Abstract
H2S is a signaling molecule associated with protection against vascular diseases, including atherosclerosis. This protection involves the stimulation of vasorelaxation, but other possible contributing mechanisms have not been extensively explored. In this study, we found that the vascular H2S-producing enzyme, cystathionine-γ-lyase (CSE), was down-regulated by oscillatory shear stress (OSS) among various vaso-regulators. Consistently, NaHS, an H2S donor, appeared to inhibit OSS-induced THP-1 cell adhesion. We also found that NaHS activated the nitric oxide (NO)-producing Akt/endothelial nitric oxide synthase (eNOS) signaling pathway in response to OSS, whereas NaHS had no effect on IκB, a well-known molecule regulating pro-inflammatory signaling pathways. Moreover, NaHS increased OSS-dependent eNOS expression and decreased expression of intercellular adhesion molecule-1 (ICAM-1). NG-nitro-L-arginine methyl ester (L-NAME), an eNOS inhibitor, abrogated the inhibitory effects of NaHS on OSS-induced endothelial ICAM-1 expression and monocyte adhesion to endothelial cells. These data suggest that down-regulation of CSE resulting in decreased levels of H2S is a key factor for OSS-associated atherogenesis and further suggest that regulation of H2S production can be a potential target for preventing cardiovascular diseases.
Keywords: cystathionine-γ-lyase, endothelial nitric oxide synthase, H2S, ICAM-1, oscillatory shear stress
INTRODUCTION
Hydrogen sulfide (H2S) is one of three gaseous mediators including nitric oxide (NO) and carbon monoxide (CO) that has anti-inflammatory effects by stimulating vaso-relaxation (Bhatia, 2005; Sun et al., 2011a; Wang, 2002). H2S is detected in tissues and biological fluids such as plasma and is synthesized in mammalian tissues through the enzymatic reactions of cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE). CBS is highly expressed in the brain, whereas CSE is mostly localized in the vascular system (Kamoun, 2004). Accumulating data suggest that H2S plays a role in protecting against cardiovascular diseases such as hypertension, blood vessel restenosis, and atherosclerosis (Mustafa et al., 2009) by inhibiting cellular inflammatory mechanisms (Bhatia, 2005). CSE knock-out and mutant mice studies have shown that a significant reduction in the amount of H2S in aorta and heart tissues stimulates hypertension in association with inhibition of endothelium-dependent vasorelaxation (Cai et al., 2007; Liu et al., 2011; Yang et al., 2008).
Although evidence supports the notion that H2S controls vascular functions, the detailed molecular mechanisms by which endothelial cells respond to H2S remain to be determined. To date, various mechanistic studies using endothelial cells have shown that H2S affects vascular functions e.g., vasodilation, resistance to hypoxia, anti-inflammation, and anti-atherogenesis through mechanisms involving ATP-sensitive potassium channel, protein kinase C, mitogen-activated protein kinases, Akt, and cytochrome c oxidase (Szabo et al., 2011).
Hemodynamic shear stress is generated by a frictional force between the endothelium and blood flow in blood vessels. Accordingly, shear stress varies depending on the shape of the vessel, flow rate, and direction of blood flow. Among these factors, the shape of the blood vessel is clinically emphasized, because atherosclerotic plaques frequently occur at branched vessels including the sinus or bulbs of the internal carotid artery (Cunningham et al., 2005; Malek et al., 1999). Oscillatory shear stress (OSS) is generated by low and disturbed flow occurring at the sinus or bulbs of the internal carotid artery and contributes to the development of atherosclerosis by stimulating inflammatory signaling mechanisms. In contrast, high and stable laminar shear stress (LSS) is generated in areas of greater curvature inside the aortic arch and has an anti-atherogenic effect by inhibiting inflammatory signaling (Davies, 1995; Malek et al., 1999).
Inflammatory responses at vessels are initiated by chemotaxis and monocyte binding to the endothelium (Dinarello, 2010). Monocyte binding to the endothelium is mediated by molecular interactions among cell adhesion molecules (CAMs) including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1) (Galkina et al., 2007). Expression of these molecules is upregulated by nuclear factor κB (NFκB) activation, consequently enhancing monocyte binding to the endothelium. Known pro-inflammatory agents such as lipopolysaccharide (LPS) and tumor necrosis factor-α (TNF-α) stimulate NFκB activation, leading to CAM overexpression (Roebuck, 1999). It has also been established that nitric oxide (NO) suppresses inflammatory processes by reducing ICAM-1 expression (Boger et al., 2000; Ramesh et al., 2011).
NO is plays an important role in shear stress-dependent vascular physiology and pathophysiology. However, the roles of H2S in shear stress-associated vascular functions remain unclear. In the present study, we performed a series of experiments to determine the role of H2S in OSS-dependent proinflammatory physiology and signaling events in the vascular system. To alter the cellular levels of H2S, we treated cells with a CSE inhibitor and an H2S donor. Then, we determined whether an alteration in the cellular level of H2S had an effect on cell signaling, CAM expression and THP-1 cell binding to endothelium in OSS-exposed bovine aortic endothelial cells (BAECs).
