Abstract
CD38, an ADP ribosyl cyclase, is a 45 kDa type II transmembrane protein having a short N-terminal cytoplasmic domain and a long C-terminal extracellular domain, expressed on the surface of various cells including macrophages, lymphocytes, and pancreatic β cells. It is known to be involved in cell adhesion, signal transduction and calcium signaling. In addition to its transmembrane form, CD38 is detectable in biological fluids in soluble forms. The mechanism by which CD38 is solubilized from the plasma membrane is not yet clarified. In this study, we found that lipopolysaccharide (LPS) induced CD38 upregulation and its extracellular release in J774 macrophage cells. Furthermore, it also increased CD38 expression at the mRNA level by activating the Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway. However, LPS decreased the levels of CD38 in the plasma membrane by releasing CD38 into the culture supernatant. LPS-induced CD38 release was blocked by the metalloproteinase-9 inhibitor indicating that MMP-9 solubilizes CD38. In conclusion, the present findings demonstrate a potential mechanism by which C38 is solubilized from the plasma membrane.
Keywords: CD38, interferon β, JAK-STAT, lipopolysaccharide, macrophages, metalloproteinase-9
INTRODUCTION
CD38 (ADP-ribosyl cyclase) leukocyte antigen is a 45 kDa type II transmembrane protein having a short N-terminal cytoplasmic domain and a long C-terminal extracellular domain (De Flora et al., 2000). It is known to be expressed in lympho-monocytic lineage cells and pancreatic β cells. Furthermore, it acts as a transmembrane cell-signalling molecule as well as an ectoenzyme. The ligation of CD38 with monoclonal antibodies (mAbs) can activate some intracellular signal transduction pathways. For instance, CD38 ligation triggers activation, proliferation and mobilization of intracellular calcium (Ca2+) in T-lymphocytes and tyrosine phosphorylation of intracellular proteins in myeloid cells (Funaro et al., 1990; Inoue et al., 1997). In addition, it could also inhibit selectin-like adhesion of leukocytes to endothelium by blocking the interaction between CD38 and CD31, a CD38 natural ligand (Dianzani et al., 1994). CD31, a well-known member of the immunoglobulin superfamily with molecular mass of 130 kDa, is involved in the lymphocyte adhesion processes (Deaglio et al., 1996). It is expressed in monocytes, neutrophils, T-cell subsets, platelets, vascular endothelial cells and solid tumor lines (Deaglio et al., 1998).
Soluble extracellular domain of CD38 is detected in biological fluids in normal conditions and in various diseases (Funaro et al., 1996; Mallone et al., 1998). CD38 solubilization is mediated by proteolytic cleavage occurring near the cell membrane (Funaro et al., 1996). Viral infection such as acquired immunodeficiency syndrome (AIDS) increases CD38 solubilization (Funaro et al., 1996), suggesting that inflammatory signals may affect CD38 solubilization. In this study, we examined the effect of lipopolysaccaride (LPS), a bacterial cell wall component, on the expression and solubilization of CD38 in macrophages. We observed that LPS stimulates CD38 expression by activating the Janus kinase-signal transducers and activators of transcription (JAK-STAT) signaling pathway, and that the expressed CD38 was released into the extracellular domain by metalloproteinase 9.
MATERIALS AND METHODS
Reagents
IFN-β, horse radish peroxidase-linked anti-mouse antibody and horse radish peroxidase-linked anti-rabbit antibody were purchased from Abcam (USA). Purified anti-mouse IFN-β was purchased from BioLegend (USA). Anti-mouse CD38 antibody was purchased from Santa Cruz Biotechnology (USA). Escherichia coli LPS from serotype 0111:B4, and anti-β-actin antibody were purchased from Sigma-Aldrich (USA). Inhibitors of PI3K (LY294002 and wortmannin), p38 (SB203580), ERK (PD098059), JNK (SP600125), NF-κB (PDTC), JAK1 and JAK3 were purchased from Calbiochem (USA).
