Abstract
Nicotine, a major component of cigarette smoking, is the important risk factor for the development of periodontal disease. However, the mechanisms that underlie the cytotoxicity of nicotine in human periodontal ligament stem cells (PDLSCs) are largely unknown. Thus, the purpose of this study was to determine the cytotoxic effect of nicotine by means of nicotinic acetylcholine receptor (nAChR) activation in PDLSCs. We first detected α7 and β4 nAChRs in PDLSCs. The gene expressions of α7 and β4 nAChR were increased by nicotine administration. Nicotine significantly decreased cell viability at a concentration higher than 10−5 M. DNA fragmentation was also detected at high doses of nicotine treatment. Moreover, the detection of sub G1 phase and TUNEL assay demonstrated that nicotine significantly induced apoptotic cell death at 10−2 M concentration. Western blot analysis confirmed that p53 proteins were phosphorylated by nicotine. Under various doses of nicotine, a decrease in the anti-apoptotic protein Bcl-2, but an increase in p53 and cleaved caspase-3 protein levels, was detected in a dose-dependent manner. However, the apoptotic effect of nicotine was inhibited by the pretreatment of α-bungarotoxin, a selective α7 nAChR antagonist or mecamylamine, a non-selective nAChR antagonist. Finally, increases in the subG1 phase and DNA fragmentation by nicotine was attenuated by each nAChR antagonist. Collectively, the presence of α7 and β4 nAChRs in PDLSCs supports a key role of nAChRs in the modulation of nicotine-induced apoptosis.
Keywords: apoptosis, nAChRs, nicotine, periodontal disease, periodontal ligament stem cells
INTRODUCTION
Periodontal disease with clinical symptoms representing loss of attachment, alveolar bone, and tooth has been currently treated by the use of bone grafts or substitutes, barrier membrane, and bioactivefactors (Pihlstrom et al., 2005). However, only a few of therapeutic methods have been considered as regenerative techniques, regardless of their unpredictable outcomes (Bosshardt and Sculean, 2009; Garraway et al., 1998; Karring et al., 1980). The fundamental concept of periodontal therapy is to stimulate or restore specific cell populations of periodontal ligament (PDL) that would result in effective periodontal regeneration (Isaka et al., 2001; McCulloch and Bordin, 1991). Recently, several studies identified a cell population with mesenchymal stem cells (MSCs) properties from human periodontal ligament (PDLSCs) capable of self renewal, clonal expansion, and multiple lineage differentiation (Seo et al., 2004). These researches have led to tissue engineering studies in the formation of PDL-like structures to assess their potential in pre-clinical applications (Liu et al., 2008; Shi et al., 2005). However, a greater understanding of the behavior of these dental stem cells under various patho-physiological conditions is a prerequisite to understand the extent of their efficacy for regenerative medicine.
Chronic exposure to nicotine typically involves the development of destructive periodontal disease (Bergstrϕm, 2004; Ojima et al., 2006). A potential mechanism for this phenomenon can be considered as that whereby nicotine binds to its own receptors with functional consequences of the nicotine-related cell damage in periodontal tissues. Moreover, the diverse effect of nicotine on non-neuronal cells through nAChRs has been recently studied with considerable attention. Nicotinic acetylcholine receptors (nAChRs) belong to the super family of ligand-gated ion channels and are multi-subunit proteins of neuromuscular and neuronal origins (Picciotto et al., 2001). A functional nAChR consists of five subunits which may differ (certain combinations of α1–9 and β1–4, γ, δ, and ε subunits) or be identical (α7–9) subunits and diverse subunit composition of nAChRs exhibit very different pharmacological and functional properties in various systems (Portugal and Gould, 2008). Recent studies have demonstrated that nicotinic receptors are expressed in undifferentiated and differentiating non-neuronal cells including embryonic stem cells, immune cells, oral epithelium and periodontal tissues (Chernyavsky et al., 2005; Resende et al., 2008; Wang et al., 2010; Wessler and Kirkpatrick, 2008), suggesting that although nAChRs primarily function as ligand-gated ion channels across synapses, they also influence other cellular activities such as cell to cell communication, survival, and apoptosis in various non-neuronal tissues (Gotti and Clementi, 2004).
To date, the biology of PDLSCs has not been fully characterized and the function of these stem cells during nicotine-related periodontal disease remains unclear. Moreover, despite the ubiquitous dispersion of the various nAChRsin tissues, subunit composition of nAChRs and their mechanisms in PDLSCs are not well understood.
Thus, the present study demonstrates (i) the effects of nicotine on apoptosis in PDLSCs, (ii) whether nicotine acts through nAChRs, and (iii) which distinct nicotine receptor subunits may mediate nicotine effects. The present study suggests not only the stem cell model system for periodontal disease derived from exposure to nicotine through cigarette smoking or nicotine supplements but also a preventive strategy for nicotine toxicity by identification of the mechanisms leading to apoptosis of PDLSCs.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS) was purchased from Gibco-BRL (USA). Nicotine, α-bungarotoxin (α-BTX), and mecamylamine were obtained from the Sigma Chemical Company (USA). The Bcl2, p53, cleaved capase3, p-p53, β-actin, goat-anti mouse, and goat-anti rabbit antibodies were supplied by Santa Cruz Biotechnology (USA). Unless otherwise specified, chemicals and laboratory wares were purchased from Sigma Chemical Company and Falcon Labware (Becton-Dickinson, USA), respectively.
