Abstract
We studied the role of a RhoA-specific guanine nucleotide exchange factor (p190RhoGEF) in dendritic cells (DCs), using transgenic (TG) mice that over-express a full gene of p190RhoGEF under the control of an invariant chain promoter. TG mice lacked localization of activated DCs to the T cell zone in the spleen and had reduced serum levels of IL-6 in response to lipopolysaccharide (LPS) injection. DCs from these mice also showed reduced surface expression of CD86, CD40, and CD205, but not MHCII, as well as reduced capability to uptake antigen. Moreover, chemokinedriven migration and secretion of IL-6, but not of IL-12, were impaired after LPS-stimulation of TG DCs. Collectively, these results suggest that over-expressing p190RhoGEF negatively regulates conventional DC function in response to bacterial LPS infection.
Keywords: dendritic cells, lipopolysaccharides, p190RhoGEF
INTRODUCTION
A specific guanine nucleotide exchange factor for RhoA, p190RhoGEF, was first identified for its role in controlling neuronal morphology (Gebbink et al., 1997). Over-expression of p190RhoGEF in neuronal cells mimicked the activity of activated RhoA and stimulated cytoskeletal contraction, preventing neurite outgrowth (Gebbink et al., 1997). Although expression of p190RhoGEF was shown to be ubiquitous (Gebbink et al., 1997), most studies on the role of this protein have been reported in neuronal systems.
We previously identified p190RhoGEF as one of the proteins whose expression is dramatically increased in B cells following CD40 stimulation (Lee et al., 2003). We showed that over-expression of p190RhoGEF or constitutive activation of RhoA mimicked the effects of CD40 stimulation in B cells (Lee et al., 2003). Our studies in B cells also showed that the changes in cellular morphology that are regulated by p190RhoGEF during CD40-mediated B cell activation were correlated with the expression of surface molecules and transcriptional regulators required for B cell maturation and differentiation (Ha et al., 2012).
We created transgenic (TG) mice, in which expression of p190RhoGEF is driven by an invariant chain (Ii) promoter, to further examine the role of p190RhoGEF in vivo (Fig. 1A). Thus, transgenes are specifically expressed in antigen presenting cells (APCs) with class II major histocompatibility complex (MHC), including B cells, macrophages, and dendritic cells (DCs).
Fig. 1.
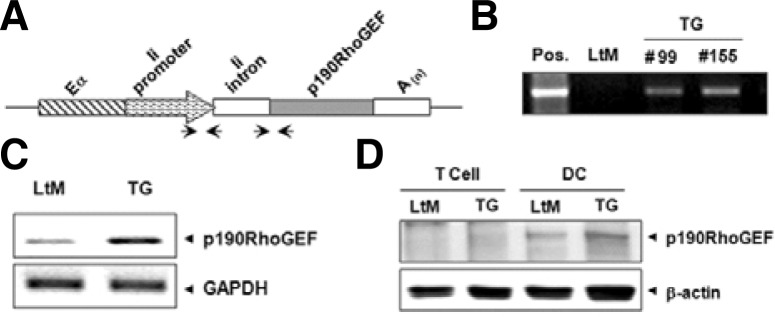
p190RhoGEF transgene expression in CD11c-expressing DCs. (A) A schematic view of p190RhoGEF transgene in the pDOI expression vector: a cassette vector for high-level expression driven by a hybrid invariant chain promoter, consisting of the promoter region spliced together with the enhancer elements (Eα) from the first intron of the Ii gene. Arrows indicate primer pairs used for the detection of the integrated transgene. (B) Transgene inclusion was analyzed by PCR on genomic tail DNA from non-TG, littermate (LtM), and two separate TG mice. (C) mRNA transcripts of p190RhoGEF in CD11c+ DCs isolated from the spleens of LtM and TG mice. mRNA transcripts of GAPDH were shown as loading controls. (D) Expression analysis of p190RhoGEF proteins in CD11c+ DCs and T cells isolated from the spleens of LtM and TG mice. Expression of β-actin was shown as loading controls. Data shown are representatives of five TG lines tested.
