Abstract
Thioredoxin reductase (TrxR) is a member of the pyridine nucleotide-disulfide reductase family, which mainly functions in the thioredoxin system. TrxR is found in all living organisms and exists in two major ubiquitous isoenzymes in higher eukaryotic cells; One is cytosolic and the other mitochondrial. Mitochondrial TrxR functions to protect mitochondria from oxidative stress, where reactive oxidative species are mainly generated, while cytosolic TrxR plays a role to maintain optimal oxido-reductive status in cytosol. In this study, we report differential physiological functions of these two TrxRs in C. elegans. trxr-1, the cytosolic TrxR, is highly expressed in pharynx, vulva and intestine, whereas trxr-2, the mitochondrial TrxR, is mainly expressed in pharyngeal and body wall muscles. Deficiency of the non-selenoprotein trxr-2 caused defects in longevity and delayed development under stress conditions, while deletion mutation of the selenoprotein trxr-1 resulted in interference in acidification of lysosomal compartment in intestine. Interestingly, the acidification defect of trxr-1(jh143) deletion mutant was rescued, not only by selenocystein-containing wild type TRXR-1, but also cysteine-substituted mutant TRXR-1. Both trxr-1 and trxr-2 were up-regulated when worms were challenged by environmental stress such as heat shock. These results suggest that trxr-1 and trxr-2 function differently at organismal level presumably by their differential sub-cellular localization in C. elegans.
Keywords: C. elegans, longevity, oxidative stress, thioredoxin reductase, V-ATPase
INTRODUCTION
Eukaryotic protein often exists as multi-gene products to perform their specialized tasks in entire organismal context. Those isomers are differentially used in different tissues, cells, cell compartments or growth conditions. The expression of those isomers derives from different cell-specific patterns of gene expression in combination with regulated protein compartmentalization and stability. As a result, isomers localized in different cellular compartments play differential physiological roles to maintain the functional and structural integrity of organisms. Therefore, it is crucial to identify protein isomers and to investigate their physical, biochemical, and molecular biological properties, in order to understand how these isomers function in entire organisms.
Reactive oxygen species (ROS) are continuously produced as normal metabolic byproducts, especially through electron leaks from electron transport system in mitochondria (Grant and Hirsh, 1999). ROS are required for normal biological functions such as development, cell differentiation and proliferation. However, excessive ROS can interfere with them by damaging various biomolecules including protein, nucleic acid, and lipid. To maintain optimum level of ROS, various ROS scavenging systems are present in the cells. The thioredoxin (Trx) system is one of hydrogen peroxide scavenging systems which has a wide range of cellular functions as a major reducing system required for nucleic acid synthesis and redox regulation as well as antioxidant defense (Mustacich and Powis, 2000). Trx is oxidized converting hydrogen peroxide to water, and then recycled by thioredoxin reductase (TrxR) that is a member of the flavoprotein family of pyridine nucleotide-disulfide oxidoreductases (Snider et al., 2010).
TrxR is evolutionarily well conserved from bacteria to human. In most eukaryotes, TrxR exists as a homodimeric protein in which each high Mr 55 kDa monomer contains an FAD prosthetic group, that captures electrons from NADPH and transfer them to a redox-active disulfide in the N-terminal CVNVGC active site. The dithiol of the reduced CVNVGC active site is oxidized by another subunit’s C-terminal redox active motif of the tetrapeptide X-Cys1-Cys2-X (X is usually Gly or Ser) where Cys1 and Cys2 form a rare vicinal disulfide bond during catalytic cycle (Kanzok et al., 2000). The Cys2 in many TrxRs in mammalian and multicellular eukaryotes is replaced with selenocystein (Sec, U in one-letter code), an analog of cysteine with substitution of sulfur for selenium. Due to the higher nucleophilicity with a low pKa of selenium than sulfur, Sec is inherently highly reactive and holds superior redox properties. Bacteria, plants, archea and most unicellular eukaryotes have a non-selenoprotein TrxR with approximate molecular weight of 35 (Arner, 2009). TrxR contains the native catalytic activity to reduce not only Trx, but also a wide variety of substrates including disulfide-containing natural proteins, such as NK-lysin (Sun et al., 1999), glutaredoxin 2 (Lee et al., 1999), TRP32 (Johnson et al., 1999), protein disulfide isomerase (PDI) (Gromer et al., 1998) and granulysin (Akamatsu et al., 1997), and several low molecular weight compounds, such as lipoic acid (Sies, 1993) and quinones (Larsen, 1993). However, the various TrxR-catalyzed reduction functions in cellular context is widely unknown.
The Trx system has a large number of functions in many biological systems by interacting with a variety of molecular targets (Han et al., 2006). Trx generates redox active cysteine residue in ribonucleotide reductase, the rate limiting enzyme in deoxyri-bonucleotide synthesis (Jee et al., 2005). The Trx system plays a critical role in cell cycle arrest and redox signaling by controlling the activity of many transcription factors such as NF-kB, p53, Ref-1, HIFa, PTEN, AP-1 and glucocorticoid receptor, etc. It regulates glucose metabolism by interacting with thioredoxin interacting protein (TXNIP), a glucose uptake regulator, and apoptosis by inactivating apoptosis signal-regulating kinase (ASK1). It also has been reported to regulate the survival of phagocytosis and culmination of the slime mold Dictyostelium discoideum by modulating of vacuolar H+-ATPase activity (Jeong et al., 2006). The broad range of functions of the Trx system reflects the fact that the redox system exists in all living organisms and has a long history of evolution and suggests that it is highly likely to emerge various other physiological functions in all living kingdoms.
In bacteria and low eukaryotes such as fungi, only one TrxR gene exists. However, in higher organisms including human, there are two major ubiquitous TrxRs: predominantly cytosolic TrxR1 and mitochondrial TrxR2. Both enzymes reduce their own counterpart Trx residing in cytoplasm and mitochondria, respectively. The studies of knockout mice showed that both TrxR1 and TrxR2 are essential for mammalian embryonic development, although the phenotypes of the knockout mice were strikingly different. In addition to the early embryonic lethality, deletion of TrxR1 in mice resulted in severe growth retardation and lack of primitive mesoderm formation, but was dispensable for cardiac development (Brenner, 1974; Radyuk et al., 2010). In contrast, deletion of TrxR2 gave insufficient proliferation of cardiomyocytes, severely impaired hematopoiesis, increased apoptosis in the liver, as well as enhanced sensitivity to oxidative stress in embryonically derived TrxR2-deficient fibroblasts (Koon and Kubiseski, 2010). Neuronal and glial precursors-targeted knockout of TrxR1 caused severe cerebellar developmental defects whereas the knockout of TrxR2 did not appear affected (Oka and Futai, 2000). Interestingly, these phenotypes of TrxR1(-/-) and TrxR2(-/-) knockout mice are clearly different from those of Trx1(-/-) and Trx2(-/-) mice. Trx1 knockout mice died shortly after implantation, clearly exhibiting worse phenotype of early embryonic lethality than the mice with deletion either of TrxR1 or TrxR2 (Mello and Fire, 1995). Trx2-deficient mice were not able to continue to develop beyond embryonic day 12.5, displaying massive apoptosis and disclosure of anterior neural tube (Ishii et al., 1990). Therefore, these cytosolic vs. mitochondrial TrxRs play physiologically different roles which involve cell- or tissue-type specific pathway that includes mechanisms independent of their ability to reduce Trx. However, the early lethality of the mammalian models makes it difficult to further analyze the differential functions of cytosolic and mitochondrial TrxRs in intact organismal context.
