Abstract
Liver fibrosis is characterized by accumulation of extracellular matrix, and activated hepatic stellate cells (HSCs) are the primary source of the fibrotic neomatrix and considered as therapeutic target cells. We previously showed that albumin in pancreatic stellate cells (PSCs), the key cell type for pancreatic fibrogenesis, is directly involved in the formation of vitamin A-containing lipid droplets, inhibiting PSC activation. In this study, we evaluated the anti-fibrotic activity of both albumin and retinol binding protein-albumin domain III fusion protein (R-III), designed for stellate cell-targeted delivery of albumin III, in rat primary HSCs and investigated the underlying mechanism. Forced expression of albumin or R-III in HSCs after passage 2 (activated HSCs) induced lipid droplet formation and deactivated HSCs, whereas point mutations in high-affinity fatty acid binding sites of albumin domain III abolished their activities. Exogenous R-III, but not albumin, was successfully internalized into and deactivated HSC-P2. When HSCs at day 3 after plating (pre-activated HSCs) were cultured in the presence of purified R-III, spontaneous activation of HSCs was inhibited even after passage 2, suggestive of a potential for preventive effect. Furthermore, treatment of HSCs-P2 with R-III led to a significant reduction in both cytoplasmic levels of all-trans retinoic acid and the subsequent retinoic acid signaling. Therefore, our data suggest that albumin deactivates HSCs with reduced retinoic acid levels and that R-III may have therapeutic and preventive potentials on liver fibrosis.
Keywords: albumin, anti-fibrotic drug, hepatic stellate cells, liver fibrosis, retinol binding protein
INTRODUCTION
Liver fibrosis, characterized by excessive production and deposition of extracellular matrix (ECM) components, is a common response to chronic liver injury (Hernandez-Gea and Friedman, 2011). There is overwhelming evidence that activated hepatic stellate cells (HSCs) are the major producers of the fibrotic neomatrix, although additional cellular sources, such as portal myofibroblasts, bone marrow-derived cells and epithelial-to-mesenchymal transition (EMT), have been reported (Forbes and Parola, 2011; Jiao et al., 2009). Upon liver injury, quiescent HSCs undergo phenotypic transition into myofibroblast-like cells, which is invariably associated with loss of cytoplasmic vitamin A-containing lipid droplets, positive staining for α-smooth muscle actin (α-SMA), and greatly increased synthesis of the ECM proteins (Friedman, 2008). Thus, HSCs are considered an attractive target for anti-fibrotic therapies (Bataller and Brenner, 2001; Li et al., 2008). Despite extensive investigations, there is, however, no effective therapy available at present for liver fibrosis and its end stage cirrhosis, except the removal of the causative agent and organ transplantation.
Retinoids (vitamin A and its metabolites) regulate multiple physiological activities, such as vision, reproduction, morphogenesis, cell proliferation and differentiation (Sporn et al., 1994). Vitamin A (retinol), acquired from the diet, circulates bound to retinol binding protein (RBP) in the bloodstream, and it is taken up initially by liver cells and transferred to HSCs via the RBP receptor (Kawaguchi et al., 2007; Senoo et al., 2010). HSCs store approximately 80% of vitamin A in the whole body as retinyl esters in cytoplasmic lipid droplets. Previous studies showed that intravenously injected RBP protein is taken up at relatively high levels by liver, especially HSCs (Gjoen et al., 1987; Senoo et al., 1990; 1993).