DCs specialize in handling antigens (Ags), from capturing and processing them to presenting their peptides to lymphocytes (Banchereau and Steinman, 1998; Banchereau et al., 2000; Guermonprez et al., 2002). DCs exist in an immature stage that is specifically primed to capture Ags. Naïve DCs undergo a complex maturation process into APCs after pathogen stimulation through the interaction between pathogen-associated molecular patterns (PAMPs) on microbes and the pattern recognition receptors (PRRs), including toll-like receptors (TLRs), on DCs (Akira et al., 2006; Janeway et al., 1989; Medzhitov and Janeway, 2002). DCs secrete a panel of chemokines and cytokines that attract various cell types (Piqueras et al., 2006) and that activate DCs themselves and induce T cell differentiation into specific lineages (Flynn et al., 1998; Ito et al., 2007). DCs also express a unique set of co-stimulatory molecules that, together with their secreted cytokines, help naïve T cells to become activated and to differentiate into different lineages (Flynn et al., 1998; Ito et al., 2007), leading to a primary immune response.
In this study, we examined the role of p190RhoGEF in DCs that highly express the CD11c surface marker. These conventional DCs were isolated from the TG mice that over-expressed p190RhoGEF specifically in APCs. The surface expression levels of CD86, CD40 and CD205 were low and Ag uptake ability was also reduced in DCs from mice over-expressing p190RhoGEF compared to those from littermate (LtM) mice. Moreover, lipopolysaccharide (LPS)-stimulated TG DCs showed impaired expression of IL-6 but not of IL-12. Similarly, LPS-stimulated TG DCs failed to localize to the T cell zone in the spleen and showed impaired IL-6 expression. Collectively, our current study suggests that over-expression of p190RhoGEF negatively regulates the functions of conventional DCs in response to bacterial LPS infection.
MATERIALS AND METHODS
Generation of p190RhoGEF-TG mice
The cDNA encoding p190RhoGEF was excised from the pcDNA3 expression vector (Lee et al., 2003; van Horck et al., 2001) by digesting with XbaI and Bgl II/NotI, respectively. The cDNA was ligated into pBluescript and pLNCX2 retroviral vectors (Clontech Laboratories, Inc., USA) and was further cloned into the pDOI-6 expression vector (van Santen et al., 2000) (a kind gift from D. Mathis, Harvard Medical School, USA), at the ClaI site. To generate TG lines, the purified fragments of the linearized expression plasmid were injected into fertilized mouse eggs (C57Bl/6) (Macrogen, Korea). The resulting mice were tested for integration of the transgene by PCR analysis of tail DNA. Five TG mice were chosen as founders.
Antibodies (Abs) and reagents
A polyclonal anti-mouse p190RhoGEF Ab (LF-r-gef) was described previously (Ha et al., 2012). The following monoclonal Abs (mAbs) were purchased from eBioscience, Inc.: purified rat anti-mouse CD4 (RM4-5), biotin-conjugated rat anti-mouse MHC class II (M5/114.15.2), CCR7 (EBI-1, 4B12), CD205 (DEC205, 205yekta), CD86 (B7-2, GL1), fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8a (53-6.7) and CD40 (HM40-3). R-phycoerythrin (PE)-conjugated hamster anti-mouse CD11c (HL3) and CD3e (145-2C11), peridinin chlorophyll protein (PerCP)-conjugated rat anti-mouse CD4 (RM4-5), purified hamster anti-mouse CD11c (HL3), FITC-conjugated and purified rat anti-mouse CD45R/B220 (RA2-6B2) were obtained from BD Pharmingen (USA). FITC-conjugated goat anti-rat IgG, FITC-, allophycocyanin- or PerCP-conjugated streptavidin and isotype control IgGs were purchased from Jackson ImmunoResearch Laboratories, Inc. (USA), eBioscience, Inc., or BD PharMingen. Ready-SET-Go cytokine ELISA (enzyme-linkied immunosorbent assay) sets for mouse IL-6 and IL-12 total p40 were obtained from eBioscience, Inc. mAb for β-actin (C4) was purchased from Santa Cruz Biotechnology, Inc. (USA). A horse radish peroxidase (HRP)-conjugated anti-rabbit IgG (Bio-Rad, USA) was used as a secondary Ab for immunoblotting (IB). Magnetic particle-labeled anti-CD11c Ab and a pan-T cell isolation kit from Miltenyi Biotec (Bergisch Gladbach, Germany) and enhanced chemiluminescence (ECL) reagents from Amersham Pharmacia Biotech Co. (USA) were used. TRIzol reagent and FITC-dextran (molecular weight 40 kDa) were purchased from Life Technologies (USA), and Oligo(dT)12–18 primer and M-MLV-reverse transcriptase were purchased from Promega (Madison, WI). Recombinant mouse CCL21 (250-13) from PeproTech Inc. (USA), LPS from Escherichia coli (serotype 055:B5) from Sigma-Aldrich Co. (USA), and collagenase D from Roche Applied Science (Germany) were used. Tissue-Tek OCT (optimal cutting temperature) compound was obtained from Sakura Finetechnical Co. Ltd. (Japan). CellTracker Orange CMTMR was purchased from Molecular Probes (USA).