In this study, we investigate the functions of the two isomeric TrxRs using Caerahnobditis elegans, a simple but powerful genetic model. Blast search revealed that there are two TrxRs in C. elegans: trxr-1 and trxr-2. Both proteins share high similarity with their mammalian orthologs (Supplementary Figs. S2A and S3A). NADPH binding domain and dimerization domain are also conserved in the worm thioredoxin reductases. trxr-1 is predicted to be the only seleno-cysteine containing protein in C. elegans (Buettner et al., 1999; Gladyshev et al., 1999; Taskov et al., 2005). According to the MitoProt II analysis, trxr-2 sequence contains predicted mitochondrial localizing sequence as expected (Arner and Holmgren, 2000; Lacey and Hondal, 2006). In the free-living soil nematode, trxr-1, the cytosolic TrxR, is required for the acidification of lysosomal compartments, whereas trxr-2, the mitochondrial TrxR, is critical for normal ageing and development under stress conditions. Interestingly, the gene expression of both trxr-1 and trxr-2 was induced by heat shock, which produces the reactive oxygen species. These results suggest that trxr-1 and trxr-2 have different physiological roles in organismal context by discrete subcellular and tissue expression, and differential gene regulation in C. elegans.
MATERIALS AND METHODS
C. elegans strains, genotyping and maintaining
Worm breeding and handling were conducted as described (Brenner, 1974). Nematodes were grown on OP50 bacterial lawn on NGM agar at 20°C. All the strains used in the study were backcrossed six times to Bristol N2 wild type. Bristol N2, daf-2(e1370)III, and daf-16(m26)I were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota. trxr-1(jh143)IV was isolated in the laboratory, as descrybed previously (Park et al., 2001). Screening of mutants from the mutagenized library was performed by nested PCR as described (Barstead, 1999b). trxr-1(jh143) that bears a 1439 bp deletion covering the second and third exons and partial of the fourth exon from TMP/UV-mutagenized worm library (Fig. S2B). The deletion and the precise location of the deleted region of both trxr mutants were confirmed by cloning and sequencing of PCR products amplified from the deletion alleles. trxr-2(tm 2047)III was obtained from National Bioresource Project Japan (NBP-Japan). A 507 bp region covering the entire first exon and a part of the second exon was deleted in tm2047 allele (Fig. S3B). To genotype the trxr-1or trxr-2 mutant, the first round PCR was performed using the 1st set of primers for each gene (Table S1). Then, the second round PCR was performed using the first round PCR products as templates with the 2nd set of primers spanning deleted region. To confirm the homozygote mutant, Inner primer was designed inside the deletion region paired with the FWD 2nd primer. Homozygous trxr-1(jh143) or trxr-2(tm2047) mutant was confirmed by a shorter band from the nested PCR, and the absence of a PCR product from the internal PCR (Fig. S2C and S3C). Rabbit polyclonal antibody raised against recombinant TRXR-1 detected double bands migrating at approximately 70 kDa in wild type worm lysates, but neither protein band with corresponding size nor transcript was detected in jh143 (Fig. S2D) or tm2047 (Fig. S3D), thus confirming that the deletion mutant was functionally null.
Constructs of C. elegans TrxRs
In order to generate a transcriptional ptrxr-1::gfp construct, 0.7 kb promoter was amplified from genomic DNA and subcloned to pPD95.77 at PstI and BamHI sites. To generate a translational ptrxr-1::trxr-1::gfp, 0.7 kb promoter and 4.7 kb full length genome of trxr-1 were amplified using genomic DNA as a template, and then the fragments were cloned into pPD95.77 at BamHI and PstI sites. In case of trxr-2 transcriptional constructs, both 1.5 kb external (EX) promoter of the operon containing trxr-2 and 0.3 kb internal (IN) promoter were amplified separately using ZK632 as a template, and then subcloned into pPD95.77 at SalI/BamHI, and HindIII/SalI, respectively. To generate a translational pEX::trxr-2::gfp, 2.9 kb full length genomic sequence was amplified and subcloned into the pEX::gfp at SalI and BamHI. To generate a translational pIN::trxr-2::gfp, the 2.9 kb full length genomic sequence with the 0.3 kb internal promoter was amplified using cosmid ZK637 as a template, and then, the amplified region was subcloned into BamHI and SalI sites of pPD95.77. In order to generate the ptrxr-1::trxr-1 s, 0.5 kb additional 3′UTR region with the full length genomic sequence of trxr-1 was amplified and subcloned into pPD95.77. All the primers used in this study are listed in Supplementary Table S1 and the descriptive cartoon of all the constructs was shown in Supplementary Figs. S1A and S1B.
GFP expression analysis
All the constructs were transformed into wild type worms to observe the expression. Microinjection was done as previously described (Mello and Fire, 1995). All GFP fusion constructs were co-injected with pRF4 selection marker at a concentration of 100 ng/μl into the N2 wild type worms to obtain transgenic progenies. Transgenic worms expressing green fluorescence were immobilized with 25 mM sodium azide and fluorescent images were captured with epi-fluorescent microscope (Zeiss Axio Imager microscope). To stain mitochondria in adult worms, L4 stage worms were transferred to NGM plates with bacterial lawn containing Mitotracker Red (Molecular Probes, 2 μg/ml) and incubated in the dark at 20°C for 12 h. They were then transferred to new bacteria-seeded NGM plates to clean off excess of dye. After 10 to 20 min, they were mounted on a slide overlaid with 2% agar and fluorescent images were captured with Olympus confocal microscope (Olympus, Japan). Images were obtained and intensity of fluorescence was measured using AxioVision software (Kim et al., 2011).
Life span analysis
Life span was performed at 20 and 25°C, as previously described (Kenyon et al., 1993). Briefly, L4 animals cultured from synchronized eggs isolated from bleached gravid hermaphrodites were placed in NGM plates in groups of 20 animals per plate. Animals were transferred to fresh plates daily until they stopped progeny production, and then continued to be transferred every other day, but the number of survived animals was monitored daily. Animals which did not respond to gentle touching and stopped pharyngeal pumping were determined as dead. Animals that crawled off the plate or died due to internal hatching or extruded gonad were statistically censored. Survival assay was repeated twice at 20°C and three times at 25°C (Supplementary Table S2). Kaplan-Meier survival curve analysis and the Mantel-Cox log-rank test were done with Oasis software (Jeong et al., 2006).