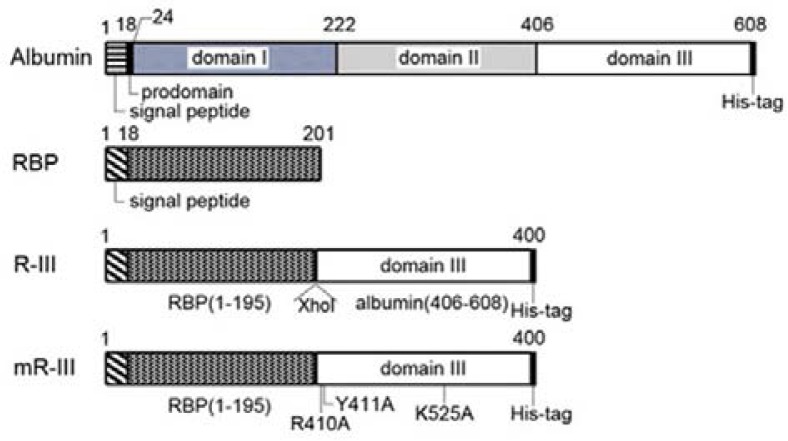
Albumin is an abundant multifunctional plasma protein synthesized primarily by liver cells (Evans, 2002). It is comprised of three homologous domains, each formed by two smaller sub-domains (He and Carter, 1992). Crystallographic analysis has revealed that albumin has five high-affinity fatty acid binding sites, three of which are asymmetrically distributed in domain III; one in subdomain IB, one between IA and IIA, two in IIIA and one in IIIB (Curry et al., 1998; Simard et al., 2005). Our previous studies showed that albumin is also expressed in quiescent hepatic and pancreatic stellate cells (PSCs), and its expression level dramatically decreases with culture activation (Kim et al., 2009; 2010). Albumin expression induces the reappearance of cytoplasmic lipid droplets in activated PSCs and inhibits PSC activation. Such antifibrotic activity of albumin requires high-affinity fatty acid-binding sites, and these findings prompted us to design a recombinant fusion protein R-III, in which albumin domain III is fused to the C-terminus of RBP for stellate cell-targeting delivery (Choi et al., 2012).
In this study, we evaluated the antifibrotic effects of R-III using culture-activated HSCs. We report that R-III is internalized into HSCs and deactivates them.
MATERIALS AND METHODS
Materials
Fetal bovine serum and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Gibco (USA). Male Sprague-Dawley rats were purchased from Orient (Charles River Korea, Korea). Animals were maintained under temperature-, humidity-and light-controlled conditions. Animal experiments were approved by the appropriate institutional committee and complied with the Guide for the Care and Use of Laboratory Animals.
HSCs isolation and culture
Primary rat HSCs were isolated as described previously (Langer et al., 2008). Briefly, livers were perfusion digested with collagenase and pronase. Parenchymal and nonparenchymal cell fractions were separated via centrifugation. HSCs were purified via density-gradient centrifugation with 15.6% and 8.2% Accudenz gradient layers. The gradient was then centrifuged at 20,000 rpm for 20 min. The band located above the 8.2% Accudenz layer was retrieved, and cells were suspended in DMEM supplemented with 10% fetal bovine serum and plated on non-coated plastic dishes. The purity of stellate cells was more than 90% as assessed by the presence of cytoplasmic lipid droplets. After reaching confluence in the primary culture, serial passages were obtained applying a 1:3 split.
Construction of expression vector for RBP-albumin fusion proteins
Expression plasmid encoding rat R-III was constructed as described previously (Choi et al., 2012). Briefly, the DNA fragments encoding RBP (1–585) and albumin (domain III: 1216–1827) were amplified with a sense primer (5′ GCGGAATTCC ACCATGGAGT GGGTGTGGGC 3′ and 5′ GGGCTCGAG GAAGAACCTAAGAACTTG 3′) and an antisense primer (5′ CCCCTCGAGT CTGCTTTGAC AGTAACC 3′ and 5′ GGCTC TAGAT TAATGATGAT GATGATGATG GGCTAAGGCT TCTT TGCT 3′), respectively. The His-tag sequence was included in the antisense primer for albumin domain III. The PCR products were double digested with EcoRI/XhoI and XhoI/XbaI, respectively, and DNA fragments purified by agarose gel electrophoresis were ligated together. The resulting DNA fragment R-III was then inserted into the expression vector pcDNA3.1+ at EcoRI and XbaI site to yield pcDNA3.1-R-III. In pcDNA3.1-R-III, the albumin-RBP coding region was located immediately upstream of 6-histidine tag coding sequence and stop codon in the same reading frame. Triple mutation (R410A/Y411A/K525A) was introduced in albumin sequence by a PCR-based method using a mutadirect site-directed mutagenesis kit (iNtRON, Korea). All constructs were then sequenced to confirm the albumin-RBP coding region using an automatic sequencer.
