Abstract
Ginseng has been shown to have memory-improving effects in human. However, little is known about the active components and the molecular mechanisms underlying its effects. Recently, we isolated novel lysophosphatidic acids (LPAs)-ginseng protein complex derived from ginseng, gintonin. Gintonin activates G protein-coupled LPA receptors with high affinity. Gintonin activated Ca2+-activated Cl− channels in Xenopus oocytes through the activation of endogenous LPA receptor. In the present study, we investigated whether the activation of LPA receptor by gintonin is coupled to the regulation of N-methyl-d-aspartic acid (NMDA) receptor channel activity in Xenopus oocytes expressing rat NMDA receptors. The NMDA receptor-mediated ion current (INMDA) was measured using the two-electrode voltage-clamp technique. In oocytes injected with cRNAs encoding NMDA receptor subunits, gintonin enhanced INMDA in a concentration-dependent manner. Gintonin-mediated INMDA enhancement was blocked by Ki16425, an LPA1/3 receptor antagonist. Gintonin action was blocked by a PLC inhibitor, IP3 receptor antagonist, Ca2+ chelator, and a tyrosine kinase inhibitor. The site-directed mutation of Ser1308 of the NMDA receptor, which is phosphorylated by protein kinase C (PKC), to an Ala residue, or co-expression of receptor protein tyrosine phosphatase with the NMDA receptor attenuated gintonin action. Moreover, gintonin treatment elicited a transient elevation of [Ca2+]i in cultured hippocampal neurons and elevated long-term potentiation (LTP) in both concentration-dependent manners in rat hippocampal slices. Gintonin-mediated LTP induction was abolished by Ki16425. These results indicate that gintonin-mediated INMDA potentiation and LTP induction in the hippocampus via the activation of LPA receptor might be responsible for ginseng-mediated improvement of memory-related brain functions.
Keywords: ginseng, gintonin, LPA receptors, N-methyl-d-aspartic acid receptor, LTP
INTRODUCTION
Glutamate is one of major excitatory neurotransmitters in the central nervous system (CNS) (Morisyoshi et al., 1991). Glutamate not only interacts with GTP-binding protein coupled receptors (i.e., metabotropic glutamate receptor subtypes) but also binds to various ionotropic ligand-gated glutamate receptors, which include α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainic acid, and N-methyl-D-aspartic acid (NMDA) receptors (Hollmann and Heinemann, 1994; Moriyoshi et al., 1991). NMDA receptors play an important role in the regulation of synaptic functions in the central nervous system (Carroll and Zukin., 2002). In the hippocampus, the activation of NMDA receptors induces long-term potentiation (LTP), which is a key contribution for learning and memory (Lisman et al., 2012). Thus, NMDA receptors have important roles in physiological functions in learning and memory. Currently, NMDA receptors are one of the target proteins for drug development for clinical applications related to learning and memory (Timofeeva and Levin, 2011).
Ginseng, the root of Panax ginseng C.A. Meyer, has been used as a general tonic against stress, fatigue, and various diseases, including cancer (Nah, 1997). Recent studies have shown that the ginseng extract has memory-improving effects in humans (Kennedy et al., 2004; Persson et al., 2004). For example, Kennedy et al. (2004) and Persson et al. (2004) demonstrated that the total ginseng extract enhanced associative and working memory. In addition, Heo et al. (2008) and Lee et al. (2008) also demonstrated that ginseng extract has cognition-enhancing effects in Alzheimer’s disease (AD) patients, indicating that ginseng might have a beneficial function in learning and memory in both normal and dysfunctional brains. However, the active ingredients and the underlying mechanisms by which ginseng extract exerts its learning-and memory-related effects are not fully understood. Recently, we isolated a unique lysophosphatidic acid (LPA)-ginseng protein complexes, namely, gintonin (Hwang et al., 2012a). We also demonstrated that gintonin activates LPA receptors with high affinity via pertussis toxin (PTX)-sensitive and PTX-insensitive G proteins in cells expressing LPA receptors endogenously or heterologously (Hwang et al., 2012a). In addition, gintonin attenuates AD-related neuropathies through the stimulation of non-amyloidogenic processes (Hwang et al., 2012b). LPA receptor expression in the brain has a crucial role in the maintenance of memory functions, since LPA receptor knockout mice showed impairment of memory functions (Castilla-Ortega et al., 2011). However, relatively much is unknown about the molecular mechanisms by which LPA receptors are involved in memory functions.
In the present study, we first investigated whether the activation of LPA receptor by gintonin is coupled to modulation of the activity of NMDA receptor channel. Here, we report that gintonin enhances INMDA through the signal transduction pathways of the activation of LPA receptor. Furthermore, gintonin-mediated INMDA enhancement involves the phosphorylation of NMDA receptor by PKC. In addition, gintonin induced a transient elevation of [Ca2+]i in hippocampal cells and long-term potentiation (LTP) in rat hippocampal slices via the activation of LPA receptor. We discuss the signal transduction pathways involved in the gintonin-mediated stimulation of INMDA and LTP induction. Finally, gintonin-mediated LTP induction by NMDA receptor modulation might be the molecular basis for the beneficial effects of ginseng on learning and memory.
MATERIALS AND METHODS
Materials
Gintonin was isolated from P. ginseng as previously described (Pyo et al., 2011), and in the present study, we used the crude gintonin fraction, which contains about 9.5% LPAs, the major LPA component of which is LPA C18:2 (Hwang et al., 2012a). The crude gintonin fraction was used without isolating the individual glycolipoproteins because of their scarcity and because of the similar ED50 values of the crude gintonin fraction in Ca2+-activated Cl− channel activation (Pyo et al., 2011). All other reagents were purchased from Sigma-Aldrich (USA). All animal experiments were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Konkuk University, KIST, or Kyung Hee University. All surgeries were performed under Zoletil-and-Rumpun combination anesthesia, and every effort was taken to minimize the suffering.
Preparation of Xenopus oocytes and microinjection
X. laevis frogs were purchased from Xenopus I (USA). To isolate the oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester, and the ovarian follicles were removed. The oocytes were separated by treatment with collagenase followed by agitation for 2 h in Ca2+-free OR2 medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2.5 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin, 5 mM HEPES (pH 7.5). Stage V-VI oocytes were collected and stored in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 50 μg/ml gentamicin. The oocyte-containing solution was maintained at 18°C with continuous gentle shaking and was changed daily. Electrophysiological experiments were performed 3–5 days after oocyte isolation. For the NMDA receptor experiments, NMDA receptor subunit-encoding cRNAs (40 nl) were injected into the animal or vegetal pole of each oocyte 1 day after isolation with a 10-μl microdispenser (VWR Scientific, USA) fitted with a tapered glass pipette tip (15–20 μm in diameter) (Pyo et al., 2011).
Data recordings
A custom-made Plexiglas net chamber was used for 2-electrode voltage-clamp recordings, as previously reported (Pyo et al., 2011). A single oocyte was constantly superfused during recording with a recording solution (96 mM NaCl, 2 mM KCl, 0.3 mM CaCl2, and 5 mM HEPES, pH 7.6) in the presence or absence of NMDA or ginsenoside metabolites. The microelectrodes were filled with 3 M KCl and had a resistance of 0.2–0.7 M Ω. Two-electrode voltage-clamp recordings were obtained at room temperature with an oocyte clamp (OC-725C; Warner Instrument, USA), and were digitized using Digidata 1200A (Molecular Devices, USA). Stimulation and data acquisition were controlled using the pClamp 8 software (Molecular Devices). For most electrophysiological data, the oocytes were clamped at a holding potential of −60 mV. To determine the current and voltage (I-V) relationship, voltage ramps were applied from −100 to +50 mV for 1 s. In the different membrane-holding potential experiments, the oocytes were clamped at the indicated holding potentials. The linear leak and capacitance currents were corrected by the leak subtraction procedure.