Periodontal ligament stem cell culture
Healthy human molars were collected from 10 young adults (15–30 year olds) under approved guidelines by the Review Board of Kyung Hee University. Written informed consent was obtained from all donors. Explants, obtained from the middle third of the root, were cultured in α-MEM (Invitrogen, USA) containing 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml) (SIGMA, USA) according to a previously described method (Seo et al., 2004). After 2 passages, the cells were subjected to magnetic isolation with antibodies to detect STRO-1 (the mesenchymal stem cell marker) antigen (Millipore, USA) and magnetic beads (MiltenyiBiotec, Germany). The resulting cell populations STRO-1(+) were cultured in α-MEM plus 10% FBS at 37°C with a humidified gas mixture of 5% CO2/95% air. All the experiments were carried out with passage 4–7 cells.
Cell viability analysis
Cytotoxicity was evaluated by using the MTT [3-(4,5-dime-thylthiazol-2yl)-2.5-diphenyltetrazolium bromide; Sigma] (5 mg/ml in PBS as stock solution) assay, which was based on the reduction of the dye MTT to formazan crystals, an insoluble intracellular blue product, by cellular dehydrogenases (Huang et al., 2004). Briefly, cells were seeded on 96-well culture plates at a density of 1 × 105 cells/ml and treated with various concentrations of nicotine (0–10−2 M) for 24 or 48 h. After exposure, 20 μl MTT was added to the cells in each well and incubated at 37°C for 4 h; then, the medium and MTT were removed and 150 μl dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The plates were agitated to ensure complete dissolution of the purple formazan crystals and the optical density was measured at the wavelength of 492 nm using an ELISA reader (Packard Instrument Co., USA).
DNA fragmentation assay
Cells were treated with various concentrations of nicotine (0–10−2 M) for 48 h; then, the level of DNA fragmentation of apoptotic cells was determined by ApoTarget™ Quick Apoptotic DNA Ladder Detection Kit (Invitrogen, USA). Briefly, the cells were lysed with 35 μl TE Lysis Buffer. Five μl Enzyme A Solution was added to the crude lysate and the samples were then incubated at 37°C in a water bath for 10 min. Another 5 μl Enzyme B Solution was treated to each sample and the samples were then incubated at 50°C for 30 min until the lysate became clear. After the procedure of precipitation, the DNA was resuspended in 30 μl of DNA suspension buffer. The extracted DNA was loaded to 25 μl of each sample onto a 1.2% agarose gel and the DNA fragmentation was visualized by transillumination with UV light and then photographed.
APO-BrdU TUNEL assays
Apoptotic cells were detected by TUNEL labeling using the APO-BrdUTUNEL Assay Kit (Molecular Probes Inc, USA) according to the manufacturer’s protocol. Briefly, after the nicotine treatments, the cells were centrifuged for 10 min at 4°C at 1,500 × g, and washed in cold PBS. Cell concentration was adjusted to 2 × 105 cells/μl in 1 ml PBS. The cells were then fixed in 1 ml 1% (w/v) paraformaldehyde for 15 min on ice, permeabilized with 70% ethanol for 30 min on ice, and incubated with 50 μl DNA-labeling solution containing TdT enzyme and Br-dUTP at 37°C for 60 min. After the labeling reaction, the cells were washed and stained with fluorescein-labeled anti-BrdU antibody for 30 min and treated with DAPI (4′,6-diamidino-2-phenylindole) and RNAse A. APO-BrdU-positive cells were analyzed using a fluorescent microscope (Olympus, USA).
Cell cycle analysis
Cells (106 cells per milliliter) were resuspended in PBS containing 0.1% BSA and were fixed for at least 1 h with 70% (w/v) ice-cold ethanol at 4°C. The cells were washed with PBS and resuspended in 1 ml of PBS containing 250 μg/ml propidium iodide (PI) and 500 U/mlRNase A for 30 min at 37°C. The DNA content was evaluated by a fluorescence-activated cell sorting (FACS) scan flow cytometer (Becton-Dickinson, USA) after staining. For cell cycle analysis, only the signals from single cells were considered (10,000 cells/sample). Finally, the analysis of the DNA content of the cells at different phases of the cell cycle was performed by using Cell Quest Software (Becton Dickinson).
RNA isolation and reverse transcription-polymerase chain reaction
The total RNA was extracted from the cells using TRIzol reagent (Invitrogen, USA), following the manufacturer’s instructions. Reverse transcription was carried out using 3 μg of RNA using a reverse transcription system kit (Bioneer, Korea) with the oligo (dT18) primers. The reverse transcription (RT) products (50 ng) were then amplified using a polymerase chain reaction (PCR) kit (Bioneer) under the following conditions: denaturation at 94°C for 5 min followed by 30 cycles at 94°C for 45 s, 55°C for 30 s, and 72°C for 30 s followed by 5 min of extension at 72°C. Each primer of nAChR is listed in Table 1.
Table 1.