Purification of (His)6-tagged R-III fusion protein
Expression vector for mouse R-III and the high producing, stably transfected 293 cell lines were prepared as described previously (Choi et al., 2012). For the purification of R-III, conditioned medium was prepared from transfected 293 cells grown in serum-free medium for 4 days, fractionated with ammonium sulfate (55%) and then subjected to His Trap affinity column. The sample was further purified by Resource Q. Purified protein was dialyzed against deionized water, freeze-dried and dissolved in saline solution. The purity of R-III exceeded 95%, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein staining.
Transfection
HSCs after passage 2 were transiently transfected using Lipofectamine 2000 (Invitrogen, USA) and analyzed after 24 h.
Western blot analysis
Cells were rinsed twice in ice-cold phosphate-buffered saline (PBS) and harvested by scraping in the lysis buffer (Kim et al., 2009). Equivalent amounts of protein were separated by SDS-PAGE, followed by immunoblot detection using the primary antibody. Primary antibodies were albumin (Bethyl Laboratories, USA), His-tag (AB frontier, Korea), RAE-1 (Santa Cruz Biotechnology, USA), RBP-4 (Affinity Bioreagents, USA), α-SMA (Sigma-Aldrich, USA), α-tubulin (Cell Signaling Technology, USA), and type I collagen (Calbiochem, USA). The bands were quantified by densitometric analysis using NIH image J software.
Analysis of gene expression using real-time RT-PCR
cDNA was synthesized from total RNA and real-time PCR was performed using LightCycler-FastStart DNA Master SYBR Green 1 (Roche, Germany). To control for variations in the reactions, PCR products were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The primers used were 5′-TGC TGG ACT CTG GAG ATG-3′ (forward primer) and 5′-GTG ATC ACC TGC CCA TC-3′ (reverse primer) for α-SMA; and 5′-GAA CGG GAA GCT CAC TGG C-3′ (forward primer) and 5′-GCA TGT CAG ATC CAC AAC GG-3′ (reverse primer) for GAPDH.
Oil red O staining
HSCs were stained with oil red O to visualize lipid droplets, essentially as described previously (Kinkel et al., 2004), except that oil red O was diluted in triethyl phosphate instead of isopropanol.
Reverse phase-high performance liquid chromatography (HPLC)
Cells were quantified and extracted as described previously (Van Merris et al., 2004). Reverse-phase HPLC (RP-HPLC) was carried out on an AKTA Explorer HPLC system (GE Healthcare Life Sciences, UK). The chromatographic conditions were as described previously (Radaeva et al., 2007): column, LC-ABZ (15 cm × 4.6 cm ID; particle size, 5 μm; reverse phase C18; Sigma-Aldrich); mobile phase, 57.5% acetonitrile−25% acetic acid (diluted to 2% with water)−17.5% methanol; flow rate, 0.8 ml/min. Acitretin (Sigma-Aldrich) was used as an internal standard.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Paired statistical analysis was done using t-tests. Comparisons were considered significant at P < 0.05 and P values were two tailed.