Primary cultures of hippocampal neurons
Rat hippocampal neuronal cultures were prepared using a modified version of technique by Kim et al. (2005). Briefly, hippocampi were isolated from 16-to 18-day-old fetal Sprague-Dawley rats and incubated with 0.25% trypsin in Leibovitz L-15 medium at 37°C for 20 min. Cells were then mechanically dissociated by trituration with fire-polished Pasteur pipettes and were plated on poly-l-lysine coated coverslips in 35-mm culture dishes or on 24-well plates. The cells were maintained in MEM culture medium containing 10% NU-serum, 2% B-27 supplement, 20 mM D-glucose, 26.2 mM sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. After 1 day in vitro, the culture medium was exchanged, and the cultures were treated with cytosine β-arabinofuranoside (1 μM). Experiments were carried out on neurons after 10–15 days in vitro.
Measurement of intracellular Ca2+ levels
We examined intracellular Ca2+ levels in hippocampal neurons exposed to gintonin. Hippocampal neurons from each group were incubated for 40–60 min at room temperature with 5 μM Fura-2/AM (Molecular Probes, USA) and 0.001% pluronic F-127 (Molecular Probes) in a HEPES-buffered solution composed of the following: 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, with the pH adjusted to 7.4 with NaOH. Cells were illuminated using a xenon arc lamp, and the excitation wavelengths (340 and 380 nm) were selected by a computer-controlled filter wheel (Sutter Instruments, USA). The emitted fluorescence was reflected through a 515-nm long-pass filter to a frame transfer-cooled CCD camera (Olympus, Japan), and the ratios of the emitted fluorescence were calculated using a digital fluorescence analyzer and converted to intracellular free Ca2+ concentrations [Ca2+]i. All imaging data were collected and analyzed using the Universal Imaging software (USA) as previously described (Kim et al., 2005).
Preparation of acute hippocampal slices
Experiments were carried out with young Sprague-Dawley male rats (3–5 weeks old) (USA). The rats were killed by fast decapitation, without anesthesia (Steidl et al., 2006). The whole brain was quickly removed by taking away the splitting bones by using a rongeur (Fine Science Tools Inc., USA) and immediately put into an ice-cold, oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (aCSF, which contains 114 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, pH 7.4). NaOH was used for pH adjustment. Hippocampal slices (400 μm) were cut with a vibratom (Camden Instruments, UK). The slices were stabilized at room temperature for at least 1 h in artificial cerebrospinal fluid before use.
Preparation of organotypic hippocampal slice culture
Organotypic hippocampal slice cultures (OHSCs) were prepared from hippocampi of 6-7-day-old Sprague-Dawley rats by the method of Stoppini et al. (1991). Briefly, hippocampi were isolated and placed immediately in cold Gey’s balanced salt solution supplemented with glucose (6.5 mg/ml; Amresco, USA). Slices (350-μm thick) were cut in parallel with the transverse axis of the hippocampus by using a McIlwain tissue chopper (Mickle Laboratory Engineering, UK). Each slice was placed on a membrane insert (polytetrafluorethylene membrane, 0.4 μm, Millicell-CM, Millipore Co., USA), which was set into a 6-well plate filled with 1 ml of culture medium containing 50% MEM medium (LM 007-01, JBI, Korea), 25% horse serum (S 104-01, JBI, Korea), 25% Hank’s balanced salt solution, 6 g/L d-glucose, 1 mM L-glutamine, 20 mM HEPES, and 1% penicillin-streptomycin. The pH was adjusted to 7.1, and the medium was changed every other day. Slices were cultured in an incubator at 36°C with 5% CO2 and used after 14 days.
Preparation of hippocampal slices on MEA probes
The hippocampal slices were removed from the membrane inserts and then placed on a multielectrode array (MEA, 8 × 8 array; interelectrode distance, 100 μm; electrode diameter, 10 μm). Each slice was positioned, and the surrounding solution was removed using a pipette, which had been precoated with 0.01% polyethylenimine. The MEA systems (Multi Channel Systems, Germany) were composed of an array, stimulator, amplifier, temperature control unit, and data acquisition hardware and software. The slice was stabilized in aCSF (which contained 114 mM NaCl, 25 mM NaHCO3, 25 mM glucose, 3 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, 20 mM HEPES, pH 7.4) for 1 h at 33°C with 95% O2 and 5% CO2 gas aeration. The MEA containing the hippocampal slice was transferred to an MEA1060 amplifier interface. The solution in the array was grounded using an Ag/AgCl pellet. Then aCSF was allowed to flow over the slices at 3 ml/min. Data were sampled from every channel at 25 kHz and recorded using Recorder-Rack software (Multi Channel Systems GmbH, Germany). The stimulating channel was disconnected during stimulation. A baseline stimulation pulse of an intensity that gave 40–65% of the maximum response was delivered at a frequency of 0.033 Hz for the remainder of the experiment. The neuronal synaptic propagation was expressed in terms of the activated area by using an 8 × 8 heatmap image matrix on the hippocampus (Moon, 2009).
Induction of LTP in acute slices in the absence or presence of gintonin
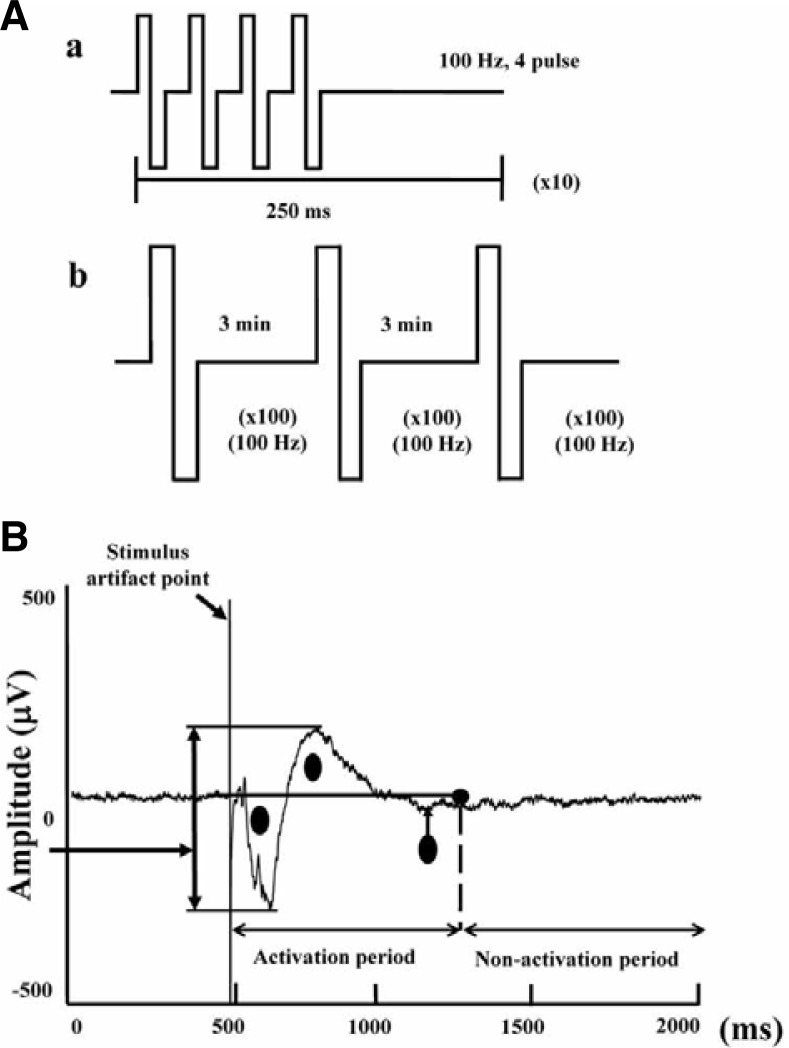
Bipolar constant current pulses were produced using the data acquisition software through a digital stimulator with a built-in isolator (Multi Channel Systems GmbH, Germany). To collect typical responses, one of the electrodes in the Schaffer collateral fibers area was selected as a stimulating electrode position while another one in the stratum radiatum of the Cornu ammonis (CA)1 was selected as a recording electrode position (Shimono et al., 2002). LTP was induced using standard protocols, which had a 100-Hz theta burst stimulation (Fig. 6Aa) or a tetanic train stimulation containing 3 bursts of 1 s at 100 Hz with 5-min intervals between each burst (Fig. 6Ab). Field potential (FP) recordings after LTP induction were performed for an additional 60–80 min period every 30 s to record the LTP condition (Figs. 7A and 7B). Stimulation and recording were carried out using the Recorder-Rack software (Multi Channel Systems GmbH, Germany). During stimulation, the stimulating channel was disconnected.