List of primers of the nAChR subunit genes
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Size (bp) |
|---|---|---|---|
| α1 | GGCTCCGAACATGAGACCCG | GCGTGACTTTGGGAGTTCCTTT | 234 |
| α2 | TGACCCACATGACCAAGGCCCA | TGGTGAACAGCAGGTACTCGCC | 536 |
| α3 | CCGAGGCCCCTCTACGGT | CACACAGCTTAGTGCTTA | 428 |
| α4 | CCTCGGCCTGTCCATCGCTCA | AAGACGGTGAGCGACAGCAGC | 668 |
| α5 | CCATCATCTTCAAAAGTCATA | CCCATTTATAAATAACAGGAAC | 453 |
| α6 | GCTGTGCAACTGAGGA | AAGACGGGTGAGC | 921 |
| α7 | CCCGGCAAGAGGAGTGAAAGGT | CCGGGCCTCTTCATTCGCAG | 442 |
| β1 | GTGTCAGGGTCAGGGTTGGT | TGCGGCGGATGATGAGGTAG | 579 |
| β2 | GTGTCCTTCTATTCCAAT | AATGATGAAGTCATACGT | 305 |
| β3 | AAGGGGAACAGAAGGGACGG | GAAGCAGTACGTCGCGGACG | 461 |
| β4 | CAACAACCTGATCCGCCCAGC | GAAGGGAAAGTACTTCACCTC | 366 |
Real time RT-PCR
The real-time quantification of RNA targets was then performed in the Rotor-Gene 2000 real-time thermal cycling system (Corbett Research, Australia) using a QuantiTect SYBR Green RTPCR kit (QIAGEN, USA). The reaction mixture (20 μl) contained 200 ng of the total RNA, 0.5 μM of each primer, the appropriate amounts of enzymes, and fluorescent dyes, as recommended by the supplier. The Rotor-Gene 2000 cycler was programmed as follows: 30 min at 50°C for reverse transcription; 15 min at 95°C for DNA polymerase activation; 15 s at 95°C for denaturing; and 45 cycles of 15 s at 94°C, 30 s at 55°C, and 30 s at 72°C. Data collection was carried out during the extension step (30 s at 72°C). The PCR reaction was followed by melting cure analysis to verify the specificity and identity of the RT-PCR products; melting curve analysis can distinguish the specific PCR products from the non-specific PCR product resulting from primer-dimer formation. The temperature of the PCR products was increased from 65 to 99°C at a rate of 1°C/5 s, and the resulting data was analyzed using the software provided by the manufacturer.
Western blot analysis
Protein extract samples (20 μg) were separated by 8–10% SDS-PAGE and blotted onto polyvinylidenedifluoride (PVDF) membranes. The blots were washed with TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20], blocked with 5% skim milk for one hour, and incubated with the appropriate primary antibody at the dilutions recommended by the supplier. The membrane was then washed, and the primary antibodies were detected with goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase. The blots were developed with enhanced chemiluminescence (ECL) (Santa Cruz Biotechnology, USA) and exposed to X-ray film (Eastman-Kodak, USA).
Statistical analysis
All data are expressed as mean ± standard deviation (S.D.). One-way ANOVA was used for multiple comparisons (Duncan’s multiple range test) using SPSS software ver. 10.0. P values < 0.05 were considered significant.
RESULTS
Nicotinic acetylcholine receptor expression in PDLSCs
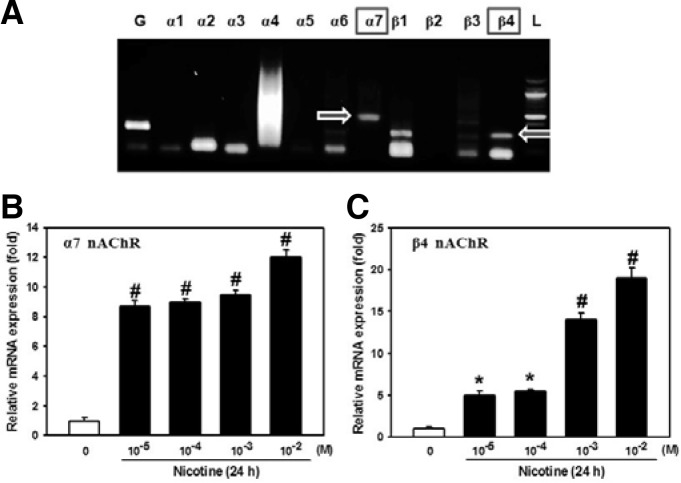
We first analyzed PDLSCs using RT-PCR to determine which nAChR subunits (among α1–7 and β1–4) are expressed. As shown in Fig. 1, the mRNA expression of α7 and β4 subunits was found in PDLSCs. When cells were exposed to various doses (0–10−2 M) of nicotine, mRNA levels for α7 and β4 subunits were increased in an adose-dependent manner (Figs. 1B and 1C). Thus, these two subunits of nAChRs may be translated and play a functional role in regulating PDLSC activity.
Fig. 1.
Effects of different concentrations of nicotine on the mRNA expression of nAChRs. (A) The mRNA expression of α1–7 and β1–4 nAChRs in PDLSCs was analyzed using RT-PCR. Cells were incubated with different concentrations of nicotine (0–10−2 M) and the mRNA levels of (B) α7 and (C) β4 nAChRs were then determined by real time RT-PCR technique. The values reported are the mean ± S.D. of three independent experiments. *P < 0.05 or #P < 0.001 vs. control value.