Mice and endotoxin injection
Mice were bred and maintained under specific pathogen-free conditions at the animal facility of Ewha Laboratory Animal Genomic Center in accordance with institutional guidelines. Ten- to thirteen-week-old mice were used for all experiments. Mice were injected intraperitoneally with 25 μg of LPS (Escherichia coli serotype 055:B5) solubilized in 200 μl of pyrogen-free PBS. The control animals were injected with the same volume of PBS. The response in mice was studied 6 h after injection. All mouse protocols were approved by Ewha Institutional Animal Care and Use Committee.
Immunohistochemistry
The spleens from the LPS-injected and control mice were removed and were embedded in Tissue-Tek OCT compound by quick freezing with liquid N2. These frozen tissues were stored at −70°C. Five to seven micrometer sections were cut on a cryostat (Leica Microsystems GmbH, Germany) and were mounted onto poly-L-lysine-coated slides. The sections were air dried for 10 min before fixing them in ice-cold acetone for 10 min, air drying them again and storing them at −20°C. The splenic sections were rehydrated with PBS and were blocked with 5% BSA in PBS for 20 min at room temperature. The sections were stained with primary Abs (PE-conjugated anti-CD11c and anti-CD3, and purified anti-B220) for 1–3 h. For B220 staining, the sections were washed gently with PBS and incubated with a FITC-conjugated secondary Ab for 30 min. The sections were washed gently with PBS before embedding in 50% glycerol and covering with a coverslip. The samples were analyzed using a Zeiss Axiovert 200 fluorescence microscope along with AxioVision or LSM software (Carl Zeiss, Germany).
Purification of DCs and T cells
To generate splenic cell suspensions, spleen pieces were incubated with collagenase D (1 mg/ml) in RPMI medium for 30 min at 37°C, as described previously (Hou and Van Parijs, 2004). The DCs and T cells were enriched using a CD11c+ magnetic isolation kit and a pan-T cell isolation kit, respectively, according to the manufacturer’s instruction.
RNA extraction and RT-PCR
CD11c-expressing DCs were purified from the spleens of LtM control and TG mice. RNA was extracted from purified DCs using TRIzol reagent. cDNA was prepared from an RNA template with Oligo(dT) primers and was subjected to PCR. A fragment of either p190RhoGEF or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified, and the PCR products were separated on a 1.2% agarose gel.
IB analysis
IB was performed as described previously (Lee and Koretzky, 1998). Whole cell lysates of CD11c+ cell-enriched fractions were prepared in 1% Nonidet P-40 lysis buffer containing protease and phosphatase inhibitors; 50 μg of the lysates were then subjected to 6% SDS-PAGE. IB analysis was performed with an anti-mouse p190RhoGEF polyclonal Ab (LF-r-gef) or an anti-mouse β-actin Ab, followed by an HRP-conjugated secondary Ab. Chemiluminescent detection was conducted with ECL reagents.
Flow cytometry
Single-cell suspensions from spleens (2 × 106/ml) or isolated DCs (1 × 105/ml) were washed in staining buffer (PBS containing 0.5% bovine serum albumin and 0.05% sodium azide) and blocked with the appropriate sera. The cells were stained with biotin- or fluorochrome-conjugated specific cell surface marker Abs in staining buffer according to standard procedures. The data were acquired on a FACSCalibur using CELLQuest software (BD Biosciences, USA). Light scattering and fluorescent signals were analyzed as dot plots of forward and side scatter (FSC-H and SSC-H) versus the fluorescence intensity channel (FL).
Uptake assay
Fresh DCs (1.5 × 105/ml) purified from splenocytes were pre-incubated in a complete RPMI medium for 30 min at 37°C and were cultured with 40 kDa FITC-dextran at a final concentration of 1 mg/ml in complete RPMI medium. After being incubated at 37°C or 4°C for 1 h, the cells were washed three times in PBS, and the FITC intake was analyzed using a FACS.
