Abstract
Cerebellar granule neurons migrate from the external granule cell layer (EGL) to the internal granule cell layer (IGL) during postnatal morphogenesis. This migration process through 4 different layers is a complex mechanism which is highly regulated by many secreted proteins. Although chemokines are well-known peptides that trigger cell migration, but with the exception of CXCL12, which is responsible for prenatal EGL formation, their functions have not been thoroughly studied in granule cell migration. In the present study, we examined cerebellar CXCL14 expression in neonatal and adult mice. CXCL14 mRNA was expressed at high levels in adult mouse cerebellum, but the protein was not detected. Nevertheless, Western blotting analysis revealed transient expression of CXCL14 in the cerebellum in early postnatal days (P1, P8), prior to the completion of granule cell migration. Looking at the distribution of CXCL14 by immunohistochemistry revealed a strong immune reactivity at the level of the Purkinje cell layer and molecular layer which was absent in the adult cerebellum. In functional assays, CXCL14 stimulated transwell migration of cultured granule cells and enhanced the spreading rate of neurons from EGL microexplants. Taken together, these results revealed the transient expression of CXCL14 by Purkinje cells in the developing cerebellum and demonstrate the ability of the chemokine to stimulate granule cell migration, suggesting that it must be involved in the postnatal maturation of the cerebellum.
Keywords: cerebellum, chemokine, CXCL14, granule cells, migration
INTRODUCTION
Chemokines are a group of small, secreted proteins that contain signal peptides and many basic amino acids. They exert their biological effects by binding to and activating G-protein-coupled receptors. The production and secretion of most chemokines are stimulated in immune cells during development and by inflammatory responses (Baggiolini et al., 1997). Therefore, chemokines have been extensively studied for their chemotactic activity and their ability to control immune cell migration. More recently, the ability of chemokines to control proliferation, differentiation, or migration in other cell types has gained interest (Sallusto and Baggiolini, 2008). In particular, it has been shown that they can control cell migration during brain development and in pathological condition (Rostene et al., 2011). For example, CXCL12 regulates neuronal progenitor cell migration by activating CXCR4, which is its cognate receptor in brain and mice with genetic deletion of either CXCL12 or CXCR4 die shortly after birth with major defects in the vasculature and central nervous system (CNS) (Li and Ransohoff, 2008; Miller et al., 2008).
CXCL14 is a breast- and kidney-expressed chemokine (BRAK). Although it is one of three orphan chemokines whose receptors has not been yet identified, many studies have suggested its functional significance in cellular responses. CXCL14 is constitutively expressed in epithelial tissues, and it is likely to confer antibacterial activity to skin (Maerki et al., 2009). It is also proposed to be involved in the recruitment and maturation of monocyte-derived macrophages and the renewal of Langerhans cells in the skin. CXCL14 has been reported to inhibit angiogenesis, which is a common function of chemokines that lack an ELR sequence adjacent to the CXC motif (Shellenberger et al., 2004). Based on these findings, CXCL14 is considered an anti-cancer peptide, even though there are some reports of opposite effects in certain cancer cells (Augsten et al., 2009; Wente et al., 2008). Studies in transgenic mice revealed that CXCL14 deficiency attenuates obesity and inhibits feeding behavior when the mice are exposed to a novel environment, implying that CXCL14 may be involved in the neural control of appetite (Tanegashima et al., 2010).
In the central nervous system, CXCL14 mRNA is broadly observed in the whole brain by northern blotting and reverse transcription polymerase chain reaction (RT-PCR). In situ hybridization analysis showed CXCL14 mRNA expression in a subset of microglia and γ-aminobutyric acid (GABA)-ergic neurons in the brain (Schmid et al., 2009). Furthermore, it is known that CXCL14 treatment represses GABAergic neurotransmission, suggesting that it can act as a neuromodulator (Banisadr et al., 2011). However, the role of CXCL14 in developing brain has not been established yet. In this study, we found that CXCL14 peptide was transiently expressed in postnatal cerebellum, and it stimulated granule cell migration in vitro, which implies that CXCL14 is involved in maturation of the developing cerebellum.
MATERIALS AND METHODS
Animals
C57BL/6 mice were purchased from DBL (www.dbl.co.kr), Korea. All experiments used the smallest number of animals necessary, and procedures were designed to be as humane as possible. All experiments were approved by the Animal Care and Use Committee of the Korea University.