Fig. 6.
Procedure for LTP induction and activity calculation in rat hippocampal slices. (A) Upper panel: The protocol for theta burst stimulation (TBS) for acute hippocampal slices (a) or tetanic train stimulation for organotypic hippocampal slices (b). (B) Activity was calculated using area measuring of field potential trajectory. The signal was divided into 2 parts: activation and non-activation. The circle area within the line was calculated as the actual activity response. The artifact as indicated by the arrow was not calculated.
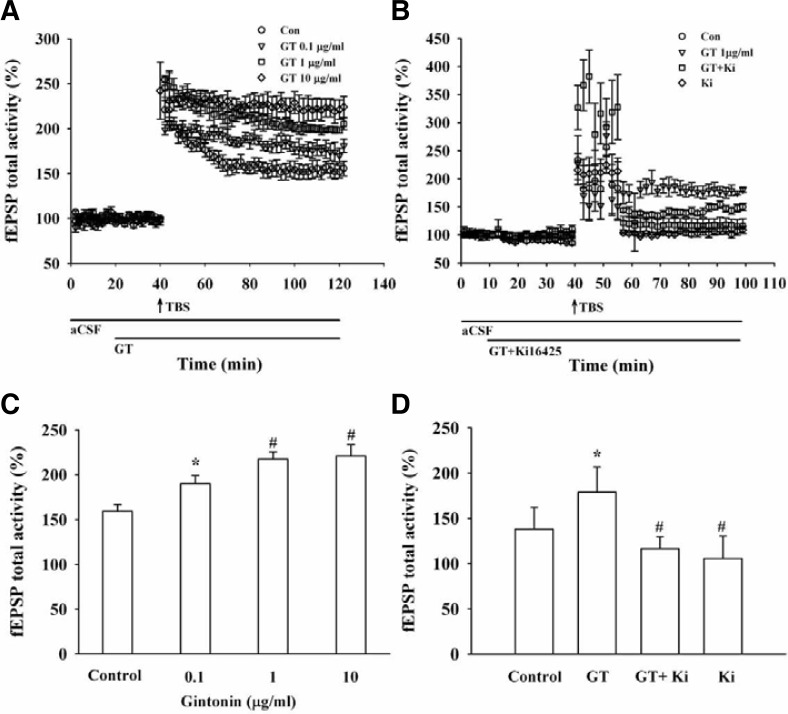
Fig. 7.
Effects of gintonin and an LPA1/3 receptor antagonist on LTP following tetanic stimulation of the Schaffer collateral (SC) area in rat hippocampal slices. LTP could be induced and maintained at CA3/CA1 (A) The LTP was increased by gintonin treatment in a concentration-dependent manner in acute hippocampal slices. Pooled data obtained from 4 different experiments. Data are expressed as percentages of control fEPSP amplitude. (B) Co-application of gintonin (1 μg/ml) with Ki16425 (1 nM), an LPA1/3 receptor antagonist, abolished gintonin-mediated LTP induction in organotypic hippocampal slices. The vertical arrow indicates the TBS addition point. Each point of the bars corresponds to the SEM (n = 4 each). (C) The summary histograms for normalized fEPSP values that show LTP levels at 40 min after TBS (*p < 0.05, compared to control; #p < 0.01, compared to control, n = 4). (D) The summary histograms for normalized fEPSP values that show LTP levels at 40 min after TBS (*p < 0.05, compared to control; #p < 0.05, compared to only gintonin treatment, n = 4).
Induction of LTP by gintonin in the absence or presence of Ki16425 in OHSC
For the LTP experiment using gintonin in the presence of Ki16425, the normal LTP-inducing stimuli repertoire could not produce a stable LTP. The triple tetanic burst program was the most successful (Fig. 6Ab). Three or four slices were used in each experimental group. A bipolar electrical stimulation was applied to the stratum radiatum of the CA2 region to stimulate the Schaffer collateral (SC)/commissural pathway. Baseline synaptic responses were evoked by stimulation at 0.033 Hz (120-μs pulse width) and were recorded for 10–20 min after at least 30 min of stabilization. LTP was then induced by a triple tetanic burst stimulation, which consists of a train of 3 × 100 Hz bursts (burst duration, 1 s; interburst interval, 5 min). After the conditioning stimulation, fEPSPs (field excitatory postsynaptic potentials) were recorded in whole sites in the hippocampus.
Data analysis
To obtain the concentration-response curve for the effect of gintonin on the inward peak (INMDA) mediated by the NMDA receptor, the INMDA peak was plotted at different concentrations of gintonin. Origin software version 8.0 (OriginLab Corp., USA) was used to fit the plot to the Hill equation: I/Imax = 1/[1 + (EC50/[A])nH], where Imax is the maximal current obtained from the ED50 value of the NMDA receptor, EC50 is the concentration of gintonin required to increase the response by 50%, [A] is the concentration of gintonin, and nH is the Hill coefficient. All values were presented as the mean ± SEM. The differences between the control and treatment data were determined using the paired Student’s t-test. A p-value of less than 0.05 was considered statistically significant. In gintonin-mediated LTP induction experiments, MC Rack (v.3.2.1.0, Multi Channel Systems) and an analyzing program (with aid from Dr. Tae-Sung Kim, department of medical-engineering Kyung-Hee University) using MatLab (v.7.0.1, The Mathworks inc.) was used to analyze the data (Moon et al., 2009). This program automatically provided the integral area of a field potential trajectory (Fig. 2). It is a better way to reveal the value of field potential than just measuring the amplitude or angle for an action potential. In one experiment with a slice, the value in an untreated sample evoked a response without TBS and was converted to % value, while the other values were converted to % value against an initial value. Data from hippocampal slice experiments were analyzed using one-way analysis of variance (ANOVA). Data were compared using the Duncan test. The alpha-type error was set to 0.05.