MATERIALS AND METHODS
Cell culture
BAECs, obtained from descending thoracic aortas, were cultured in DMEM (Wel GENE Inc., Korea) containing 20% fetal bovine serum (FBS, Wel GENE Inc.) and antibiotics (penicillin/streptomycin) at 37°C and 5% CO2. Cells from passage 5 to 10 were used. Mouse aortic endothelial cells (MAECs) were cultured in DMEM (Gibco, USA) containing 10% FBS, 1% bovine brain extract, 1% MEM non-essential amino acid solution (Gibco) and antibiotics (penicillin/streptomycin) at 37°C and 5% CO2. THP-1 cells were grown in RPMI 1640 medium (Wel GENE inc.) containing 10% FBS and antibiotics (streptomycin and penicillin) in a 5% CO2 incubator at 37°C.
Preparation of cell lysates
Cells were washed in ice-cold phosphate buffered saline (PBS) and placed in a lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM vanadate, 10% glycerol, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride) containing a protease inhibitor cocktail (Roche Molecular Biochemicals, USA). Soluble lysates were fractionated by centrifugation. Total protein amounts in the cell lysates were measured using a Bio-Rad DC assay kit (Bio-Rad, USA).
Shear stress exposure
BAECs, grown to 90% confluency in 10-cm plates, were exposed to LSS or OSS in the growth medium by rotating a Teflon cone (0.5° cone angle) as described previously (Sorescu et al., 2003). In brief, BAECs grown in 100-mm tissue culture dishes were positioned to a cone rotating with one directional 400 rpm (15 dyn/cm2) for LSS or with directional changes of flow at 1 Hz cycle (± 5 dyn/cm2) for OSS. OSS was generated by rotating the cone back and forth using a stepping servo motor and a computer program (DC Motor Company, USA). BAECs were pretreated with none, 1 mM NaHS alone, and 1 mM NaHS in the presence of 2 mM DL-propargyl glycine or 2.5 mM L-NAME for 0–20 h prior to OSS exposure. Then, cells were exposed to OSS for the indicated periods of time.
Western blotting
If necessary, BAECs were pretreated with 20 ng/ml TNF-α, 100 ng/ml lipopolysaccharide (LPS), or 1 mM H2O2 for at least 18 h and then lysed as described above. Proteins (25 μg) in the soluble lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Millipore, USA), and blotted with antibodies specific to IκB (Cell Signaling Technology, USA), Akt (Cell Signaling Technology), p-Akt (Cell Signaling Technology), pS1177-eNOS (Cell Signaling Technology), eNOS (Calbiochem, USA), ICAM-1 (Santa Cruz Biotechnology, USA), VCAM-1 (Santa Cruz Biotechnology), or PECAM-1 (Santa Cruz Biotechnology). Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies and developed using the enhanced chemiluminescence detection method (Amersham, USA).
Adhesion of THP-1 monocytes to BAECs
Serum-starved BAECs were exposed to OSS (5 dyn/cm2) for 24 h in the presence of none, 1 mM NaHS or 1 mM NaHS plus 2.5 mM L-NAME. THP-1 cells (5–6 × 106 cells/ml) labeled with 10 μM calcein AM (Sigma-Aldrich, USA) for 45 min (37°C) were added to BAEC. One hour after adding the THP-1 cells, unbound cells were washed away. Adherent THP-1 cells to BAEC were observed under a confocal laser scanning fluorescent microscope (Carl Zeiss-LSM710, Germany) and quantified by counting adhesive THP-1 cells in the same visual field with Alpha Ease FC software (Alpha Innotech Co. version 3.2.1.).
H2S Measurement
Serum-starved BAECs were exposed to OSS (5 dyn/cm2) for 24 h, trypsinized and harvested by centrigugation (3,000 rpm, 3 min). Then BAECs were thoroughly washed with PBS and lysed by ultrasonication. Soluble cell lysates were fractionated by centrifugation. Finally, H2S amounts in cell lysates were measured using an H2S Elisa kit (BlueGene Biotech, China).
NO Measurement
Intracellular NO amount was monitored via the fluorescent intensity of DAF-2 (Calbiochem). Cells were pre-incubated with a HEPES buffer (5 mmol/L HEPES, 140 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 5 mmol/L Glucose, pH7.4) containing 1 μmol/L Ca2+ ionophore, A23187 (Sigma) for 20 minutes. Then, cells were incubated at 37°C and 5% CO2 with 0.1 μmol/L DAF-2 for 15 min. Harvested cells were lysed by sonication. Supernatants were collected following centrifugation and DAF-2 fluorescence was measured by a spectrofluorophotometer (RF 5301PC Shimadzu) at excitation and emission of 495 and 515 nm (slit 10 nm), respectively. NO was calculated via DAF-2 fluorescence intensity (Kim et al., 2008).
Reverse transcription-polymerase chain reaction (RT-PCR)
Prior to OSS exposure, BAECs were pretreated with various concentrations of NaHS. Then, total RNA was extracted from BAECs and reversed transcribed, as described previously (Jung et al., 2010). After reverse transcription, cDNAs were amplified by PCR using the following conditions; denaturation at 98°C for 30 s, annealing at 60.5°C for 30 s, and extension at 72°C for 90 s. The primers for PCR were synthesized as following sequences:
-
ICAM-1: 5′-GCTGAACTGTAGCCGGAAAG-3′ (forward)
5′-ACCCAAGATGAGGGTCACAG-3′ (reverse)
-
GAPDH: 5′-CCAACGTGTCTGTTGTGGATCTGA-3′ (forward)
5′-CAACCTGGTCCTCAGTGTAGCCTA-3′ (reverse)
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) PCR product was used as an internal control.