Paraquat assay
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) assay was performed as previously described with slight modification (Ishii et al., 1990). Synchronized young adult worms were incubated in 1 × PBS buffer containing 40 mM paraquat for 24 h, and then the number of dead worms was scored. Synchronized eggs were placed on the seeded NGM plates covered with paraquat to final concentration of 0.1 mM, and then cultured at 20°C. Proportion of adult worms to total was scored on the fifth day of treatment. Data were collected from four independent experiments using at least one hundred animals for each genotype.
Acridine orange staining
Acridine orange staining was slightly modified from a previously described method (Oka and Futai, 2000). Adult worms were incubated for 30 min in staining buffer (40 mM acridine orange and 1% dimethyl sulfoxide) in the presence or absence of 25 mM bafilomycin A1 (Sigma). After washing with phosphate buffer three times, animals were mounted on agar pad and images were obtained. Intensity of fluorescence was measured and quantified using AxioVision software (Lambers Heerspink et al., 2009).
Measurement of hydrogen peroxide
Worms cultured for 3 days at 20°C after hatch were collected and homogenized in PBST (1× PBS/0.1% Tween20). The 100 ul of 1× PBST containing 60 intact worms or 100 μl of worm extract were mixed with 100 μl 100 mM 2,7-dichlorodihydro-fluorescein diacetate (H2DCF-DA, Invitrogen), and incubated for 120 min at room temperature. Fluorescence was measured every 20 min at 485 nm of excitation and 530 nm of emission with SpectraMax M2 fluorometer (Molecular Devices), and then the RFU was normalized with protein concentration.
Stress and qRT-PCR
To stress worms, translational transgenic worms were incubated at 30°C for 8 to 16 h, or treated with 0.1 mM or 2 mM paraquat for 8 h. Worms were harvested and mRNA was extracted, and then reverse transcription was carried out using Sensiscript RT Kit (QUIAGEN) and Oligo (dT)12–18 primer (Invitrogen) to synthesize the cDNA. Real time PCR was done by using SYBR® Premix Ex Taq (Perfect Real Time) (Takara) and Thermal Cycler Dice® Real Time System Takara. The results were quality-controlled by confirming single peak of the dissociation curve of the PCR products and sequencing them.
RESULTS
TRXR-1 is a cytoplasmic protein whereas TRXR-2 is located in mitochondria
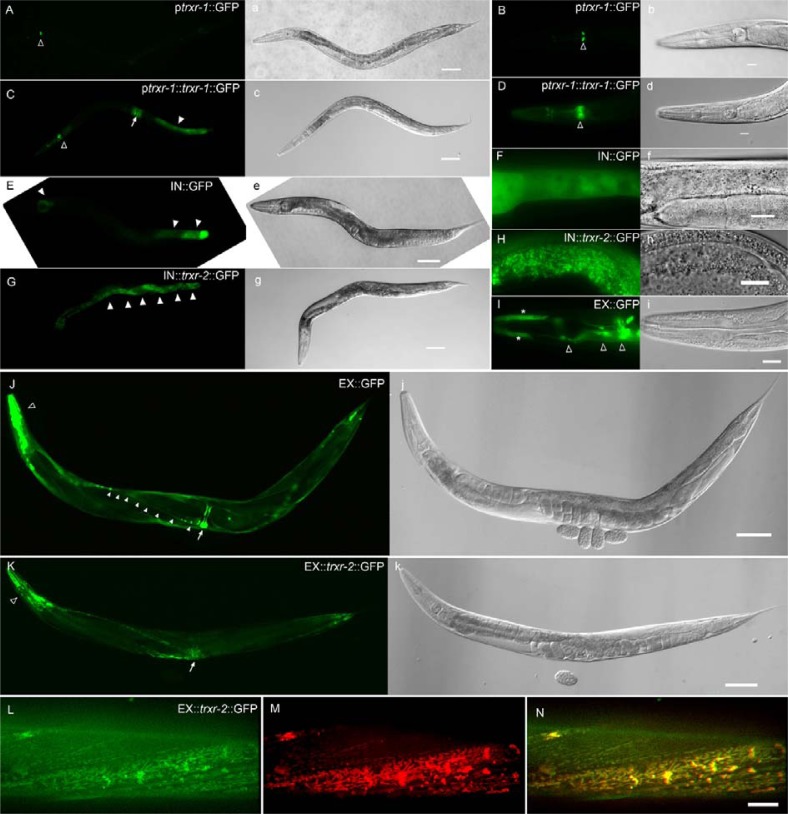
In order to examine the expression pattern of TRXRs, we constructed plasmids expressing only GFP or GFP fusion of TRXRs driven by their own promoters, and generated the transgenic worms by injecting the plasmids into wild-type young adult worms and isolating transformed progenies showing roller phenotype and GFP fluorescence. In case of trxr-2 which is present in an operon including two additional genes of unc-32 and tpx-1, we constructed two transcriptional and translational expression plasmids driven by either external operon promoter (pEX) or internal promoter (pIN) adjacent to the coding region of trxr-2, because internal promoters also can drive gene expression (Meder et al., 2011). A transcriptional GFP expression construct driven by trxr-1 promoter was expressed only in M2 (Figs. 1A and 1B). The expression level of translational TRXR-1::gfp was especially high in intestine, vulva and pharynx (Figs. 1C and 1D). A transcriptional construct with the internal trxr-2 promoter was mostly expressed in intestine (Figs. 1E and 1F), while the one with the external trxr-2 promoter, identical to the unc-32 promoter, was expressed in head neurons, nerve cord, and vulva as previously shown (Figs. 1I and 1J) (Barstead, 1999a; Pujol et al., 2001). TRXR-2::gfp was prominent in intestine when driven by internal trxr-2 promoter (Figs. 1G and 1H), and in muscles by external promoter (Figs. 1K∼1N). Obviously, we observed punctate and sometimes elongated pattern of TRXR-2::gfp expression which often indicates particular sub-cellular organelle localization (Figs. 1I and 1L). Considering the predicted mitochondrial localizing motif found in trxr-2, we tested whether those puncta of TRXR-2::gfp are present in mitochondria. Mitotracker staining was well co-localized with the punctuated expression of trxr-2, demonstrating that TRXR-2 is mitochondrial protein as predicted (Figs. 1M and 1N).
Fig. 1.