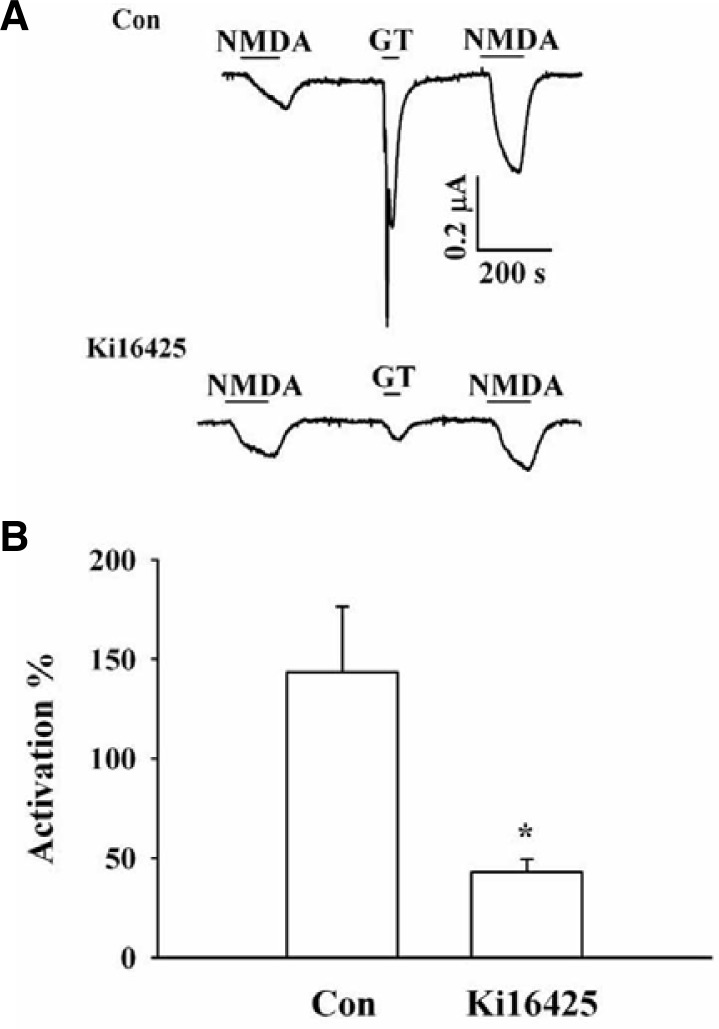
Fig. 2.
Effects of an LPA 1/3 receptor antagonist on gintonin-mediated potentiation of INMDA. (A) Representative traces of gintonin (1 μg/ml) (GT)-mediated potentiation of INMDA in the absence (upper) or presence (lower) of Ki16425. Treatment with Ki16425 attenuated gintonin-mediated potentiation of INMDA as well as the Ca2+-activated Cl− current. (B) Summary of the percent potentiation induced by gintonin in the absence or presence of Ki16425. Oocytes were voltage-clamped at a holding potential of −60 mV. Data represent the mean ± SEM. (n = 6–7/group).
RESULTS
Effect of gintonin on INMDA in oocytes expressing the rat NMDA receptor
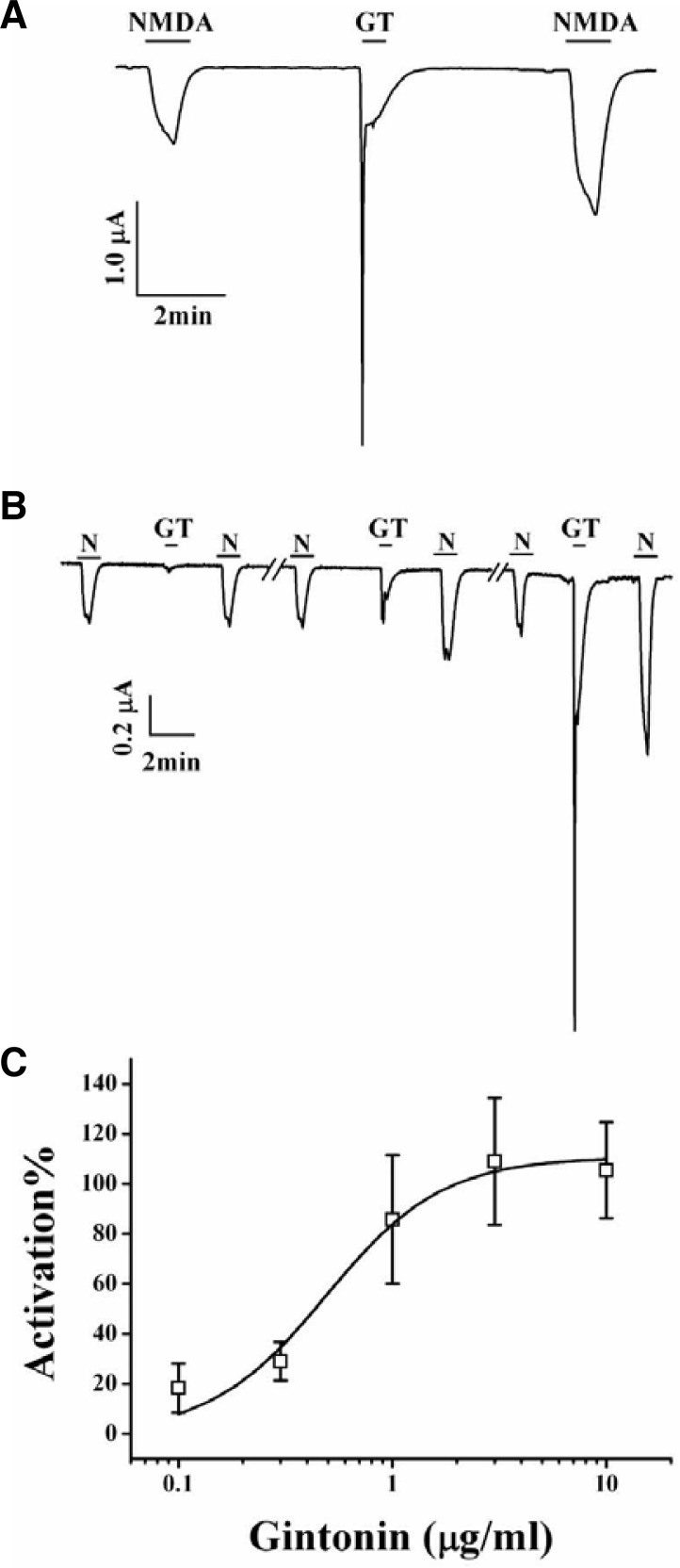
In the present study, we examined the effects of gintonin on the activity of NMDA receptor channel. For this, we expressed the rat NMDA receptor NR1b and NR2B subunits in Xenopus oocytes (Zheng et al., 1997). As shown in Fig. 1A, the addition of NMDA with glycine, a co-agonist required for efficient opening of the ion channel (Durand et al., 1993), to the bathing solution induced a large inward current (INMDA) in oocytes injected with rat NMDA receptor subunit cRNAs at a holding potential of −60 mV. In H2O-injected control oocytes, the application of NMDA did not induce any inward currents (data not shown). Gintonin treatment of the same oocyte induced a large Ca2+-activated Cl− channel (CaCC) current (Pyo et al., 2011). Interestingly, after gintonin treatment, subsequent NMDA application induced further enhancement of INMDA compared to that of the first NMDA treatment (Fig. 1A, n = 6–9 from 3 different frogs). Gintonin treatment also further potentiated a second INMDA in oocytes expressing NR1b and NR2A subunits (data not shown). In concentration-dependent experiments, gintonin treatment enhanced INMDA in a concentration-dependent manner in oocytes expressing the NMDA receptor (Fig. 1B). Gintonin at 0.1, 0.3, 1, 3, and 10 μM increased INMDA in oocytes expressing the NMDA receptor by 18.4 ± 9.8, 29.1 ± 7.7, 85.7 ± 25.8, 109.1 ± 25.4, and 105.5 ± 19.2%, respectively. The EC50 for an effect of gintonin on INMDA was 0.49 ± 0.1 μg/ml (Fig. 1C, n = 10–11, with samples taken from 3 different frogs for each point).