Statistical analysis
Differences in data between control and a sample were analyzed using a Student’s t-test. The ICAM-1 protein levels and THP-1 cell adhesions among control and various sample groups were compared with one way ANOVA.
RESULTS
OSS markedly reduces CSE expression
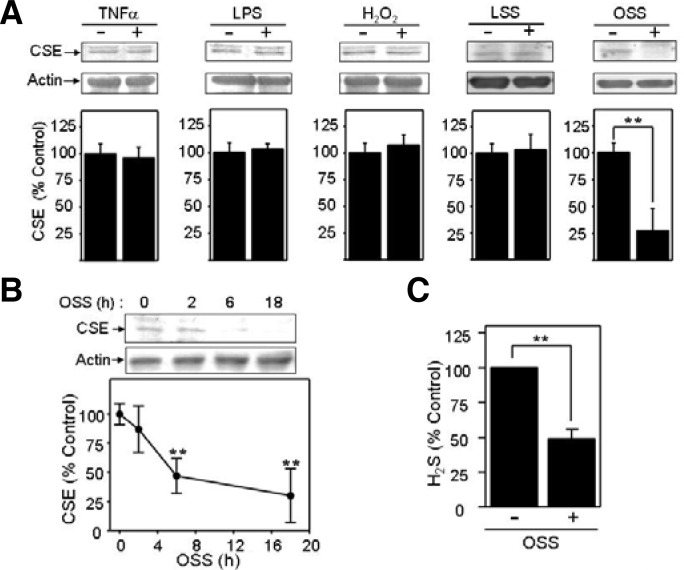
TNF-α and LPS are strong pro-inflammatory agents, whereas H2O2 is an oxidizing agent (Gloire et al., 2006). These molecules all have pro-atherogenic activity by different mechanisms (Gloire et al., 2006). Additionally, OSS is a well-known pro-atherogenic factor, acting at branched vessels (Cunningham et al., 2005; Malek et al., 1999), whereas LSS is known as an anti-atherogenic mechanical force (Davies, 1995; Malek et al., 1999). Little is known about whether or how H2S is associated with these atherogenic or anti-atherogenic stimuli. Accordingly, to determine (a) role(s) for H2S in the vascular physiology or pathophysiology, we first tested if the expression of CSE, an H2S-producing enzyme, was altered by proatherogenic stimuli, such as TNF-α, LPS, H2O2, LSS or OSS. As shown in Fig. 1A, OSS markedly reduced CSE expression by 4-fold, whereas other stimuli including TNF-α, LPS, H2O2, and LSS had no effect on CSE expression. We executed time-course experiments with OSS to confirm the inhibitory effect of OSS. As a result, we found that the amount of CSE protein was more decreased by the longer exposure of BAECs to OSS (Fig. 1B). The CSE protein amount was decreased by 50% at 6 h and 18 h of OSS exposure. These data indicate that OSS is a stimulus to decrease endothelial [H2S] through a reduction in CSE expression. Additionally, we measured H2S amounts of BAECs under static or OSS condition. As shown in Fig. 1C, the amount of H2S appeared to be decreased in BAECs exposed to OSS by ∼50% less than that in static control.
Fig. 1.
Oscillatory shear stress (OSS) specifically downregulates cystathionine-γ-lyase (CSE) in bovine aortic endothelial cells (BAECs). (A) Serum-starved BAECs were treated with 20 ng/ml tumor necrosis factor (TNF)-α, 100 ng/ml lipopolysaccharide (LPS), 1 mM H2O2, LSS (15 dyn/cm2), or OSS (± 5 dyn/cm2) for 18 h. Then, immunoblotting was performed by probing with antibodies specific to CSE (top panels). In the bottom panels, densitometry was performed and data were plotted as bar graphs (mean ± standard error [SE], n = 3). **P < 0.03 (B) Serum-starved BAECs were exposed to OSS (± 5 dyn/cm2) for 0–18 h. Immunoblots using CSE-specific antibody are shown in the top panel, and quantified data were plotted as the line graph (mean ± SE, n = 3). **P < 0.03 (C) H2S amounts were assessed in BAECs exposed to OSS or none using an H2S Elisa Kit. Data were plotted as bar graphs (mean ± SE, n = 6). **P < 0.03.