Apoptotic effect of nicotine on PDLSCs
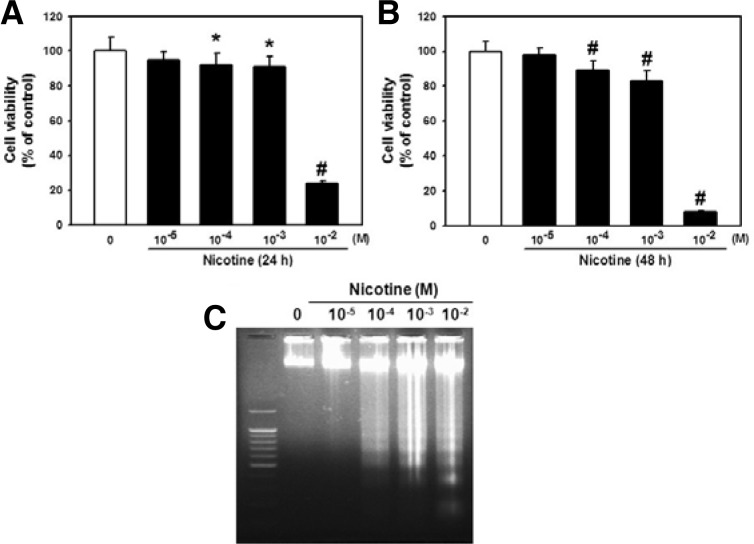
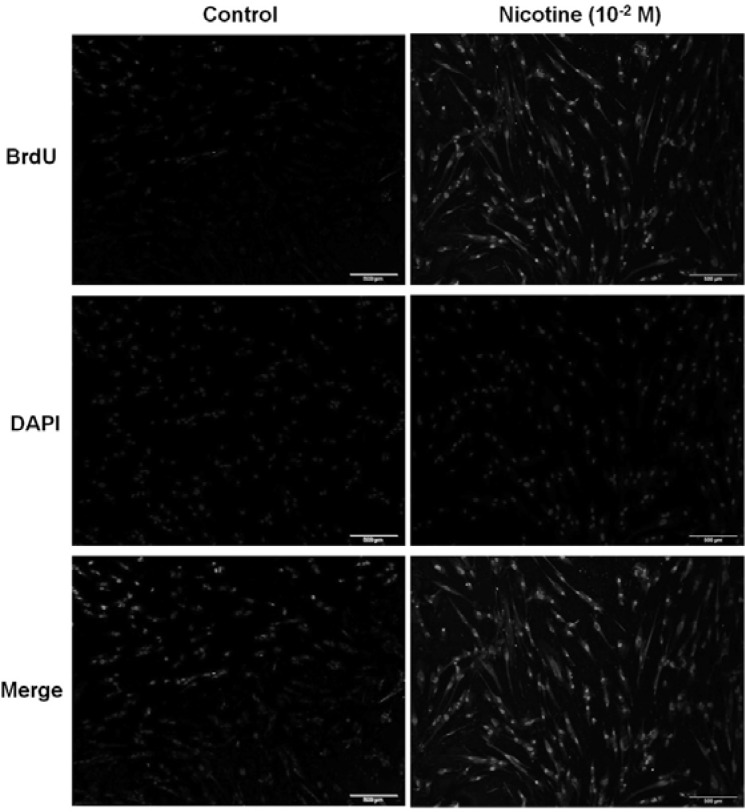
To examine the effect of nicotine on cell viability, the cells were treated with different concentrations of nicotine (0–10−2 M) for 24 or 48 h each. As shown in Figs. 2A and 2B, more than 10−4 M of nicotine significantly attenuated cell survival. Particularly, a marked cytotoxic effect was shown upon treatment with 10−2 M of nicotine (76% decrease vs. control; p < 0.01 after 24 h incubation and 92% decrease vs. control; p < 0.01 after 48 h incubation). With the increase in concentration, a more intense pattern of DNA fragments was detected. Efficient DNA fragmentation was observed at 10−4–10−2 M nicotine treatment for 48 h (Fig. 2C). No fragmentation was observed with DNA extracted from untreated cells. For further evidence of apoptosis, cells were subjected to APO-BrdU TUNEL staining after 10−2 M nicotine exposure for 48 h and observed using a fluorescent microscope. Figure 3 shows that the detection of BrdU incorporation at DNA fragmentation sites is significantly higher in the 10−2 M nicotine group than in the untreated group (Fig. 3).
Fig. 2.
Effects of nicotine on cell viability. Cells were incubated with different concentrations of nicotine (0–10−2 M) for (A) 24 or (B) 48 h each then cell viability was assessed as described in “Materials and Methods”. (C) Cells were treated with various concentrations of nicotine (0–10−2 M) for 48 h and the level of DNA fragmentation of apoptotic cells was then determined. The values reported are the mean ± S.D. of seven independent experiments. *P < 0.05 or #P < 0.001 vs. control value.
Fig. 3.
Evaluation of apoptosis by Apo-BrdU TUNEL assay. Cells were subjected to APO-BrdU TUNEL staining after 10−2 M nicotine exposure for 48 h. The nuclei were counterstained with DAPI. Each image shown is a representative of five separate experiments. The size bars on the panels represent 500 μm.
