Abstract
Reactive oxygen species (ROS) generation is linked to dynamic actin cytoskeleton reorganization, which is involved in tumor cell motility and metastasis. Thus, inhibition of ROS generation and actin polymerization in tumor cells may represent an effective anticancer strategy. However, the molecular basis of this signaling pathway is currently unknown. Here, we show that the Ecklonia cava-derived antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells. Steady-state intracellular ROS levels were higher in malignant B16F10 cells than in parental, nonmetastatic B16F0 cells. Elevation of ROS by H2O2 treatment increased migration and invasion ability of B16F0 cells to level similar to that of B16F10 cells, suggesting that intracellular ROS signaling mediates the prometastatic properties of B16 mouse melanoma cells. ROS levels and the cell migration and invasion ability of B16 melanoma cells correlated with Rac1 activation and WAVE2 expression. Overexpression of dominant negative Rac1 and depletion of WAVE2 by siRNA suppressed H2O2-induced cell invasion of B16F0 and B16F10 cells. Similarly, dieckol attenuates the ROS-mediated Rac1 activation and WAVE2 expression, resulting in decreased migration and invasion of B16 melanoma cells. In addition, we found that dieckol decreases association between WAVE2 and NADPH oxidase subunit p47phox. Therefore, this finding suggests that WAVE2 acts to couple intracellular Rac1/ROS signaling to the invasive migration of B16 melanoma cells, which is inhibited by dieckol.
Keywords: dieckol, invasion, migration, ROS, WAVE2
INTRODUCTION
Cancer cells migrate and invade to change position within tissues, and increased motility and invasiveness are key steps for metastasis of tumor cells. Reorganization of the actin cytoskeleton is fundamental for cancer cell migration and invasion, since highly dynamic actin polymerization provides the driving force for protrusion of the plasma membrane at the leading edge of migrating cells. This process is regulated by members of Rho family of small GTPases, Wiskott-Aldrich syndrome proteins (WASPs), and by the Arp2/3 complex. Rho family proteins, in particular Rac and Cdc42, are activated in response to various extracellular stimuli. WASP family proteins subsequently relay signals produced by the activation of Rac and Cdc42 to the actin-nucleation complex Arp2/3 (Pollard and Borisy, 2003). Thus, when cells are exposed to extracellular stimuli, WASP family proteins induce rapid actin polymerization through activation of the Arp2/3 complex, leading to dynamic cell migration and invasion. Mammalian WASP family proteins identified to date include WASP; N-WASP; and WAVE-1, 2, and 3 (Derry et al., 1994; Miki et al., 1996; 1998b; Suetsugu et al., 1999). A number of studies have shown that WASP and N-WASP induce filopodium formation downstream of Cdc42 (Miki et al., 1998; Symons et al., 1996), whereas WAVE-1, 2, and 3 mediate lamellipodium formation downstream of Rac (Miki et al., 2000).
Reactive oxygen species (ROS) have been shown to act as signaling molecules to mediate various biological responses, including cell growth and gene expression (Bedard and Krause, 2007). Moreover, recent reports have indicated the involvement of ROS in tumor cell migration and invasion (Bae et al., 2011; Wu, 2006). NADPH oxidases are one of the major enzymatic sources of ROS in various tissues (Bedard and Krause, 2007). Rac1, a member of the Rho family of small GTPases that is essential for cytoskeletal reorganization, forms a key component of the NADPH oxidase complex and is necessary for NADPH oxidase activation (Bedard and Krause, 2007). It has been reported that ROS derived from Rac1-dependent NADPH oxidase are involved in vascular endothelial growth factor (VEGF)- and angiotensin-1 (Ang-1)-mediated endothelial migration (Harfouche et al., 2005; Ushio-Fukai et al., 2004). Growth factors and tumor promoters such as EGF and TPA induce expression of matrix metalloproteinases (MMPs) in a Rac1/ROS-dependent manner, which is required for tumor cell invasion (Binker et al., 2009; Steinbrenner et al., 2005). Therefore, it is likely that Rac1-induced ROS generation is essential for actin cytoskeleton reorganization, which is responsible for cell migration and invasion of various tumor cells. However, intermediates downstream of Rac1 and ROS in tumor cell migration and invasion are poorly understood.
Since ROS play an important role in migration and invasion of cancer cells, investigation of antioxidants to reduce intracellular oxidative stress has been proposed as anticancer strategy. We have previously isolated dieckol - a nutrient polyphenol compound with antioxidant acitivity - from the brown alga Ecklonia cava (Lee et al., 2010). Therefore, in the present study, we examined the effect of dieckol on intracellular ROS generation and on the migration and invasion of B16 mouse melanoma cells. We found that the antioxidant dieckol inhibited migration and invasion of B16 melanoma cells by suppressing the expression of WAVE2, downstream of Rac1/ROS. Our results suggest that WAVE2 is an intermediate that links ROS signaling to actin cytoskeletal reorganization and that this pathway is inhibited by dieckol which mediates ROS scavenging and Rac1 inactivation and decreases WAVE2 expression.