RESULTS
Expression of albumin or R-III induces lipid droplet formation in activated HSCs
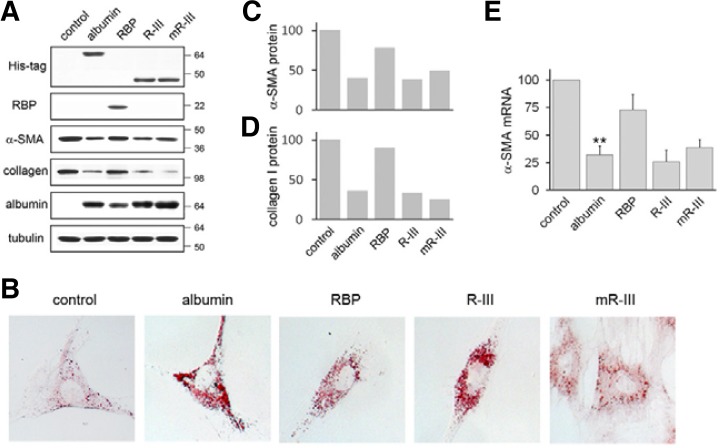
We previously showed that expression of albumin or recombinant fusion protein, RBP1-195a.a.-albumin406-608a.a. (R-III) (Fig. 1), induces lipid droplet formation and deactivates pancreatic stellate cells (Choi et al., 2012). In this study, we sought to evaluate the anti-fibrotic activity of albumin and R-III in HSCs, because HSCs and PSCs are distinct with different tissue microenvironments although they reportedly display similar cellular behavior (Erkan et al., 2010). HSCs were isolated from rat livers and spontaneous culture-induced activation was determined by increased levels of α-SMA, a marker for the activated HSC phenotype (Van de Bovenkamp et al., 2007). HSCs after passage 2 (HSCs-P2; activated HSCs) were transiently transfected with either empty vector or expression plasmids for His-tagged albumin, RBP or His-tagged R-III, and cell lysates were analyzed by Western blotting. It was previously shown that albumin is expressed in quiescent HSCs, but its level markedly declines with culture activation (Kim et al., 2009). Anti-His tag antibody detected expression of R-III at the expected size of approximately 45 kDa (Fig. 2A). Control HSCs-P2 displayed elongate fibroblastoid morphology with only a few lipid droplets, whereas expression of albumin or R-III induced lipid droplet formation (Fig. 2B). Such a phenotypic change was accompanied with a decrease in levels of α-SMA and type I collagen (Figs. 2C and 2D), indicating that albumin (domain III) deactivates HSCs. α-SMA mRNA levels correlated with its protein levels (Fig. 2E). RBP expression also slightly increased lipid droplet formation, as compared with control HSCs, and led to a marginal decrease in α-SMA levels. In order to gain mechanistic insight, three high-affinity fatty acid binding sites (Arg410, Tyr411 and Lys525) in albumin domain III were substituted by an alanine residue (Fig. 1). HSCs expressing mutant R-III displayed a flattened morphology and enlarged cell size, with only a slight increase in lipid droplet formation (Fig. 2B), suggesting that direct binding to fatty acids is critical for their action. The effects of mutant R-III are considered further in the “Discussion”.
Fig. 1.
Construction of recombinant RBP-albumin fusion proteins. Schematic diagram of RBP-albumin fusion proteins, R-III and mutant R-III, compared to albumin and RBP.
Fig. 2.
Expression of R-III deactivates HSCs. (A) HSCs after passage 2 (HSCs-P2) were transiently transfected with either empty vector (control) or expression plasmids for His-tagged albumin, RBP, His-tagged R-III or mutant R-III (mR-III), and cell lysates were analyzed by Western blotting. The Western blots are representative of three independent experiments from separate cell preparations. α-tubulin serves as loading control. (B) Transfected HSCs were subjected to oil red O staining. (C, D) Quantitative result of Western blots of α-SMA (C) and collagen type I (D) using image J software. (E) Total RNA was isolated from transfected HSCs and analyzed for α-SMA by real-time PCR. The data are expressed as the percentage of control HSCs and represent the means with standard deviation (n = 3). **P < 0.01 compared with control HSCs.
R-III is internalized into and deactivates HSCs
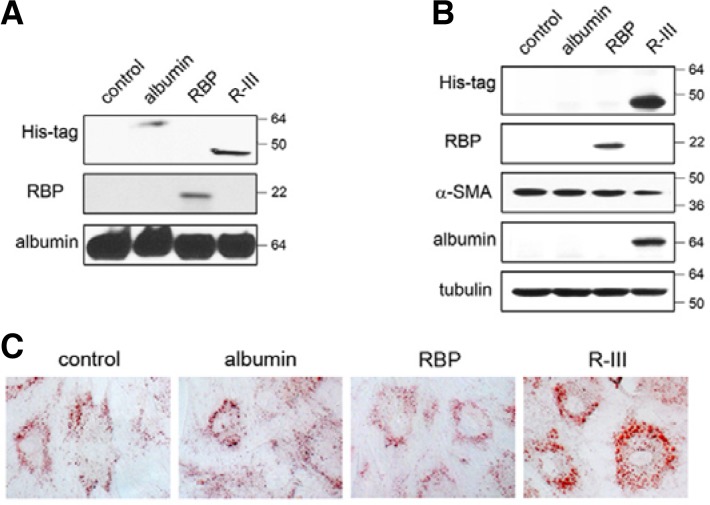
To confirm the biological activity of the RBP moiety of R-III, we first established a stable transfectant of 293 cells expressing His-tagged albumin, RBP or His-tagged R-III, and conditioned medium of each transfectant was analyzed by Western blotting (Fig. 3A). Each conditioned medium was added to HSCs-P2 and the effects were assessed after 24 h. Interestingly, R-III and RBP, but not albumin, were incorporated into HSCs (Fig. 3B); of these, only R-III induced fat-storing phenotype and reduced α-SMA levels (Fig. 3C). This finding suggests that the two parts constituting the R-III fusion protein are functional; RBP for stellate cell-targeting and albumin III for stellate cell-deactivation. Albumin levels were found elevated in R-III-treated cells, which is consistent with our previous finding that albumin is required for the acquisition and maintenance of fat-storing phenotype in stellate cells (Kim et al., 2009; 2010).
