Abstract
Mitogen-activated protein kinase (MAPK) is activated by various biotic and abiotic stresses. Salt stress induces two well-characterized MAPK activating signaling molecules, phosphatidic acid (PA) via phospholipase D and phospholipase C, and reactive oxygen species (ROS) via nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase. In our previous study, the activity of soybean MAPK, GMK1 was strongly induced within 5 min of 300 mM NaCl treatment and this early activity was regulated by PA. In this study, we focused on the regulation of GMK1 at the later stage of the salt stress, because its activity was strongly persistent for up to 30 min. H2O2 activated GMK1 even in the presence of PA generation inhibitors, but GMK1 activity was greatly decreased in the presence of diphenyleneiodonium, an inhibitor of NADPH-oxidase after 5 min of the treatment. On the contrary, the n-butanol and neomycin reduced GMK1 activity within 5 min of the treatment. Thus, GMK1 activity may be sustained by H2O2 10 min after the treatment. Further, GMK1 was translocated into the nucleus 60 min after NaCl treatment. In the relationship between GMK1 and ROS generation, ROS generation was reduced by SB202190, a MAPK inhibitor, but was increased in protoplast overexpressing TESD-GMKK1. However, these effects were occurred at prolonged time of NaCl treatment. These data suggest that GMK1 indirectly regulates ROS generation. Taken together, we propose that soybean GMK1 is dually regulated by PA and H2O2 at a time dependant manner and translocated to the nucleus by the salt stress signal.
Keywords: GMK1, hydrogen peroxide, phosphatidic acid, salt stress, soybean
INTRODUCTION
High salt concentration is harmful to plants because it results in hyperosmotic stress and ionic toxicity (Tester and Davenport, 2003). Plants protect themselves from this stress by recognizing and transferring signals to the inside of the cell via signaling pathways such as mitogen-activated protein kinase (MAPK) pathway.
MAPK is activated by various biotic and abiotic stresses and regulated by the upstream regulator MAPK kinase (MAPKK), which is, in turn, regulated by MAPKK kinase (MAPKKK). This modulation is conserved in all eukaryotes. Active site phosphorylation of MAPK is essential for its activation. In mammalian cells, most activated MAPKs are translocated to the nucleus to regulate gene expression. In plants, few studies have been conducted to examine MAPK translocations. Parsley MAPKs, PcMPK3 and PcMPK6 are translocated to the nucleus by elicitor treatment (Lee et al., 2004), and Arabidopsis MAPKs, AtMPK3 and AtMPK6 are translocated by ozone treatment (Ahlfors et al., 2004).
Phosphatidic acid (PA) is a secondary messenger that is generated directly from phospholipase D (PLD) and indirectly by phospholipase C (PLC). Neomycin, a well-known PLC inhibitor, and n-butanol reduces PA generation by PLD. PA is generated for a short period of time in response to various stresses (Raho et al., 2011; Yu et al., 2010; Zhang et al., 2009). PA also directly activates MAPK (Lee et al., 2001; Zhang et al., 2003) and nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (Ogasawara et al., 2008).
H2O2 is another well-known MAPK activator that regulates various responses to biotic and abiotic stresses. For example, H2O2 directly activates the two Arabidopsis MAPKs through the action of oxidative signal-inducible 1 (OXI1) (Rentel et al., 2004); further, abscisic acid (ABA)-induced H2O2 activates MAPK in maize (Jiang et al., 2006). Moreover, H2O2 alters diverse cellular processes in a dose-dependent manner. High H2O2 concentration triggers cellular response of programmed cell death (Alvarez et al., 1998; Delledonne et al., 2001), whereas low H2O2 concentration blocks cell cycle progression and regulates plant development and stress response (Foyer and Noctor, 2005; Neill et al., 2002; Reichheld et al., 1999). Several hormones also increase the H2O2 levels, leading to enhanced stress tolerance (Dat et al., 1998; Xia et al., 2009; Yang et al., 2001).
Many stresses result in the generation of signaling molecules, such as PA and H2O2, and their direct involvement in MAPK activation has been well-established. However, their involvement in regulating MAPK activity is not largely understood.
In our previous study, we found that soybean MAPK, GMK1 activity was strongly induced within 5 min of 300 mM NaCl treatment; this early activity is regulated by PA (Im et al., 2012). In this study, we examined regulation of GMK1 at late time periods of the treatment, because we have already shown that GMK1 activity is retained for up to 30 min. We found that GMK1 is dually regulated by PA and H2O2 at different time points and is translocated to the nucleus under salt stress.
