Abstract
RANKL induces the formation of osteoclasts, which are responsible for bone resorption. Herein we investigate the role of the transmembrane adaptor proteins in RANKL-induced osteoclastogenesis. LAT positively regulates osteoclast differentiation and is up-regulated by RANKL via c-Fos and NFATc1, whereas LAB and LIME act as negative modulators of osteoclastogenesis. In addition, silencing of LAT by RNA interference or overexpression of a LAT dominant negative in bone marrow-derived macrophage cells attenuates RANKL-induced osteoclast formation. Furthermore, LAT is involved in RANKL-induced PLCγ activation and NFATc1 induction. Thus, our data suggest that LAT acts as a positive regulator of RANKL-induced osteoclastogenesis.
Keywords: gene expression, LAT, osteoclast, RANKL, transmembrane adaptor
INTRODUCTION
Bone is continuously remodeled by two processes, formation by osteoblasts and resorption by osteoclasts. These two processes are tightly regulated by various hormones and osteotropic factors. Excess bone resorption causes bone diseases such as osteoporosis and periodontitis. Macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) induce osteoclast differentiation from monocyte/macrophage lineage cells (Walsh et al., 2006). Binding of RANKL to its receptor, RANK, initiates signals mediated by TNF receptor-associated factor (TRAF) adaptors such as TRAF6. TRAF6 activates downstream signaling pathways, including NF-κB, c-Jun N-terminal kinase, p38 mitogen-activated protein (MAP) kinase, extracellular signal-related kinase (ERK), and AKT.
During osteoclastogenesis, RANKL strongly stimulates c-Fos gene induction. The binding of c-Fos to NFATc1 promoter region induces NFATc1 gene expression (Boyle et al., 2003; Takayanagi et al., 2002). RANKL also activates Ca2+ signaling through the activation of phospholipase Cγ (PLCγ). Calcineurin, which is activated by cellular calcium concentration, causes dephosphorylation of NFATc1 and induces translocation of NFATc1 into the nucleus. NFATc1 induces the expression of target genes by binding to the NFAT-binding sites in the promoter region of such genes as: tartrate-resistant acid phosphatase (TRAP), cathepsin K, and osteoclast-associated receptor (OSCAR), which are important for osteoclast differentiation or function (Kim et al., 2002; 2005a; Walsh et al., 2006).
Costimulatory signals mediated by the immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors, Fc receptor common γ chain (FcRγ) and DNAX-activating protein (DAP) 12, cooperate with RANKL, and their activation enhances the induction of NFATc1 via calcium signaling during osteoclastogenesis (Asagiri and Takayanagi, 2007; Koga et al., 2004). ITAM signaling is coupled to the downstream signaling pathway through Syk tyrosine kinase.
Immunoreceptors, including T-cell receptor (TCR), B-cell receptor (BCR), and Fc receptor (FcR), play important roles in the adaptive immune response. These receptors mediate signals through a complex of adaptor proteins, which contain ITAMs. Upon ligand binding to these receptors, the ITAM is phosphorylated by Src family kinases, leading to Src homology 2 (SH2)-domain mediated recruitment and activation of the Syk tyrosine kinase family and other adaptor molecules (Fodor et al., 2006; Humphrey et al., 2005). The activation of these molecules leads to downstream signaling that includes phospholipase Cγ (PLCγ) activation, calcium mobilization, and NFAT activation (Samelson, 2002).
Transmembrane adaptor proteins in membrane microdomains have been shown to be important regulators of immunoreceptor signaling (Horejsi, 2004). Several palmitoylated membrane-associated adaptor proteins have been identified: linker for activation of T cells (LAT) is indispensable for TCR signaling, linker for activation of B cells (LAB) appears to play a LAT-like-role in signaling initiated by BCR, and Lck-interacting molecule (LIME) moderately enhances TCR-mediated signals (Horejsi, 2004; Simeoni et al., 2004). Tyrosine phosphorylation of transmembrane adaptors mediated by Src or Syk kinases leads to recruitment and activation of other downstream signaling molecules. Although transmembrane adaptor proteins are known to play important roles in immunoreceptor signaling, their role in RANKL-induced osteoclastogenesis has not yet been described.