Fig. 1.
Effects of gintonin on INMDA. (A) A representative trace of gintonin (1 μg/ml) (GT)-mediated potentiation of INMDA in oocytes expressing the rat NMDA receptor. The trace represents 6 separate oocytes. (B) Concentration-dependent effect of gintonin on INMDA potentiation. The trace represents 6 separate oocytes. Application of NMDA (300 μM) and glycine (10 μM) elicited INMDA. Gintonin application after NMDA (300 μM) and glycine (10 μM) potentiated INMDA. (C) Concentration-dependent potentiating effects of gintonin on INMDA. Oocytes were voltage-clamped at a holding potential of −60 mV. Each point represents the mean ± SEM (n = 4–5/group).
Effects of LPA1/3 receptor antagonist on gintonin-mediated enhancement of INMDA
Xenopus oocytes express the endogenous LPA1 receptors (Kimura et al., 2001), and we have also previously demonstrated that gintonin activates CaCC via the activation of LPA receptor (Hwang et al., 2012a). We examined the effect of an LPA1/3 receptor antagonist, Ki16425, on gintonin-mediated INMDA enhancement. In the absence of Ki16425, gintonin treatment enhanced the second INMDA. However, in the presence of Ki16425, gintonin-mediated CaCC activation was greatly attenuated (Fig. 2A) and gintonin-mediated enhancement of INMDA was also significantly attenuated from 148.5 ± 33.0% to 43.0 ± 6.4% (*p < 0.05 compared to control) (Fig. 2B). This result indicates that gintonin-mediated enhancement of INMDA as well as CaCC activation was achieved through the activation of LPA receptors that endogenously express LPA1 receptors in Xenopus oocytes.
Signal transduction in gintonin-mediated enhancement of INMDA
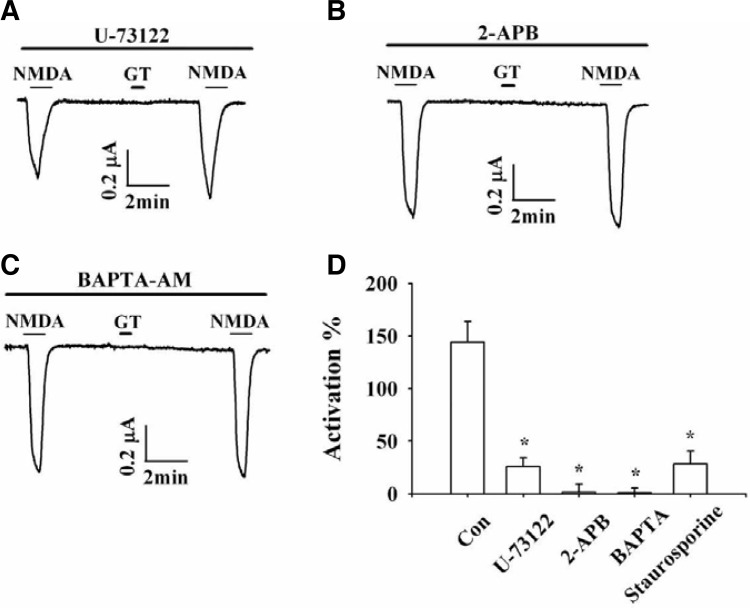
Next, we examined the signaling pathways involved in the gintonin-mediated stimulation of INMDA. We first examined the involvement of phospholipase C (PLC), because PLC activation is involved in gintonin-mediated CaCC activation in Xenopus oocytes (Pyo et al., 2011). To determine whether the PLC signaling pathway is involved in gintonin-mediated INMDA enhancement, we examined the effect of U-73122, an active PLC inhibitor, on gintonin action. The presence of U-73122 not only abolished the gintonin-mediated CaCC activation, but also attenuated gintonin-mediated enhancement of INMDA from 144.2 ± 19.6% to 25.6 ± 8.7% (*p < 0.05 compared with gintonin-untreated cells) (Fig. 3A).
Fig. 3.
Effects of a PLC inhibitor, IP3 receptor antagonist, or BAPTA on gintonin-mediated potentiation of INMDA. (A–C) Representative traces of gintonin (1 μg/ml) (GT)-mediated potentiation of INMDA in the presence of an active PLC inhibitor U-73122, IP3 receptor antagonist 2-APB, or intracellular Ca2+ chelator BAPTA. (D) Summary of the percent potentiation induced by gintonin in the absence (Con) or presence of various inhibitors or calcium chelator (U-73122, 2-APB, stauroporine, or BAPTA). Oocytes were voltage-clamped at a holding potential of −60 mV. Data represent the mean ± SEM (n = 6–7/group).
To determine whether gintonin action on INMDA enhancement proceeded through inositol-3-phosphate (IP3) receptor activation (Pyo et al., 2011), we examined the effect of gintonin on INMDA enhancement by pretreatment with 2-APB, an IP3 receptor antagonist (Fig. 3B). Gintonin-induced INMDA enhancement was abolished after pretreatment with 2-APB (*p < 0.05 compared with GT). In a previous report, we demonstrated that gintonin induces the [Ca2+]i transient via the activation of LPA receptor (Hwang et al., 2012a). The [Ca2+]i transient is a key element of INMDA enhancement by GPCR ligands (Zheng et al., 1997). We examined the role of the [Ca2+]i transient during gintonin-mediated INMDA enhancement and found that the gintonin-mediated INMDA enhancement was abolished in cells pretreated with 1,2-bis(o-aminophenoxy)ethane tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM), a membrane-permeable Ca2+ chelator (Fig. 3C). These results indicated that the gintonin-mediated [Ca2+]i transients via the PLC and IP3 receptor pathways are coupled to INMDA enhancement. In addition, treatment with staurosporine, a PKC inhibitor, blocked the gintonin-mediated INMDA enhancement from 144.2 ± 19.6% to 28.4 ± 12.3% (Fig. 3D). These results indicated that gintonin-mediated INMDA enhancement involved the PLC, PKC, and IP3 receptor pathways.