RT-PCR
Total RNA was extracted from several regions of adult mouse brain and various parts of the body using an RNeasy mini kit (Qiagen, Germany). First-strand cDNAs were synthesized from 2 μg total RNA with a reverse transcription kit containing Moloney murine leukemia virus reverse transcriptase (M-MLVRT) (Promega, USA). Reaction mixtures were diluted and subjected to PCR amplification with sense and antisense primers specific for mouse CXCL14 and GAPDH. The PCR primer set for CXCL14 is shown below. Upper and lower sequences were 5′-AGG CTC CTG GCG GCC GCG CTG-3′ and 5′-CAC CCT ATT CTT CGT AGA CC-3′, respectively, covering a distance of 297 bp (382–678 bp; accession number, NM_019568). Primers for GAPDH were as follows: 5′-GGT GAA GGT CGG TGT GAA CG-3′ and 5′-CTC GCT CCT GGA AGA TGG TG-3′. After 30 cycles of amplification, PCR products were electrophoresed on a 1.5% agarose gel and visualized using ethidium bromide.
Western blotting
Tissues were solubilized by ultrasonification in lysis buffer containing Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.2% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Roche, Switzerland). Lysates were clarified by centrifugation at 15,000 rpm for 15 min at 4°C, and supernatants were transferred to new tubes. Protein concentrations were determined by the Bradford method. Total protein extracts (20 μg) were loaded into each well of an SDS-PAGE gel and separated by electrophoresis. The proteins were transferred onto a nitrocellulose membrane and probed with anti-CXCL14 antibody or anti-actin antibody, and signals were detected using an enhanced chemiluminescence assay kit.
In situ hybridization
Mice were sacrificed by cervical dislocation, and their brains were removed and quickly frozen in isopentane on dry ice. Tissue sections were cut to 20 μm thickness with a cryostat, thaw mounted on Superfrost Plus slides (Thermo Fisher Scientific, USA), and stored at −70°C until use. Sections were fixed in 4% paraformaldehyde (PFA), washed with phosphate-buffered saline (PBS), and acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% NaCl (pH 8.0). Samples were hybridized overnight with a radiolabeled probe (1.2 × 106 cpm) and washed four times with 2× standard sodium citrate (SSC). A template for the CXCL14 probe was prepared by subcloning the RT-PCR products into a pGEM-T vector (Promega). Sense and antisense riboprobes were prepared using an in vitro transcription system (Promega) in the presence of [α-35S] UTP (Amersham Pharmacia Biotech, USA). After RNase A treatment, slides were rinsed with 2×, 1×, 0.5×, and 0.1× SSC containing 1 mM dithiothreitol for 10 min each at room temperature, then washed with 0.1× SSC at 60°C. The samples were dehydrated in ethanol and exposed to X-ray film (Biomax MR, Kodak, USA).
Immunohistochemistry
Immunohistochemistry using brain slices was performed as described previously (Kim et al., 2011). In brief, brains from neonatal (P1 and P8) and adult (6 weeks old) mice were removed after cardiac perfusion with 4% PFA. All tissue was postfixed in 4% PFA overnight at 4°C, cryoprotected in 20% sucrose in PBS until it sank, and frozen in optimal cutting temperature (O.C.T.) compound. Sections on slides were dried, washed with PBS, and blocked with blocking buffer (10% normal goat serum and 0.2% Triton X-100 in PBS) for 1 h at room temperature (RT). Then the slices were incubated in primary antibody overnight at 4°C. Primary antibodies included rabbit polyclonal CXCL14 (1:300; Abcam, UK), mouse monoclonal TAG1 (1:100; Developmental Studies Hybridoma Bank, USA), and mouse monoclonal calbindin (1:500; Sigma-Aldrich, USA). Primary antibodies were removed, and sections were rinsed in PBS containing 0.2% Triton X-100 (PBS-T) before incubation in secondary antibodies for 2 h at room temperature. Secondary antibodies were either conjugated to Alexa Fluor 488 (1:1,000; Invitrogen, USA) or Cyanine 3.5 (1:500; Abcam). The nucleus was stained with Hoechst 33342 (1:20,000; Invitrogen). Sections were rinsed with PBS and coverslipped (Marienfeld, Germany). All staining patterns were observed under an LSM510 confocal microscope or a fluorescence microscope (Carl Zeiss Microimaging, Inc., Germany).