Fig. 3.
R-III is incorporated into and induces phenotypic changes in HSCs. (A) 293 cells were stably transfected with expression vector for His-tagged albumin, RBP or His-tagged R-III and conditioned medium collected from each stable transfectant was analyzed by Western blotting. (B, C) HSCs after passage 2 were incubated with the conditioned media for 24 h and subjected to Western blotting (B) and oil red O staining (C).
Albumin-mediated lipid droplet formation correlates with reduction of RA in HSCs
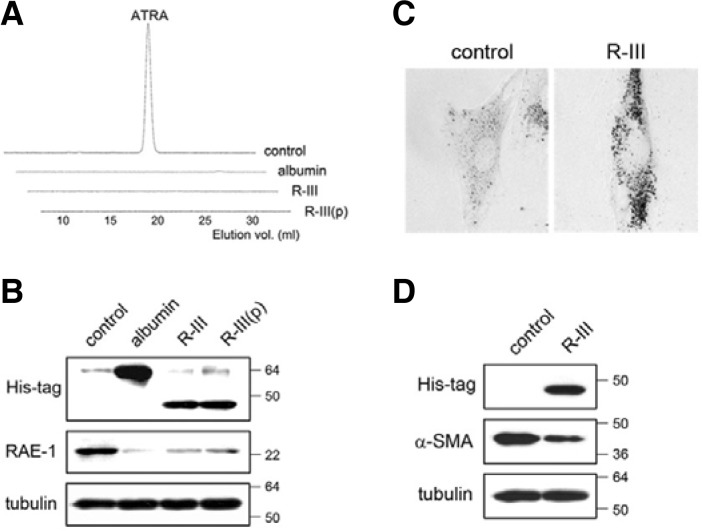
It is widely hypothesized that loss of vitamin A-containing lipid droplets, a hallmark of HSC activation, leads to the secretion of retinol and its subsequent metabolic conversion to retinoic acids (RA), which in turn serve as profibrogenic mediators. To examine the possibility that albumin may modulate cytoplasmic levels of RA, HSCs-P2 were transiently transfected with expression plasmids for His-tagged albumin or R-III and the level of all-trans retinoic acid (ATRA), a biologically active form of vitamin A, was measured from whole-cell extracts. ATRA production was detected in control HSCs as previously reported (Radaeva et al., 2007), but disappeared in transfectant cells (Fig. 4A). Similar effects were seen when HSCs were treated with FPLC-purified R-III (0.15 μM). We failed to detect any significant changes in retinol levels (data not shown). Such decrease in RA levels was accompanied with reduced expression levels of RA early inducible-1 gene (RAE-1) (Nomura et al., 1994) (Fig. 4B), suggesting that albumin and R-III downregulate RA signaling in HSCs. We then examined whether culture-activation of HSCs could be inhibited by exogenous R-III. HSCs at 3 days after plating (pre-activated HSCs) were incubated with R-III (0.15 μM) for 9 days and examined for phenotypic changes. Untreated control HSCs underwent spontaneous activation, whereas R-III-treated cells retained fat-storing phenotype with reduced α-SMA levels (Figs. 4C and 4D).
Fig. 4.