Cells
The J774A.1 murine macrophage cell line was obtained from the American Type Culture Collection (USA) and cultured at 37°C in Dulbecco’s Modified Eagle’s Media (DMEM, Gibco-BRL, USA) supplemented with 10% heat-inactivated and sterile-filtered fetal bovine serum (FBS), 2 mM glutamine, 100 U/mlpenicillin, and 100 μg/ml streptomycin. The cells were passaged twice a week, and cells older than 15 passages were not used.
Western blotting
Cells were rinsed twice with ice-cold phosphate buffered saline and lysed in a mammalian cell lysis solution (Thermo scientific, USA) containing protease inhibitor cocktail (Roche, USA) for 40 min, and centrifugated to separate the soluble supernatants. Protein extracts (20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gels electrophoresis at 100 V with a running time of 120 min and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% non-fat milk in Tris-buffered saline (pH 7.4) with 0.1% Tween 20 for 1 h at room temperature and probed overnight at 4°C with primary antibodies against CD38 and β-actin. After wash, the membranes were further incubated with species-specific horseradish phosphatase-conjugated secondary antibody for 40 min at room temperature. Immunoreactive proteins were detected by using an enhanced chemiluminescence detection system (G&E Amersham life science, USA) according to the manufacturer’s recommendations.
RNA extraction and quantitative real time PCR
Total RNA was extracted using RNeasy mini kit (Qiagen, USA) according to the manufacturer’s instructions. Real-time PCR primer sets for mouse CD38 and GAPDH were purchased from SABiosciences (USA). Total RNA was treated with DNase I to remove residual DNA. RNA was treated with RNase-free DNase for 20 min at room temperature before reverse transcription. RNA concentrations were determined at OD260 absorbance. Reverse transcription was performed with 1 μg of RNA in a 20 μl reaction volume by using ImProm-II™ Reverse Transcription System (Promega). Real-time quantitative PCR was then performed in a 20 μl reaction volume using SYBR premix (TaKaRa, Japan) with 10 μM of primer sets (the CD38 upstream primer, 5′-TTG CAA GGG TTC TTG GAA AC-3′, the CD38 downstream primer, 5′-CGC TGC CTC ATC TAC ACT CA-3′, the GAPDH upstream primer, 5′-CGT GCC GCC TGG AGA AAC C-3′, and the GADPH downstream primers 5′-TGG AAG AGT GGG AGT TGC TGT TG-3′) and 1 μl cDNA in a 7900HT Realtime PCR machine (Applied Biosystems, USA). PCR conditions consisted of 30 s hot start at 95°C, followed by 40 cycles of 5 s at 95°C and 30 s at 60°C, with a final 15 s at 72°C. The average threshold cycle (Ct) for each gene was determined from triplicate reactions, and the levels of gene expression relative to GAPDH were determined.
Immunoprecipitation and Western blot
For CD38 immunoprecipitation (IP), lysates were incubated overnight at 4°C with 2 μg/ml of anti-mouse CD38 antibody (eBioscience) with a 20 μl slurry of protein G-plus agarose (USA) and phosphatase inhibitor cocktail. Immunoprecipitates were loaded on 7.5% polyacrylamide gels, electrophoresed and transferred. The membranes were blocked with 5% skim milk with tris-buffered saline (pH 7.4) with 0.1% Tween 20 and incubated with goat polyclonal anti-CD38 antibody (USA) for 4 h at 4°C followed by donkey anti-goat IgG-HRP (USA) for 1 h at 4°C. Proteins were developed using chemiluminescence (Amersham Pharmacia Biotech, UK).
Statistical analysis
Data are presented as means ± SEM. Statistical significance was determined using a two-tailed Student’s t-test and one-way ANOVA test followed by Scheffe’s post-hoc test.