Involvement of protein kinase C, tyrosine kinase, and Src kinase on gintonin-mediated enhancement of INMDA
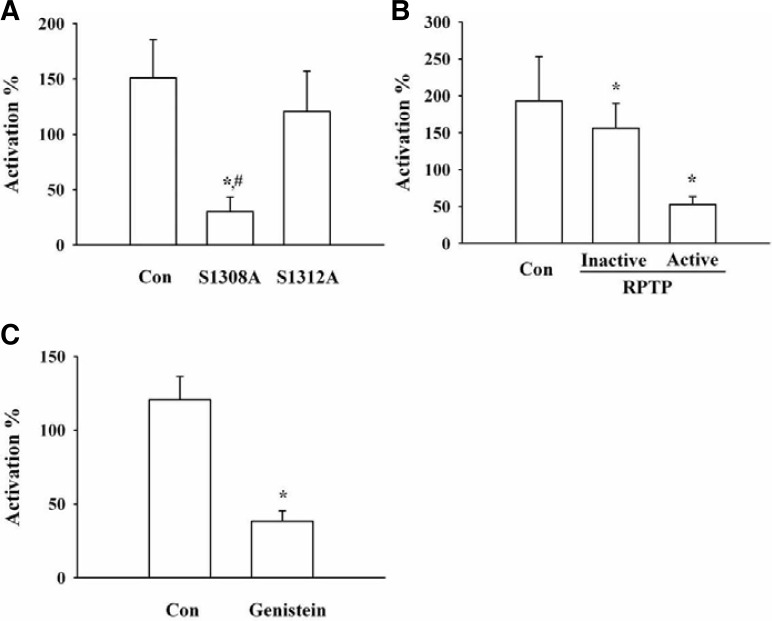
As shown in the above results, treatment with a PKC inhibitor blocked the gintonin action on INMDA enhancement, indicating that the phosphorylation of NMDA receptor by PKC activation is involved in gintonin-mediated INMDA enhancement. To demonstrate the involvement of the phosphorylation of NMDA receptor by PKC in gintonin action, we constructed a mutant NMDA receptor and examined the effect of gintonin on INMDA enhancement. As shown in Fig. 4A, the Ser1308Ala mutation of the NMDA receptor, where the Ser residue is phosphorylated by PKC (Jones and Leonard, 2005; Liao et al., 2001), greatly attenuated the gintonin action. Co-expression of the active receptor protein tyrosine phosphatase (RPTP) with the NMDA receptor, but not the co-expression of the inactive RPTP also attenuated gintonin action on the NMDA receptor (Fig. 4B). Next, we examined the involvement of tyrosine kinase in gintonin stimulation of the activity of NMDA receptor channel. Treatment with the tyrosine kinase inhibitor, genistein, also significantly attenuated the gintonin action (Fig. 4C). These results indicate that gintonin-mediated enhancement of INMDA is achieved through the phosphorylation of the NMDA receptor by PKC and through tyrosine kinase activation following the activation of LPA receptor.
Fig. 4.
Effects of mutation of the PKC phosphorylation site, use of a tyrosine kinase inhibitor, or co-expression of receptor protein tyrosine phosphatase on gintonin-mediated potentiation of INMDA. A summary of the percent potentiation induced by gintonin in site-directed mutation of the PKC phosphorylation site (A), or co-expression of receptor protein tyrosine phosphatase (B), or in the absence (Con) or in the presence of tyrosine kinase (C). Oocytes were voltage-clamped at a holding potential of −60 mV. A, *p < 0.05, compared to control; #p < 0.05, compared to S1312A mutant. B, *p < 0.05, compared to control. C, *p < 0.05, compared to control; Data represent the mean ± SEM (n = 4–5/group).
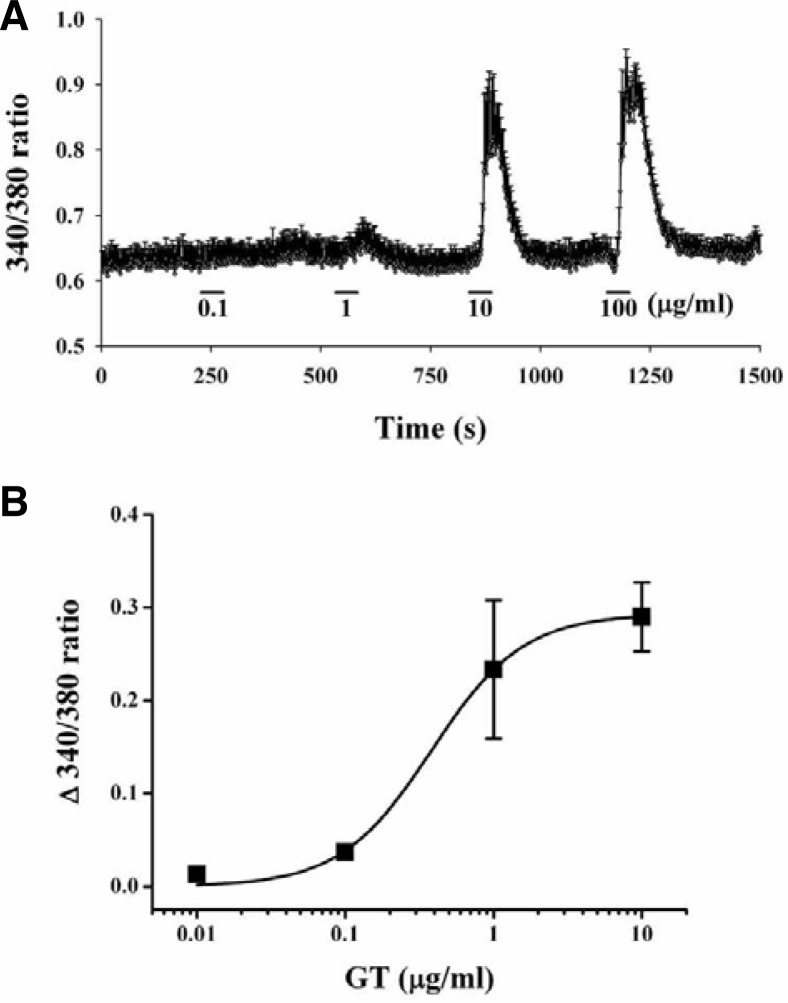
Gintonin induces [Ca2+]i transients in hippocampal neurons
Since gintonin enhanced INMDA in oocytes expressing NMDA receptors by transient [Ca2+]ivia the LPA receptor, we examined the effects of gintonin on intracellular Ca2+ levels in cultured hippocampal neurons. As shown in Fig. 5, treatment with gintonin induced [Ca2+]i transients in a concentration-dependent manner. The EC50 for gintonin on [Ca2+]i transients in hippocampal neurons was 0.4 ± 0.07 μg/ml (Fig. 5B, n = 10–11, with samples taken from 3 different batches of cells). However, an LPA1/3 receptor antagonist, Ki16425, again blocked gintonin action on [Ca2+]i transients (data not shown). These results indicate that gintonin induces transient elevation of intracellular Ca2+ levels via LPA receptors in hippocampal neurons.
Fig. 5.
Effects of gintonin on [Ca2+]i transients in cultured rat hippocampal neurons. (A) Representative traces in the absence (Con) or presence of various concentrations of gintonin. (B) Concentration-dependent effects of gintonin on [Ca2+]i transients. Each point represents the mean ± SEM (n = 7–10).