H2S inhibits THP-1 cell adhesion to BAECs in response to OSS
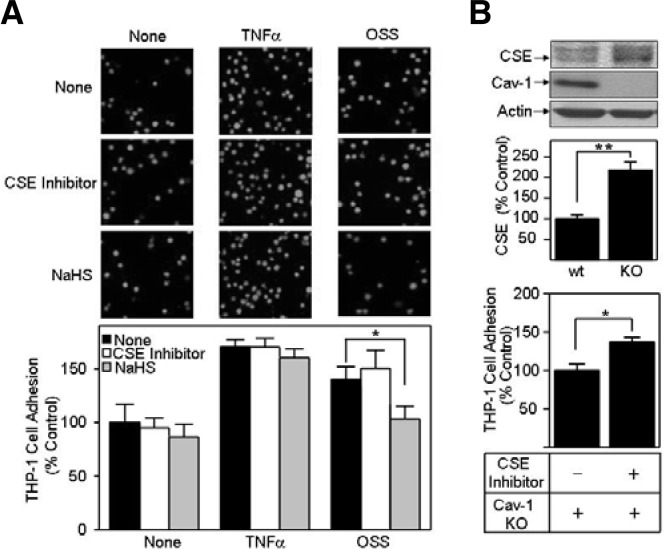
OSS is an established pro-atherogenic factor. We thereby conjectured that the inhibitory effect of OSS on both CSE expression and H2S production could be associated with a pro-atherogenic process. Additionally, early and crucial events among various atherosclerotic developmental stages include monocyte binding to endothelium (Dinarello, 2010). Accordingly, we first examined whether H2S had an effect on monocyte adhesion to endothelium. We used DL-propargyl glycine (a CSE inhibitor) and NaHS (an H2S donor) to reduce and elevate the cellular H2S concentration, respectively (Dam et al., 2012). As shown in Fig. 2A, NaHS decreased monocyte adhesion by OSS exposure, whereas DL-propargyl glycine appeared to have no effect. In contrast, both DL-propargyl glycine and NaHS had no effect on monocyte adhesion to TNF-α-stimulated BAECs (Fig. 2A). The inhibitory effect of NaHS on OSS-induced monocyte adhesion appeared to be caused by a recovery of H2S from OSS-induced H2S depletion. In contrast, no effect of H2S on the THP-1 cell binding to TNF-α-stimulated BAECs suggests that H2S is not linked to TNF-α-induced monocyte binding. Consequently, our data support the notion that H2S plays an important role in OSS-induced increase in monocyte binding by TNF-α-independent mechanisms.
Fig. 2.
NaHS inhibits oscillatory shear stress (OSS)-enhanced THP-1 cell adhesion to bovine aortic endothelial cells (BAECs). (A) BAECs were exposed to OSS or treated with tumor necrosis factor (TNF)-α (100 ng/ml) for 18 h in the presence of none, 2 mM DL-propargyl glycine (marked as cystathionine-γ-lyase [CSE] inhibitor) or 1 mM NaHS. Then, calcein AM-labeled THP-1 monocytes were added to shear-exposed BAECs and unbound THP-1 cells to BAECs were removed by washing the cells with PBS. Attached THP-1 cells were observed under a confocal microscope (top panel). THP-1 cell attachment was quantified by counting the number of cells in a visual field and the number of attached cells was plotted as bar graphs (bottom panel, mean ± standard error [SE], n = 3). *P < 0.05 (B) Proteins were obtained from wild-type (wt) and caveolin-1 knock-out (KO) mouse aortic endothelial cells (MAECs) and immunoblotted with antibodies specific for CSE, caveolin-1 (Cav-1), or actin (top panel). In the middle panel, CSE immunoblots were quantified and plotted as bar graphs (means ± SE, n = 3). **P < 0.03. In the bottom panel, THP-1 cell adhesion to Cav-1 KO MAECs was carried out as mentioned above. Data are plotted as bar graphs (mean ± SE, n = 3). *P < 0.05.
Our additional mechanism study confirms the crucial role of H2S in monocyte binding to endothelium. As shown in Fig. 2B, CSE was increased in caveolin-1 knocked-out MAECs. This finding is not surprising since a previous study showed that an administration of NO donor enhances CSE mRNA expression by 3-fold in arterial tissue (Liu et al., 2012). Given the inhibitory role of caveolin-1 for NO production (Mineo et al., 2012), our finding of CSE level in caveolin-1 knocked-out MAECs suggests that CSE expression is stimulated by NO but inhibited by caveolin-1. In addition, DL-propargyl glycine, a CSE inhibitor, significantly promoted THP-1 cell adhesion to caveolin-1 knocked-out MAECs, suggesting that elevated cellular H2S level inhibits monocyte binding to endothelium and further protects atherosclerotic development.