MATERIALS AND METHODS
Cell culture
B16F0 and B16F10 mouse melanoma cells, obtained from the American Type Culture Collection (ATCC), were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco), supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/ml penicillin, and 100 mg/ml streptomycin, in 5% CO2 at 37°C. For use in experiments, cells were passaged at least 3 times and detached with trypsin-EDTA. Matrigel was a product from BD Biosciences (USA). Chemicals and reagents were purchased form Sigma if not differently stated. pEF-Myc-Bos construct (Myc-Rac1t17N) were described previously (Miki et al., 1998).
Cell viability (MTT) assay
Cells were seeded in 96-well plates at a density of 1 × 103 cells/well in DMEM containing 10% fetal bovine serum. Twenty-four hours after seeding, the medium was replaced with serum-free DMEM, and the cells were incubated with 100 μM H2O2 for 48 h. The cells were subsequently incubated with or without 25 μg/ml dieckol for 24 h. Thereafter, the medium was carefully removed, and 100 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (1 mg/ml final concentration) was added to each well prior to incubation for another 3 h at 37°C in 5% CO2. Absorbance was measured at 540 nm on a microplate reader (iMark Bio-Rad).
Cell migration and invasion assay
Cell migration was determined using a wound-healing scratch assay, as previously described (Meng et al., 2006). Briefly, cells were seeded in 3.5-cm dishes and grown overnight. After serum starvation for 24 h, cells were preincubated with 100 μM H2O2 for 48 h and then incubated with or without 25 μg/ml dieckol for 16 h. A sterile 200-μl pipette tip was used to scratch the cells to form a wound. Migration of the cells to the wound was visualized with an inverted Olympus phase-contrast microscope and representative fields were photographed. The healing rate was quantified by measuring the gap size after culture. Ten different areas in each assay were chosen to measure the distance of migrating cells to the origin of the wound.
For the invasion assay, the undersurface of the porous membranes in Matrigel Invasion Chambers (BD Biosciences, USA) was coated with fibronectin (25 μg/ml) at room temperature for 1 h and washed 3 times in DMEM containing 0.1% bovine serum albumin (DMEM-BSA). DMEM-BSA was added to the lower compartment of the chamber. Cells were starved in DMEM-BSA overnight and treated with H2O2 and/or dieckol (as described above), trypsinized, and collected. Subsequently, 200 μl of each cell suspension (2 × 105 cells/well in DMEM-BSA) was added to the upper compartment of the chamber and incubated at 37°C in a humidified atmosphere with 5% CO2 for 24 h. Cells on the upper surface of the membrane were removed, whereas cells that had migrated to the lower surface of the membrane were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS), stained with crystal violet (0.4% dissolved in 10% ethanol) for 15 min, washed 2 times with PBS, and counted under a phase-contrast microscope with a 10 × objective lens. The number of cells in 9 randomly selected fields from triplicate chambers was counted in each experiment.
Measurement of ROS
Dichlorofluorescein diacetate (DCF-DA) was used to evaluate the generation of ROS in response to oxidative stress. Cells (4 × 104 cells/well) in 24-well plates were incubated with H2O2 for 48 h and subsequently incubated with or without dieckol for 24 h. The cells were washed with PBS and incubated with 10 μM DCF-DA for 30 min at room temperature. Fluorescence was measured with a fluorescence plate reader.
Transient transfection of RNAi
WAVE2 siRNA and a non-specific siRNA control were obtained from Invitrogen. The WAVE2 siRNA sequence used for the experiments described in this study was 5′-AAGTGCCTTTG CCTCCCGAGT-3′ (nt 174–194 relative to the start codon). Transient transfection of siRNA was accomplished by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. To obtain a sufficient level of suppression by RNAi, we carried out a second transfection 24 h later.
Small GTPase Rac1 activity assay
GST-PAK-CRIB fusion protein was expressed as previously described (Miki et al., 2000) and immobilized on glutathione-Sepharose beads (Amersham Biosciences, USA). Cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1% NP-40, 10% glycerol, 200 mM NaCl, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM DTT, and 1 mM PMSF. A total of 5–10 μl of cell lysate was retained for use in Western blotting as a gel loading control, and 1 ml of cell lysate was mixed with 50 μl (bed volume) of GST-PAK CRIB beads and rotated at 4°C for 40 min. The beads were washed 3 times with cold wash buffer containing 25 mM Tris-HCl (pH 7.5), 30 mM MgCl2, 40 mM NaCl, 1% NP-40, 1 mM DTT, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mM PMSF, and 20 μl of sodium dodecyl sulfate (SDS) sample buffer containing 50 mM Tris-HCl (pH 6.8), 2% SDS, 6% 2-mercaptoethanol, 10% glycerol, and 0.5 mg/ml bromophenol blue was added. Samples were separated by electrophoresis, and Rac1-GTP was detected by Western blotting.