MATERIALS AND METHODS
Plant material
Glycine max L. seeds were sterilized using bleach solution (0.2% bleach) for 5 min, followed by 5 washes with sterilized distilled water. The seeds were then placed on a wet paper towel for germination in a growth chamber (25°C, 60% humidity) for 7 days under dark conditions. Before chemical treatment, all seedlings were stabilized in B & D solution (Broughton and Dilworth, 1971) for at least 4 h. After treatment, seedling samples, excluding cotyledons and hypocotyls, were immediately frozen in liquid nitrogen and pulverized using mortars and pestles. Ground samples were stored as powder at −80°C until use.
Preparation of protein extracts and in-gel kinase assay
The 200 μg of tissue powders were dissolved in 200 μl of extraction buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and 1 mg/ml aprotinin, leupeptin, and pepstatin) in 1.5 ml centrifuge tubes. The samples were incubated on ice for 5 min and homogenized for 10 s by vortexing. After centrifugation at 15,000 rpm for 15 min at 4°C, the supernatants were transferred into clean tubes. After 2 additional centrifugations, the concentrations of protein samples were determined using the Bradford method. The 30 ug of total protein was loaded to acrylamide gel and performed an in-gel kinase assay as described previously (Lee et al., 2008).
Immunoprecipitation assay
For the immunoprecipitation assay, 400 μg of total protein samples were incubated with anti-GMK1 antibody at 4°C for 2 h, and then precipitated using protein A sepharose (GE healthcare, Sweden). After washing with washing buffer (Lee et al., 2001), the beads were eluted using SDS sample buffer at 95°C for 3 min and subjected to in-gel kinase assay.
H2O2 and O2− measurement
H2O2 content was measured using 5-(and-6)-chloromethyl-2,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA). Soybean seedlings were treated with 10 μM CM-H2DCFDA (Molecular Probes™) for 60 min. After the seedlings had been treated with chemical inhibitors and/or NaCl, H2O2 signals were detected using the fluorescein isothiocyanate (FITC) channel of a fluorescence microscope (Carl Zeiss).
To detect O2−, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT; Sigma, USA) was used. The chemical inhibitor or mock-treated soybean seedling roots were embedded in XTT and 300 mM NaCl solution, and then 200 μl of the solution was used for measuring XTT reduction using a spectrophotometer at an absorbance wavelength of 470 nm (A470).
Protoplast isolation and polyethylene glycol transfection
The protoplast isolation method used in this study has been previously described (Yoo et al., 2007). Briefly, roots and hypocotyls of 7-day-old seedlings that were grown in dark conditions were cut to a size of 1 mm and transferred to an enzyme solution [1% w/v of cellulase RS (YAKULT, Japan) and macerozyme R-10 (MB cell, Korea), 0.4 M mannitol, 20 mM KCl and MES, 10 mM CaCl2, and 0.1% BSA]. After the solution was incubated at room temperature for 3 h, it was diluted with an equal volume of W5 solution (150 mM NaCl, 125 mM CaCl2, 5 mM KCl, and MES; pH 5.7). The solution was then filtered through an 80-μm nylon mesh, centrifuged at 200 × g for 3 min, and resuspended in W5 solution. W5 solution was removed and the cells were resuspended in MMG solution (0.4 M mannitol, 15 mM MgCl2, and 4 mM MES; pH 5.7). PEG transfection was performed as described previously (Yoo et al., 2007).
Immunolocalization assay
Paraffin-embedded roots were cut and fixed onto a slide glass, and the paraffin was removed. The samples were treated using xylene and ethanol for rehydration and washed with phosphate buffered saline (PBST; 137 mM NaCl, 1.5 mM KH2PO4, 2.7 mM KCl, 8 mM Na2HPO4, and 0.5 ml Tween 20). The slides were incubated in blocking buffer [1% (w/v) non-fat milk powder in PBST] for 45 min and washed using PBST. After the slides were treated with the anti-GMK1 antibody, washed 3 times using PBST, an FITC-conjugated antibody was added. The slides were washed and the signal was measured using the z-stack method of a confocal microscope (Carl Zeiss-LSM510). 0.25 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) was used to detect nucleus.