RESULTS
LPS increases CD38 expression at mRNA level in J774 macrophage cells
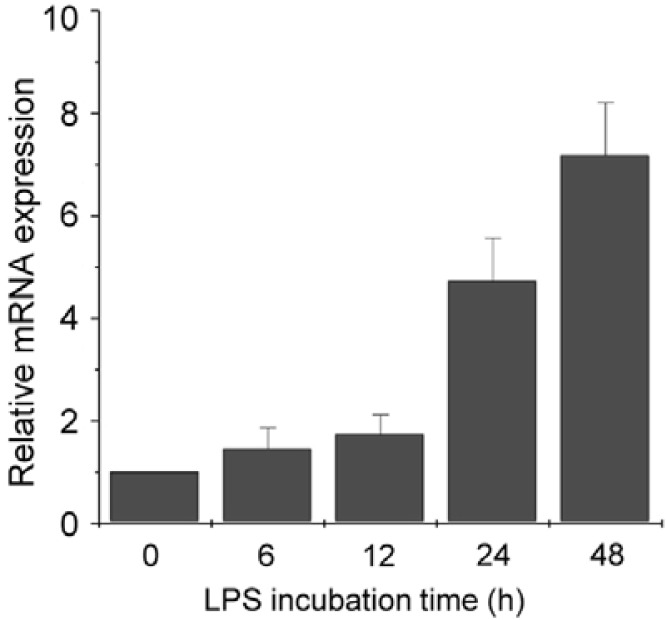
To analyze the effect of LPS on CD38 expression in J774 cells, cells were treated with LPS (100 ng/ml) and harvested according to a time-course. The levels of CD38 mRNA were measured by quantitative real-time PCR. Real-time PCR analysis showed over 4-fold induction of CD38 mRNA expression 24 h after LPS exposure (Fig. 1).
Fig. 1.
LPS increases CD38 expression at mRNA level in J774 macrophage cells. J774 cells were treated with 100 ng/ml LPS for the indicated times. CD38 mRNA levels were measured by real-time PCR. Data are expressed as means ± SEM.
LPS-induced CD38 expression is mediated by the JAK-STAT signaling pathway
To elucidate the mechanism of LPS-induced CD38 expression, the effect of several signal transduction inhibitors on LPS-induced CD38 expression was examined. J774 macrophage cells were pretreated with inhibitors of mitogen activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphoinositide 3 kinase (PI3K), Janus kinase (JAK) 1 and JAK3, for 1 h and stimulated with LPS for 24 h. Wortmannin, a PI3K inhibitor, and SB203580, a p38 inhibitor, slightly decreased LPS-induced CD38 expression whereas LY294002, a PI3K inhibitor, slightly increased it (Fig. 2). Interestingly, JAK-3 inhibitor completely abolished LPS-induced CD38 expression (Fig. 2). These results suggest that LPS-induced CD38 expression is mediated by the JAK-STAT pathway.
Fig. 2.
LPS-induced CD38 expression is mediated by the JAK-STAT signaling pathway. J774 cells were pretreated with 10 μM PD098059 (PD), 10 μM SB203580 (SB), 10 μM SP600125 (SP), 10 μM PDTC, 1 μM wortmannin (WT), 5 μM LY294002 (LY), 5 μM JAK1 inhibitor, or 10 μM JAK3 inhibitor. One hour later, they were treated with 100 ng/ml LPS for 24 h and CD38 mRNA levels were analyzed using real-time PCR. Data are expressed as means ± SEM. *P < 0.01.
IFN-β increases CD38 expression at mRNA level in J774 macrophage cells
Previous studies have shown that LPS mediates IFN-β induction and secretion through interferon regulatory factor (IRF)-3 (Sakaguchi et al., 2003). It has also been demonstrated that IFN-β activates the JAK-STAT signaling pathway (Horvath, 2004). In view of these findings, it is possible that IFN-β is involved in CD38 expression. To investigate this possibility, we treated J774 macrophage cells with IFN-β and measured CD38 mRNA expression levels by real-time PCR. IFN-β treatment dose-dependently increased CD38 mRNA levels (Fig. 3), indicating t h at I FN-β signaling may be involved in LPS-induced CD38 expression in J774 macrophage cells.
Fig. 3.