RESULTS
Dieckol suppresses migration and invasion of B16 melanoma cells
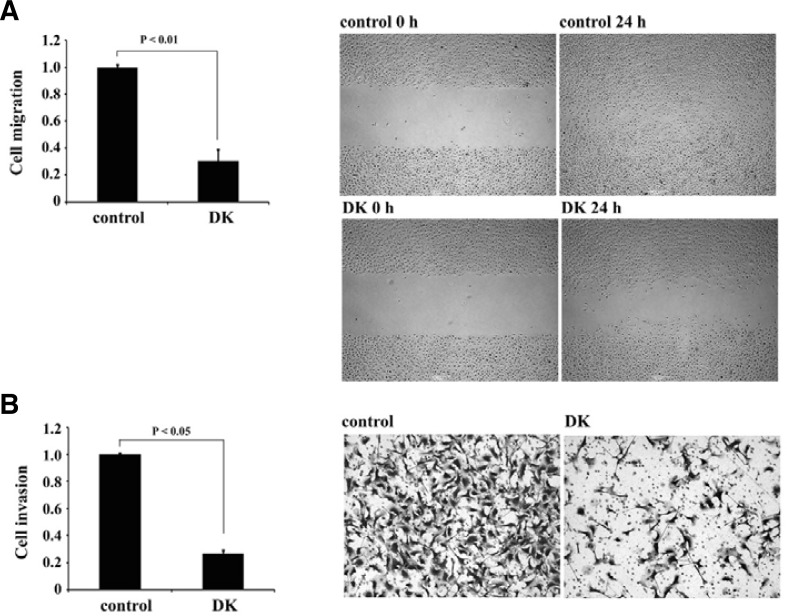
A number of reports have shown that polyphenolic compounds, including dieckol, possess antitumor and antioxidant activities (Cottart et al., 2006; Kandaswami et al., 2005). However, the molecular mechanisms responsible for the antitumor activity of polyphenols are not fully understood. Therefore, we investigated the effect of the antioxidant dieckol on metastatic phenotypes of cancer cells, such as dynamic migration and invasion. Dieckol did not affect viability of B16 mouse melanoma cells at the concentrations of below 50 μg/ml (Fig. 2B; Supplementary Fig. 1). As shown in Fig. 1A and Supplementary Fig. 1B, treatment of highly metastatic B16F10 melanoma cells with non-cytotoxic 25 μg/ml dieckol decreased cell motility in wound-healing assays. Invasion of dieckol-treated B16F10 cells into Matrigel chambers also reduced to approximately 23% that of untreated control cells (Fig. 1B; Supplementary Fig. 1B). Therefore, these results suggest that dieckol regulates an intracellular signaling cascade involved in the migration and invasion of B16F10 cells.
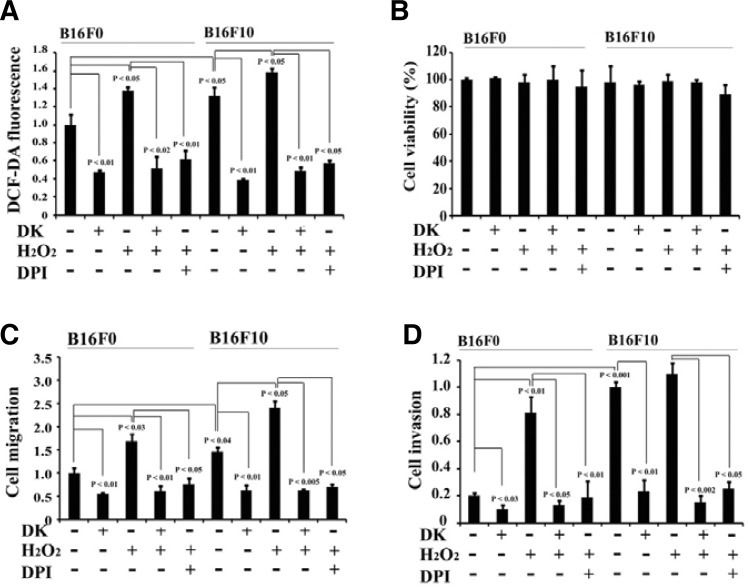
Fig. 2.