Expression pattern of trxr-1 and trxr-2 in C. elegans. A transcriptional construct ptrxr-1::gfp is mainly expressed in M2 neurons [open arrowheads in (A) and (B)]. A translational construct ptrxr-1::trxr-1::gfp is expressed in post intestine [arrowhead in (C)], vulva [arrow in (C)], and post pharyngeal bulb [open arrowheads in (C) and (D)]. Both transcriptional pIN::gfp and translational pIN::trxr-2::gfp constructs are mostly expressed in intestine [arrowheads in (E) and (G), respectively]. The transcriptional fluorescence signal is diffused (F), whereas the translational expression reveals the punctate and reticular mitochondrial morphology (H). The other tran-scriptional pEX::gfp is expressed in vulva [arrow in (J)], nerve cord [arrowheads in (J)], many head neurons [open arrowheads in (I) and (J)], and pharyngeal hypodermis cells [asterisks in (I)]. A translational pEX::trxr-2::gfp is mainly expressed in muscles including pharynx (open arrowheads) and vulva (arrow). TRXR-2::gfp signals in body wall muscle (L) were colocalized with Mitotracker staining (M), as shown in the merged image (N). A corresponding DIC image to each fluorescent image is shown in (A) to (K). Scale bar: 50 μm in (A), (C), (E), (G), (J), and (K); 10 μm in (B), (D), (F), (H), (I), and (N).
Brood size of trxr-2(tm2047);trxr-1(jh143) double mutants was reduced
Both cytosolic and mitochondrial thioredoxin reductases have been shown to be essential for viability in mammalian systems, but the nematodal deletion mutants, trxr-1(jh143) and trxr-2 (tm2047), seemed to be normal in terms of viability at the standardized culture condition. In order to see whether either of the gene is required for normal nematodal reproduction, we measured the brood sizes of the mutants and the double mutant of both genes, trxr-2(tm2047);trxr-1(jh143). The brood sizes of the single mutants were not changed at both 20 and 25°C, whereas the double mutant showed reduced brood size at 25°C (Table 1). This result suggests that trxr-1 and trxr-2 be redundantly involved in either fertility or early development.
Table 1.
Brood size
| 20°C | n | 20°C | n | |
|---|---|---|---|---|
| N2 | 330 ± 9 | 22 | 235 ± 4 | 43 |
| trxr-1(jh143) | 309 ± 11 | 21 | 221 ± 5 | 46 |
| trxr-2(tm2047) | 338 ± 5 | 22 | 210 ± 7 | 46 |
| trxr-2(tm2047); trxr-1(jh143) | 317 ± 8 | 22 | 174 ± 4* | 45 |
p < 0.001
Life span of trxr-2(tm2047) was reduced, when cultured at 25°C
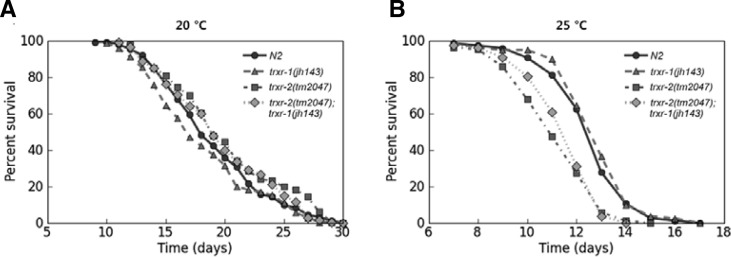
Oxidative stress has been widely accepted as one of the major causes for aging process, and the thioredoxin system plays a pivotal role to scavenge hydrogen peroxide, a type of ROS that can damage biomacromolecules, such as nucleic acid, protein, and lipid. The previous study reported that trx-1(jh127) mutant, that has a deletion in the gene coding thioredoxin, a primary substrate of TrxR, lived shorter than wild type animals (Jee et al., 2005). Therefore, we tested whether TRXRs also play roles in the maintenance of normal longevity, as postulated, by comparing the life span of trxr mutants to that of wild-type animals. The life spans of both trxr mutants and of the double mutants trxr-2(tm2047);trxr-1(jh143) were not different from that of wild-type at 20°C (Supplementary Fig. 2A and Table S2). However, at 25°C, the condition usually generating more ROS, not trxr-1(jh143), but trxr-2(tm2047) lived shorter than wild type, and the double mutants trxr-2(tm2047);trxr-1(jh143) showed similarly reduced life span to trxr-2(tm2047) (Fig. 2B, Table 2). We also examined the pharyngeal pumping rate as the worms aged, because the pumping reduces as the worms senesce. trxr-2 (tm2047) and trxr-2(tm2047);trxr-1(jh143) showed the descending tendency of the pumping rate, although they were not statistically significant (Supplementary Fig. S4). Altogether, these data suggest that there is no cumulative effect on aging process by loss of both TrxRs in C. elegans.
Fig. 2.
Longevity of thioredoxin reductase mutants. (A) Life span analysis at 20°C. There was no change in longevity between wild type and thioredoxin reductases mutants. (B) trxr-2(tm2047) and trxr-1(jh143);trxr-2(tm2047) worms lived shorter than wild type at 25°C (Mantel-Cox log-rank test, p-values < 0.0001). One representative data out of three independent experiments is shown, and the data of individual experiments are shown in Supplementary Table S2.
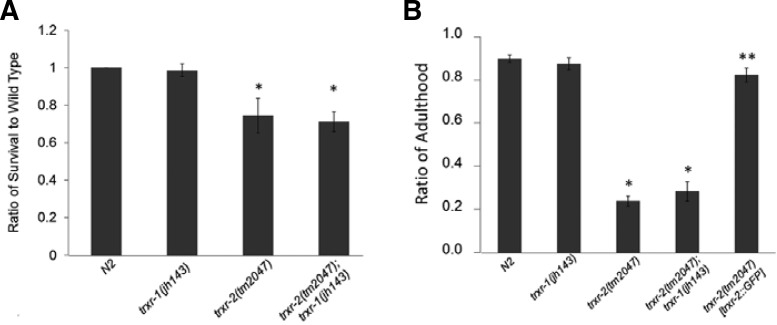
The treatment of paraquat delayed development of trxr-2(tm2047)
Because life span of trxr-2(tm2047) is reduced, but not trxr-1(jh143), the different life span of those two mutants may be due to differential sensitivity to oxidative stress. Paraquat stimulates superoxide production in mitochondria by accepting electrons from complex I to oxygen. This superoxide production causes extensive mitochondrial oxidative damage. Therefore, we tested whether those two different trxr mutants show different sensitivity to paraquat. After incubated for 24 h in PBS buffer containing 40 mM paraquat, the survival of both trxr-2 (tm2017) and trxr-2(tm2017);trxr-1(jh143) was significantly reduced, compared to wild type (Fig. 3A). We examined the developmental rates, because we observed that trxr-2[trxr-2::gfp] transgenic line tends to show worm bag phenotype carrying hatched embryos in uterus, which is not adequate for the rescue of survival. When cultured on NGM plates treated with paraquat, the growth rate of trxr-2(tm2017) was significantly lower than that of wild types, whereas that of trxr-1(jh143) was similar (Fig. 3B). Development of TRXR-2::gfp rescue line was comparable to wild type, indicating that the delayed development is indeed the result of trxr-2 deficiency. As in the case of life span, the double mutants trxr-2(tm2047);trxr-1(jh143) also showed slower growth rate than wild type.
Fig. 3.