Transwell migration assay
A suspension of granule cells was obtained by trypsin-EDTA treatments for 10 min at 37°C and subsequent trituration with a Pasteur pipette. Before the migration assay, transwell inserts with 8-μm pores (Corning, Inc., USA) were coated with laminin for 2 h at 37°C. Cells (2 × 104) in Neurobasal medium supplemented with B27 were added to transwell inserts. Medium containing different concentrations of CXCL14 was placed in the bottom wells. The plates were maintained in a humidified incubator with 5% CO2 at 37°C for 24 h. Transwell inserts were washed in PBS, and the non-migrated cells in the inner well were removed with a cotton swab. The membranes were fixed in 4% formalin, stained with hematoxylin, and migrated cells were counted in five high-power microscope fields.
Cell spreading assay
Microexplants were prepared for the in vitro cell-spreading assay as described previously (Nagata and Nakatsuji, 1990). In brief, the EGL of P5 mice were freed from meninges, cut into pieces (300–400 μm), and plated on dishes coated with poly-L-lysine (10 μg/ml) and laminin (25 μg/ml). The explants were maintained in Neurobasal media supplemented with B27 for 24 h and then treated with CXCL14. Then, images were captured in the same field at 24-h intervals. Microscope image analysis software from Metamorph (Molecular Devices, Inc., USA) was used to measure the distance between neuronal cell bodies and the farthest cells migrated from the explant margin. Distances were measured in at least 10 explants to obtain mean values. To explore blocking effect of antibodies on CXLC14-stimulated spreading, the peptides were mixed with anti-CXCL14 antibodies before treatment. After 48 h, spreading distances were measured as described above. All experiments were repeated more than three times.
RESULTS AND DISCUSSION
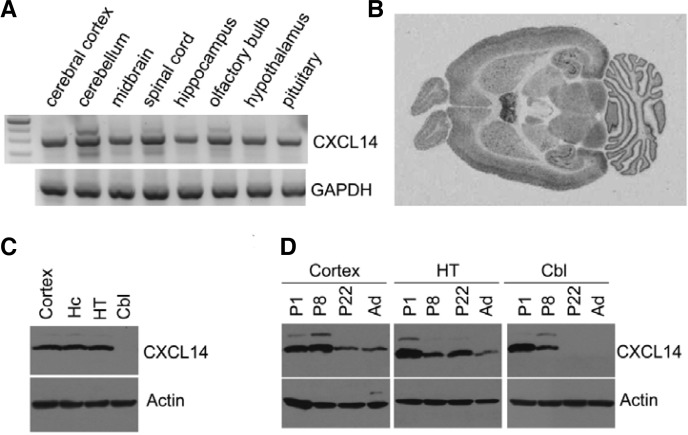
CXCL14 mRNA was detected in most brain regions by RT-PCR (Fig. 1A), which is in agreement with a previously published northern blot analysis (Sleeman et al., 2000). In situ hybridization also revealed that it was highly expressed in most brain regions, including septum, hippocampus, and cerebellum (Fig. 1B). By contrast, Western blotting with a specific antibody against CXCL14 showed that it was in cerebral cortex, hippocampus, and hypothalamus, but not in the cerebellum of adult mice (Fig. 1C). We further investigated CXCL14 expression in brains from mice of different ages with Western blotting. In cerebral cortex and hypothalamus, CXCL14 was highly expressed in neonates, was sustained until P8-22, and then decreased in adult brain. However, in the cerebellum, the expression level of CXCL14 declined more sharply, and CXCL14 protein was not detected in P22 or adult brain (Fig. 1D).
Fig. 1.
Regional expression of CXCL14 in the mouse brain on different postnatal days. (A) CXCL4 mRNA expression was detected by RT-PCR in various brain regions. (B) Autoradiogram of in situ hybridized brain slices of adult mice. (C) CXCL14 expression was examined in cortex, cerebral cortex; Hc, hippocampus; HT, hypothalamus; and Cbl, cerebellum (D) Extracts from cerebral cortex, hypothalamus, and cerebellum of different postnatal day mice (P1, P8, P22, adult) were subjected to Western blotting. 1, blot with anti-CXCL14 antibody; 2, blot with anti-actin antibody.