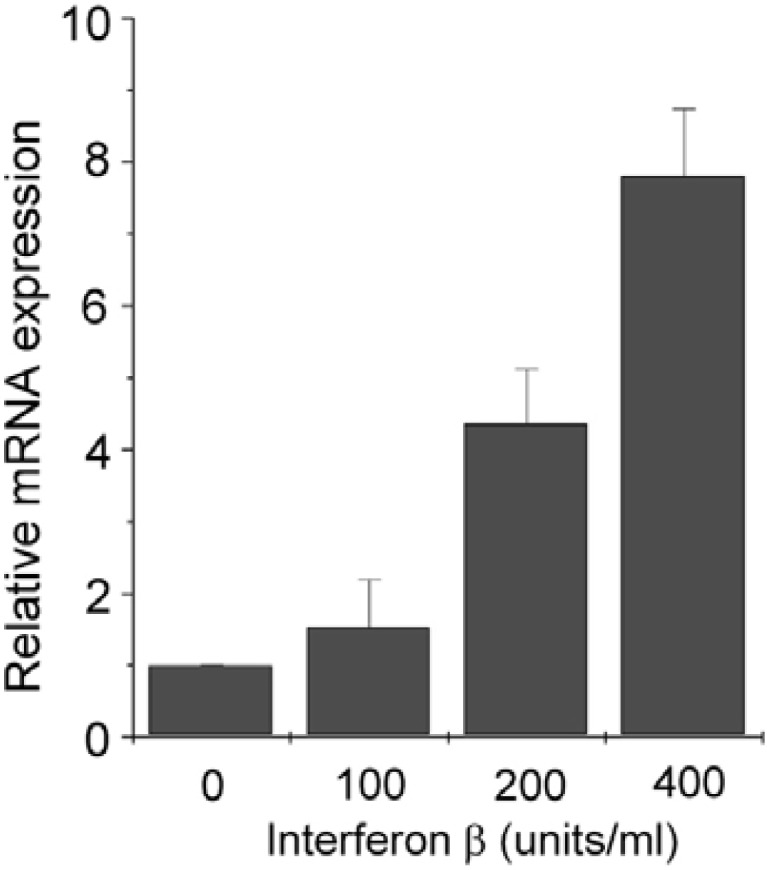
IFN-β increases CD38 expression at mRNA level in J774 macrophage cells. J774 cells were treated with indicated doses of INF-β for 24 h. CD38 mRNA levels were measured by real-time PCR. Data are expressed as means ± SEM.
LPS releases CD38 to the extracellular space in J774 macrophage cells
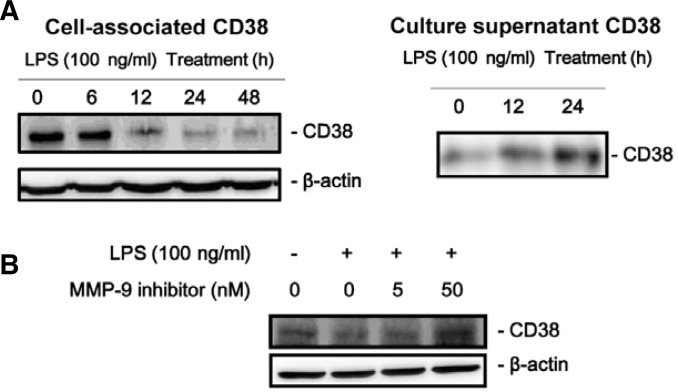
We observed that LPS stimulation decreased cell-associated CD38 protein levels (Fig. 4A, left) but increased CD38 mRNA levels (see Fig. 1) suggesting that CD38 protein might be degraded or released from the cells after protein synthesis. To confirm this, we checked CD38 protein levels in the culture supernatant after LPS stimulation. The results showed that CD38 were released to the culture supernatant by LPS treatment (Fig. 4A, right).
Fig. 4.