We report here that LAT is up-regulated by RANKL through c-Fos and NFATc1 and positively regulates RANKL-induced osteoclastogenesis. By contrast, LAB and LIME are down-regulated by RANKL and act as negative regulators of RANKL-mediated osteoclast formation. In addition, we demonstrate that LAT is involved in RANKL-induced PLCγ activation and NFATc1 induction. Therefore, our data suggest that LAT is a modulator of RANKL-induced osteoclastogenesis.
MATERIALS AND METHODS
Constructs
Expression constructs encoding c-Fos and constitutively active NFATc1 were described previously (Kim et al., 2009). Fulllength LAT, LAB, LIME, as well as the LAT transmembrane domain deletion mutant (LAT-ΔTM: amino acid 31–242) were amplified by RT-PCR and subcloned into pMX-IRES-EGFP. Site-directed PCR mutagenesis was used to introduce the missense changes S27A and S30A into the LAT sequence (LAT-C27/ 30A). For LAT knockdown experiments, siRNA oligonucleotides were generated by targeting 19-base sequences (LAT-Sh362: 5-AGA ATG TGG ATG CAG ATG A-3, LAT-Sh525: 5-CGT GAA TGT TCC TGA GAG T-3) of the mouse LAT gene. Sense and anti-sense oligonucleotides were annealed and ligated into the pSuper-retro vector.
Osteoclast formation and TRAP staining
Spleen cells from c-Fos-deficient mice and littermates were provided by Dr. Koichi Matsuo (Keio University, Japan) (Kim et al., 2009; Matsuo et al., 2004). Murine osteoclasts were prepared from bone marrow cells as previously described (Kim et al., 2007). Femurs were aseptically removed from 6–8-week-old ICR mice and bone marrow cells were flushed out with a sterile 21-gauge syringe. The cells were cultured in α-minimal essential medium (α-MEM; Hyclone, USA) containing 10% FBS (Hyclone) with M-CSF (30 ng/ml) for 3 days. Floating cells were removed and adherent cells (BMMs) were used as osteoclast precursors. To generate osteoclasts, BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 3 days. Cultured cells were fixed and stained for TRAP. TRAP-positive multinuclear cells (TRAP+ MNCs), containing more than three nuclei, were counted as osteoclasts.
Retroviral gene transduction
Retroviral infection was performed as previously described (Kim et al., 2010). To generate retroviral stock, recombinant plasmids and the parental pMX vector were transfected into the packaging cell line Plat E using FuGENE 6 (Roche Applied Sciences, USA). Plat E cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 2 × 105 cells per well were seeded in a 6-well plate. On the following day, the medium was changed to α-MEM containing 10% FBS. Viral supernatant was collected from cultured media 24–48 h after transfection. BMMs were incubated with viral supernatant for 8 h in the presence of 10 μg/ml polybrene (Sigma-Aldrich, USA). After removing the viral supernatant, BMMs were further cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 3 days.
Semiquantitative RT-PCR
RT-PCR was performed as previously described (Kim et al., 2005b). Primers used were the following: LAT sense, CAGAAT TCAGATGATGCCAACAGTG; LAT antisense, CTCCTGGGA GTTCACAGAGGCCAG; LAB sense, TTCTACA AAGGAAG TAACCAGGAG; LAB antisense, CCTCTTGGACTCTCGCCA CTGATG; LIME sense, GCTATGGGCACTGTGCACAGCCTG; LIME antisense, AAT AAGTGGCCTCAGAGCTAGTAG; LAX sense, AACTCAGAGCCCAGCACTCG GCAG; LAX antisense, ATGAACGGACACTCTATGCATCTG. Primer sequences of TRAP, OSCAR, NFATc1, HPRT, and GAPDH were previously described (Lee et al., 2006).