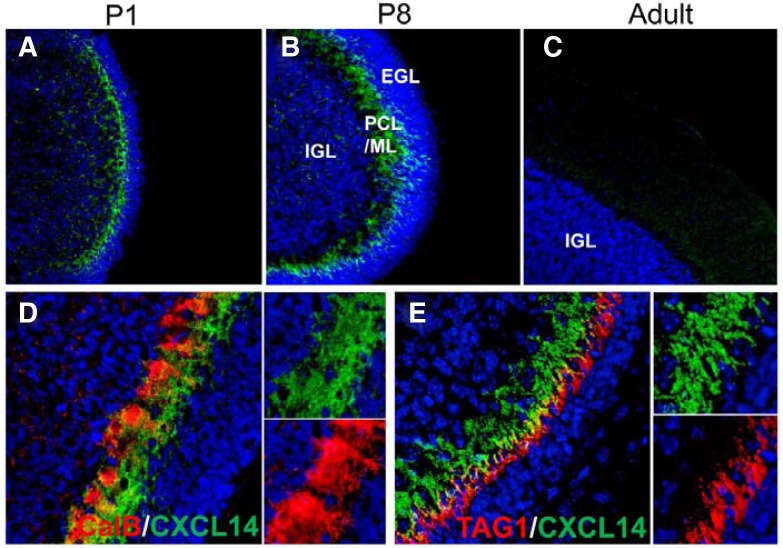
Next, we explored specific cell-type CXCL14 protein expression in the cerebellum (Fig. 2). Although CXCL14 was localized in the Purkinje cell layers (PCL)/molecular layer (ML) of P1 and P8 cerebellum, there was no CXCL14 signal in the adult cerebellum (Figs. 2A–2C). Double immunofluorescent labeling further revealed that CXCL14 was expressed in the dendrites of calbindin-expressing Purkinje cells localized in the ML (Fig. 2D). Some CXCL14 signal was also observed in extracellular regions near the dendrites, suggesting that CXCL14 is secreted from Purkinje cell dendrites and associates with the extracellular matrix. TAG1 is considered as a marker of early migrating granule cells (Xenaki et al., 2011). As shown in Fig. 2E, CXCL14 was closely localized with TAG1-expressing early migrating granule cells.
Fig. 2.
Regional CXCL14 expression in the mouse cerebellum on different postnatal days. (A, B, and C) Slices of cerebellum were immunostained with anti-CXCL14 antibody, and nuclei were stained with Hoechst 33342. CXCL14 was localized in the molecular layer on P1 and P8, but it was not detected in adult cerebellum. (D and E) Co-staining of CXCL14 with neuronal markers. Slices of P8 cerebellum were stained with anti-CXLC14 antibodies (green) and antibodies for calbindin or TAG1 (red). Nuclei were stained with Hoechst 33342. IGL, Internal granular layer; EGL, external granular layer; PCL/ML, Purkinje cell layers/molecular layer.
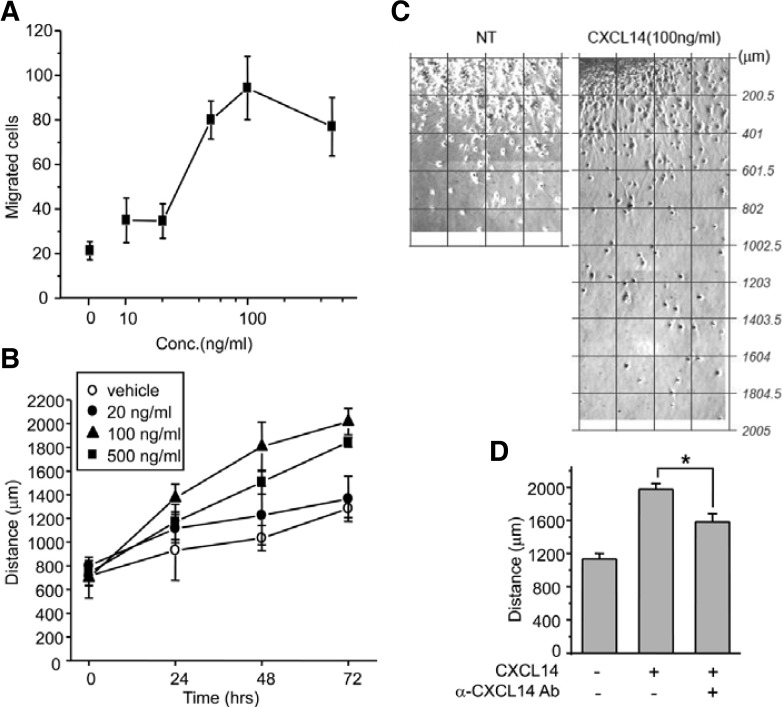
The spatiotemporal expression of CXCL14 in the developing cerebellum suggests that it may contribute to granule cell migration. Therefore, we examined whether CXCL14 influences granule cell migration using two different migration assays. In the transwell assay, we found that CXCL14 induced cell migration with maximum activity at a concentration of 100 ng/ml, suggesting that CXCL14 induces granule cell chemotaxis (Fig. 3A). Similar to how granule cells migrate from the EGL to the IGL due to in vivo factors, granule cells migrate away from EGL explants in vitro, independent of glial cells (Nagata and Nakatsuji, 1990). To examine the effect of CXCL14 on granule cell spreading, we treated explants with different concentrations of CXCL14 and found that it enhanced the granule cell migration in a dose-dependent manner. The maximum effect was observed in 100 ng/ml CXCL14-treated explants, which had a 1.75-fold increase in migration distance compared with control explants at 48 h (1807 ± 206 μm versus 1033 ± 105 μm, p < 0.01) (Figs. 3B and 3C). Since specific antibodies for peptide ligands are likely to block cell-stimulating activity of the peptides, we mixed CXCL14 with anti-CXCL14 antibodies before applying them to the explants. Interestingly, the antibody-mixed peptides partially decreased the migration (at 48 h, 1977 ± 122 μm versus 1583 ± 172 μm, p < 0.05) (Fig. 3D), suggesting that the pro-migration activity of CXCL14 was specific for cerebellar granule cells.
Fig. 3.