Gintonin induces long-term potentiation (LTP) in rat hippocampal slices
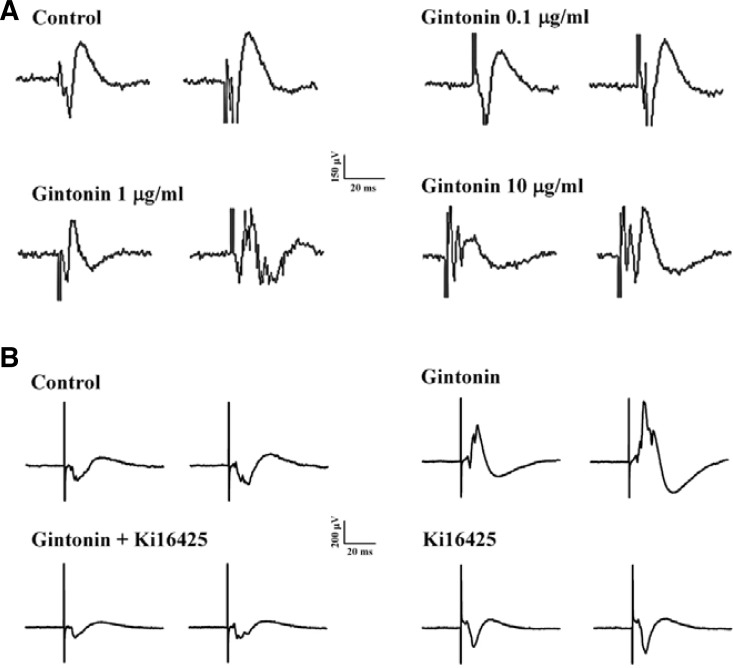
Since gintonin could enhance INMDA in oocytes expressing NMDA receptors and induce [Ca2+]i transients in hippocampal neurons, we further investigated whether gintonin-mediated INMDA potentiation and [Ca2+]i transients could be coupled to LTP in the rat hippocampus. According to the protocol of Fig. 6Aa, we induced a tetanic stimulation for the field excitatory postsynaptic potential (fEPSP) activity in hippocampal slices. The fEPSP dramatically decreased after 20 min in the absence of gintonin (Fig. 7A). However, gintonin-treated slices first exhibited stronger fEPSP activity than the controls, and gintonin-induced LTP maintained fEPSP activity with a slight attenuation of activity with time (Figs. 7 and 8A). Gintonin-induced LTP was concentration-dependent. Thus, gintonin remarkably increased fEPSPs by 180.90 ± 7.53, 205.32 ± 6.40, and 214.14 ± 11.55% at 0.1, 1, and 10 μg/ml, respectively (n = 4 each) (Figs. 7A and 7C). Next, we examined whether gintonin-induced LTP is blocked by an LPA1/3 receptor antagonist, Ki16425 (Ohta et al., 2003). According to the protocol of Fig. 6Ab, we also induced a tetanic stimulation for the field excitatory postsynaptic potential (fEPSP) activity in organotypic hippocampal slice culture. Although 1 nM Ki16425 alone treatment did not significantly affect fEPSP activity compared to control, the effect of gintonin (1 μg/ml) on LTP induction was abolished by Ki16425 (Figs. 7B, 7D, and 8B), indicating that gintonin-mediated LTP induction was achieved through LPA 1/3 receptor activation in rat hippocampal slices.
Fig. 8.
Representative traces from control, gintonin alone, or gintonin + Ki16425 in rat hippocampal slices. (A) Traces were obtained 50 min after TBS in control or with different concentrations of gintonin. (B) Traces were obtained 50 min after TBS in control, gintonin, gintonin + Ki16425, or Ki16425 alone. Sample traces show fEPSP evoked by test pulse stimulation.
DISCUSSION
LPA is a known neurolipid in the nervous system (Veloso et al., 2011). LPA participates in cell proliferation, migration, growth, differentiation, and morphological changes during brain development through G protein-coupled LPA receptors (Chun et al., 2010). LPA also plays an important role in memory, since LPA1 receptor knockout animals showed memory dysfunctions (Castilla-Ortega et al., 2011). However, relatively little is known about the molecular mechanisms by which LPA receptors are coupled to memory functions. The previous reports showed that ginseng extract improves cognition and memory functions (Kennedy et al., 2004, Persson et al., 2004). Recently, we demonstrated that ginseng contains a unique LPA receptor ligand, gintonin, which elicits transient [Ca2+]i increases through the activation of LPA receptor with a higher affinity than LPA (Hwang et al., 2012a). In the present study, we investigated how gintonin-mediated activation of LPA receptor is coupled to NMDA receptor activation and furthermore to LTP induction.
In the present study, we made 4 key observations indicating that gintonin modulates the activity of NMDA receptor channel and further induces LTP in the rat hippocampus through LPA receptors. First, we observed that gintonin enhanced INMDA in a concentration-dependent manner through the activation of LPA receptor. Second, the gintonin-mediated INMDA enhancement was linked to events upstream of the PLC, IP3 receptor, and Ca2+ pathway. Third, the activation of PKC and tyrosine kinases was involved in gintonin-mediated INMDA enhancement because inhibitors of these kinases or site-directed mutation of the PKC phosphorylation site blocked gintonin action. Fourth, gintonin-mediated INMDA enhancement and [Ca2+]i transients in hippocampal neurons via the activation of LPA receptor were further coupled to LTP induction in the rat hippocampus. These results indicate that gintonin-mediated NMDA receptor activation is coupled to LTP in the rat hippocampus. In addition, repeated treatment with gintonin induced a dramatic decrease in INMDA potentiation in oocytes expressing NMDA receptors, indicating that repeated treatment with gintonin might cause a rapid desensitization of LPA receptors (data not shown).
The previous reports demonstrated that activation of several types of G protein-coupled receptors, which are coupled to Gαq/11 proteins, potentiate the activity of NMDA receptor channel (Macdonald et al., 2006). Furthermore, NMDA receptor activation by Gαq/11-coupled receptor stimulations are linked to LTP induction through a sequential signal pathway composed of PLC, PKC, IP3 receptor, or Ca2+ (Sevilla and Buno, 2010). Thus, transient elevation of [Ca2+]i and the following activation of the downstream kinases by activation of Gαq/11-coupled receptors play an important role in the potentiation of the activity of NMDA receptor channel and LTP induction (MacDonald et al., 2006). In the present study, although we currently do not know whether LPA receptors are co-localized with NMDA receptors at synapses of the same neurons, we found that gintonin-mediated INMDA potentiation is coupled to the induction of LTP in the hippocampus through a sequential signal pathway of activation of LPA receptor.
The LPA1 receptor was abundantly expressed during the brain developmental period and decreased after birth and is maintained at low levels in adulthood (Hecht et al., 1996). The LPA1 receptor plays an important role in brain neurogenesis during nervous system development (Hecht et al., 1996). However, a recent study demonstrated the possibility that LPA receptors may also participate in brain functions in adulthood. Veloso et al. (2011) showed that LPA receptors in the human hippocampus were as abundant as the cannabinoid 1 receptor. In addition, LPA1 receptor knockout mice exhibited reduced neurogenesis and showed more damage after chronic stress, accompanied by spatial memory impairment (Castilla-Ortega et al., 2011; Matas-Rico et al., 2008), implying that the LPA receptor might also play an important role in memory function as well as neurogenesis in adults. Supporting this notion, we demonstrated in a previous report that short-term oral administration gintonin rescued β-amyloid-induced acute memory dysfunction in adult mice. Furthermore, in a transgenic mouse model for Alzheimer’s disease, we also showed that long-term oral administration of gintonin attenuated senile short-and long-term memory impairment (Hwang et al., 2012b). Thus, gintonin-mediated LTP induction via NMDA receptor activation could be one contribution for the restoration of memory in memory-related brain dysfunction in neurodegenerative diseases like Alzheimer’s disease.