Effect of nicotine on cell cycle regulation in PDLSCs
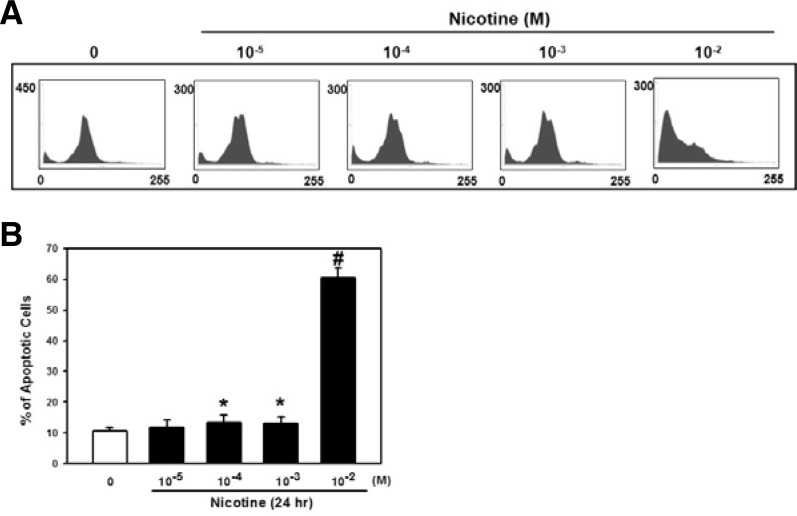
We next examined the subG1 phase, an indication of apoptotic cells, and detected an increased percentage of cells in the subG1 phase by nicotine exposure for 24 h (Fig. 4A). In spite of no significant increase in the subG1 phase percentage with doses of 10−5–10−3 M nicotine compared with the control, a certain increase in the fraction of the subG1 cells was precisely shown in the cells exposed to 10−2 M nicotine (50% increase vs. control; p < 0.01) (Fig. 4B).
Fig. 4.
Effect of nicotine on apoptotic cell death in PDLSCs. (A) Cells were treated with various concentrations of nicotine (0–10−2 M) for 24 h and the DNA contents of cells were then measured by flow cytometry. (B) The bars denote the percentage of cells in the subG1 phase. The values reported are the mean ± S.D. of five independent experiments. *P < 0.05 or #P < 0.001 vs. control value.
Nicotine activates apoptotic pathways during cell death
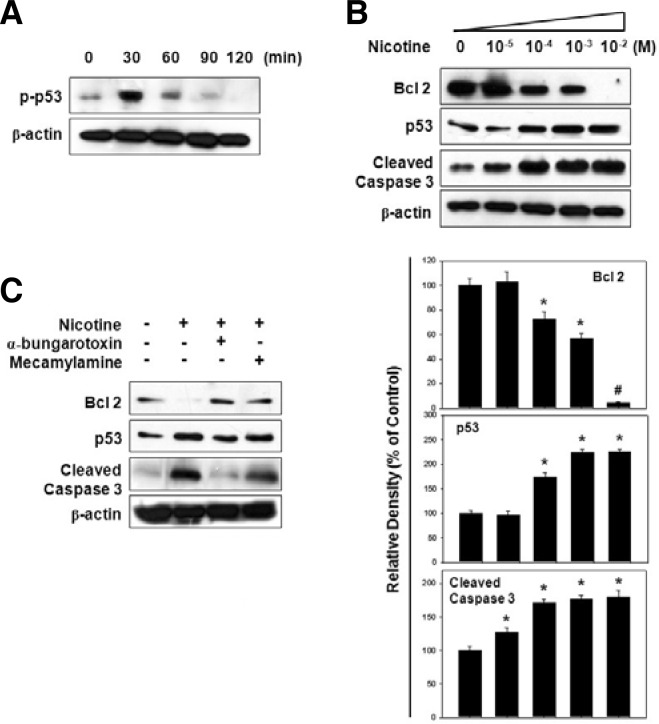
We also verified the apoptotic pathway profile during cell death induction by nicotine. Figure 5A shows that the phosphorylation of p53 was increased to 30 min and decreased gradually by nicotine (10−2 M) treatment. After incubation of the cells for 48 h with various doses of nicotine, we observed a decrease in the Bcl-2 anti-apoptotic protein, but an increase in p53 and cleaved caspase-3 protein levels dose-dependently (Fig. 5B). These results suggest that nicotine promotes the mitochondrial apoptotic pathway during nicotine-induced cell death.
Fig. 5.
Effect of nicotine on Bcl-2, p53, and caspase-3 protein levels. (A) Cells were subjected to Western blot analysis after 10−2 M nicotine treatment for various amounts time (0–120 min) and then phosphorylation of p53 was determined. (B) Cells were treated with nicotine (0–10−2 M) for 48 h then the protein levels of Bcl-2, p53, and cleaved caspase-3 were assessed using total protein lysates. The panels (bars) denote the mean ± S.D. of five experiments for each condition determined from densitometry relative to β-actin. *P < 0.05 or #P < 0.001 vs. control value. (C) Cells were pretreated with each α-BTX or mecamylamine for 1 h before the addition of 10−2 M nicotine. After 48 h of incubation, each apoptotic signal protein was analyzed.