LPS releases CD38 into the extracellular space through MMP-9 in J774 macrophage cells. (A) CD38 release by LPS treatment. J774 cells were treated with 100 ng/ml LPS for the indicated times. Cell-associated CD38 protein levels as well as culture supernatant CD38 were measured by Western blotting after immunoprecipitation. (B) MMP-9 inhibitor blocks LPS-induced CD38 release. J774 cells were treated with 100 ng/ml LPS for 24 h in the presence of 5 nM or 50 nM MMP-9 inhibitor. CD38 protein levels in the cell pellets were measured by Western blotting.
Matrix metalloproteinase (MMP)-9 releases CD38 to the extracellular space in J774 macrophage cells
CD38 is an ecto-enzyme whose catalytic domain is localized outside of the cells (De Flora et al., 2000). MMP-9, a neutral endopeptidase, participates in diverse physiologic and pathologic processes (Deryugina and Quigley, 2006; Opdenakker et al., 2001; Parks et al., 2004) including degradation of extracellular matrix components. In addition to degrading ECM, the biological actions of MMP-9 result from its ability to degrade and modify the activities of cytokines and chemokines, growth factors, and proteinase inhibitors (Opdenakker et al., 2001; Parks et al., 2004). In view of these findings, it is likely that CD38 might be extracellularly released by MMP-9 in J774 macrophage cells when stimulated with LPS. To test this possibility, we pretreated cells with MMP-9 inhibitor and analyzed the effect of the inhibitor on LPS-induced extracellular release of CD38. The results showed that MMP-9 inhibitor dose-dependently blocked LPS-induced extracellular release of CD38 (Fig. 4B).
DISCUSSION
CD38 is known to be involved in activities typical of cell surface receptors, such as signaling for activation and proliferation events and heterotypic cell adhesion (Dianzani et al., 1994; Funaro et al., 1990). It is a bifunctional ectoenzyme catalyzing the cyclization of NAD+ into cyclic ADP-ribose (cADPR) and the hydrolysis of cADPR into ADPR (Howard et al., 1993). cADPR is widely involved in the regulation of intracellular Ca2+ levels in several cell systems (Lee and Aarhus, 1991). It has been demonstrated that extracellularly generated cADPR is transported into the cytosol via connexin 43 hemichannels (Song et al., 2008; 2011). In this study, we observed that the levels of cell-associated CD38, of which expression is increased by LPS, is decreased through MMP9-mediated cleavage of its extracellular domain. Thus, it is possible that the released CD38 binds to its ligand CD31, supplies cADPR into the cytosol and mediates the increase in intracellular Ca2+ levels although further studies are needed to confirm this.
Some reports show that in addition to a membrane-bound CD38, a soluble form of CD38 is detected in the cell culture supernatant or biological fluid (Funaro et al., 1996; Zielinska et al., 2004). Soluble CD38 is also detected in vivo in normal and pathological biological fluids (Funaro et al., 1996). However, to date, it is unclear how CD38 is solubilized into the extracellular space. Our findings suggest that CD38 is solubilized by MMP-9 in certain conditions such as inflammation. LPS is a main mediator of inflammation in bacterial infection (Lee et al., 2009). Accordingly, CD38 knockout mice were shown to be more sensitive to bacterial infection than their wild-type counterparts (Partida-Sanchez et al., 2001). Therefore, LPS-mediated CD38 expression and solubilization may be involved in the defense against bacterial infection.
Acknowledgments
This study was supported by a grant [KRF-2008-E00171] from Korea Research Foundation.