Effect of CXCL14 on granule cell migration and EGL microexplant spreading. (A) Transwell migration assay. Isolated granule cells were placed in upper wells coated with laminin, and different concentrations of CXCL14 (0, 10, 20, 100, or 500 ng/ml) were added to the bottom wells. After 24 h of incubation, migrated cells were fixed, stained with hematoxylin, and counted in five high-power microscope fields. The numbers are the average of three experiments. (B) EGL microexplant spreading rates. Small pieces of EGL were seeded on laminin-coated culture dishes. Twenty-four hours later, explants were treated with CXCL14, and the distances of neuronal cell bodies from explant margins were measured using the scale bar function of Metamorph in images taken of the same fields at 24-h intervals. (C) Representative microexplant pictures. Several images captured under 200× magnification were combined into a single picture. Values with lines were converted with the scale bar function of Metamorph. NT, non-treat. (D) EGL microexplants were treated with CXCL14 (100 ng/ml) or premixture of CXCL14 (100 ng/ml) and anti-CXCL14 antibodies (2 μg/ml). After 48 h, spreading distance of neuronal cells was measured. n = 9–10, *p < 0.05. All experiments were performed more than 3 times.
Here, we demonstrate that CXCL14 expression in Purkinje cells is spatiotemporally regulated during cerebellar development. Even though the CXCL14 gene is transcribed into mRNA in Purkinje cells throughout postnatal stages, CXCL14 protein expression is restricted to early postnatal stages when granule cell migration is active. It is not clear whether CXCL14 mRNA translation is inhibited by microRNA or if the peptide is easily degraded by specific amino acid sequences in the mature cerebellar environment (Peterson et al., 2006). Immunohistochemical studies indicated that Purkinje cells seem to express and release CXCL14 through dendrites that project to the molecular layer. Because CXCL14 can easily attach to glycoproteins that are enriched in the plasma membrane and the extracellular matrix (Shellenberger et al., 2004), it is likely that CXCL14 in the PCL/ML induces granule cell migration. This hypothesis was confirmed by transwell migration assays and microexplant culture experiments. CXCL14 stimulated migration of a crude population of EGL cells and enhanced the spreading rate cells from EGL microexplants.
During cerebellar development, granule cells in the EGL migrate to the IGL through the ML and PCL; this process is completed by approximately P14 (Komuro and Yacubova, 2003). Chemokines may be an important factor for this process. For instance, CXCL12 is expressed in the pia mater and is known to be responsible for EGL formation and immature granule cell maintenance in the EGL by chemoattracting them toward the pia mater (Zou et al., 1998). CXCL12 gene knockout results in the premature migration of immature granule cells into the IGL. Interestingly, CXCL14 is localized in the PCL/ML, which is the primary route of granule cell migration. It is known that Purkinje cells play a key role in the control of granule cell proliferation and migration from the EGL to the IGL (Feddersen et al., 1992) and the present data indicate that CXCL14 released by Purkinje cells may have a unique function in the control of granule cell migration; by stimulating an unknown receptor expressed on migrating granule cells.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean Government (Ministry of Education, Science and Technology) (no. 2009-0073875).