RESULTS
Reactive oxygen species were generated in salt-treated soybean roots
Our previous study suggested that the PA signaling controls GMK1 activity up to 5 min of NaCl treatment (Im et al., 2012). However, salt-inducible GMK1 activity was persistent up to 30 min. Therefore, it is reasonable to speculate that another modulator has a role in regulation at the latter stage of salt stress.
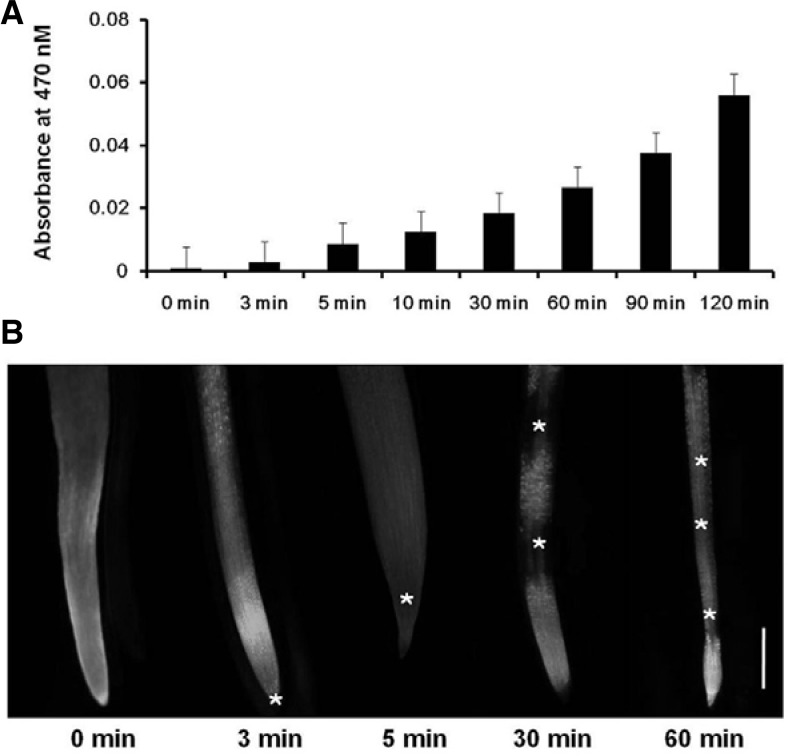
Reactive oxygen species (ROS) is a well-characterized MAPK modulator. Salt stress has known to induce oxidative bursts (Katsuhara et al., 2005). To examine ROS generation in soybean root by NaCl treatment, XTT and CM-H2DCFDA were used to detect superoxide (O2−) and H2O2, respectively. As shown in Fig. 1A, O2− increased continuously with 300 mM NaCl treatment. Hydrogen peroxide also increased, beginning at the secondary root tip by 3 min, spreading throughout to peripheral region of the tip by 5 min, and then to other regions of the secondary roots by 30 min. Finally, H2O2 was generated in all regions of the secondary root by 60 min after the treatment (Fig. 1B). This phenomenon was also detected in whole seedling root (data not shown).
Fig. 1.
ROS generation in soybean root during NaCl treatment. (A) Soybean seedlings were treated with 300 mM NaCl for the indicated times, and then O2− generation was measured using XTT-formazan using a spectrophotometer at A470. Mean ± SD of 2 samples. (B) Soybean seedlings were treated with 10 μM CM-H2DCFDA for 60 min, followed by 300 mM NaCl treatment for the indicated times. H2O2 generation signals in secondary roots were detected using the FITC channel of a fluorescence microscope. (*) indicates H2O2 signal. Scale bar = 1 mm.
To examine whether NADPH-oxidase is the source of ROS production, the production was tested with/without an NADPH-oxidase inhibitor, diphenyleneiodonium (DPI). H2O2 production was significantly reduced compared to the control (Supplementary Fig. S1). These results suggest that ROS generation by high salts in soybean roots is produced by NADPH-oxidase.