Dieckol suppresses migration and invasion of B16F10 cells by reducing intracellular ROS. (A) Dieckol attenuates intracellular ROS. B16F0 and B16F10 cells (4 × 104 cells/well) were incubated in the presence or absence of 100 μM H2O2 for 48 h, followed by incubation with or without dieckol (25 μg/ml) or DPI (20 μM), a NADPH oxidase-dependent ROS-quenching agent, for 24 h. Cellular ROS levels were assessed by DCF-DA. All data are presented as mean ± SD. Statistical significance by Student’s t-test is shown. (B) Effects of H2O2, dieckol, and/or DPI on cell viability. Cells were treated with H2O2 (100 μM), dieckol (25 μg/ml), and/or DPI (20 μM) for 48 h and assessed using MTT assay. The results of 3 independent experiments were averaged. (C, D) Cells were treated as shown in (A). Wound-healing scratch assays and Matrigel invasion assays were performed for 16 h. All data are presented as mean ± SD. Statistical sig-nificance by Student’s t-test is shown.
Fig. 1.
Dieckol inhibits migration and invasion of B16F10 cells. (A) Inhibitory effect of dieckol on the migration of B16F10 cells. Wound-healing scratch assays were performed with B16F10 cells plated onto fibronectin-coated dishes. After serum starvation, cells were incubated in the absence or presence of 25 μg/ml (34 μM) dieckol for 24 h. A sterile 200-μl pipette tip was used to scratch the cells to form a wound. Cell migration was quantified by measurement of the gap size of 4 different images at 0 and 16 h; representative images are shown. The results of 3 independent experiments were averaged, and PBS-treated cells were used as a control. Statistical significance by Student’s t-test is shown. (B) Dieckol inhibits invasion of B16F10 cells. Matrigel invasion assays were performed with B16F10 cells incubated with 25 μg/ml dieckol. Statistical significance by Student’s t-test is also shown.
ROS mediate migration and invasion of B16 melanoma cells, which is downregulated by the scavenging activity of dieckol
Both the contribution of ROS to cell migration and invasion and the antioxidant properties of dieckol have been reported by many recent studies (Heo et al., 2009; Lee et al., 2010; Wu, 2006). Thus, we examined the effect of dieckol on the level of ROS in malignant B16F10 cells, as well as the ability of dieckol to regulate B16F10 cell migration and invasion due to its antioxidant properties. As shown in Fig. 2A, dieckol treatment attenuated ROS levels in B16F10 cells. Moreover, the antioxidant activity of dieckol was associated with decreased migration and invasion of B16F10 cells (Figs. 2C and 2D; Supplementary Fig. 1B), suggesting that the reduction in intracellular ROS following dieckol treatment plays a role in the decreased migration and invasion of B16F10 cells. To confirm this hypothesis, we treated B16F10 cells with 100 μM H2O2 and subsequently incubated cells with or without dieckol and then evaluated the effect on cell migration and invasion. In addition, we compared the effect of H2O2 and dieckol on the different metastatic potential of tumor cells by using parental, nonmetastatic B16F0 melanoma cells (Fidler and Kripke, 1977; Fidler and Nicolson, 1976). In both cell types, cellular exposure to a relatively low concentration of H2O2 (100 μM) over 2 days did not affect cell viability (Fig. 2B; Supplementary Fig. 1). We found that the steady-state intracellular ROS level in B16F10 cells was 137% of the level in B16F0 cells (Fig. 2A), suggesting that intracellular ROS levels may correlate with metastatic capacity. While H2O2 treatment increased the cellular ROS level in both B16F0 and B16F10 cells, the relative increase ratio in cellular ROS was greater in B16F0 cells, and the resulting ROS level in B16F0 cells was comparable to that in B16F10 cells. Similar results were observed for migration and invasion assays with B16F0 and B16F10 cells (Figs. 2C and 2D). Malignant B16F10 cells displayed increased migration and invasion in comparison with nonmetastatic B16F0 cells. However, elevated ROS levels in response to H2O2 treatment increased migration and invasion, not only of B16F10 cells, but also of B16F0 cells. In particular, H2O2 treatment enhanced the invasive capacity of B16F0 cells by approximately 4-fold, as compared to the untreated control cells, which represents 80% of the invasion potential of B16F10 cells. Thus, it is likely that metastatic potential of B16 melanoma cells is contributed by high concentration of intracellular ROS. In contrast, antioxidant dieckol inhibited this H2O2-induced increase in cell migration and invasion in both B16F0 and B16F10 cells (Figs. 2C and 2D). In addition, similar results have been observed by using diphenyleneiodonium chloride (DPI), a NADPH oxidase-dependent ROS-quenching agent (Figs. 2A, 2C, and 2D). DPI treatment effectively reduced H2O2-induced cell migration and invasion in both cell types. Therefore, these results indicate that ROS mediates migration and invasion of B16 melanoma cells, whereas the antioxidant dieckol downergulates cell migration and invasion by reducing ROS levels.