H2S reverses OSS-induced inactivation of Akt and eNOS
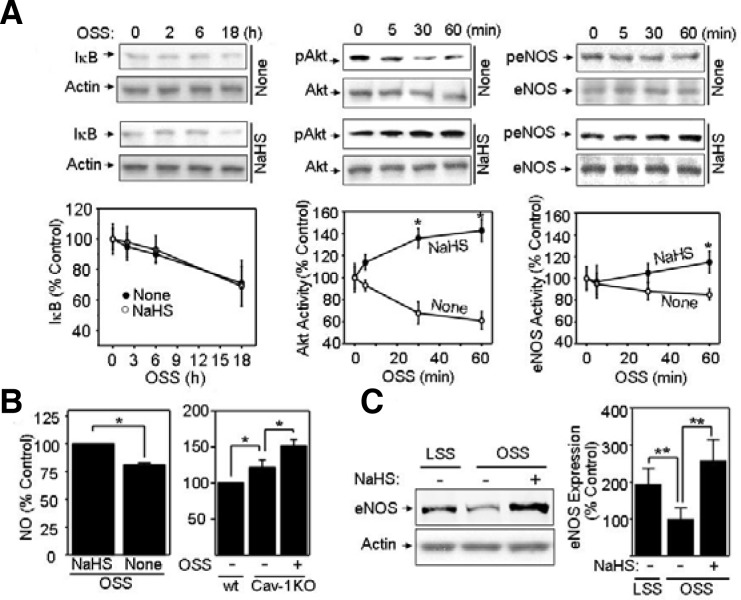
We found that OSS controls CSE expression and the monocyte adhesion to endothelium. Then, we investigated which signaling pathways are involved in OSS-induced monocyte binding to endothelium. NFκB is most likely to be a target signaling molecule, because it has been established that activating NFκB mediates CAM expression and monocyte binding to endothelium (Roebuck, 1999). Additionally, IκB inhibits NFκB by direct interaction with NFκB (Roebuck, 1999). Therefore, we determined if NaHS regulates OSS-controlled IκB stability (Roebuck, 1999). However, our data showed that NaHS had no effect on OSS-associated IκB stability (Fig. 3A), suggesting that H2S has no effect on the NFκB activation triggered by OSS. These findings suggest that H2S is involved in OSS-mediated atherogenesis by NFκB-independent mechanisms. We also found that H2S controls the Akt/eNOS pathways during OSS exposure. As shown in Fig. 3A, OSS substantially increased Akt phosphorylation in the presence of NaHS, whereas OSS decreased Akt phosphorylation in the absence of NaHS. Akt is a well-known upstream regulator of eNOS activation by stimulating eNOS phosphorylation (Go et al., 2001). Accordingly, we examined whether NaHS had an effect on eNOS activation by OSS. Consistent with Akt activation by NaHS, OSS increased eNOS activation only in the presence of NaHS. Taken together, NaHS-induced activation of Akt/eNOS during OSS exposure led us to hypothesize that H2S protects against atherogenic development via the Akt/eNOS-mediated production of NO. To test this hypothesis, we measured NO levels in none or NaHS-treated BAECs under acute (1 h) OSS exposure. As shown in Fig. 3B (left panel), NaHS treatment under OSS exposure appeared to enhance NO production by ∼130% compared to that in untreated BAECs. In MAECs, basal level of NO was higher in caveolin-1 knocked-out cells than wild-type cells (Fig. 3B, right panel). Interestingly, OSS stimulated NO production in caveolin-1 knocked-out MAECs (Fig. 3B, right panel). These data confirmed a stimulatory effect of H2S on eNOS activation under OSS exposure (Fig. 3A), because the H2S-producing CSE level was higher in caveolin-1 knocked-out MAECs than that in wildtype cells. In addition, NaHS up-regulated the eNOS expression under prolonged OSS exposure (Fig. 3C). Previously, Cai et al. (2004) reported that OSS upregulates the expression of eNOS mRNA by calcium/calmodulin-dependent protein kinase II (CaMK II)-controlled mechanism, whereas pathways for upregulation of eNOS by LSS are CaMK II-independent (Cai et al., 2004). As for eNOS enhancement level, our present result showed that LSS-induced eNOS expression was remarkably higher than eNOS expression by OSS (Fig. 3C). Moreover, our results showed that NaHS significantly enhanced the OSS-induced eNOS expression. Together, these data strongly support the hypothesis that NO plays a crucial role in a H2S-mediated reversal effect of OSS-elevated monocyte binding.
Fig. 3.
NaHS stimulates activation of Akt/endothelial nitric oxide synthase (eNOS) and enhances eNOS expression. (A) Bovine aortic endothelial cells (BAECs) pretreated with 1 mM NaHS or none were exposed to oscillatory shear stress (OSS) (± 5 dyn/cm2). Then, cells were analyzed for IκB, p-Akt, and pS1177-eNOS by Western blotting. The blots were probed with antibodies specific for IκB, total or p-Akt, and total or pS1177-eNOS. Quantification was performed using densitometry and data are plotted as line graphs shown in the lower panels (mean ± standard error [SE], n = 3). *P < 0.05 (B) BAECs were treated with none or 1 mM NaHS under acute (1 h) OSS (left panel). Wild-type (wt) or caveolin-1 knocked-out (Cav-1 KO) MAECs were exposed to none or OSS (right panel). Then, cells were pre-incubated with HEPES buffer containing 1 μmol/L Ca2+ ionophore, A23187, for 20 min and incubated with 0.1 μmol/L DAF-2 for 15 min. Finally, intracellular NO was measured as described in “Materials and Methods”. Data are plotted as bar graphs (mean ± SE, n = 3). *P < 0.05 (C) BAECs were pretreated with none or 1 mM NaHS followed by LSS or OSS for 24 h. Cell lysates were immunoblotted with anti-eNOS antibody. Equal amounts of protein loading were shown by probing the blot with anti-actin antibody. Quantification was performed using densitometry, and data are plotted as bar graphs (mean ± SE, n = 3). **P < 0.03.