Involvement of α7 and β4 nAChR in nicotine-induced apoptosis
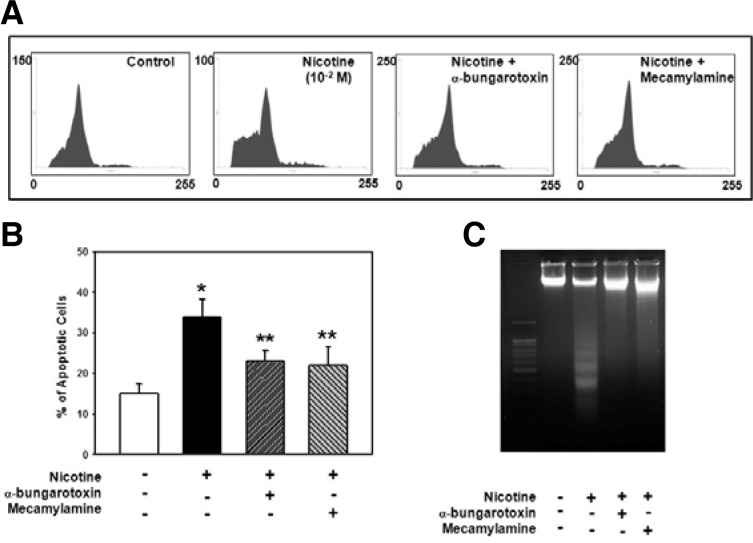
Finally, we examined whether the activation of α7 and β4 nAChR with nicotine leads to apoptotic cell death. In this experiment, cells were pretreated with different nAChR antagonists such as α-BTX, a selective α7 nAChR antagonist or mecamylamine, a nonselective nAChR antagonist prior to nicotine treatment. As shown in Fig. 5C, the pretreatment of each nAChR antagonist inhibited the nicotine effect on the decrease of Bcl-2 or increase of p53 and the cleaved caspase-3 protein levels. Moreover, all antagonists also exerted a protective effect against nicotine-induced apoptosis, such that the increased percentage of cells in subG1 cells (Figs. 6A and B) and DNA fragmentation (Fig. 6C) was found to be attenuated by α-BTX or mecamylamine treatment. These results indicate that nAChRs play an important role in nicotine-induced apoptotic cell death.
Fig. 6.
Effect of nAChR antagonists on nicotine-induced apoptosis in PDLSCs. Cells were incubated with each α-BTX or mecamylamine for 1 h before 10−2 M nicotine treatment and (A) the DNA contents and (C) the level of DNA fragmentation of apoptotic cells were then determined. (B) The data shows the percentage of cells in the subG1 phase. The values reported are the mean ± S.D. of four independent experiments. *P < 0.05 vs. control value, **P < 0.05 vs. nicotine treatment.
DISCUSSTION
The relationship between smoking and periodontal diseases has been studied over the past 60 years (Bergstrϕm, 1989; Haber, 1994; Preber and Bergstrϕm, 1990). However, there is no effective treatment and smokers contribute to the vast majority of therapeutic failures or refractory cases (MacFarlane et al., 1992; Magnusson et al., 1994). Thus, to accelerate clinical outcomes, therapeutic strategies first need to be developed based on endogenous regeneration to stimulate latent self-repair mechanisms in smoker patients. Recently, several populations of cells with stem cell properties have been isolated from different parts of the tooth (Gronthos et al., 2000; Miura et al., 2003; Morsczeck et al., 2005; Seo et al., 2004; Sonoyama et al., 2008). Particularly, the identification of PDL-derived MSC-like populations, which exhibit osteogenic, adipogenic, and chondrogenic characteristics under defined culture conditions (Gay et al., 2007; Lindroos et al., 2008; Nam et al., 2011; Xu et al., 2009), has contributed considerably in the efforts of reconstructive therapy to return the tissues to their original state before the periodontal tissue destruction started. Despite appropriate cell sources for periodontal tissue regeneration, many techniques with PDLSCs were not based on biological principles and were eventually found to have a limited value. In this respect, a biological approach with the characterization of PDLSCs’ own functions during disease states can be the first consideration for any other studies. Thus, the present study examined cellular and molecular events of PDLSCs using an experimental model, which can represent tobacco product, nicotine-mediated periodontal disease.
Nicotine is a lipophilic molecule of which the effects on neuronal nicotinic acetylcholine receptors (nAChR) have been primarily focused on its physiologic impact within the confines of the nervous system (Mao et al., 2011). However, many studies have recently found neuronal nAChRs to be expressed on many different non-neuronal cell types throughout the body (Chernyavsky et al., 2005; Resende et al., 2008; Wang et al., 2010; Wessler and Kirkpatrick, 2008). Recently, studies have found that nAChRs can be expressed on oral gingival and periodontal tissues (Nguyen et al., 2000; Wang et al., 2010). The expression of α7 nAChR in periodontal tissues can initiate the function of the regulation IL-1β in the process of periodontitis (Wang et al., 2010). It has been reported that nicotine substantially affects the levels of α5 and α7 nAChR expressions in oral keratinocytes, and it has been suggested that nAChRs have an important pathophysiological effect on determining the consequences of nicotine use on the diseased state (Arredondo et al., 2008). Moreover, exposures to nicotine can change the nAChR structure and function in both neuronal and non-neuronal cells (Huang and Winzer-Serhan, 2006; Zia et al., 1997). The present study demonstrated that PDLSCs prominently express the mRNA of α7 and β4 nAChRs more than any other nAChR subunits; in addition, it was shown that they were respectively increased by nicotine treatment in a dose-depen-dent manner. Even though the effects of nicotine on specific nAChR subunits varies from one cell type to another, based on previous and present studies, the nAChRs can be regarded as a novel molecular target to stimulate the progression of nicotine-related cell damage and disease pathways.