WAVE2 couples Rac1/ROS signaling to migration and invasion of B16 melanoma cells, and dieckol attenuates the ability of ROS to increase WAVE2 expression
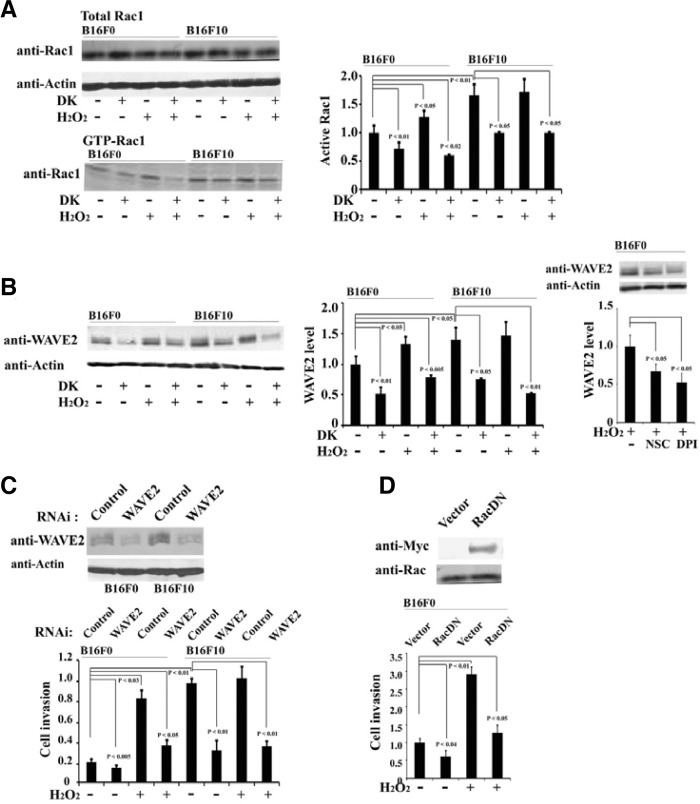
The observed changes in cell migration and invasion in response to oxidative stress implicate the involvement of a small GTPase, Rac1, which is an upstream regulator required for actin reorganization, cell migration, and invasion. Generation of ROS by a variety of physiological conditions is associated with Rac1 activation (Nimnual et al., 2003; Werner and Werb, 2002). To examine endogenous Rac1 activation, a GST-PAK binding assay was performed with cell lysates. Rac1 activity in B16F10 cells was 1.6-fold higher than that in B16F0 cells. H2O2 treatment effectively increased activation of Rac1 in B16F0 cells rather than in B16F10 cells. In contrast, dieckol treatment clearly decreased Rac1 activation in either the presence or absence of H2O2 treatment (Fig. 3A). This data suggests that Rac1 activity is correlated with both cellular ROS levels and the migration and invasion capacity of B16 melanoma cells.
Fig. 3.
Dieckol attenuates Rac1 activation and WAVE2 expression. (A) B16F0 and B16F10 cells were incubated in the presence or absence of 100 μM H2O2 for 48 h, followed by incubation with or without 25 μg/ml dieckol for 24 h. Lysates of B16F0 and B16F10 cells were incubated with a purified GST-PAK-PBD fusion protein. Bound proteins were collected and GTP-bound Rac1 was detected by western blot by using an anti-Rac1 antibody. The images are representative of 3 independent experiments. The results of 3 independent experiments were averaged. All data are presented as mean ± SD. Statistical significance by Student’s t-test is shown. (B) B16F0 and B16F10 cells were treated with H2O2 (100 μM), dieckol (25 μg/ml), a Rac inhibitor NSC (1 mM), or DPI (20 μM). Cell lysates were analyzed for WAVE2 and β-actin. Representative image among 3 independent experiments was shown. The results of 3 independent experiments were averaged. Statistical significance by Student’s t-test is shown. (C) B16F0 and B16F10 cells were transfected with WAVE2 siRNA or the same amount of control siRNA. After 48 h, cell lysates were prepared, and WAVE2 expression was analyzed by Western blot. Following transfection with WAVE2 siRNA, the cells were incubated for 48 h in the presence or absence of 100 μM H2O2, and cells were subsequently harvested for invasion assays. Representative image among 3 independent experiments was shown. The results of 3 independent experiments were averaged. Statistical significance by Student’s t-test is also shown. (D) Invasion of B16F0 cells exogenously expressing dominant negative Rac1 was examined.