In vitro migration assay
An in vitro DC chemotaxis assay was performed using Costar Transwell inserts (5 μm pores) in 48-well plates. Fresh DCs were purified from splenocytes and were incubated in complete RPMI medium with or without LPS (100 ng/ml) at 37°C. After being incubated for 24 h, the cells were labeled with CMTMR for 10 min. These DCs (1.5 × 105) were suspended in serum-free RPMI medium and were put in the upper Transwell insert in a volume of 100 μl. The lower well contained 600 μl serum-free RPMI medium containing a recombinant murine chemokine, CCL21 (100 ng/ml). The plates were incubated at 37°C for 90 min, and the migrated cells on the lower chamber were harvested. Analyses of cell density were performed by counting the cells that were collected for 60 s using flow cytometry. The results were further confirmed by reading the emission from the harvested cells at 565 nm using a microplate reader (Molecular Devices, Spectra MAX 190).
ELISA
For measurements of mouse IL-12 or IL-6, Ready-SET-Go cytokine ELISA sets were used according to the manufacturer’s instructions. Isolated DCs (5 × 104) were stimulated with 200 μl of medium in the presence or absence of LPS (100 ng/ml) in a 96-well U-bottomed culture plate. After incubating the cells for 24 h, the culture supernatants were collected. Sera were also obtained from the mice that were injected with LPS (25 μg in 200 μl PBS) for 6 h and were diluted serially. Culture supernatants and serum samples were added to each well of 96-well flat-bottomed ELISA plates that were prepared for the assay. At the end of the assay, the plates were read at 450 nm using a microplate reader (Molecular Devices, Spectra MAX 190).
Statistical analysis
Comparisons between samples were performed using paired or two-tailed Student’s t-test. Statistics were determined using Prism software (GraphPad Software, Inc.). Values of p < 0.05 were considered significant.
RESULTS
Expression of p190RhoGEF in mouse DCs expressing CD11c
Plasmids were constructed by inserting the entire coding region of p190RhoGEF into the pDOI-6 vector (van Santen et al., 2000) under the control of an invariant chain (Ii) promoter to allow specific transgene expression in APCs (Fig. 1A). Five founder mice that were transgene-positive were established by probing genomic DNA with primers for the Ii promoter sequence. Figure 1B shows the representative genomic DNA screening results from two lineages. Studies were performed in mice after 10-generation back-crossing and similar results were repeated from three TG lineages at least.
We tested whether p190RhoGEF transgenes are expressed in the DCs from these mice because the DCs are professional APCs that express class II MHC molecules. Conventional DCs expressing the CD11c surface marker were isolated from the spleens of LtM control, or p190RhoGEF TG mice. The transcriptional levels of p190RhoGEF were compared by RT-PCR (Fig. 1C), and the protein expression was analyzed by Western blotting on DC cellular extracts using an anti-p190RhoGEF Ab (Fig. 1D). To demonstrate specific transgene expression in APCs, the expression of p190RhoGEF in T cells that do not express class II MHC molecules was also shown (Fig. 1D). As shown in Figs. 1C and 1D, endogenous expression of p190RhoGEF was seen in DCs isolated from the spleens of LtM control mice, and the expression of p190RhoGEF transgene was confirmed in DCs isolated from the spleens of TG mice.
DC function in p190RhoGEF TG mice after LPS injection
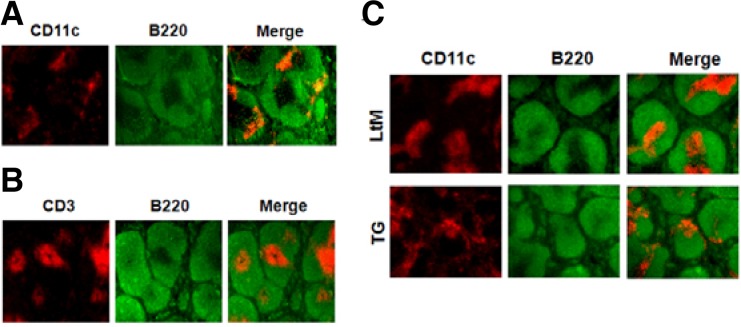
We first examined whether the expression of p190RhoGEF transgene in DCs changed their characteristic functions in vivo. To test this, LtM control or p190RhoGEF TG mice were injected with LPS and changes in the spleens were examined by histology. Although how DC precursors enter the spleen has not been characterized in detail, DCs are found in an immature stage in the marginal zone of the spleen and mobilize to the T cell zone of the splenic white pulp during maturation and activation (Blanco et al., 2008; Randolph et al., 2005; 2008). Figure 2A shows a spleen section from a control mouse. DCs were stained with anti-CD11c Ab, and the marginal zone was indicated by anti-B220 staining (Fig. 2A). When the images of the CD11c and B220 staining were merged, DCs were found scattered in regions outside of the white pulp or in the marginal zone (Fig. 2A). CD3 staining for the T cell zone merged with B220 staining for the marginal zone of the splenic white pulp was also presented in Fig. 2B. As shown by immunohistochemistry of splenic sections of mice after LPS injection (Fig. 2C), activated DCs were localized to the T cell zone in the splenic sections from LtM control mice. In contrast, DCs did not localize to the T cell zone in spleens from LPS-injected TG mice (Fig. 2C).