Immunoprecipitation and Western blot analysis
Immunoprecipitation and Western blot analyses were performed as previously described (Kim et al., 2008). Cells from transfected 293Ts, BMMs, or osteoclasts were harvested after washing with ice-cold PBS and then lysed in extraction buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 0.01% protease inhibitor cocktail). Samples immunoprecipitated with the indicated antibodies and whole cell lysates were subjected to SDS-PAGE and Western blotting. Primary antibodies included 4G10, OSCAR, actin, Flag, LAT, LAB, LIME, Syk, NFATc1, PLCγ2, IκB, and p-PLCγ2. Horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) were probed and developed with ECL solution (Amersham Biosciences). Signals were detected and analyzed by LAS3000 luminescent image analyzer (Fuji photo film Co.).
RESULTS
RANKL induces the expression of LAT via c-Fos and NFATc1
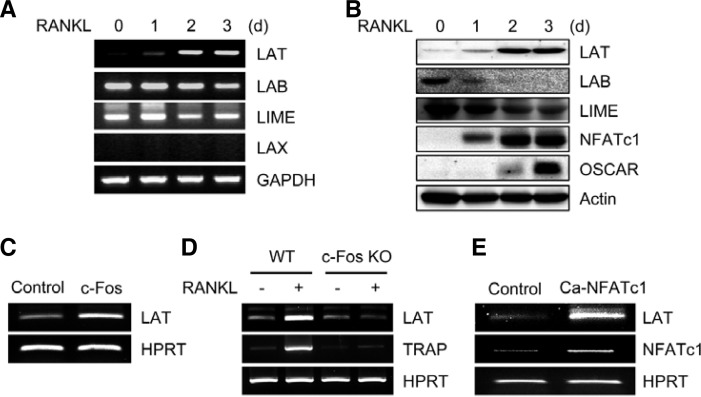
To investigate the role of membrane-associated adaptors in RANKL-induced osteoclastogenesis, we first examined the expression patterns of several transmembrane adaptors that are known to play an important role in lymphocyte activation. RANKL strongly induced LAT expression at both the mRNA and protein levels, whereas expression levels of LAB and LIME were down-regulated by RANKL during osteoclastogenesis. Expression of LAX was undetectable (Figs. 1A and 1B). Next, we examined whether RANKL-induced LAT expression is mediated by c-Fos and NFATc1, which are important transcription factors in osteoclast differentiation. Retroviral overexpression of c-Fos induced LAT expression in BMMs (Fig. 1C), whereas RANKL-mediated LAT induction was strongly attenuated in c-Fos-deficient cells (Fig. 1D). In addition, overexpression of constitutively active NFATc1 strongly induced LAT expression in BMMs (Fig. 1E). Together, these results suggest that RANKL induces LAT expression through c-Fos and NFATc1 during osteoclastogenesis.
Fig. 1.
Expression of transmembrane adaptor proteins during osteoclastogenesis. (A, B) BMMs were cultured for the indicated times in the presence of RANKL. RT-PCR (A) or Western blot analysis (B) was performed for the expression of the indicated genes. (C) BMMs were transduced with control (pMX-IRES-EGFP) or c-Fos retrovirus. (D) Spleen cells from wild type (WT) and c-Fos-deficient mice were cultured with M-CSF alone or M-CSF and RANKL for 4 days. (E) BMMs were transduced with control (pMX-IRES-EGFP) or constitutively active NFATc1 (Ca-NFATc1) retrovirus. (C-E) RT-PCR was performed to assess the expression of the indicated genes.