Cell migration is regulated by actin cytoskeleton reorganization and is required for tumor invasion and metastasis. WAVE2 has been shown to act as the primary downstream effector of Rac1 to promote invasion and metastasis of B16 melanoma cells (Kurisu et al., 2005). Therefore, we investigated whether WAVE2 can couple ROS-mediated Rac1 signaling to migration and invasion of B16 melanoma cells. As shown in Fig. 3B, increased WAVE2 expression correlated with increased metastatic properties of cells; WAVE2 levels in B16F10 cells were approximately 1.5-fold higher than that in B16F0 cells. In addition, treatment of cells with H2O2 increased WAVE2 expression in B16F0 cells to a level similar to that in B16F10 cells. In contrast, dieckol treatment of both types of B16 melanoma cells decreased expression of WAVE2, suggesting that increased B16 cell migration and invasion induced by ROS may be mediated by WAVE2. In addition, treatment with NSC, a Rac inhibitor, decreased the H2O2-induced increased WAVE2 expression as well as DPI did (Fig. 3B), indicating that Rac1 and ROS signaling pathway is involved in H2O2-induced regulation of WAVE2 expression. Furthermore, the relationship between the expression of WAVE2 and H2O2-induced cell invasion was determined by WAVE2 siRNA. B16F0 and B16F10 cells were transfected with WAVE2 siRNA and subsequently treated with H2O2 for 2 days. Transfected WAVE2 siRNA sequences attenuated WAVE2 expression (Fig. 3C). In addition, WAVE2 siRNA attenuated the invasive migration capacity of both B16F0 and B16F10 cells, thereby indicating an essential role of WAVE2 in H2O2-induced B16 melanoma cell invasion (Fig. 3C). We also observed similar inhibitory effects by overexpression of dominant negative Rac1 in B16F0 cells (Fig. 3D). Therefore, these results suggest that WAVE2 mediates migration and invasion of B16 melanoma cells downstream of Rac1/ROS signal and that this pathway is suppressed by dieckol treatment.
Dieckol decreases association between WAVE2 and NADPH oxidase subunit p47phox
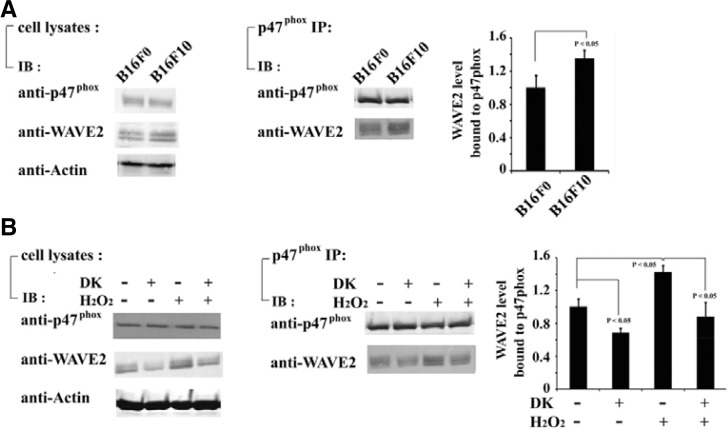
Rac1 is a component of the NADPH oxidase complex that is necessary for NADPH oxidase activation. ROS derived from NADPH oxidase have been shown to mediate cell migration in a Rac1-dependent manner (Modovan et al., 2000; Ushio-Fukai et al., 2002). Furthermore, activation of Rac1 regulates actin cytoskeleton reorganization by association with WAVE2 in a variety of cells (Takenawa and Miki, 2001). The results of Figs. 3B and 3D have shown that inhibition of Rac1 reduced the H2O2-mediated increase of WAVE2 expression and cell invasion. Therefore, we examined the association between WAVE2 and NADPH oxidase. B16F0 and B16F10 cells lysates were immunoprecipitated with an antibody specific to the NADPH oxidase subunit p47phox. We found that a greater amount of endogenous WAVE2 coprecipitated with p47phox in B16F10 cells compared with B16F0 cells (Fig. 4A). While H2O2 treatment increased the association between WAVE2 and p47phox in B16F0 cells, dieckol treatment decreased the association of WAVE2 with p47phox following H2O2 treatment (Fig. 4B). These findings suggest that the complex of WAVE2 and NADPH oxidase may regulate actin cytoskeletal reorganization in response to ROS signaling, which is required for migration and invasion of B16 melanoma cells.
Fig. 4.