Paraquat sensitivity assay. (A) Survived worms were scored, after they were incubated in 40 mM paraquat for 24 h. Data were collected from four independent experiments using at least one hundred animals for each genotype (*t-test, p-value < 0.0001). (B) Percentage of adulthood was measured five days after the treatment with 0.1 mM paraquat. (n = 4, t-test, *p-value < 0.0001, **p-value > 0.0001).
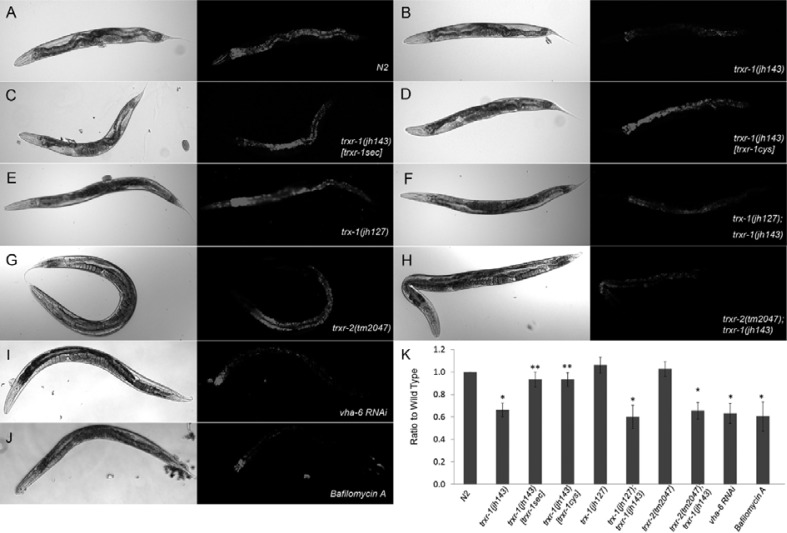
Dye-uptake into lysosomal compartments is reduced in trxr-1(jh143)
TRXRs are promiscuous enzymes capable of reducing a variety of physiological substrates (Mustacich and Powis, 2000). A study using Dictyostelium has suggested that thioredoxin regulates Vacuolar (H+)-ATPase (V-ATPase) activity by controlling of disulfide bond formation (Jeong et al., 2006). Acridine orange is a fluorescent dye that accumulates in acidic intracellular compartments, and its uptake in acidic compartments in intestine of C. elegans depends on V-ATPase activity and is blocked by bafilomycin A, a specific inhibitor for V-ATPase. In order to see whether V-ATPase activity was affected by either mutation of TRXRs in worms, we examined acidification of lysosomal compartments in intestine by acridine orange vital staining (Ji et al., 2006; Lee et al., 2010; Li et al., 2010; Oka and Futai, 2000). The level of acridine orange was significantly dropped in trxr-1(jh143) (Figs. 4A and 4B). The level of fluorescence was comparable to V-ATPase (vha-6) RNAi- or bafilomycin A-treated worms, indicating that V-ATPase activity in trxr-1 mutant background was interfered (Figs. 4I and 4J). Interestingly, the vital dye uptake defect in trxr-1(jh143) was reverted by not only selenocystein-containing TRXR-1 (Fig. 4C), but also cystein-containing TRXR-1, in which the penultimate amino acid seleno-cysteine in active site is substituted with cysteine (Fig. 4D). Selenocystein is essential for TRXR-1 to reduce thioredoxin, and the thioredoxin system is crucial for normal nematodal development (Stenvall et al., 2011). Therefore, we asked whether the reduced V-ATPase activity in trxr-1(jh143) requires thioredoxin system by observing acridine orange uptake in the condition absent of cytoplasmic thioredoxin, TRX-1 (Jee et al., 2005). The fluorescent level of the vital dye in trx-1 (jh127) was comparable to wild type whereas that in trx-1(jh127);trxr-1(jh143) double mutants was significantly reduced to similar level in trxr-1(jh143) (Figs. 4E and 4F). In contrast, acridine orange uptake was not affected in trxr-2 (tm2047) and trxr-2(tm2047);trxr-1(jh143) (Figs. 4G and 4H). Altogether, these results suggest that V-ATPase activity be regulated by cytosolic thioredoxin reductase activity that does not involve TRX-1.
Fig. 4.
Acridine orange uptake assay. Reduced dye uptake in trxr-1(jh143) (B) was rescued by expressing either selenocystein- or cystein-containing TRXR-1. DIC and fluorescence images of acridine orange were shown for N2 (A), trxr-1(jh143) (B), trxr-1(jh143) [trxr-1sec] (C), trxr-1(jh143)[trxr-1cys] (D), trx-1(jh127) (E), trx-1(jh127); trxr-1(jh143) (F), trxr-2(tm2047) (G), trxr-2(tm2047); trxr-1(jh143) (H), vha-6RNAi (I), and bafilomycin A (J) treated worms. Intensity of acridine orange fluorescence was quantified (K; n = 20, t-test, *p-value < 0.01, **p-value > 0.01).
H2O2 production was increased in worm TrxR mutants
Trx system is an effective hydrogen peroxide scavenging systems, and malfunction of the system often causes increased level of H2O2 accompanying a variety of physiological defects. To see whether the loss of thioredoxin reductase activity caused the change in the level of hydrogen peroxide, we treated either the intact young adult trxr mutant worms or the trxr mutant extracts with H2DCF-DA, the H2O2 indicator, and measured the emitted fluorescence in time course manner, different measuring strategy from the previously reported (Barstead, 1999a). All the three mutant animals exhibited tendency to show higher fluorescence than wild type, in spite of statistical insignificance (Fig. 5A). In case of the experiments using cell extracts, both trxr-1 and trxr-2 mutants showed increased fluorescence at the 120 min measuring time point, and the effect was not cumulative in the double mutants, trxr-2;trxr-1, indicating that the level of hydrogen peroxide was comparably increased in those three mutants, when compared to wild type (Fig. 5B).
Fig. 5.
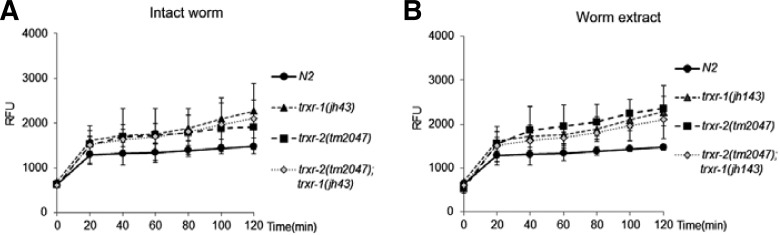
Hydrogen peroxide is over-produced in the TrxR mutant worms. Intact worms (A) or worm extracts (B) were treated with H2DCF-DA, and the fluorescence was measured every 20 min for 120 min. Data were collected and quantified from three independent experiments (t-test, *p < 0.01).