The issue that nicotine induces apoptosis in non-neuronal cells has been contradictory. A previous study has shown that α7 nAChR stimulation by nicotine inhibits the cytotoxic effect of glutamate on cultured cortical neurons and that phosphatidylinositol 3-kinase (PI3K)/Akt survival signal transduction contributes to the neuroprotective effect (Kihara et al., 2001). Moreover, nicotine has a negative effect on the apoptotic potential of anti-cancer agents in the human lung cancer cell line (Dasgupta et al., 2006). In one stem cell study, the activation of nAChRs and the downstream signaling pathways improved the survival and angiogenic effect of human embryonic stem cell-derived endothelial cells (hESC-ECs) in vivo and in vitro (Yu et al., 2009). On the other hand, the nicotinic effect mediated by α3 nAChR in keratinocytes demonstrated the increase of not only cell cycle regulatory proteins and anti-apoptotic protein, Bcl-2, but also the expression of caspase3 (Arredondo et al., 2003). This phenomenon could suggest that nicotine probably activates compensatory death and survival mechanisms in each biological condition. As a consequence of the findings of α7 and β4 nAChR existence in PDLSCs, we have found that nAChRs can play a significant role in the nicotine-induced apoptosis of PDLSCs that were blocked with α-BTX and mecamylamine. It is believed that these compounds would be useful for the treatment of conditions associated with decreased nicotinic transmission by acting as a selective α7 nAChR antagonist (α-BTX) and non-specific antagonist (mecamylamine) to block the ability of nicotine to bind to all subclasses of nAChRs (Shytle et al., 2002). In monitoring the antagonizing action of these compounds, the pretreatment of each nAChR antagonist prevented the nicotine-induced increase of apoptotic signal pathways, the fraction of subG1 cells, and DNA fragmentation. These findings indicate that nicotine stimulates apoptosis in PLDSCs by facilitating the functions of α7 and β4 nAChRs. Thus, effective blocking of periodontal nAChRs may offer preventive/therapeutic approaches to restore the PDLSC population that would result in periodontal regeneration for smoking-related periodontal disease.
In conclusion, nicotine can modulate PDLSC function and influence apoptosis, indicating that PDLSCs can be considered as a good model system for nicotine-involved periodontal disease. Moreover, our data provide a good starting point that will be useful to address molecular mechanisms that mediate the effects of nicotine on apoptosis in PDLSCs and can help to find target molecules and pathways which can restore a patients’ regenerative capacity.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013595).
REFERENCES
- Arredondo J., Hall L.L., Ndoye A., Nguyen V.T., Chernyavsky A.I., Bercovich D., Orr-Urtreger A., Beaudet A.L., Grando S.A. Central role of fibroblast alpha3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab. Invest. 2003;83:207–225. doi: 10.1097/01.lab.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- Arredondo J., Chernyavsky A.I., Jolkovsky D.L., Pinkerton K.E., Grando S.A. Receptor-mediated tobacco toxicity: acceleration of sequential expression of alpha5 and alpha7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB. J. 2008;22:1356–1368. doi: 10.1096/fj.07-9965.com. [DOI] [PubMed] [Google Scholar]
- Bergstrϕm J. Cigarette smoking as risk factor in chronic periodontal disease. Community Dent. Oral Epidemiol. 1989;17:245–247. doi: 10.1111/j.1600-0528.1989.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Bergstrϕm J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. doi: 10.1007/s10266-004-0043-4. [DOI] [PubMed] [Google Scholar]
- Bosshardt D.D., Sculean A. Does periodontal tissue regeneration really work? Periodontol. 2009;2000;51:208–219. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- Chernyavsky A.I., Arredondo J., Karlsson E., Wessler I., Grando S.A. The Ras/Raf-1/MEK1/ERK signaling pathway coupled to integrin expression mediates cholinergic regulation of keratinocyte directional migration. J. Biol. Chem. 2005;280:39220–39228. doi: 10.1074/jbc.M504407200. [DOI] [PubMed] [Google Scholar]
- Dasgupta P., Kinkade R., Joshi B., Decook C., Haura E., Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc. Natl. Acad. Sci. USA. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway R., Young W.G., Daley T., Harbrow D., Bartold P.M. An assessment of the osteoinductive potential of commercial demineralized freeze-dried bone in the murine thigh muscle implantation model. J. Periodontol. 1998;69:1325–1336. doi: 10.1902/jop.1998.69.12.1325. [DOI] [PubMed] [Google Scholar]
- Gay I.C., Chen S., MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofac. Res. 2007;10:149–160. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- Gotti C., Clementi F. Neuronal nicotinic receptors from structure to pathology. Prog. Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. Smoking is a major risk factor for periodontitis. Curr. Opin. Periodontol. 1994;1:12–18. [PubMed] [Google Scholar]
- Huang L.Z., Winzer-Serhan U.H. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006;1113:94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Huang J., Best S.M., Bonfield W., Brooks R.A., Rushton N., Jayasinghe S.N., Edirisinghe M.J. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004;15:441–445. doi: 10.1023/b:jmsm.0000021117.67205.cf. [DOI] [PubMed] [Google Scholar]