Dieckol decreases association between WAVE2 and p47phox. (A) Expression of WAVE2 and p47phox in B16F0 and B16F10 cells were determined using antibodies specific to WAVE2 and p47phox. Cell lysates were also immunoprecipitated with antip47phox antibody, and bound proteins were assayed using antibodies specific to WAVE2 and p47phox. Representative image among 3 independent experiments was shown. The results of 3 independent experiments were averaged. Data are presen-ted as mean ± SD. (B) B16F0 cells were incubated in the presence or absence of 100 μM H2O2 for 48 h and subsequently incubated with or without 25 μg/ml dieckol for 24 h. Expression level of WAVE2 and p47phox under each condition and amount of WAVE2 bound to p47phox were determined using antibodies specific to WAVE2 and p47phox. The results are representative of 3 independent experiments. The results of 3 independent experiments were averaged. Data are presented as mean ± SD.
DISCUSSION
ROS production has been linked to actin cytoskeletal reorganization, which is required for cellular motility. Treatment of a variety of cell types with H2O2 results in an increase in actin polymerization (Hinshaw et al., 1986; 1991; Omann et al., 1994), whereas treatment with NADPH oxidase inhibitors and superoxide scavengers abolishes actin monomer incorporation at the fast-growing barbed ends of filaments and blocks migration of endothelial cells. These results correlate with oxidant activity in membrane ruffles of migrating cells (Moldovan et al., 2000). Recently, several actin cytoskeletal regulatory proteins, including TRAF4, IQGAP, and WAVE1, have been reported to be associated with NADPH oxidase (Ikeda et al., 2005; Usatyuk et al., 2009; Wu et al., 2003; 2005). TRAF4 directly binds to p47phox. Furthermore, an active mutant of TRAF4 activates NADPH oxidase downstream of Rac1 and PAK and initiates robust membrane ruffling (Wu et al., 2005). Hyperoxia-mediated IQGAP1 activation through Rac1 causes redistribution of Src, cortactin, and p47phox to the membrane ruffles (Usatyuk et al., 2009). WAVE1 is also associated with p47phox in response to VEGF stimulation in endothelial cells, which leads to NADPH oxidase activation and ruffle formation (Wu, 2003). These results suggest that intracellular ROS generation is required for cell migration in a variety of cells types. In addition, recruitment of p47phox to actin cytoskeletal regulatory protein complexes implicates ROS generation at critical subcellular sites such as membrane ruffle in the leading edge of migratory cells.
WAVE2 is found at the tips of lamellipodia that protrude towards the direction of movement (Suetsugu et al., 2003). Moreover, WAVE2 acts downstream of Rac1 to mediate the migration and invasive and metastatic phenotypes of B16 mouse melanoma cells (Kurisu et al., 2005). Depletion of WAVE2, but not of WAVE1, by RNAi significantly suppressed membrane ruffling, cell invasion, and pulmonary metastasis of B16F10 cells to the lungs. Rac1/WAVE2-mediated actin cytoskeleton reorganization is responsible for the highly motile phenotype of B16F10 cells. However, the regulatory mechanism responsible for the role of WAVE2 in B16F10 cell invasion remains unclear. In this study, we found that H2O2 stimulation induced not only Rac1 activation, but also increased WAVE2 expression, which correlated with migration and invasion of B16 melanoma cells. In contrast, overexpression of dominant negative Rac1, or depletion of WAVE2 by RNAi, suppressed the H2O2-mediated increase in the invasive migration of B16 melanoma cells. These results suggest that WAVE2 functions downstream of Rac1 as an important mediator between ROS signaling and actin cytoskeletal reorganization, which leads to invasive migration. In parallel with these results, the antioxidant dieckol reduced intracellular ROS levels and suppressed Rac1/WAVE2 signaling, which inhibited the migration and invasion of B16 melanoma cells. Furthermore, interestingly, the steady-state intracellular ROS level was higher in B16F10 cells than in B16F0 cells. The effect of H2O2 stimulation on invasive migration was more pronounced in B16F0 parental cells, which are neither invasive nor metastatic, than in malignant B16F10 cells. The ROS level and invasive potential of B16F0 parent cells following H2O2 treatment was similar to that of B16F10 cells. Therefore, it is likely that H2O2-mediated oxidative stress is involved in the acquisition of metastatic properties by B16 melanoma cells. Rac1/ROS signaling pathway may already be active in B16F10 cells. We also found that association between WAVE2 and p47phox in B16F10 cells, or in B16F0 cells treated with H2O2, was decreased by dieckol treatment. This indicates the presence of a molecular complex that controls oxidant-related actin cytoskeletal reorganization in B16 melanoma cells. Further studies on the interaction between WAVE2 and p47phox are required to understand the precise role of WAVE2 in the signaling pathway that links intracellular ROS generation to invasive migration of B16 malignant cells
Dieckol, a natural polyphenol, has been shown to possess antitumor and antioxidant activites (Heo et al., 2009; Lee et al., 2010). However, the cancer chemopreventive properties of dieckol have previously been linked to its protective role against pleiotropic effects, including proliferation defects and DNA damage induced by several oxidative stress conditions. Here, we demonstrate a new potential mechanism underling the chemopreventive properties of dieckol, whereby dieckol inhibits cancer progression and metastasis by intracellular ROS scavenging, WAVE2 signal suppression, and inhibition of invasive cell migration of melanoma cells. These findings provide a basis for further investigation into the use of the antioxidant dieckol as a treatment for cancer.