REFERENCES
- Augsten M., Hagglof C., Olsson E., Stolz C., Tsagozis P., Levchenko T., Frederick M.J., Borg A., Micke P., Egevad L., et al. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc. Natl. Acad. Sci USA. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Human chemokines: an update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Banisadr G., Bhattacharyya B.J., Belmadani A., Izen S.C., Ren D., Tran P.B., Miller R.J. The chemokine BRAK/CXCL14 regulates synaptic transmission in the adult mouse dentate gyrus stem cell niche. J. Neurochem. 2011;119:1173–1182. doi: 10.1111/j.1471-4159.2011.07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddersen R.M., Ehlenfeldt R., Yunis W.S., Clark H.B., Orr H.T. Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron. 1992;9:955–966. doi: 10.1016/0896-6273(92)90247-b. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Kim H., Sun W. Spontaneous reactive astrogliosis in the dentate gyrus of Bax-deficient mice. Mol Cells. 2011;31:379–383. doi: 10.1007/s10059-011-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H., Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol. Life Sci. 2003;60:1084–1098. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Ransohoff R.M. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog. Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerki C., Meuter S., Liebi M., Muhlemann K., Frederick M.J., Yawalkar N., Moser B., Wolf M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J. Immunol. 2009;182:507–514. doi: 10.4049/jimmunol.182.1.507. [DOI] [PubMed] [Google Scholar]
- Miller R.J., Rostene W., Apartis E., Banisadr G., Biber K., Milligan E.D., White F.A., Zhang J. Chemokine action in the nervous system. J. Neurosci. 2008;28:11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata I., Nakatsuji N. Granule cell behavior on laminin in cerebellar microexplant cultures. Brain Res. Dev. Brain Res. 1990;52:63–73. doi: 10.1016/0165-3806(90)90222-k. [DOI] [PubMed] [Google Scholar]
- Peterson F.C., Thorpe J.A., Harder A.G., Volkman B.F., Schwarze S.R. Structural determinants involved in the regulation of CXCL14/BRAK expression by the 26 S proteasome. J. Mol. Biol. 2006;363:813–822. doi: 10.1016/j.jmb.2006.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostene W., Dansereau M.A., Godefroy D., Van Steenwinckel J., Reaux-Le Goazigo A., Melik-Parsadaniantz S., Apartis E., Hunot S., Beaudet N., Sarret P. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J. Neurochem. 2011;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Baggiolini M. Chemokines and leukocyte traffic. Nat. Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- Schmid C.D., Melchior B., Masek K., Puntambekar S.S., Danielson P.E., Lo D.D., Sutcliffe J.G., Carson M.J. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J. Neurochem. 2009;109:117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellenberger T.D., Wang M., Gujrati M., Jayakumar A., Strieter R.M., Burdick M.D., Ioannides C.G., Efferson C.L., El-Naggar A.K., Roberts D., et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- Sleeman M.A., Fraser J.K., Murison J.G., Kelly S.L., Prestidge R.L., Palmer D.J., Watson J.D., Kumble K.D. B cell- and monocyte-activating chemokine (BMAC), a novel non-ELR alpha-chemokine. Int. Immunol. 2000;12:677–689. doi: 10.1093/intimm/12.5.677. [DOI] [PubMed] [Google Scholar]
- Tanegashima K., Okamoto S., Nakayama Y., Taya C., Shitara H., Ishii R., Yonekawa H., Minokoshi Y., Hara T. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. PLoS One. 2010;5:e10321. doi: 10.1371/journal.pone.0010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente M.N., Mayer C., Gaida M.M., Michalski C.W., Giese T., Bergmann F., Giese N.A., Buchler M.W., Friess H. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett. 2008;259:209–217. doi: 10.1016/j.canlet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Xenaki D., Martin I.B., Yoshida L., Ohyama K., Gennarini G., Grumet M., Sakurai T., Furley A.J. F3/contactin and TAG1 play antagonistic roles in the regulation of sonic hedgehog-induced cerebellar granule neuron progenitor proliferation. Development. 2011;138:519–529. doi: 10.1242/dev.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]