Both trxr-1 and trxr-2 are induced by environmental stresses
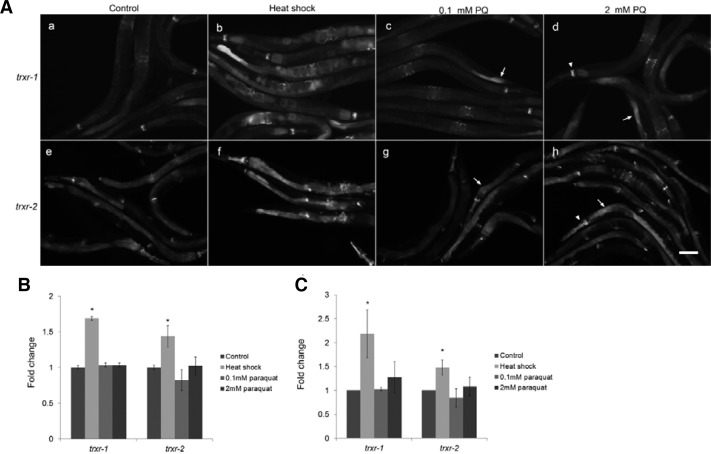
In order to see whether the gene expression of trxr-1 or trxr-2 is regulated by environmental stress, the translational gfp expression transgenic lines were treated with heat shock or paraquat. The GFP fluorescence of TRXR-1:gfp and TRXR-2:gfp fusion proteins were dramatically increased by heat shock (Figs. 6A and 6B). Although the total mRNA level was not changed by the treatment of paraquat treatment (Fig. 6C), the gfp expression is upregulated in some tissues such as intestine and pharynx (arrows and arrowheads in Fig. 6A). This suggests that the gene expression of TrxR in worms is highly regulated by the environmental stress.
Fig. 6.
Gene regulation of trxr-1 and trxr-2 under stress (A) GFP fluorescence in translational trxr-1 and trxr-2 transgenic worms was significantly increased when challenged with heat shock. The paraquat treatment increased the fluorescence in intestine (arrow) and pharynx (arrowhead). (B) Fluorescence intensity of entire worm in (A) was quantified. t-test, *p < 0.001 (C) Endogenous mRNA level in each experimental condition was measured using qRT-PCR, and data from three independent experiments were quantified. t-test, *p < 0.001.
DISCUSSION
Two major ubiquitous TrxRs exist in multi-cellular eukaryotic cells as compartmentalized enzymes; one is cytosolic and the other is mitochondrial. In order to investigate the physiological roles of TrxRs in organismal contexts, we examined trxr-1 and trxr-2 C. elegans mutants in which the genes encoding cytosolic selenoprotein TrxR or mitochondrial one was deleted, respectively. In contrast to mammalian knock-out models of TrxR1 and TrxR2, both of which were embryonic lethal, the counterpart worm mutants, trxr-1(jh143) and trxr-2(tm2047), are viable and their brood size was little changed (Table 1), thus make it possible to assess the roles of those two major ubiquitous TrxRs in various physiological processes. These two mutants showed obvious different phenotypes in longevity, development, subcellular organelle activity, and gene expression, suggesting that those two TrxR genes have discrete functions in the nematode model system.
Mitochondria are major ROS producers in eukaryotes and mitochondrial dysfunction that leads to overproduction of ROS has been postulated as one of the main causes for aging and related diseases (Gromer et al., 2003). Not trxr-1(jh143), but trxr-2(tm2047) and the double mutants of trxr-2(tm2047);trxr-1 (jh143) showed developmental defect and reduced life span under the environmental oxidative stress or at the high temperature, the conditions that generate excessive ROS. Mitochondria may play a dominant role in aging process and development in worms, and mitochondrial TrxR may contribute to protect mitochondria from the oxidative damage. In those regards, it has been shown that the deficiency of mitochondrial redox enzymes results in the increased sensitivity to the oxidative stress and mitochondrial defects in C. elegans (Kern et al., 2003). Interestingly, the depletion of trxr-2, but not trx-2 has been reported to aggravate aging-dependent paralysis caused by the muscle-specific expression of human β-amyloid, which forms pathological plaque aggregates in the brain of Alzheimer’s disease patients where the oxidative stress is a prominent factor of the disease etiology (Barstead, 1999a). Therefore, the protective role of trxr-2 for ageing process may involve multiple pathways possibly including Trx system-independent one.
The regulation of TrxR genes seems to be of complexity, contributed by their complicated genomic structure involving overlapping open reading frames and various alternative splicing (reviewed in) (Arner, 2009). Human TrxR1 gives a rise to a number of different alternative transcripts, which are potentially expressed in a tissue-, cell-, or growth condition-specific manner, and is driven by a promoter showing a typical characteristics of a housekeeping gene, i.e. being TATA-less with transcription driven by Sp1/Sp3 and Oct-1. However, the human TrxR1 promoter also contains Nrf-2-regulated antioxidant responsive element, thus is able to be activated in oxidizing condition such as oxidative stress or electrophilic chemical treatment. On the other hand, the gene regulation of human TrxR2 seems to be more complex, because its open reading frame is overlapped with catechol-O-methyltransferase, encoded in opposite direction from the other DNA strand, in addition to the extensive alternative splicing (Gururajan et al., 2010). In C. elegans, a single transcript arises from trxr-1 or trxr-2 gene, and trxr-2 is transcribed as a polycistronic transcript arising from an operon (www.wormbase.org). Our gene expression regulation study indicates that the expression of both trxr-1 and trxr-2 are highly induced by heat shock and oxidative stress in a tissue-specific manner. Analysis of promoter region revealed no heat shock response element, but one putative skn-1 (the worm ortholog of Nrf-2) binding site in the promoter of trxr-2 (data not shown). Currently, it is not clear how and why nematodal TrxRs are induced by heat shock. Interestingly, thioredoxin reductase from E. coli has been reported to be capable to serve as a molecular chaperone by interacting to renature denatured proteins (Xu et al., 2010). TrxRs in worms under heat shock stress may contribute to recovering the damaged macromolecular systems utilizing multiple properties of the reductase enzyme.