In conclusion, our results suggest that WAVE2 is an intermediate that links Rac1/ROS signaling to the migration and invasion of B16 mouse melanoma cells and that this pathway is clearly inhibited by dieckol. Dieckol should be a potential therapeutic agent for cancer treatment.
Supplementary Material
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0003665) and (2011-0005476).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Bae Y.A., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Binker M.G., Binker-Cosen A.A., Richards D., Oliver B., Cosen-Binker L.I. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem. Biophys. Res. Commun. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- Cottart C.H., Nivet-Antoine V., Laguillier-Morizot C., Beaudeux J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- Derry J.M., Ochs H.D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Fidler I.J., Kripke M.L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I.J., Nicolson G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J. Natl. Cancer Inst. 1976;57:1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- Harfouche R., Malak N.A., Brandes R.P., Karsan A., Irani K., Hussain S.N. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- Heo S.J., Ko S.C., Cha S.H., Kang D.H., Park H.S., Choi Y.U., Kim D., Jung W.K., Jeon Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro. 2009;23:1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Hinshaw D.B., Sklar L.A., Bohl B., Schraufstatter I.U., Hyslop P.A., Rossi M.W., Spragg R.G., Cochrane C.G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am. J. Pathol. 1986;123:454–464. [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D.B., Burger J.M., Beals T.F., Armstrong B.C., Hyslop P.A. Actin polymerization in cellular oxidant injury. Arch. Biochem. Biophys. 1991;288:311–316. doi: 10.1016/0003-9861(91)90200-3. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Yamaoka-Tojo M., Hilenski L., Patrushev N.A., Anwar G.M., Quinn M.T., Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler. Thromb. Vasc. Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- Kandaswami C., Lee L.T., Lee P.P., Hwang J.J., Ke F.C., Huang Y.T., Lee M.T. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- Kurisu S., Suetsugu S., Yamazaki D., Yamaguchi H., Takenawa T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene. 2005;24:1309–1319. doi: 10.1038/sj.onc.1208177. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Han J.S., Heo S.J., Hwang J.Y., Jeon Y.J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro. 2010;24:375–381. doi: 10.1016/j.tiv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Meng Q., Xia C., Fang J., Rojanasakul Y., Jiang B.H. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell. Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Miki H., Miura K., Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- Miki H., Sasaki T., Takai Y., Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998a;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- Miki H., Yamaguchi H., Suetsugu S., Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Moldovan L., Moldovan N.I., Sohn R.H., Parikh S.A., Goldschmidt-Clermont P.J. Redox changes of cultured endothelial cells and actin dynamics. Circ. Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- Omann G.M., Harter J.M., Burger J.M., Hinshaw D.B. H2O2-induced increases in cellular F-actin occur without increases in actin nucleation activity. Arch. Biochem. Biophys. 1994;308:407–412. doi: 10.1006/abbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Ryu B., Li Y., Qian Z.J., Kim M.M., Kim S.K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling implication for chronic articular disease. Chem. Biol. Interact. 2009;179:192–201. doi: 10.1016/j.cbi.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Steinbrenner H., Ramos M.C., Stuhlmann D., Mitic D., Sies H., Brenneisen P. Tumor promoter TPA stimulates MMP-9 secretion from human keratinocytes by activation of superoxide-producing NADPH oxidase. Free Radic. Res. 2005;39:245–253. doi: 10.1080/10715760500053487. [DOI] [PubMed] [Google Scholar]
- Suetsugu S., Miki H., Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem. Biophys. Res. Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- Symons M., Derry J.M., Karlak B., Jiang S., Lemahieu V., McCormick F., Francke U., Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Miki H. WASP and WAVE family proteins key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Usatyuk P.V., Gorshkova I.A., He D., Zhao Y., Kalari S.K., Garcia J.G., Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47 phox translocation and reactive oxygen species generation in lung endothelial cells. J. Biol. Chem. 2009;284:15339–15352. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M., Alexander R.W. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P) H oxidase. Mol. Cell. Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- Wu W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Wu R.F., Gu Y., Xu Y.C., Nwariaku F.E., Terada L.S. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J. Biol. Chem. 2003;278:36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- Wu R.F., Xu Y.C., Ma Z., Nwariaku F.E., Sarosi G.A., Jr, Terada L.S. Subcellular targeting of oxidants during endothelial cell migration. J. Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.