Gintonin consists of LPAs-ginseng protein complexes. Gintonin contains about 9.5% LPAs, which mainly consist of LPA C18:2; LPAs act as functional ligands for G protein-coupled LPA receptors (Hwang et al., 2012a). LPA evoked [Ca2+]i transients and exhibited neurotransmitter-like activities in cortical neurons (Dubin et al., 1999; 2010). In the present study, we further showed that the activation of LPA receptor by gintonin was associated with INMDA enhancement, [Ca2+]i transients, and LTP induction in the hippocampus. Furthermore, gintonin-induced LTP in the hippocampus was blocked by a low concentration of Ki16425 (Fig. 7), indicating that gintonin-induced LTP is achieved through LPA1/3 receptors. In addition, we observed that gintonin itself improved the learning and memory functions and that it ameliorates scopolamine-induced learning and memory damage (data not shown). Thus, we speculate that gintonin might be a useful candidate for functional food or drug development for improvement of learning and memory.
In conclusion, we demonstrated for the first time that gintonin-mediated activation of LPA receptor is coupled to the potentiation of NMDA receptor channel currents and to the induction of LTP in rat hippocampal slices. Finally, we suggest that the gintonin-mediated LTP induction via the activation of LPA receptor is the molecular basis for the beneficial effect of ginseng on learning and memory functions.
Acknowledgments
This work was supported by the Basic Science Research Program (2011-0021144) and the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012-0006686), Veterinary Science Research Institute of the Konkuk University, and Brain Korea 21 to S.-Y. Nah.
REFERENCES
- Carroll R.C., Zukin R.S. NMDA-receptor trafficking and targeting, implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Castilla-Ortega E., Hoyo-Becerra C., Pedraza C., Chun J., Rodríguez De Fonseca F., Estivill-Torrús G., Santín L.J. Aggravation of chronic stress effects on hippocampal neurogenesis and spatial memory in LPA1 receptor knockout mice. PLoS One. 2011;6:e25522. doi: 10.1371/journal.pone.0025522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Hla T., Lynch K.R., Spiegel S., Moolenaar W.H. International union of basic and clinical pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin A.E., Bahnson T., Weiner J.A., Fukushima N., Chun J. Lysophosphatidic acid stimulates neurotransmitter-like conductance changes that precede GABA and L-glutamate in early, presumptive cortical neuroblasts. J. Neurosci. 1999;19:1371–1381. doi: 10.1523/JNEUROSCI.19-04-01371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin A.E., Herr D.R., Chun J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J. Neurosci. 2010;30:7300–7309. doi: 10.1523/JNEUROSCI.6151-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand G.M., Bennett M.V., Zukin R.S. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc. Natl. Acad. Sci USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J.H., Weiner J.A., Post S.R., Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H., et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer’s disease-related neuropathies: involvement of non-amyloidogenic processing. J. Alzheimers Dis. 2012a;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W., et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012b;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.L., Leonard J.P. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2 B subunits. J. Neurochem. 2005;92:1431–1438. doi: 10.1111/j.1471-4159.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- Kennedy D.O., Haskell C.F., Wesnes K.A., Scholey A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav. 2004;79:401–411. doi: 10.1016/j.pbb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim S., Yoon I.S., Lee J.H., Jang B.J., Jeong S.M., Lee J.H., Lee B.H., Han J.S., Oh S., et al. Protective effects of ginseng saponins on 3-nitropropionic acid-induced striatal degeneration in rats. Neuropharmacology. 2005;48:743–756. doi: 10.1016/j.neuropharm.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Schmitt A., Fukushima N., Ishii I., Kimura H., Nebreda A.R., Chun J. Two novel Xenopus homologs of mammalian LP(A1)/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J. Biol. Chem. 2001;276:15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- Lee S.T., Chu K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008;22:222–224. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Park J.H., Won R., Lee H., Nam T.S., Lee B.H. Inhibition of hexokinase leads to neuroprotection against excitotoxicity in organotypic hippocampal slice culture. J. Neurosci. Res. 2011;89:96–107. doi: 10.1002/jnr.22525. [DOI] [PubMed] [Google Scholar]
- Liao G.Y., Wagner D.A., Hsu M.H., Leonard J.P. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol. Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.F., Jackson M.F., Beazely M.A. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit. Rev. Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- Matas-Rico E., García-Diaz B., Llebrez-Zayas P., López-Barroso D., Santín L., Pedraza C., Smith-Fernández A., Fernández-Llebrez P., Tellez T., Redondo M., et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell. Neurosci. 2008;39:342–355. doi: 10.1016/j.mcn.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E., Her Y., Lee J.B., Park J.H., Lee E.H., Kim S.H., Oh M.K., Jang C.G., Kim S.Y. The multi-herbal medicine Gongjin-dan enhances memory and learning tasks via NGF regulation. Neurosci. Let. 2009;466:114–119. doi: 10.1016/j.neulet.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nah S.Y. Recent advances in ginseng research. Korean J. Ginseng Sci. 1997;21:1–12. [Google Scholar]
- Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J., Kimura T., Tobo M., Yamazaki Y., Watanabe T. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Persson J., Bringlöv E., Nilsson L.G., Nyberg L. The memory-enhancing effects of Ginseng and Ginkgo biloba in healthy volunteers. Psychopharmacology (Berl) 2004;172:430–434. doi: 10.1007/s00213-003-1675-8. [DOI] [PubMed] [Google Scholar]
- Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M., Lim Y., Kim D., Nah S.Y. Novel glycoproteins from ginseng. J. Ginseng Res. 2011;35:92–103. [Google Scholar]
- Sevilla F.D., Buño W. The muscrarinic long-term enhancement of NMDA and AMPA receptor-mediated transmission at Schaffer collateral synapses develop through different intracellular mechanisms. J. Neurosci. 2010;30:11032–11042. doi: 10.1523/JNEUROSCI.1848-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K., Baudry M., Ho L., Taketani M., Lynch G. Long-term recording of LTP in cultured hippocampal slices. Neural Plast. 2002;9:249–254. doi: 10.1155/NP.2002.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl E.M., Neveu E., Bertrand D., Buisson B. The adult rat hippocampal slice revisited with multi-electrode arrays. Brain Res. 2006;1096:70–84. doi: 10.1016/j.brainres.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Stoppini L., Buchs P.A., Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Timofeeva O.A., Levin E.D. Glutamate and nicotinic receptor interactions in working memory: importance for the cognitive impairment of schizophrenia. Neuroscience. 2011;195:21–36. doi: 10.1016/j.neuroscience.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Veloso A., Fernández R., Astigarraga E., Barreda-Gómez G., Manuel I., Giralt M.T., Ferrer I., Ochoa B., Rodríguez-Puertas R., Fernández J.A. Distribution of lipids in human brain. Anal. Bioanal. Chem. 2011;401:89–101. doi: 10.1007/s00216-011-4882-x. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhang L., Wang A.P., Bennett M.V., Zukin R.S. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J. Neurosci. 1997;17:8676–8686. doi: 10.1523/JNEUROSCI.17-22-08676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]