The Trx-reducing activity of mammalian TrxR is totally selenium-dependent, which implies that all of the Trx-dependent systems, in fact, are selenium-dependent (Cocheme and Murphy, 2008). The lower pKa of a selenol and the higher nucleophilicity of a selenolate confers a type of “catalytic advantage” over Cys, when attacked position of thiol-disulfide interchange reaction. Replacement of Sec with Cys in TRXR-1 completely eliminated catalytic activity to reduce thioredoxin (Stenvall et al., 2011). Interestingly, the same Sec replacement in TRXR-1 as well as selenolate TRXR-1 successfully restored vital-dye staining defect, dependent on V-ATPase activity, which did not require thioredoxin. It has been reported that the activity of V-ATPase is regulated by redox status of its disulfide bonding (Feng and Forgac, 1994). The enhanced oxidizing power in cytosol due to the increased level of H2O2 in trxr-1 mutants directly or indirectly altered V-ATPase activity through unknown mechanisms. Alternatively, V-ATPase may be a direct substrate for thioredoxin reductase like other previously known substrates including protein disulfide isomerase, glutaredoxin, and glutathione peroxidase. Then, how is the TRXR-1 with Cys substitution possibly able to be catalytic, although it lost the activity for Trx? It has been reported that replacement of Cys for Sec in TrxR still preserves some catalytic activity (Zhong and Holmgren, 2000). One possible mechanism is that nearby amino acid residues may increase the nucleophilicity of the Cys2 by lowering its pKa as previously proposed in Drosophila melanogaster TrxR (Williams et al., 2000). At a physiological pH, most Cys with pKa∼8.3 will be found in the protonated and thereby rather inert forms, while Sec would mainly be deprotonated and thus more more prone to engage in chemical reaction (Cocheme and Murphy, 2008). However, the actual pKa values of Cys present in protein can be significantly lowered by the combined effects of a number of other residues in the protein, depending on the microenvironment of the polypeptide structure. The specific composition of amino acids surrounding the redox active motif in TRXR-1 may contribute to the maintaining of its catalytic activity. The other possible mechanism would lie on the unique eight-membered ring structure consisted of C-terminal redox active motives in a dimer of the high Mr TrxR. The ring structure of the dyad seems to be more critical in Cys-TrxR than Sel-TrxR for the enzymatic activity (Kanzok et al., 2000). The conformation of TRXR-1 may be compensatory for the Cys substitution for Sel reserving the catalytic activity of the promiscuous enzyme.
Cystein can occur at UGA codon instead of selenocystein at 10 to 50% of the selenocystein levels in mammalian TrxR1 maintained on a diet with normal amounts of selenium and at 50% in liver TR1 of mice maintained on a selenium deficient diet (Xu et al., 2010). This can be achieved by the synthesis of Cys-tRNA[Ser]Sec which is catalyzed by selenophosphate synthetase 2 (SPS2) using thiophosphate as a substrate instead of selenophosphate. Therefore, the Cys insertion to TRXR-1 may be mostly compensatory for the regulation of V-ATPase in the condition of selenium deficiency.
Why does trxr-1 have selenoprotein but trxr-2 does not? The lower pKa of a selenol and the higher nucleophilicity of a selenolate contribute to the high reactivity of the enzyme and to the broad substrate specificty, because they provide catalytic advantage over Cys in the attacking position of thiol-disulfide interchange reaction (Bonilla et al., 2008). The existence of Sec may be an “ancient relic of the anaerobic world”, because Se is more sensitive to oxidation than S (Cho et al., 2010). Sec-containing enzymes are resistant to irreversible inactivation, in part because of the ease with which an oxidized Sec residue in the form of seleninic acid (Sec-SeO2) can be converted back to a selenol in comparison to the very slow chemical conversion of cysteine-sulfinic acid (Cys-SO2) to cysteine thiol (Cys-SH) in a Cys-containing enzyme (Hondal amino acid 2010). Cys-TrxR may provide a more reliable catalytic machinery in mitochondria which is a main source for the ROS in worms, where the broad substrate specificity could be compensated for the stability of the Trx system.
The expression of the two major TrxR isomers, trxr-1 and trxr-2, in C. elegans are compartmentalized in cytoplasm and mitochondria, respectively. The null mutations of the isomers resulted in differential physiological defects in entire organismal context as shown in mammals where more diverse forms of TrxR isomers are employed. The functional compartmentalization of worm TrxRs strongly indicates that different worm cells with different dimension of compartmentalization may have distinctive properties of their redox systems, which in turn supports the cell-specific functions. The study on the expression and regulation patterns of TrxRs in multicellular eukaryotic C. elegans can help understand the roles of the TrxR isoenzymes in humans, as well as hopefully form the basis for improved therapeutic strategies including drug targeting of TrxR in clinical conditions where impaired TrxR function may contribute to the pathogenesis.
Supplementary Material
Acknowledgments
We appreciate Caenorhabditis Genetics Center for worms used in this study. All the pPD vectors were kind gifts provided by Andy Fire. We specially thank the Research Institute at Seoul Medical Center for allowing us to use their research facilities. Dr. Sue Goo Rhee is specially appreciated for his valuable discussion. This research was supported by the World Class University program (no. R33-2008-000-10026-0) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 201200000001029).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Akamatsu Y., Ohno T., Hirota K., Kagoshima H., Yodoi J., Shigesada K. Redox regulation of the DNA binding activity in transcription factor PEBP2. The roles of two conserved cysteine residues. J. Biol. Chem. 1997;272:14497–14500. doi: 10.1074/jbc.272.23.14497. [DOI] [PubMed] [Google Scholar]
- Arner E.S. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Arner E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Barstead R.J. Hope I.A., editor. Reverse genetics. C. elegans: a practical approach. 1999a. pp. 97–118.
- Bonilla M., Denicola A., Novoselov S.V., Turanov A.A., Protasio A., Izmendi D., Gladyshev V.N., Salinas G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J. Biol. Chem. 2008;283:17898–17907. doi: 10.1074/jbc.M710609200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C., Harney J.W., Berry M.J. The Caenorhabditis elegans homologue of thioredoxin reductase contains a selenocysteine insertion sequence (SECIS) element that differs from mammalian SECIS elements but directs selenocysteine incorporation. J. Biol. Chem. 1999;274:21598–21602. doi: 10.1074/jbc.274.31.21598. [DOI] [PubMed] [Google Scholar]
- Cho C.S., Lee S., Lee G.T., Woo H.A., Choi E.J., Rhee S.G. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocheme H.M., Murphy M.P. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Feng Y., Forgac M. Inhibition of vacuolar H(+)-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J. Biol. Chem. 1994;269:13224–13230. [PubMed] [Google Scholar]