R-III-mediated lipid droplet formation correlates with reduction of RA in HSCs. (A, B) HSCs after passage 2 were either transfected with expression plasmids for His-tagged albumin or R-III, or treated with purified R-III [0.15 μM, R-III(p)], and the levels of all-trans retinoic acid (ATRA) from whole-cell extracts were measured by reverse-phase-HPLC (A). Cell lysates of treated HSCs were studied for RAE-1 by Western blot analysis (B). (C, D) HSCs at 2 days after plating were continuously treated for 9 days with R-III (0.15 μM) and subjected to oil red O staining (C) and Western blotting (D). R-III-treated cells retain fat-storing phenotype.
These findings show that R-III may deactivate HSCs and also help to maintain fat-storing phenotype, suggestive of therapeutic and preventive effects on liver fibrosis.
DISCUSSION
HSCs play pivotal roles in the development of liver fibrosis (Bataller and Brenner, 2001; Jiao et al., 2009). Treatments that aim to deactivate HSCs, downregulate the proliferative and fibrogenic responses of HSCs and promote HSC apoptosis have been reported, but the efficacy of most treatments has not yet been proven in humans, due to the lack of cell specificity (Ghiassi-Nejad and Friedman, 2008; Poelstra and Schuppan, 2011). In this study, we investigated the anti-fibrotic properties of the recombinant fusion protein RBP-albumin domain III (R-III), which was designed for stellate cell-targeted delivery. Forced expression of R-III or exogenous addition of purified R-III protein induced the reappearance of cytoplasmic lipid droplets and deactivated HSCs, suggesting that R-III is a good candidate for antifibrotic therapy.
It has been hypothesized that, due to the collapse of lipid droplets observed during stellate cell activation, the retinoid contents are released and part of them metabolized to RA isomers, such as all-trans-RA (ATRA) and 9-cis-RA. These molecules may then bind to their nuclear cognate receptors and upregulate fibrosis-associated target genes. This is supported by the previous findings that ATRA content is increased in activated stellate cells as compared with preactivated stellate cells, whereas the contents of retinyl ester and retinol decrease (D’Ambrosio et al., 2011; Radaeva et al., 2007). We showed in this study that albumin and R-III deactivate HSCs with concomitant reduction of ATRA levels (Fig. 4A). On the other hand, studies with Lrat-deficient mice raised a question whether loss of lipid droplets causally contributes to liver fibrosis (Kluwe et al., 2011). Thus, further studies are required to elucidate the underlying mechanisms of HSC activation and to examine how RA synthesis and metabolism are regulated in the livers of Lrat-deficient mice and during liver regeneration (Xu et al., 2011).
It is interesting to note that albumin-, retinol-, peroxisome proliferator-activated receptor-γ-C/EBP-α-or R-III-induced lipid droplet formation is accompanied with an increase in albumin expression levels (Fig. 2) (Kim et al., 2009; 2010). This finding strengthens our notion that albumin is required for the acquisition and maintenance of fat-storing phenotype and suggests that albumin can be used as a reliable marker for the activation status of stellate cells. Critical role of albumin in cytoplasmic lipid droplet formation was also demonstrated during 3T3-L1 adipocyte differentiation (Yoo et al., 2010), and its expression was recently reported in human adipocytes (Sirico et al., 2012). It remains unclear how albumin promotes lipid droplet formation in 3T3-L1 and HSCs. Further studies are needed to address this issue.
Forced expression of RBP in HSCs led to a slight induction of lipid droplet formation (Fig. 2B), suggesting that secreted RBP may bind retinol and subsequently affect HSCs. We previously showed that retinol-mediated stellate cell deactivation requires albumin (Kim et al., 2009). On the contrary, conditioned medium of RBP-transfected 293 cells failed to show any significant effect (Fig. 3). It may be due to the concentration difference of RBP available to the cells. We also found that mutant R-III-expressing HSCs display an enlarged and flattened morphology with only a slight increase in lipid droplet formation and yet express low levels of α-SMA and collagen type I (Fig. 2). These changes were similar to those found in cells expressing mutant albumin (R410A/Y411A/K525A) (Kim et al., 2009). Mutant albumin expression produced a senescent-like phenotype with increased p53 levels, which was reportedly associated with a decrease in collagen and α-SMA levels (Schnabl et al., 2003).