LAT positively regulates RANKL-induced osteoclastogenesis
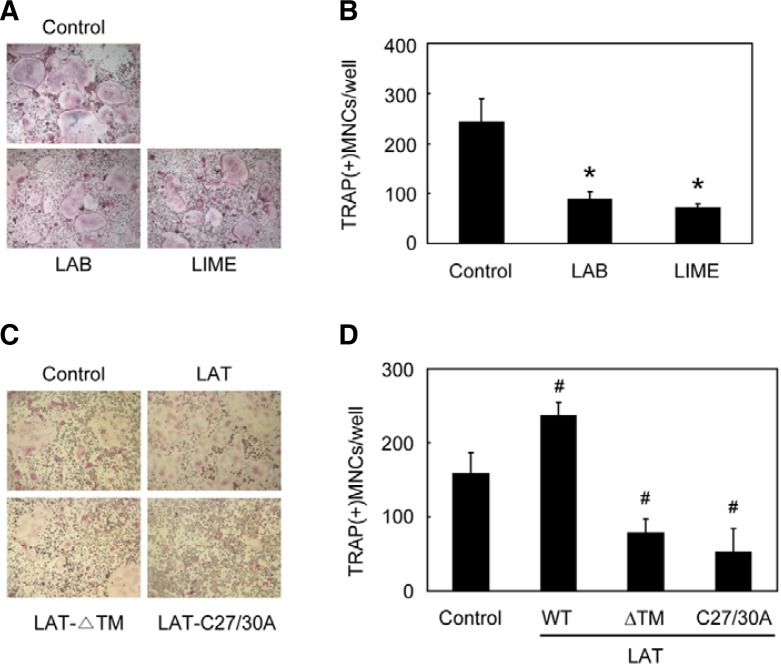
Next, we investigated the effect of transmembrane adaptors on RANKL-induced osteoclastogenesis by retroviral overexpression. Compared to the control vector, overexpression of LAB or LIME attenuated RANKL-induced osteoclastogenesis (Figs. 2A and 2B). Compared to the control vector, overexpression of wild type (WT) LAT in BMMs increased RANKL-induced osteoclast formation, whereas overexpression of each of two dominant negative forms of LAT, a transmembrane domain-deleted LAT (LAT-ΔTM) (Torgersen et al., 2001; Zhang et al., 1998) and a LAT mutant with cysteine-to-alanine mutations at residues 27 and 30 (LAT-C27/30A) (Zhang et al., 1998), led to significantly attenuated osteoclast formation (Figs. 2C and 2D). These results suggest that LAT positively regulates RANKL-induced osteoclastogenesis, whereas LAB and LIME negatively modulate this process.
Fig. 2.
Differential effects of transmembrane adaptor proteins on osteoclastogenesis. (A, B) BMMs were transduced with control (pMX-IRESEGFP), LAB, or LIME retrovirus, and were further cultured for 3 days with M-CSF and RANKL. (C, D) BMMs were transduced with control (pMX-IRES-EGFP), wild type LAT, transmembrane domain-deleted LAT (LAT-ΔTM), or a LAT mutant with cysteine-to-alanine mutations at amino acid residues 27 and 30 (LAT-C27/30A) retrovirus, and were further cultured for 3 days with M-CSF and RANKL. (A, C) cultured cells were fixed and stained for TRAP. (B, D) numbers of TRAP-positive multinucleated cells were counted. Data represent means ± SDs of triplicate samples. #P < 0.05, *P < 0.01 vs. control vector. Results are representative of at least three independent sets of similar experiments.
Silencing of LAT attenuates osteoclast differentiation and induction of NFATc1 and OSCAR
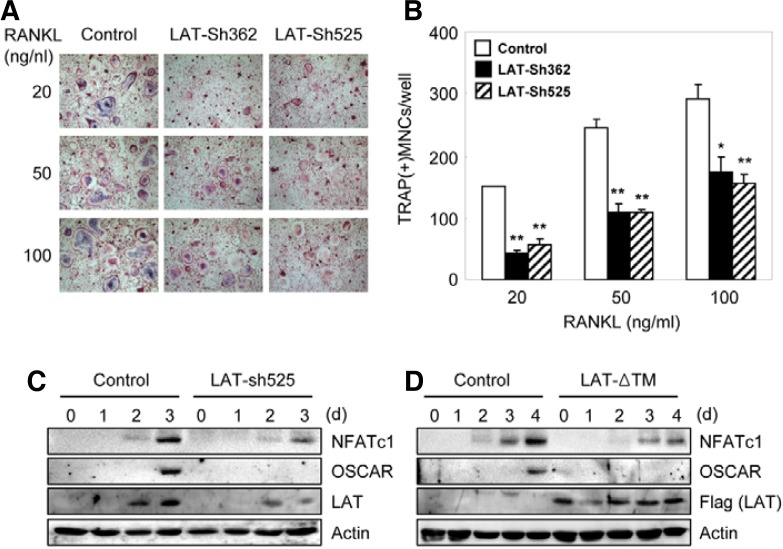
To investigate the role of endogenous LAT in osteoclastogenesis, we used LAT shRNA retroviral constructs (LAT-Sh362 and LAT-Sh525) to suppress the expression of LAT. Both LAT shRNA constructs significantly inhibited RANKL-induced osteoclast formation (Figs. 3A and 3B). The overexpression of LAT-Sh525 or LAT-ΔTM strongly attenuated RANKL-mediated induction of NFATc1 and OSCAR (Figs. 3C and 3D), which are important for osteoclast differentiation. These results suggest that RANKL-induced induction of NFATc1 and OSCAR as well as RANKL-induced osteoclastogenesis is at least, in part, dependent upon LAT.
