijo.15.2.245. [DOI] [PubMed] [Google Scholar]
- Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jeong S.H., Jo W.S., Song S., Suh H., Seol S.Y., Leem S.H., Kwon T.K., Yoo Y.H. A novel resveratrol derivative, HS1793, overcomes the resistance conferred by Bcl-2 in human leukemic U937 cells. Biochem. Pharmacol. 2009a;77:1337–1347. doi: 10.1016/j.bcp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Jeong S.H., Lee J.S., Jeong N.Y., Kim T.H., Yoo K.S., Song S., Suh H., Kwon T.K., Park B.S., Yoo Y.H. A novel resveratrol analogue HS-1793 treatment overcomes the resistance conferred by Bcl-2 and is associated with the formation of mature PML nuclear bodies in renal clear cell carcinoma Caki-1 cells. Int. J. Oncol. 2009b;35:1353–1360. doi: 10.3892/ijo_00000453. [DOI] [PubMed] [Google Scholar]
- Joe A.K., Liu H., Suzui M., Vural M.E., Xiao D., Weinstein I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E.A., Caldwell S.D., Napper A., Curtis R., DiStefano P.S., Fields S., et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kang D., Kim S.H., Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Kim Y.A., Choi B.T., Lee Y.T., Park D.I., Rhee S.H., Park K.Y., Choi Y.H. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 2004;11:441–446. [PubMed] [Google Scholar]
- Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Larsson N.G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G.S., Clayton D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Luo J., Nikolaev A.Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Mahyar-Roemer M., Kohler H., Roemer K. Role of Bax in resveratrol-induced apoptosis of colorectal carcinoma cells. BMC Cancer. 2002;2:27. doi: 10.1186/1471-2407-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M.C., Divecha N., Lemieux M., Kamel C., Chen D., Gu W., Bultsma Y., McBurney M., Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Muth V., Nadaud S., Grummt I., Voit R. Acetylation of TAF(I)68, a subunit of TIF-IB/SL1 activates RNA polymerase I transcription. EMBO J. 2001;20:1353–1362. doi: 10.1093/emboj/20.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Narayanan B.A., Narayanan N.K., Re G.G., Nixon D.W. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- Qian W., Van Houten B. Alterations in bioenergetics due to changes in mitochondrial DNA copy number. Methods. 2010;51:452–457. doi: 10.1016/j.ymeth.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Reddy M.A., Jain N., Yada D., Kishore C., Vangala J.R., R.P.S., Addlagatta A., Kalivendi S.V., Sreedhar B. Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J. Med. Chem. 2011;54:6751–6760. doi: 10.1021/jm200639r. [DOI] [PubMed] [Google Scholar]
- Savagner F., Mirebeau D., Jacques C., Guyetant S., Morgan C., Franc B., Reynier P., Malthiery Y. PGC-1-related coactivator and targets are upregulated in thyroid oncocytoma. Biochem. Biophys. Res. Commun. 2003;310:779–784. doi: 10.1016/j.bbrc.2003.09.076. [DOI] [PubMed] [Google Scholar]
- Schneider Y., Vincent F., Duranton B., Badolo L., Gosse F., Bergmann C., Seiler N., Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine on human colonic cancer cells. Cancer Lett. 2000;158:85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- Shadel G.S., Clayton D.A. Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- Sun N.J., Woo S.H., Cassady J.M., Snapka R.M. DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J. Nat. Prod. 1998;61:362–366. doi: 10.1021/np970488q. [DOI] [PubMed] [Google Scholar]
- Szekeres T., Saiko P., Fritzer-Szekeres M., Djavan B., Jager W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann. N Y Acad. Sci. 2011;1215:89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Dessain S.K., Ng Eaton E., Imai S.I., Frye R.A., Pandita T.K., Guarente L., Weinberg R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Garnier A., Veksler V. Energy metabolism in heart failure. J. Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F., Akoglu B., Clausnitzer A., Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J. Nutr. 2001;131:2197–2203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- Yan Y., Yang J., Chen G., Mou Y., Zhao Y., Pan L., Ma C., Liu X., Wu C. Protection of resveratrol and its analogues against ethanol-induced oxidative DNA damage in human peripheral lymphocytes. Mutat. Res. 2011;721:171–177. doi: 10.1016/j.mrgentox.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Bao Y., Li J. Nuclear respiratory factor-1 is involved in mitochondrial dysfunction induced by benzo(a) pyrene in human bronchial epithelial cells. Basic Clin. Pharmacol. Toxicol. 2011;109:115–122. doi: 10.1111/j.1742-7843.2011.00697.x. [DOI] [PubMed] [Google Scholar]