Hydrogen peroxide activates GMK1 independently of PA
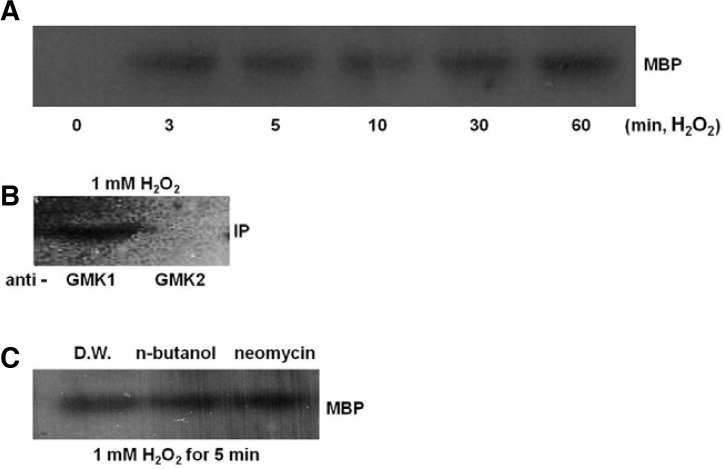
To examine whether ROS activates GMK1 or not, soybean seedlings were treated with in 1 mM H2O2 for 60 min and activity was measured using myelin basic protein (MBP) as a MAPK substrate. H2O2 strongly activated 47-kDa MAPK as early as 3 min after treatment, and its activity was maintained for up to 60 min (Fig. 2A). In soybean, GMK1 and GMK2 (GmMPK3) are orthologs of AtMPK6 and AtMPK3, respectively. The two MAPKs are activated by H2O2 in Arabidopsis (Rental et al., 2004). To identify H2O2-activated MAPK in soybean, immunoprecipitation and an in-gel kinase assay were performed with these two kinds of MAPK antibodies, respectively. The anti-GMK1 antibody-precipitated MAPK was activated by H2O2 treatment (Fig. 2B), suggesting that GMK1 is activated by H2O2.
Fig. 2.
H2O2 activated GMK1 independently of PLD and PLC. (A) Soybean seedlings were treated with 1 mM H2O2 for indicated time points and subjected to an in-gel kinase assay. (B) Total protein was extracted from 5 min sample of A and subjected to immunoprecipitation and an in-gel kinase assay using anti-GMK1 and anti-GMK2 antibodies. (C) Seedlings were treated with 1% n-butanol or 15 μM neomycin for 60 min before treatment with 1 mM H2O2 for 5 min. Total protein extracted from these seedlings was subjected to an in-gel kinase assay. Myelin basic protein (MBP) was used as a MAPK substrate.
In our previous study, GMK1 is activated by PA (Im et al., 2012). Therefore, it is reasonable to speculate that GMK1 activation is regulated by both PA and H2O2. To examine whether GMK1 activation by H2O2 is mediated by PA, soybean seedlings were treated with n-butanol or neomycin for 60 min and then treated with 1 mM H2O2 for 5 min. Both chemicals did not affect GMK1 activity induced by H2O2 (Fig. 2C), suggesting that H2O2 activates GMK1 independently of PA generated by PLD and PLC.
Activity of GMK1 is regulated by PA and hydrogen peroxide at different time points in salt stress
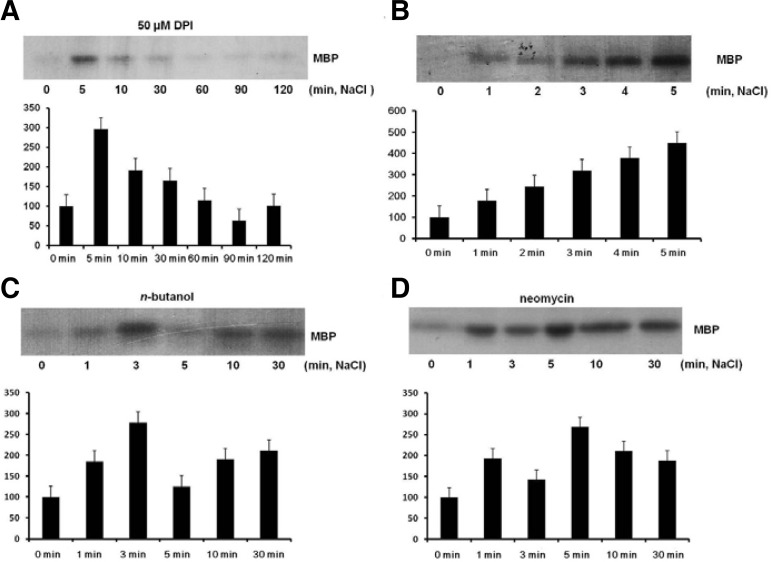
Previous studies indicate that both PA and ROS activate GMK1. In salt stress, PA reached its maximum level at 5 min (Im et al., 2012) but H2O2 was significantly increased even after 30 min of NaCl treatment (Fig. 1B). Therefore, it could be speculated that PA and H2O2 activate GMK1 with different time points in salt stress. To investigate the effect of endogenous ROS on GMK1 activity, soybean seedlings were treated with DPI for 60 min and then treated with 300 mM NaCl for up to 240 min. DPI treatment decreased GMK1 activity from 10 min after treatment (Fig. 3A). This result suggested that NADPH-oxidase dependant ROS was likely to be involved in maintaining GMK1 activity from 10 min of the NaCl treatment.