REFERENCES
- De Flora A., Franco L., Guida L., Bruzzone S., Usai C., Zocchi E. Topology of CD38. Chem. Immunol. 2000;75:79–98. doi: 10.1159/000058764. [DOI] [PubMed] [Google Scholar]
- Deaglio S., Dianzani U., Horenstein A.L., Fernandez J.E., van Kooten C., Bragardo M., Funaro A., Garbarino G., Di Virgilio F., Banchereau J., et al. Human CD38 ligand. A 120-kDa protein predominantly expressed on endothelial cells. J. Immunol. 1996;156:727–734. [PubMed] [Google Scholar]
- Deaglio S., Morra M., Mallone R., Ausiello C.M., Prager E., Garbarino G., Dianzani U., Stockinger H., Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J. Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- Deryugina E.I., Quigley J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Funaro A., DiFranco D., Garbarino G., Bragardo M., Redoglia V., Buonfiglio D., De Monte L.B., Pileri A., Malavasi F. Interaction between endothelium and CD4+CD45 RA+ lymphocytes. Role of the human CD38 molecule. J. Immunol. 1994;153:952–959. [PubMed] [Google Scholar]
- Funaro A., Spagnoli G.C., Ausiello C.M., Alessio M., Roggero S., Delia D., Zaccolo M., Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J. Immunol. 1990;145:2390–2396. [PubMed] [Google Scholar]
- Funaro A., Horenstein A.L., Calosso L., Morra M., Tarocco R.P., Franco L., De Flora A., Malavasi F. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int. Immunol. 1996;8:1643–1650. doi: 10.1093/intimm/8.11.1643. [DOI] [PubMed] [Google Scholar]
- Horvath C.M. The Jak-STAT pathway stimulated by interferon α or interferon β. Sci. STKE. 2004;2004:tr10. doi: 10.1126/stke.2602004tr10. [DOI] [PubMed] [Google Scholar]
- Howard M., Grimaldi J.C., Bazan J.F., Lund F.E., Santos-Argumedo L., Parkhouse R.M., Walseth T.F., Lee H.C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kontani K., Tsujimoto N., Kanda Y., Hosoda N., Hoshino S., Hazeki O., Katada T. Protein-tyrosine phosphorylation by IgG1 subclass CD38 monoclonal antibodies is mediated through stimulation of the FcgammaII receptors in human myeloid cell lines. J. Immunol. 1997;159:5226–5232. [PubMed] [Google Scholar]
- Lee H.C., Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., An H.J., Kim J.L., Lee H., Paik S.G. Inhibitory effect of a phosphatidyl ethanolamine derivative on LPS-induced sepsis. Mol Cells. 2009;27:251–255. doi: 10.1007/s10059-009-0049-4. [DOI] [PubMed] [Google Scholar]
- Mallone R., Ferrua S., Morra M., Zocchi E., Mehta K., Notarangelo L.D., Malavasi F. Characterization of a CD38-like 78-kilodalton soluble protein released from B cell lines derived from patients with X-linked agammaglobulinemia. J. Clin. Invest. 1998;101:2821–2830. doi: 10.1172/JCI1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Van den Steen P.E., Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S., Cockayne D.A., Monard S., Jacobson E.L., Oppenheimer N., Garvy B., Kusser K., Goodrich S., Howard M., Harmsen A., et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Negishi H., Asagiri M., Nakajima C., Mizutani T., Takaoka A., Honda K., Taniguchi T. Essential role of IRF-3 in lipopolysaccharide-induced interferon-β gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 2003;306:860–866. doi: 10.1016/s0006-291x(03)01049-0. [DOI] [PubMed] [Google Scholar]
- Song E.K., Lee Y.R., Yu H.N., Kim U.H., Rah S.Y., Park K.H., Shim I.K., Lee S.J., Park Y.M., Chung W.G., et al. Extracellular NAD is a regulator for FcgR-mediated phagocytosis in murine macrophages. Biochem. Biophys. Res. Commun. 2008;367:156–161. doi: 10.1016/j.bbrc.2007.12.131. [DOI] [PubMed] [Google Scholar]
- Song E.K., Rah S.Y., Lee Y.R., Yoo C.H., Kim Y.R., Yeom J.H., Park K.H., Kim J.S., Kim U.H., Han M.K. Connexin-43 hemichannels mediate cyclic ADP-ribose generation and its Ca2+-mobilizing activity by NAD+/cyclic ADP-ribose transport. J. Biol. Chem. 2011;286:44480–44490. doi: 10.1074/jbc.M111.307645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska W., Barata H., Chini E.N. Metabolism of cyclic ADP-ribose: Zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 2004;74:1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]