Fig. 2.
DC localization in the spleens of LtM control or p190RhoGEF TG mice after LPS injection. (A) A frozen mouse spleen section was stained with PE-conjugated anti-mouse CD11c and FITC-conjugated anti-mouse B220 Abs. When merged together, CD11c+ DCs (red) were shown scattered in the marginal zone of B cell area (green) outside of T cell zone. (B) By staining a frozen mouse spleen section using PE-conjugated anti-CD3 and FITC-conjugated anti-mouse B220 Abs, the T cell zone (red) was discriminated from the B cell area (green). (C) Frozen spleen sections were prepared from LtM control or TG mice that were injected with LPS (25 μg) for 6 h. By staining sections with PE-conjugated anti-mouse CD11c and FITC-conjugated anti-mouse B220 Abs, the localizations of DCs were compared. When merged together, the DCs (red) were stained within the T cell zone in the LtM mice. In contrast, the DCs (red) were shown scattered in the marginal zone of B cell area (green) outside of the T cell zone in the TG mice. Experiments were performed in three distinct TG lineages at least. The data shown are representative of at least six separate experiments.
The sera of these LPS-injected mice were also evaluated for cytokine secretion. The level of secreted IL-12 did not significantly differ for LtM and TG (Fig. 3A). However, IL-6 secretion varied after LPS injection: TG mice had significantly reduced IL-6 secretion after LPS injection compared to the LtM control mice (Fig. 3B).
Fig. 3.
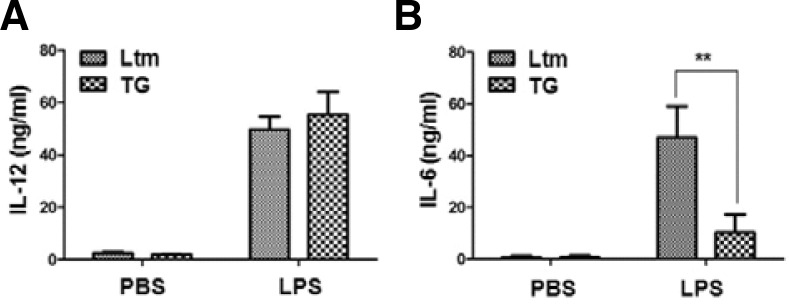
Cytokine secretion in p190RhoGEF TG mice after LPS injection. Age-matched mice (LtM control or p190RhoGEF TG, three mice in each group) were injected with either PBS or LPS (25 μg) for 6 h as described in the “Materials and Methods”. Each mouse serum was collected by eye bleeding and was diluted serially. The level of IL-12 (A) or IL-6 (B) in the serum of each mouse was compared by ELISA. Experiments were performed in three distinct TG lineages at least and repeated more than three times. The data are the mean ± SD of three independent experiments. Statistical significance was analyzed by two-way ANOVA with Bonferroni multiple comparison tests. Significant comparisons are noted as **, p < 0.01.