Fig. 3.
DPI and PA generation inhibitors reduced GMK1 activity at different time points during NaCl treatment. (A) After soybean seedlings were treated with 50 μM DPI for 60 min, 300 mM NaCl was introduced to the seedlings at the indicated times, followed by an in-gel kinase assay. (B) Soybean seedlings were pre-treated with 300 mM NaCl at the indicated times. Total protein from the seedlings was subjected to an in-gel kinase assay. (C) Soybean seedlings were treated with 1% n-butanol for 30 min, then treated with 300 mM NaCl for the indicated times and subjected to an ingel kinase assay. (D) Treatment is the same as for (C) except for 15 μM neomycin replacing n-butanol. All of the MAPK activities were measured by relative intensity of the band compared to control. The intensity was measured by LAS-3000 and Multi gauge 4.0 (Fujifilm, Japan). Control band intensity was set as a 100. Mean ± SD of 2 samples.
For a detailed examination of GMK1 activity regulated by PA under salt stress, PLD and PLC dependant GMK1 activity was monitored at early time points. Activity of GMK1 induced within 1 min was gradually increased up to 5 min of 300 mM NaCl treatment (Fig. 3B). The n-butanol pre-treatment, however, reduced GMK1 activity from 5 min after the treatment. Especially, GMK1 activity at 5 min was strongly affected by n-butanol (Fig. 3C). Neomycin treatment also reduced the activity in a time-dependant manner, with a strong reduction at 3 min after NaCl treatment (Fig. 3D). These results suggested that GMK1 activity in the initial 5 min of NaCl treatment was regulated by the PA.
Based on these results, we propose that GMK1 activity may be induced by PA generated by PLD and PLC during early stages of salt stress but maintained by H2O2 at later stages.
GMK1 is translocated to the nucleus following NaCl treatment
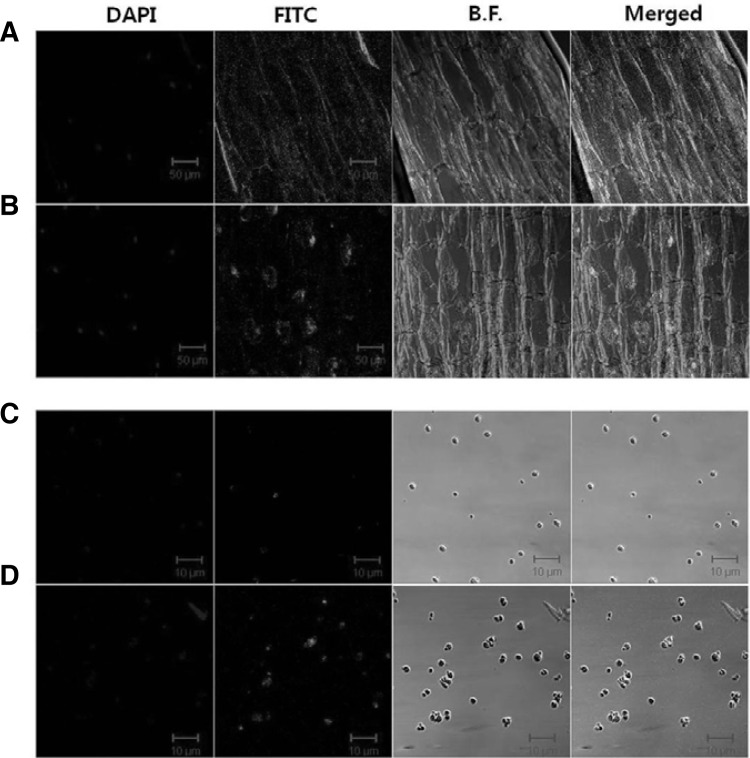
MAPK seems to be translocated to the nucleus upon activation in mammalian and yeast cells. Localization of salt stress-activated GMK1 was determined using immunostaining assay. Under normal conditions, GMK1 was distributed in the cytosol or near the plasma membrane (Fig. 4A). However, GMK1 was detected in the nucleus 60 min after NaCl treatment (Fig. 4B). To further verify this phenomenon, we isolated the nuclei and examined GMK1 localization in the nucleus using the anti-GMK1 antibody. The MAPK was not detected in the nuclei of untreated seedling, but was detected in those of NaCl-treated seedling (Figs. 4C and 4D). This result suggests that GMK1 is likely to be translocated to the nucleus upon activation under salt stress.