H2S prevents OSS-stimulated inflammatory signaling via NO production
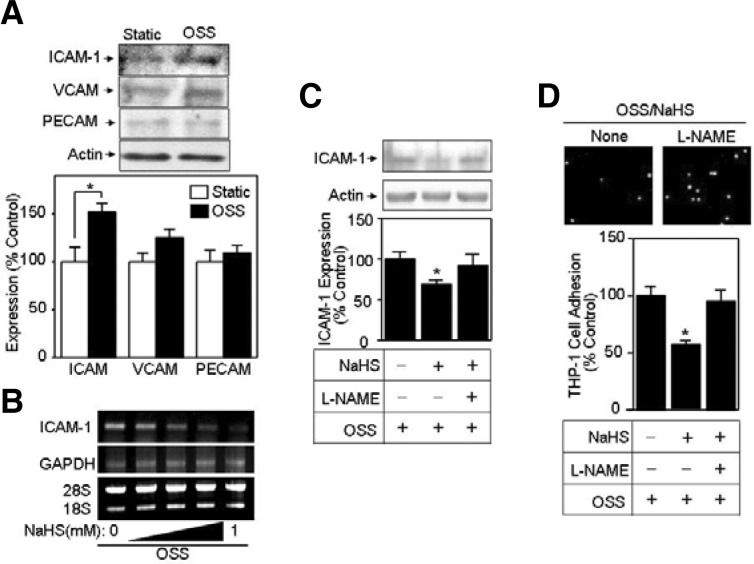
Prolonged OSS exposure plays an important role in atherogenesis-associated cellular processes involving expression of cell adhesion molecules, i.e., ICAM-1, VCAM-1, and PECAM-1 (Galkina et al., 2007). In the present study, we found that OSS significantly enhanced ICAM-1 expression when compared to VCAM-1 and PECAM-1 (Figs. 4A and 4B). Additionally, promotion of the endothelial NO level as a consequence of eNOS activation elicits a variety of vascular functions including inhibition of monocyte binding to endothelium (Boger et al., 2000; Ramesh et al., 2011). Accordingly, we also examined whether NO is involved in H2S/OSS-induced CAM expression and THP-1 cell adhesion. As shown in Fig. 4C, we found that the NaHS-reduced ICAM-1 amount was elevated by treatment with L-NAME, indicating that the depletion of NO is a critical factor for NaHS-induced reduction in ICAM-1 level. Next, we tested whether L-NAME had an effect on NaHS-reduced THP-1 cell adhesion during OSS exposure. As shown in Fig. 4D, L-NAME appeared to reverse the NaHS-induced reduction in THP-1 cell adhesion during OSS exposure. These data confirmed that NO plays a crucial role in H2S-controlled vascular function during OSS exposure.
Fig. 4.
NaHS blocks up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression and THP-1 cell adhesion, but L-NAME reverses the inhibitory effect of NaHS. (A) Serum-starved bovine aortic endothelial cells (BAECs) were exposed to oscillatory shear stress (OSS) (± 5 dyn/cm2) for 24 h. Then, immunoblotting was performed by probing with antibodies specific to various CAMs, such as ICAM-1, vascular cell adhesion molecule-1 (VCAM-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1). Bar graphs represent quantified Western data (mean ± standard error [SE], n = 3). *P < 0.05. (B) Confluent BAECs were incubated with various concentrations (0, 1, 10, 100, 1,000 μM) of NaHS under OSS. Then, after cells were lysed, mRNAs were extracted and used for RT-PCR. Finally, PCR products were separated by the 1% agarose gel electrophoresis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR product and rRNA (28S and 18S) were used as controls. (C, D) BAECs pretreated with none, 1 mM NaHS, or 1 mM NaHS plus 2.5 mM L-NAME were exposed to OSS (± 5 dyn/cm2). (C) Western blotting and quantification were performed as described in Fig. 3 (means ± SE, n = 3). *P < 0.05 (D) THP-1 cell adhesion and quantification were performed as described in Fig. 2 (mean ± SE, n = 3). *P < 0.05.
DISCUSSION
Although H2S acts as a vasoregulator controlling vessel diameter, little is known about its role in monocytic binding to endothelium, a pro-inflammatory response in the early stage of atherosclerotic development (Dinarello, 2010). Here, we found that OSS downregulated CSE expression, thereby reducing the vascular concentration of H2S. Consequently, due to the CSE decrease, OSS possibly removes the atheroprotective agent, particularly H2S, from endothelium. These observations provide an insight into the protective roles of H2S in OSS-exposed vessels. Moreover, we found that a CSE inhibitor promoted monocyte adhesion to CSE-overexpressed endothelial cells (see Fig. 2B), strongly supporting that deprivation of H2S from endothelium accelerates atherosclerosis. Similarly, the vascular protective role of H2S was confirmed by the reversal effect of adding NaHS, an H2S donor.
Our findings also demonstrate that H2S-dependent functions of OSS appear to be highly specific in the vascular system, because H2S had no effect on THP-1 cell adhesion induced by other pro-inflammatory agents including TNF-α. This OSS-specific vascular activity was also shown in our other findings that CSE expression was altered only by OSS, but not by other pro-inflammatory agents including TNF-α, LPS, or hydrogen peroxide. Taken together, our results support the postulation that H2S plays a specific inhibitory role in OSS-mediated atherogenesis.