- Gladyshev V.N., Krause M., Xu X.M., Korotkov K.V., Kryukov G.V., Sun Q.A., Lee B.J., Wootton J.C., Hatfield D.L. Selenocysteine-containing thioredoxin reductase in C. elegans. Biochem. Biophys. Res. Commun. 1999;259:244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- Grant B., Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromer S., Arscott L.D., Williams C.H., Jr., Schirmer R.H., Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- Gromer S., Johansson L., Bauer H., Arscott L.D., Rauch S., Ballou D.P., Williams C.H., Jr, Schirmer R.H., Arner E.S. Active sites of thioredoxin reductases: why selenoproteins? Proc. Natl. Acad. Sci. USA. 2003;100:12618–12623. doi: 10.1073/pnas.2134510100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan M., Haga C.L., Das S., Leu C.M., Hodson D., Josson S., Turner M., Cooper M.D. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010;22:583–592. doi: 10.1093/intimm/dxq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.M., Lee T.H., Mun J.Y., Kim M.J., Kritikou E.A., Lee S.J., Han S.S., Hengartner M.O., Koo H.S. Deleted in cancer 1 (DICE1) is an essential protein controlling the topology of the inner mitochondrial membrane in C. elegans. Development. 2006;133:3597–3606. doi: 10.1242/dev.02534. [DOI] [PubMed] [Google Scholar]
- Ishii N., Takahashi K., Tomita S., Keino T., Honda S., Yoshino K., Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat. Res. 1990;237:165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Jee C., Vanoaica L., Lee J., Park B.J., Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes Cells. 2005;10:1203–1210. doi: 10.1111/j.1365-2443.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- Jeong S.Y., Choi C.H., Kim J.S., Park S.J., Kang S.O. Thioredoxin reductase is required for growth and regulates entry into culmination of Dictyostelium discoideum. Mol. Microbiol. 2006;61:1443–1456. doi: 10.1111/j.1365-2958.2006.05329.x. [DOI] [PubMed] [Google Scholar]
- Ji Y.J., Choi K.Y., Song H.O., Park B.J., Yu J.R., Kagawa H., Song W.K., Ahnn J. VHA-8, the E subunit of V-ATPase, is essential for pH homeostasis and larval development in C. elegans. FEBS Lett. 2006;580:3161–3166. doi: 10.1016/j.febslet.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Johnson F.B., Sinclair D.A., Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Kanzok S.M., Schirmer R.H., Turbachova I., Iozef R., Becker K. The thioredoxin system of the malaria parasite Plasmodium falciparum. Glutathione reduction revisited. J. Biol. Chem. 2000;275:40180–40186. doi: 10.1074/jbc.M007633200. [DOI] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kern R., Malki A., Holmgren A., Richarme G. Chaperone properties of Escherichia coli thioredoxin and thioredoxin reductase. Biochem. J. 2003;371:965–972. doi: 10.1042/BJ20030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.W., Sung H., Shin D., Shen H., Ahnn J., Lee S.K., Lee S. Differential physiological roles of ESCRT complexes in Caenorhabditis elegans. Mol. Cells. 2011;31:585–592. doi: 10.1007/s10059-011-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon J.C., Kubiseski T.J. Developmental arrest of Caenorhabditis elegans BRAP-2 mutant exposed to oxidative stress is dependent on BRC-1. J. Biol. Chem. 2010;285:13437–13443. doi: 10.1074/jbc.M110.107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey B.M., Hondal R.J. Characterization of mitochondrial thioredoxin reductase from C. elegans. Biochem. Biophys. Res. Commun. 2006;346:629–636. doi: 10.1016/j.bbrc.2006.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers Heerspink H.J., Agarwal R., Coyne D.W., Parving H.H., Ritz E., Remuzzi G., Audhya P., Amdahl M.J., Andress D.L., de Zeeuw D. The selective vitamin D receptor activator for albuminuria lowering (VITAL) study: study design and baseline characteristics. Am. J. Nephrol. 2009;30:280–286. doi: 10.1159/000225903. [DOI] [PubMed] [Google Scholar]
- Larsen P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.R., Kim J.R., Kwon K.S., Yoon H.W., Levine R.L., Ginsburg A., Rhee S.G. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999;274:4722–4734. doi: 10.1074/jbc.274.8.4722. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Li W., Ryu S.E., Rhim T., Ahnn J. Vacuolar (H+)-ATPases in Caenorhabditis elegans: what can we learn about giant H+ pumps from tiny worms? Biochim. Biophys. Acta. 2010;1797:1687–1695. doi: 10.1016/j.bbabio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Li L., Hsiao W.W., Nandakumar R., Barbuto S.M., Mongodin E.F., Paster B.J., Fraser-Liggett C.M., Fouad A.F. Analyzing endodontic infections by deep coverage pyrosequencing. J. Dent. Res. 2010;89:980–984. doi: 10.1177/0022034510370026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder B., Haas J., Keller A., Heid C., Just S., Borries A., Boisguerin V., Scharfenberger-Schmeer M., Stahler P., Beier M., et al. Targeted next-generation sequencing for the molecular genetic diagnostics of cardiomyopathies. Circ. Cardiovasc. Genet. 2011;4:110–122. doi: 10.1161/CIRCGENETICS.110.958322. [DOI] [PubMed] [Google Scholar]
- Mello C., Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Mustacich D., Powis G. Thioredoxin reductase. Biochem. J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- Oka T., Futai M. Requirement of V-ATPase for ovulation and embryogenesis in Caenorhabditis elegans. J. Biol. Chem. 2000;275:29556–29561. doi: 10.1074/jbc.M002756200. [DOI] [PubMed] [Google Scholar]
- Park B.J., Lee D.G., Yu J.R., Jung S.K., Choi K., Lee J., Kim Y.S., Lee J.I., Kwon J.Y., Singson A., et al. Calreticulin, a calcium-binding molecular chaperone, is required for stress response and fertility in Caenorhabditis elegans. Mol. Biol. Cell. 2001;12:2835–2845. doi: 10.1091/mbc.12.9.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N., Bonnerot C., Ewbank J.J., Kohara Y., Thierry-Mieg D. The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J. Biol. Chem. 2001;276:11913–11921. doi: 10.1074/jbc.M009451200. [DOI] [PubMed] [Google Scholar]
- Radyuk S.N., Rebrin I., Klichko V.I., Sohal B.H., Michalak K., Benes J., Sohal R.S., Orr W.C. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic. Biol. Med. 2010;49:1892–1902. doi: 10.1016/j.freeradbiomed.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Snider G., Grout L., Ruggles E.L., Hondal R.J. Metha-neseleninic acid is a substrate for truncated mammalian thioredoxin reductase: implications for the catalytic mechanism and redox signaling. Biochemistry. 2010;49:10329–10338. doi: 10.1021/bi101130t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvall J., Fierro-Gonzalez J.C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arner E.S., Persson O.P., Miranda-Vizuete A., Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.A., Wu Y., Zappacosta F., Jeang K.T., Lee B.J., Hatfield D.L., Gladyshev V.N. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J. Biol. Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- Taskov K., Chapple C., Kryukov G.V., Castellano S., Lobanov A.V., Korotkov K.V., Guigo R., Gladyshev V.N. Nematode selenoproteome: the use of the selenocysteine insertion system to decode one codon in an animal genome? Nucleic Acids Res. 2005;33:2227–2238. doi: 10.1093/nar/gki507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.H., Arscott L.D., Muller S., Lennon B.W., Ludwig M.L., Wang P.F., Veine D.M., Becker K., Schirmer R.H. Thioredoxin reductase two modes of catalysis have evolved. Eur. J. Biochem. 2000;267:6110–6117. doi: 10.1046/j.1432-1327.2000.01702.x. [DOI] [PubMed] [Google Scholar]
- Xu X.M., Turanov A.A., Carlson B.A., Yoo M.H., Everley R.A., Nandakumar R., Sorokina I., Gygi S.P., Gladyshev V.N., Hatfield D.L. Targeted insertion of cysteine by decoding UGA codons with mammalian selenocysteine machinery. Proc. Natl. Acad. Sci. USA. 2010;107:21430–21434. doi: 10.1073/pnas.1009947107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J. Biol. Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.