Fig. 3.
Silencing of LAT attenuates osteoclast formation and induction of NFATc1 and OSCAR. (A, B) BMMs were transduced with control (pSuper-Retro) or pSuper-Retro LAT shRNA-expressing (LAT-Sh362 and LAT-Sh525) retrovirus, respectively. Cells were cultured with MCSF and RANKL. (A) Cultured cells were fixed and stained for TRAP. (B) Numbers of TRAP-positive multinucleated cells were counted. Data represent means ± SDs of triplicate samples. *P < 0.01, **P < 0.001 vs. control vector. Results are representative of at least three independent sets of similar experiments. (C) BMMs were transduced with control (pSuper-Retro) or pSuper-Retro LAT shRNA-expressing (LAT-Sh525) retrovirus, respectively. Cells were cultured with M-CSF and RANKL f or the indicated times. Lysates were pro bed with NFATc1, OSCAR, LAT, and actin antibodies. (D) BMMs were transduced with control (pMX-IRES-EGFP) or pMX-transmembrane domain-deleted LAT (LAT-ΔTM)-IRES-EGFP retrovirus, and were further cultured with M-CSF and RANKL for the indicated times. Lysates were probed with NFATc1, OSCAR, Flag (for exogenous LAT), and actin antibodies.
LAT is involved in RANKL-induced PLCγ2 activation
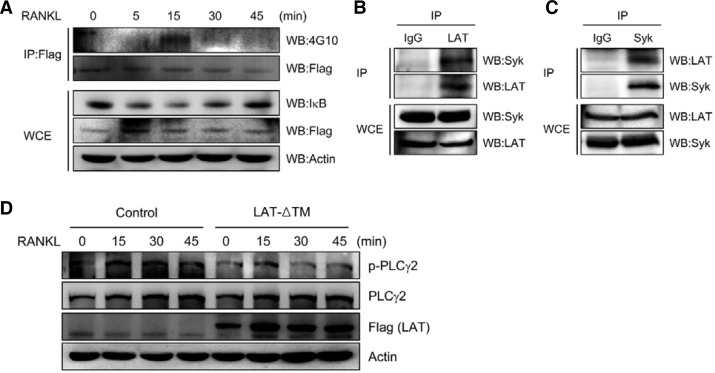
To investigate whether LAT is involved in RANKL-mediated signaling cascades, we tested whether RANKL is able to phosphorylate LAT. BMMs were transduced with Flag-tagged LAT and treated with RANKL for the indicated times. Whole cell extracts were immunoprecipitated with anti-Flag followed by Western blotting with anti-phosphotyrosine antibody 4G10. RANKL treatment resulted in LAT phosphorylation in BMMs (Fig. 4A, upper panel). As expected, upon RANKL treatment we also observed IκB degradation, which is known to lead to NF-κB activation (Fig. 4A, lower panel).
Fig. 4.
LAT is involved in RANKL-induced PLCγ activation. (A) BMMs transduced with Flag-tagged LAT were stimulated with RANKL for the indicated times. Lysates were immunoprecipitated (IP) with anti-Flag antibody. Immunoprecipitated samples (upper panel) or whole cell extracts (WCE; lower panel) were subjected to Western blotting for detection of phosphotyrosine (4G10), LAT (Flag), IκB, and actin. (B, C) Lysates from pre-osteoclasts were immunoprecipitated (IP) with anti-LAT (B) or anti-Syk (C) antibodies. Immunoprecipitated samples (upper panel) or whole cell extracts (WCE; lower panel) were subjected to Western blotting for detection of Syk and LAT. (D) BMMs were transduced with control (pMX-IRES-EGFP) or LAT-ΔTM retrovirus, and were further cultured for 2 days with M-CSF and RANKL. The starved preosteoclasts were stimulated with RANKL for the indicated times. Lysates were probed with anti-phospho-PLCγ2, anti-PLCγ2, anti-Flag (for LAT), and anti-actin.