Expression of surface markers in splenic DCs of p190RhoGEF TG mice
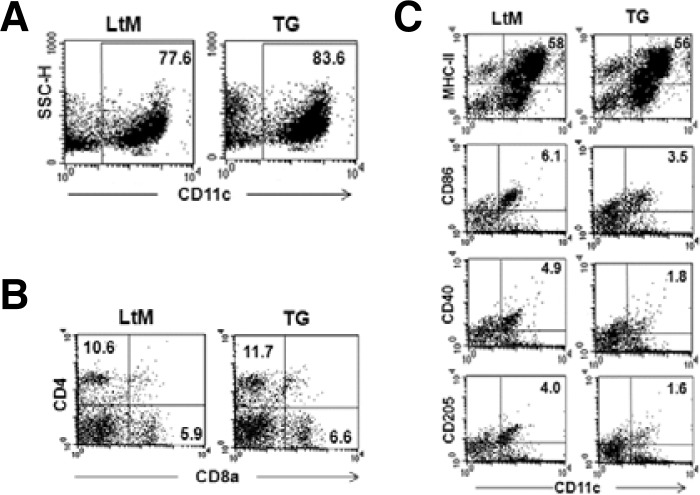
We next examined whether over-expressing p190RhoGEF altered the expression levels of surface co-receptor molecules in DCs (Fig. 4). Because DCs isolated from mouse spleen mostly contain CD4+ myeloid and CD8a+ lymphoid DCs (Banchereau et al., 2000), flow cytometric analyses of these subsets were compared for LtM and p190RhoGEF TG mice. The overall percentage of isolated CD11c-expressing DCs was similar, although TG mice had slightly higher numbers (Fig. 4A). Similarly, the percentage of CD4+ myeloid and CD8a+ lymphoid subsets in CD11c-expressing DCs was not much different (Fig. 4B). However, TG mice showed between 40–60% reduction in the population of DCs expressing CD86, CD40 and CD205 (Fig. 4C). In contrast, DC population showing class II MHC expression was similar in LtM and TG (Fig. 4C).
Fig. 4.
Analyses of subset populations and surface marker expression in DCs isolated from the spleens of p190RhoGEF TG mice. CD11c+ DCs were isolated from the spleens of each genotype group and were purified as described in the Methods section. Purified DCs were labeled with PE-conjugated anti-CD11c mAb along with combinations of mAbs for different surface co-receptor molecules followed by three-color flow cytometric analyses. (A) The results are presented as a dot plot, showing the cellular change (SSC-H) vs. CD11c staining. The CD11c+ DC population was compared as a percentage of the total cells. (B) For the cell populations gated by CD11c expression, dot plots are presented for CD4 (PerCP) vs. CD8a expression (FITC). The CD4+ myeloid and CD8a+ lymphoid DC populations were compared by the percentage of total cells. (C) Dot plots compare the expression of MHC-II, CD86, CD40 or CD205 vs. CD11c. The populations of CD11c+ DCs expressing each surface molecule were reported as the percentage of total cells. The fluorescence intensities of the cells stained with an isotype control Ab or antiserum were all corrected (data not shown). Experiments were performed in three distinct TG lineages at least. The data are representative of three separate experiments.
Functional analyses of splenic DCs from p190RhoGEF TGc mice
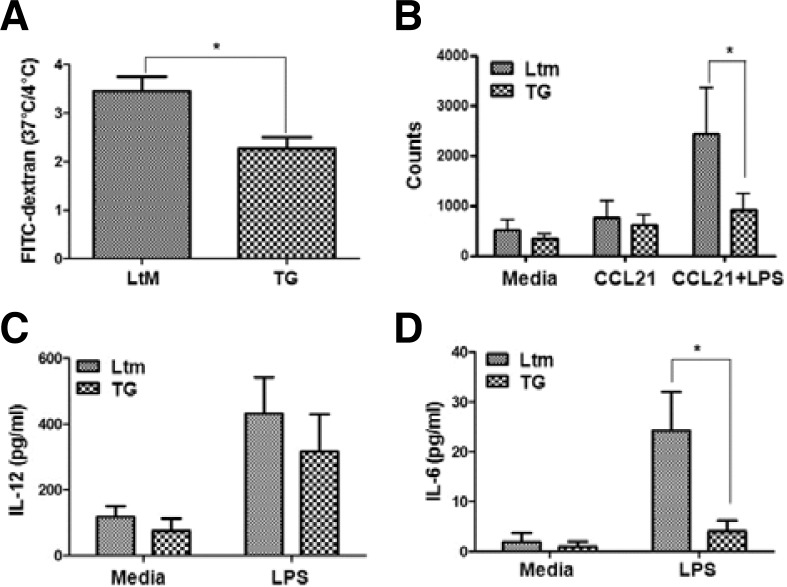
DCs that reside in the spleen are found in an immature stage and retain the capacity to internalize Ag. DCs from the spleens of LtM or p190RhoGEF TG mice were incubated with FITC-conjugated dextran (40 kDa) at 4°C or 37°C for 1 h to examine their capacity to uptake Ag. The uptake capacity of DCs was compared as the mean fluorescence intensity of dextran that was internalized into DCs at 37°C, and the value was normalized by the basal fluorescence level of dextran uptake at 4°C, which was similar for DCs from LtM and TG mice (Fig. 5A). The dextran internalization capacity, presented as the fluorescence ratio at 37°C over the fluorescence at 4°C, was significantly reduced in DCs from TG spleens, compared to the uptake capacity of splenic DCs from LtM mice (Fig. 5A). These results suggest that over-expression of p190RhoGEF in DCs negatively regulates the ability to capture Ag, which may correlate with the relatively low CD205 expression, which is an Ag uptake receptor in DCs, in TG mouse spleens (Fig. 4C).