The retinoid-storing stellate cells also exist in extrahepatic organs such as pancreas, kidney, spleen, intestine and lung (Nagy et al., 1997). They show striking similarities in morphology and perivascular location, which suggests that activated stellate cells may contribute to the myofibroblast cells seen in the fibrotic extrahepatic tissues. Interestingly, we observed that intravenously injected R-III also accumulate in extrahepatic organs where stellate cells exist (Choi et al., 2012). Thus, it may be worth evaluating the anti-fibrotic potential of RBP-albumin fusion protein R-III in liver and extrahepatic tissues, such as pancreas and kidney.
In summary, our study demonstrated that the recombinant RBP-albumin fusion protein R-III deactivates culture-activated HSCs.
Acknowledgments
We thank Suhyun Kim and Ah-Young Chung for technical assistance. We are also grateful to Hyeyeun Jeong for proofreading the manuscript. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2012R1A1A2043349).
REFERENCES
- Bataller R., Brenner D.A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- Choi S., Park S., Kim S., Lim C., Kim J., Cha D.R., Oh J. Recombinant fusion protein of albumin-retinol binding protein inactivates stellate cells. Biochem. Biophys. Res. Commun. 2012;418:191–197. doi: 10.1016/j.bbrc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Curry S., Mandelkow H., Brick P., Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998;5:827–835. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio D.N., Walewski J.L., Clugston R.D., Berk P.D., Rippe R.A., Blaner W.S. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLoS One. 2011;6:e24993. doi: 10.1371/journal.pone.0024993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan M., Weis N., Pan Z., Schwager C., Samkharadze T., Jiang X., Wirkner U., Giese N.A., Ansorge W., Debus J., et al. Organ-, inflammation-and cancer specific transcriptional fingerprints of pancreatic and hepatic stellate cells. Mol Cancer. 2010;9:88. doi: 10.1186/1476-4598-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.W. Review article: albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002;16(Suppl 5):6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- Forbes S.J., Parola M. Liver fibrogenic cells. Best Pract. Res. Clin. Gastroenterol. 2011;25:207–217. doi: 10.1016/j.bpg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Friedman S.L. Hepatic stellate cells: protean, multifunctional and enigmatic cells of the liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiassi-Nejad Z., Friedman S.L. Advances in anti-fibrotic therapy. Expert Rev. Gastroenterol. Hepatol. 2008;2:803–816. doi: 10.1586/17474124.2.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoen T., Bjerkelund T., Blomhoff H.K., Norum K.R., Berg T., Blomhoff R. Liver takes up retinol-binding protein from plasma. J. Biol. Chem. 1987;262:10926–10930. [PubMed] [Google Scholar]
- He X.M., Carter D.C. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Jiao J., Friedman S.L., Aloman C. Hepatic fibrosis. Curr. Opin. Gastroenterol. 2009;25:223–229. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kim N., Yoo W., Lee J., Kim H., Lee H., Kim Y.S., Kim D.U., Oh J. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut. 2009;58:1382–1390. doi: 10.1136/gut.2008.170233. [DOI] [PubMed] [Google Scholar]
- Kim N., Choi S., Lim C., Lee H., Oh J. Albumin mediates PPAR-gamma or C/EBP-alpha-induced phenotypic changes in pancreatic stellate cells. Biochem. Biophys. Res. Commun. 2010;391:640–644. doi: 10.1016/j.bbrc.2009.11.112. [DOI] [PubMed] [Google Scholar]