It has been shown that activated ITAM adaptors signal via phosphorylation of Syk kinase and PLCγ2 (Humphrey et al., 2005; Mao et al., 2006). To examine the interaction of LAT with Syk in osteoclasts, preosteoclast cell lysates were immunoprecipitated with anti-LAT or anti-Syk followed by Western blot analysis. Our data show that LAT can interact with Syk in preosteoclasts (Figs. 4B and 4C). In addition, RANKL-induced PLCγ2 activation, as evidenced by phosphorylation, was attenuated by overexpression of dominant negative LAT (Fig. 4D). Collectively, these data suggest that LAT is involved in RANKL-mediated signaling cascades during osteoclastogenesis.
DISCUSSION
ITAM-containing adaptor proteins and their receptors are critical regulators of the immune system and bone remodeling. Mice deficient in both DAP12 and FcRγ are severely osteopetrotic due to defective osteoclastogenesis (Koga et al., 2004), suggesting that costimulatory signaling is required for RANKL-induced osteoclast differentiation. Activation of ITAM adaptors induces phosphorylation of Syk and PLCγ2 (Mao et al., 2006; Mocsai et al., 2004), resulting in activation of NFATc1, a master regulator of osteoclastogenesis. In this study, we demonstrate that LAT is involved in RANKL-mediated PLCγ2 activation and NFATc1 induction during osteoclast differentiation. Our data show that RANKL induced LAT expression via c-Fos and NFATc1. In addition, LAT positively regulated RANKL-mediated NFATc1 induction. These data suggest that a positive feedback circuit of RANKL-NFATc1-LAT is necessary for efficient differentiation of osteoclasts.
Transmembrane adaptor proteins, including LAT, LAB, and LIME, are similar in structure. Each contains a short N-terminal extracellular peptide, a transmembrane domain followed by a palmitoylation motif, and a cytoplasmic domain containing tyrosine residues that are phosphorylation targets for Src or Syk family members. Despite these structural similarities, our data demonstrate that these molecules vary in their expression patterns and roles in osteoclastogenesis. In contrast to LAT, we found that LAB and LIME are down-regulated during RANKL-induced osteoclastogenesis. In further contrast, overexpression of LAB or LIME in BMMs attenuates rather than enhances osteoclast formation. Consistent with our results, LAT and LAB displayed distinct functions in mast cells (Saitoh et al., 2000; Volna et al., 2004; Zhu et al., 2004). In these cells, LAT overexpression was shown to induce FcεRI-mediated phosphorylation of PLCγ, MAPK activation, Ca2+ flux, and degranulation. By contrast, LAB deficiency enhances these responses in mast cells (Volna et al., 2004), suggesting that LAB negatively regulates mast cell signaling and function. Upon BCR or FcR engagement, LAB is phosphorylated and then associates with the cytoplasmic signaling molecules Grb2, Gab1, and c-Cbl, but not with PLCγ or SLP76, both of which are key components of LAT signaling in the context of TCR activation (Brdicka et al., 2002; Horejsi et al., 2004). These data suggest that LAB-mediated signaling pathways are different from LAT-induced signaling cascades. Of note, Volna et al. reported that LAT and LAB are located in distinct, nonoverlapping regions of the plasma membrane in both resting and activated cells, although both LAB and LAT are considered to be localized in membrane rafts (Volna et al., 2004). In aggregate, these data suggest that (Volna et al., 2004). In aggregate, these data suggest that LAB is functionally and topographically distinct from LAT.