Fig. 5.
Functional analyses of splenic DCs from p190RhoGEF TG mice. (A) For Ag uptake, the DCs from the spleens of LtM control and p190RhoGEF TG mice were incubated with FITC-dextran (40 kD) at 4°C or at 37°C for 1 h. The uptake capacity was compared as a ratio of the fluorescence at 37°C over the fluorescence at 4°C. Experiments were performed at least in three distinct TG lineages and repeated more than three times. The data are the mean ± SD of three independent experiments. Statistical significance was analyzed by a paired t-test. Significant comparison is noted as *, p < 0.05. (B) Splenic DCs from LtM control or WT p190RhoGEF TG mice were cultured either in the media with or without LPS (25 μg/ml) overnight. CCL21-dependent migration of these DCs was compared by counting the migrated cells on the lower chamber as described in the “Materials and Methods.” Experiments were performed at least in three distinct TG lineages and repeated more than three times. The data are the mean ± SD of three independent experiments. Statistical significance was analyzed by two-way ANOVA with Bonferroni multiple comparison tests. Significant comparisons are noted as *, p < 0.05. (C, D) Splenic DCs from LtM control or p190RhoGEF TG mice were cultured either in the media alone or with LPS (25 μg/ml) overnight. The level of IL-12 (A) or IL-6 (B) in the supernatants was compared by ELISA. Experiments were performed in three distinct TG lineages at least and repeated more than three times. The data are the mean ± SD of three independent experiments with triplet sample settings. Statistical significance was analyzed by two-way ANOVA with Bonferroni multiple comparison tests. Significant comparisons are noted as *, p < 0.05.
We examined whether the expression of p190RhoGEF in the DCs of TG mice altered the chemokine-induced migratory ability of DCs because DCs upregulate CCR7 and become responsive to the CCL21 ligand during maturation and terminal activation into APCs (Randolph et al., 2005; 2008). To compare the CCL21-dependent migration of DCs from LtM and TG mouse spleens, the density of migrated cells was analyzed by flow cytometry (Fig. 5B). DCs from LtM mice migrated toward CCL21; this migration was further enhanced after LPS stimulation. In contrast, less migration was seen in DCs from p190RhoGEF TG mice compared to those from the LtM control mice (Fig. 5B). These results are not due to different levels of CCR7 expression because the DCs from LtM and p190RhoGEF TG mice showed similar CCR7 expression levels (data not shown). The results from this experiment further support that overe-xpression of p190RhoGEF in DCs negatively regulates their ability to migrate.
During maturation and activation, DCs secrete pro-inflammatory cytokines that further activate themselves and also cause T cells to differentiate into specific lineages (Blanco et al., 2008). Among these cytokines, we examined the production of IL-12 and IL-6 by DCs from LtM or TG mouse spleens. Figure 5C shows that the DCs isolated from the spleens of LtM and TG mice produced similar levels of IL-12 after LPS stimulation. However, the LPS-induced secretion of IL-6 was significantly reduced in the DCs from the TG spleen (Fig. 5D). These results coincide with the reduced serum levels of IL-6 after LPS injection in vivo (Fig. 3B).
DISCUSSION
In this study, we examined whether p190RhoGEF was expressed in DCs and whether constitutive expression of p190RhoGEF affected DC function. In contrast to B cells, in which p190RhoGEF expression is induced only in mature/activated B cells (Ha et al., 2012; Lee et al., 2003), CD11c-expressed conventional DCs expressed p190RhoGEF endogenously, both at the RNA and protein levels (Figs. 1C and 1D). Expression of the p190RhoGEF transgene in the DCs did not change the total DC population or either subset population (CD4+ myeloid and CD8a+ lymphoid DCs) (Figs. 4A and 4B).