Anti-atherogenic function of H2S in the vascular system is closely associated with its blood concentration. According to Lim et al.’s study, H2S concentration in mammalian tissues and blood is 1–160 μM (Lim et al., 2008). Another previous study showed that 50–1,000 μM H2S treatment gradually stimulated vasodilation in rat aorta without a toxic effect (Sun et al., 2011b). Therefore, our findings from the current study using 1 mM NaHS confirms beneficial roles of H2S in the vascular function and further provides an insight into pharmaceutical or therapeutic applications for vascular diseases.
Adhesion of THP-1 to endothelial cells is closely linked to inflammatory responses (Dinarello, 2010) and controlled by NFκB activation (Roebuck, 1999). However, our data show that the H2S-induced reduction of THP-1 cell adhesion was not mediated by NFκB activation, but by NO. This finding is consistent with previous reports showing that NO is an inhibitory agent for monocyte adhesion to endothelial cells (Boger et al., 2000; Ramesh et al., 2011). Furthermore, we determined that NO-induced suppression of ICAM-1 inhibited the OSS-induced monocyte binding. Although detailed mechanisms remain to be studied, NO-suppressed ICAM-1 expression was shown by NFκB-independent way in both in vitro endothelial cells and in vivo animal model (Glushakova et al., 2008). Possible mechanisms could involve multiple transcription initiation sites in the ICAM-1 promoter including binding sites for transcription factors, AP-1 and Ets (Roebuck et al., 1999). Accordingly, there is a possibility that NO has an effect on mitogen-activated protein (MAP) kinases, further suppressing ICAM-1 expression. Taken together, we hypothesize that H2S reverses OSS-induced depression of NO production, thereby reducing the ICAM-1 expression in endothelial cells and THP-1 cell adhesion to endothelial cells. THP-1 cell adhesion to endothelial cells possibly occurs through the interaction between ICAM-1 of endothelial cells and integrins (LFA-1 and Mac-1) of THP-1 cells (Roebuck et al., 1999). In summary, our data confirm the NO-mediated inhibition of inflammatory responses in the vascular system. First, NaHS appeared to stimulate the activation of NO-producing Akt/eNOS signaling. Second, NaHS markedly enhanced eNOS expression during OSS exposure. Third, L-NAME, an eNOS inhibitor, reversed NaHS-induced inhibition of ICAM-1 expression and THP-1 cell adhesion. Fourth, NO production was higher in caveolin-1 knocked-out cells than in wild-type cells (Fig. 3B left panel), whereas THP-1 cell adhesion was significantly decreased in caveolin-1 knocked-out cells (Manuscript submitted). These findings also suggest that both H2S and NO have inhibitory effect on THP-1 cell adhesion under OSS exposure.
Given that OSS accelerates atherosclerosis development (Malek et al., 1999), our finding that H2S appeared to reverse OSS-dependent signaling is therapeutically applicable. However, molecular and biochemical mechanisms by which H2S negatively regulates OSS-induced signaling are not completely understood. A previous study by Yan et al. suggested that H2S may modulate redox tone and redox signaling (Yan et al., 2006). We also observed that NaHS reduced cystine (oxidized disulfide form of cysteine) to cysteine by an acute reaction mechanism (data not shown). These results led us to postulate a reductant/antioxidant role of H2S in pro-inflammatory OSS signaling. As OSS but not LSS is closely associated with oxidative signaling, H2S may react with reactive oxygen/nitrogen species generated by OSS, resulting in a substantial decrease in oxidative and inflammatory cellular responses. However, the role of H2S in controlling redox signaling remains to be identified.
Controlling vascular doses of H2S will be an option to utilize H2S as a protective agent against vascular diseases. H2S is endogenously generated in biological systems via reactions catalyzed by CBS and CSE (Abe et al., 1996; Kamoun, 2004). CSE, dominantly expressed in the vascular system and associated with hypertension and vasorelaxation, is essential for H2S production in the vascular system. Regulation of CSE gene/protein expression under physiological and pathophysiological conditions is critical to alter vascular H2S level (Li et al., 2007; Xiaohui et al., 2005), further regulating vascular functions. Treatment with exogenous H2S donors affects endogenous H2S production by altering CSE expression and activity (Ma et al., 2011). Therefore, how to endogenously or exogenously control H2S concentrations should be more systematically investigated to develop therapeutics. Conclusively, the current study showed that exogenous adding H2S controlled OSS-stimulated signaling pathways in vascular endothelial cells and subsequently inhibited inflammatory responses to OSS. These findings confirm that H2S acts as a vascular health enhancer rather than a toxicant in the vascular system and provide an insight into novel medicinal applications for H2S, such as protecting mechanical force-associated vascular diseases.
Acknowledgments
This study was supported by research funds of Dankook University in 2010.