In this study, we have provided further insight into the specific role of LAT in RANKL-induced osteoclastogenesis. We demonstrate that LAT positively regulates RANKL-induced osteoclastogenesis via PLCγ activation and NFATc1 induction. Understanding the molecular mechanisms underlying LAT regulation through these and future studies holds promise for the development of novel therapeutic approaches to a variety of bone diseases.
Acknowledgments
We thank Aeran Ko for technical assistance. This work was supported by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (Grant 2011-0013512 to K.K.) and the Korean Science and Engineering Foundation (KOSEF) National Research Laboratory Program funded by the Korean government (MEST) (Grant R0A-2007-000-20025-0 to N.K.).
REFERENCES
- Asagiri M., Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Brdicka T., Imrich M., Angelisova P., Brdickova N., Horvath O., Spicka J., Hilgert I., Luskova P., Draber P., Novak P., et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S., Jakus Z., Mocsai A. ITAM-based signaling beyond the adaptive immune response. Immunol. Lett. 2006;104:29–37. doi: 10.1016/j.imlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Horejsi V. Transmembrane adaptor proteins in membrane microdomains: important regulators of immunoreceptor signaling. Immunol. Lett. 2004;92:43–49. doi: 10.1016/j.imlet.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Horejsi V., Zhang W., Schraven B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. 2004;4:603–616. doi: 10.1038/nri1414. [DOI] [PubMed] [Google Scholar]
- Humphrey M.B., Lanier L.L., Nakamura M.C. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Lett. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- Kim N., Takami M., Rho J., Josien R., Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim J.H., Lee J., Jin H.M., Lee S.H., Fisher D.E., Kook H., Kim K.K., Choi Y., Kim N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005a;280:35209–35216. doi: 10.1074/jbc.M505815200. [DOI] [PubMed] [Google Scholar]
- Kim N., Kadono Y., Takami M., Lee J., Lee S.H., Okada F., Kim J.H., Kobayashi T., Odgren P.R., Nakano H., et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J. Exp. Med. 2005b;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim J.H., Lee J., Jin H.M., Kook H., Kim K.K., Lee S.Y., Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- Kim K., Lee S.H., Kim J.H., Choi Y., Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol. Endocrinol. 2008;22:176–185. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Jin H.M., Kim K., Song I., Youn B.U., Matsuo K., Kim N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim K., Jin H.M., Song I., Youn B.U., Lee S.H., Choi Y., Kim N. Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J. Biol. Chem. 2010;285:5224–5231. doi: 10.1074/jbc.M109.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., Iwata T., Ohnishi H., Matozaki T., Kodama T., et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim K., Kim J.H., Jin H.M., Choi H.K., Lee S.H., Kook H., Kim K.K., Yokota Y., Lee S.Y., et al. Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood. 2006;107:2686–2693. doi: 10.1182/blood-2005-07-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D., Epple H., Uthgenannt B., Novack D.V., Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Galson D.L., Zhao C., Peng L., Laplace C., Wang K.Z., Bachler M.A., Amano H., Aburatani H., Ishikawa H., et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- Mocsai A., Humphrey M.B., Van Ziffle J.A., Hu Y., Burghardt A., Spusta S.C., Majumdar S., Lanier L.L., Lowell C.A., Nakamura M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S., Arudchandran R., Manetz T.S., Zhang W., Sommers C.L., Love P.E., Rivera J., Samelson L.E. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- Samelson L.E. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- Simeoni L., Kliche S., Lindquist J., Schraven B. Adaptors and linkers in T and B cells. Curr. Opin. Immunol. 2004;16:304–313. doi: 10.1016/j.coi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Torgersen K.M., Vaage J.T., Rolstad B., Tasken K. A soluble LAT deletion mutant inhibits T-cell activation: reduced recruitment of signalling molecules to glycolipid-enriched microdomains. Cell. Signal. 2001;13:213–220. doi: 10.1016/s0898-6568(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Volna P., Lebduska P., Draberova L., Simova S., Heneberg P., Boubelik M., Bugajev V., Malissen B., Wilson B.S., Horejsi V., et al. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 2004;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.C., Kim N., Kadono Y., Rho J., Lee S.Y., Lorenzo J., Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Zhu M., Liu Y., Koonpaew S., Granillo O., Zhang W. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]