Although the exact mechanisms remain to be resolved, the level of maturation/activation of DCs may be directly or indirectly regulated by the activity of p190RhoGEF. Over-expression of p190RhoGEF affected the levels of surface expression of CD205, CD86, and CD40 in the DCs, as well as their ability to take up Ag and to migrate in a chemokine-dependent manner. Furthermore, the p190RhoGEF over-expression in DCs may stimulate cytoskeletal contraction by activating RhoA and preventing migratory protrusions, resulting in defective DC mobilization to the T cell zone after LPS injection, as shown in neuronal cells (Gebbink et al., 1997).
Interestingly, the over-expression of p190RhoGEF differentially regulated the secretion of IL-12 and IL-6 by DCs. These results suggest that these two cytokines may be produced by DCs at different stages of maturation/activation and that IL-6 secretion may depend on the complete activation of DCs and, thus, their localization to the T cell zone of the white pulp in the spleen. The relatively low levels of surface expression of CD86 and CD40 in the DCs of TG mice also suggest possibilities for the regulation of immunomodulatory cytokine production by the signals from these co-receptors. IL-6 is known to induce the differentiation and activation of many immune cell types, including B and T lymphocytes, macrophages, and megakaryocytes (Kishimoto et al., 1995). More importantly, recent studies have also described the role of IL-6 in the differentiation of a novel T cell subset, Th17, which displays pro-inflammatory functions (Harrington et al., 2005; Park et al., 2005). Thus, IL-6 is likely to be involved in the pathogenesis of inflammatory diseases.
Here, we show for the first time that the over-expression of p190RhoGEF negatively regulates DC function in response to LPS by using TG mice over-expressing p190RhoGEF. Although the regulatory mechanisms of p190RhoGEF remain to be studied, these results may provide new insights into the regulation of DCs in the pathogenesis of various inflammatory and autoimmune diseases. Moreover, considering our previous report in B cells (Ha et al., 2012; Lee et al., 2003), p190RhoGEF plays distinct roles in the regulation and activation of B cells and DCs. Identifying novel factors that interact with the C-terminal region of p190RhoGEF may help explain the distinct regulation of DC or B cell functions by p190RhoGEF.
Acknowledgments
We thank Drs. W. Moolenaar and D. Mathis for providing p190RhoGEF plasmid and pDOI expression vector, respectively. This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090743) and grants (2009-0067361, 2012-0000952) from the National Research Foundation of Korea funded by the Korean government. H.J.S., Y.R.A., H.M.S., and Y.J.H. were supported in part by the second stage of the Brain Korea 21 Program of the Korea Ministry of Education.
REFERENCES
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Blanco P., Palucka A.K., Pascual V., Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S., Toellner K.M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink M.F., Kranenburg O., Poland M., van Horck F.P., Houssa B., Moolenaar W.H. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J. Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P., Valladeau J., Zitvogel L., Thery C., Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Ha Y.J., Jeong J.H., Park Y., Lee J.R. Increased p190RhoGEF expression in activated B cells correlates with the induction of the plasma cell program. Exp. Mol. Med. 2012;44:138–148. doi: 10.3858/emm.2012.44.2.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hou W.S., Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 2004;5:583–589. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- Ito T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A. Plasmacytoid dendritic cells prime IL-10-productin T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Jr, Yagi J., Conrad P.J., Katz M.E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol. Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Narazaki M., Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- Lee J.R., Koretzky G.A. Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur. J. Immunol. 1998;28:4188–4197. doi: 10.1002/(SICI)1521-4141(199812)28:12<4188::AID-IMMU4188>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lee J.R., Ha Y.J., Kim H.J. Cutting edge: Induced expression of a RhoA-specific guanine nucleotide exchange factor, p190RhoGEF, following CD40 stimulation and WEHI 231 B cell activation. J. Immunol. 2003;170:19–23. doi: 10.4049/jimmunol.170.1.19. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producting interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras B., Connolly J., Freitas H., Palucka A.K., Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G.J., Angeli V., Swartz M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–618. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Randolph G.J., Ochando J., Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- van Horck F.P., Ahmadian M.R., Haeusler L.C., Moolenaar W.H., Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J. Biol. Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- van Santen H.-M., Benoist C., Mathis D. A cassette vector for high-level reporter expression driven by a hybrid invariant chain promoter in transgenic mice. J. Immunol Methods. 2000;245:133–137. doi: 10.1016/s0022-1759(00)00276-3. [DOI] [PubMed] [Google Scholar]