- Kinkel A.D., Fernyhough M.E., Helterline D.L., Vierck J.L., Oberg K.S., Vance T.J., Hausman G.J., Hill R.A., Dodson M.V. Oil red-O stains non-adipogenic cells: a precautionary note. Cytotechnology. 2004;46:49–56. doi: 10.1007/s10616-004-3903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe J., Wongsiriroj N., Troeger J.S., Gwak G.Y., Dapito D.H., Pradere J.P., Jiang H., Siddiqi M., Piantedosi R., O’Byrne S.M., et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- Langer D.A., Das A., Semela D., Kang-Decker N., Hendrickson H., Bronk S.F., Katusic Z.S., Gores G.J., Shah V.H. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–1993. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.T., Liao Z.X., Ping J., Xu D., Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008;43:419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- Nagy N.E., Holven K.B., Roos N., Senoo H., Kojima N., Norum K.R., Blomhoff R. Storage of vitamin A in extrahepatic stellate cells in normal rats. J. Lipid Res. 1997;38:645–658. [PubMed] [Google Scholar]
- Nomura M., Takihara Y., Shimada K. Isolation and characterization of retinoic acid-inducible cDNA clones in F9 cells: one of the early inducible clones encodes a novel protein sharing several highly homologous regions with a Drosophila polyhomeotic protein. Differentiation. 1994;57:39–50. doi: 10.1046/j.1432-0436.1994.5710039.x. [DOI] [PubMed] [Google Scholar]
- Poelstra K., Schuppan D. Targeted therapy of liver fibrosis/cirrhosis and its complications. J. Hepatol. 2011;55:726–728. doi: 10.1016/j.jhep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Radaeva S., Wang L., Radaev S., Jeong W.I., Park O., Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G809–816. doi: 10.1152/ajpgi.00212.2007. [DOI] [PubMed] [Google Scholar]
- Schnabl B., Purbeck C.A., Choi Y.H., Hagedorn C.H., Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- Senoo H., Stang E., Nilsson A., Kindberg G.M., Berg T., Roos N., Norum K.R., Blomhoff R. Internalization of retinol-binding protein in parenchymal and stellate cells of rat liver. J. Lipid Res. 1990;31:1229–1239. [PubMed] [Google Scholar]
- Senoo H., Smeland S., Malaba L., Bjerknes T., Stang E., Roos N., Berg T., Norum K.R., Blomhoff R. Transfer of retinol-binding protein from HepG2 human hepatoma cells to cocultured rat stellate cells. Proc. Natl. Acad. Sci USA. 1993;90:3616–3620. doi: 10.1073/pnas.90.8.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo H., Yoshikawa K., Morii M., Miura M., Imai K., Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol. Int. 2010;34:1247–1272. doi: 10.1042/CBI20100321. [DOI] [PubMed] [Google Scholar]
- Simard J.R., Zunszain P.A., Ha C.E., Yang J.S., Bhagavan N.V., Petitpas I., Curry S., Hamilton J.A. Locating high-affinity fatty acid-binding sites on albumin by x-ray crystallography and NMR spectroscopy. Proc. Natl. Acad. Sci USA. 2005;102:17958–17963. doi: 10.1073/pnas.0506440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirico M.L., Guida B., Procino A., Pota A., Sodo M., Grandaliano G., Simone S., Pertosa G., Riccio E., Memoli B. Human mature adipocytes express albumin and this expression is not regulated by inflammation. Mediators Inflamm. 2012;2012:236796. doi: 10.1155/2012/236796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M.B., Roberts A.B., Goodman D.S. The retinoids: biology, chemistry, and medicine. 2nd ed. Raven Press; New York: 1994. [Google Scholar]
- Van de Bovenkamp M., Groothuis G.M., Meijer D.K., Olinga P. Liver fibrosis in vitro: cell culture models and precision-cut liver slices. Toxicol In Vitro. 2007;21:545–557. doi: 10.1016/j.tiv.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Van Merris V., Meyer E., Duchateau L., Blum J., Burvenich C. All-trans retinoic acid is increased in the acute phase-related hyporetinemia during Escherichia coli mastitis. J. Dairy Sci. 2004;87:980–987. doi: 10.3168/jds.S0022-0302(04)73243-9. [DOI] [PubMed] [Google Scholar]
- Xu C., Chen X., Chang C., Wang G., Wang W., Zhang L., Zhu Q., Wang L., Zhang F. Analysis of gene expression profiles of liver stellate cells during liver regeneration in rats. Mol Cells. 2011;31:17–23. doi: 10.1007/s10059-011-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo W., Lee J., Park S., Kim Y.S., Lim C., Yoon E., Hur G., Oh J. Albumin expression is required for adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2010;397:170–175. doi: 10.1016/j.bbrc.2010.05.067. [DOI] [PubMed] [Google Scholar]