- Isaka J., Ohazama A., Kobayashi M., Nagashima C., Takiguchi T., Kawasaki H., Tachikawa T., Hasegawa K. Participation of periodontal ligament cells with regeneration of alveolar bone. J. Periodontol. 2001;72:314–323. doi: 10.1902/jop.2001.72.3.314. [DOI] [PubMed] [Google Scholar]
- Karring T., Nyman S., Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J. Clin. Periodontol. 1980;7:96–105. doi: 10.1111/j.1600-051x.1980.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Kihara T., Shimohama S., Sawada H., Honda K., Nakamizo T., Shibasaki H., Kume T., Akaike A. α7 Nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block a β-amyoidinduced neurotoxicity. J. Biol. Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- Lindroos B., Maenpaa K., Ylikomi T., Oja H., Suuronen R., Miettinen S. Characterization of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 2008;368:329–335. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zheng Y., Ding G., Fang D., Zhang C., Bartold P.M., Gronthos S., Shi S., Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane G.D., Herzberg M.C., Wolff L.F., Hardie N.A. Refractory periodontitis associated with abnormal polymorphonuclea leukocytephagocytosis and cigarette smoking. J. Periodontol. 1992;63:908–913. doi: 10.1902/jop.1992.63.11.908. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Low S.B., McArthur W.P., Marks R.G., Walker C.B., Maruniak J., Taylor M., Padgett P., Jung J., Clark W.B. Treatment of subjects with refractory disease. J. Clin. Periodontol. 1994;21:628–637. doi: 10.1111/j.1600-051x.1994.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Mao D., Gallagher K., McGehee D.S. Nicotine potentiation of excitatory inputs to ventral segmental area dopamine neurons. J. Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch C.A., Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J. Periodontal. Res. 1991;26(3 Pt 1):144–154. doi: 10.1111/j.1600-0765.1991.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C., Gotz W., Schierholz J., Zeilhofer F., Kuhn U., Mohl C., Sippel C., Hoffmann K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nam H., Kim J., Park J., Park J.C., Kim J.W., Seo B.M., Lee J.C., Lee G. Expression profile of the stem cell markers in human Hertwig’s epithelial root sheath/Epithelial rests of Malassez cells. Mol. Cells. 2011;31:355–360. doi: 10.1007/s10059-011-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.T., Hall L.L., Gallacher G., Ndoye A., Jolkovsky D.L., Webber R.J., Buchli R., Grando S.A. Choline acetyltransferase, acetylcholinesterase and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J. Dent. Res. 2000;79:939–949. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- Ojima M., Hanioka T., Tanaka K., Inoshita E., Aoyama H. Relationship between smoking status and periodontal conditions findings from national databases in Japan. J. Periodontal Res. 2006;41:573–579. doi: 10.1111/j.1600-0765.2006.00915.x. [DOI] [PubMed] [Google Scholar]
- Picciotto M.R., Caldarone B.J., Brunzell D.H., Zachariou V., Stevens T.R., King S.L. Neuronal nicotinic acetylcholine receptor subunit knockout mice physiological and behavioral phenotypes and possible clinical implications. Pharmacol. Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Portugal G.S., Gould T.J. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction converging evidence from human and animal research. Behav. Brain Res. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preber H., Bergstrϕm J. Effect of cigarette smoking on periodontal healing following surgical therapy. J. Clin. Periodontol. 1990;17:324–328. doi: 10.1111/j.1600-051x.1990.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Resende R.R., Alves A.S., Britto L.R., Ulrich H. Role of acetylcholine receptors in proliferation and differentiation of P19 embryonal carcinoma cells. Exp. Cell Res. 2008;314:1429–1443. doi: 10.1016/j.yexcr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shi S., Bartold P.M., Miura M., Seo B.M., Robey P.G., Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac. Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Shytle R.D., Penny E., Silver A.A., Goldman J., Sanberg P.R. Mecamylamine (Inversine) an old antihypertentensive with new research directions. J. Hum. Hypertens. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- Sonoyama W., Liu Y., Yamaza T., Tuan R.S., Wang S., Shi S., Huang G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth a pilot study. J. Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Liu Y.F., Wang Q.Y., Tsuruoka M., Ohta K., Wu S.X., Yakushiji M., Inoue T. Functional expression of alpha 7 nicotinic acetylcholine receptors in human periodontal ligament fibroblasts and rat periodontal tissues. Cell Tissue Res. 2010;340:347–355. doi: 10.1007/s00441-010-0949-9. [DOI] [PubMed] [Google Scholar]
- Wessler I., Kirkpatrick C.J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang W., Kapila Y., Lotz J., Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487–496. doi: 10.1089/scd.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Huang N.F., Wilson K.D., Velotta J.B., Huang M., Li Z., Lee A., Robbins R.C., Cooke J.P., Wu J.C. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS One. 2009;4:e7040. doi: 10.1371/journal.pone.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia S., Ndoye A., Nguyen V.T., Grando S.A. Nicotine enhances expression of the α3, α4, α5, and α7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res. Commun. Mol. Pathol. Pharmacol. 1997;97:243–262. [PubMed] [Google Scholar]