REFERENCES
- Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–606. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- Böger R.H., Bode-Böger S.M., Tsao P.S., Lin P.S., Chan J.R., Cooke J.P. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J. Am. Coll. Cardiol. 2000;36:2287–2295. doi: 10.1016/s0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- Cai H., McNally J.S., Weber M., Harrison D.G. Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J. Mol. Cell. Cardiol. 2004;37:121–125. doi: 10.1016/j.yjmcc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y. C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Cunningham K.S., Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- Dam V. P., Scott J.L., Ross A., Kinobe R.T. Inhibition of cystathionine gamma-lyase and the biosynthesis of endogenous hydrogen sulphide ameliorates gentamicin-induced nephrotoxicity. Eur. J. Pharmacol. 2012;685:165–173. doi: 10.1016/j.ejphar.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Anti-inflammatory agents:present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- Gloire G., Legrand-Poels S., Piette J. NF-kB activation by reactive oxygen species fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Glushakova O., Kosugi T., Roncal C., Mu W., Heinig M., Cirillo P., Sánchez-Lozada L.G., Johnson R.J., Nakagawa T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y.M., Boo Y.C., Park H., Maland M.C., Patel R., Pritchard K.A., Jr., Fujio Y., Walsh K., Darley-Usmar V., Jo H. Protein kinase B/Akt activates c-Jun NH(2)-terminal kinase by increasing NO production in response to shear stress. J. Appl. Physiol. 2001;91:1574–1581. doi: 10.1152/jappl.2001.91.4.1574. [DOI] [PubMed] [Google Scholar]
- Jung W.S., Cho J., In K., Kim J., Cho K.H., Park J.M., Moon S. K., Kim K.W., Park S.U., Pyee J., et al. Chunghyul-dan acts as an anti-inflammatory agent in endothelial cells by regulating gene expression. Animal Cells Syst. 2010;14:275–282. [Google Scholar]
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- Kim J., Park J., Choi S., Chi S.G., Mowbray A.L., Jo H., Park H. X-linked inhibitor of apoptosis protein is an important regulator of vascular endothelial growth factor-dependent bovine aortic endothelial cell survival. Circ. Res. 2008;102:896–904. doi: 10.1161/CIRCRESAHA.107.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Du J., Jin H., Tang X., Bu D., Tang C. The regulatory effect of endogenous hydrogen sulfide on pulmonary vascular structure and gasotransmitters in rats with high pulmonary blood flow. Life Sci. 2007;81:841–849. doi: 10.1016/j.lfs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Lim J.J., Liu Y.H., Khin E.S., Bian J.S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008;295:C1261–C1270. doi: 10.1152/ajpcell.00195.2008. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Yan C.D., Bian J.S. Hydrogen sulfide: a novel signaling molecule in the vascular system. J. Cardiovasc. Pharmacol. 2011;58:560–569. doi: 10.1097/FJC.0b013e31820eb7a1. [DOI] [PubMed] [Google Scholar]
- Liu W.L., Hu Y.F., Li T. Effect of nitric oxide on the preterm neonatal rabbit ductus arteriosus cyatathionine-gammalyase/hydrogen sulfide pathway. Zhonghua Er Ke Za Zhi. 2012;50:136–140. [PubMed] [Google Scholar]
- Ma K., Liu Y., Zhu Q., Liu C.H., Duan J.L., Tan B.K., Zhu Y.Z. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek A.M., Alper S.L., Izumo S. Hemodynamic shear stress and its role in atherosclerosis. J. Am. Med. Assoc. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Mineo C., Shaul P.W. Regulation of eNOS in caveolae. Adv. Exp. Med. Biol. 2012;729:51–62. doi: 10.1007/978-1-4614-1222-9_4. [DOI] [PubMed] [Google Scholar]
- Mustafa A.K., Gadalla M.M., Snyder S.H. Signaling by gasotransmitters. Sci. Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh S., Morrell C.N., Tarango C., Thomas G.D., Yuhanna I.S., Girardi G., Herz J., Urbanus R.T., de Groot P.G., Thorpe P.E., et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J. Clin. Invest. 2011;121:120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck K.A., Finnegan A.J. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. Leukoc. Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Sorescu G.P., Sykes M., Weiss D., Platt M.O., Saha A., Hwang J., Boyd N., Boo Y.C., Vega J.D., Taylor W.R., et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J. Biol. Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- Sun Y., Tang C.S., DU J.B., Jin H.F. Hydrogen sulfide and vascular relaxation. Chin. Med. J (Engl) 2011a;124:3816–3819. [PubMed] [Google Scholar]
- Sun Y., Tang C.S., Jin H.F., Du J.B. The vasorelaxing effect of hydrogen sulfide on isolated rat aortic rings versus pulmonary artery rings. Acta Pharmacol. Sin. 2011b;32:456–464. doi: 10.1038/aps.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G., Veres G., Radovits T., Gero D., Módis K., Miesel-Gröschel C., Horkay F., Karck M., Szabó C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide. 2011;25:201–210. doi: 10.1016/j.niox.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Xiaohui L., Junbao D., Lin S., Jian L., Xiuying T., Jianguang Q., Bing W., Hongfang J., Chaoshu T. Down-regulation of endogenous hydrogen sulfide pathway in pulmonary hypertension and pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Circ. J. 2005;69:1418–1424. doi: 10.1253/circj.69.1418. [DOI] [PubMed] [Google Scholar]
- Yan S.K., Chang T., Wang H., Wu L., Wang R., Meng Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006;351:485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]