Fig. 4.
Immunolocalization of GMK1 during NaCl treatment. (A) As a control, longitudinal sections of soybean root were treated with anti-GMK1 serum, and then exposed to a FITC-conjugated antibody. (B) Soybean seedling treated with 300 mM NaCl for 60 min was longitudinally sectioned and was treated with anti-GMK1 serum and FITC-conjugated antibody. (C) Nuclei were isolated from control seedlings using PARTEC (Germany) nuclear isolation reagents and then treated with anti-GMK1 antibody and FITC-conjugated antibody. (D) Nuclei were isolated from 300 mM NaCl-treated soybean seedlings and were handled in same manner as those in (C). All images were obtained using a confocal microscope. Nucleus was seen as blue with DAPI staining. B.F. indicates bright field.
Reactive oxygen species generation is indirectly regulated by GMK1
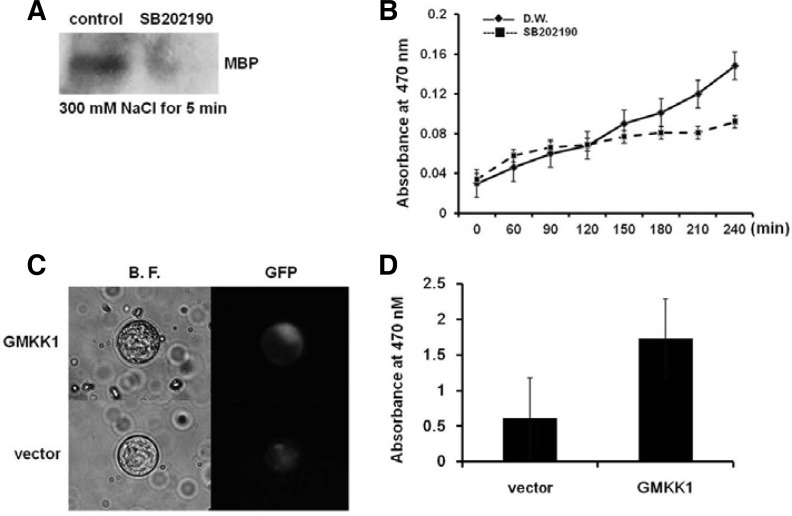
Previous reports suggested the activation of MAPK by ROS, but ROS generation was also regulated by MAPK (Pitzschke and Hirt, 2009). To elucidate the relationship between GMK1 activation and ROS generation under salt stress, we used SB202190, a specific inhibitor of p38 MAPK, to inhibit GMK1 activity and measured ROS generation. GMK1 activity was decreased by the SB202190 application under NaCl treatment (Fig. 5A). Super oxide generation detected by XTT in NaCl-treated seedlings increased slightly up to 120 min after the treatment compared to control seedlings, and then reduced (Fig. 5B), suggesting indirect effect of GMK1 on ROS generation. To further examine this relationship, a constitutively activated form of GMKK1 (GenBank accession No. BF598140) was expressed in protoplasts from soybean root. GMKK1 activated GMK1 in vitro (data not shown). Constitutively activated GMKK1 was manufactured by substituting two amino acids (T211 → E and S217 → D; hereafter, TESD-GMKK1). TESD-GMKK1 was transfected into protoplasts from soybean roots and hypocotyls using PEG transfection method. After incubation for 10 h, protoplasts were treated with XTT and incubated for another 2 h. TESD-GMKK1-transfected protoplasts generated more O2− than vector-transfected protoplasts (Fig. 5C). However, the XTT-formazan signal was very weak. After additional 8 h of incubation, TESD-GMKK1-transfected protoplasts showed 3 times higher generation of O2− than the vector control (Fig. 5D). These data also suggest that ROS generation is indirectly regulated by GMK1 under salt stress.
Fig. 5.
The effects of SB202190 and a constitutively activated GMKK1 on ROS generation du-ring salt stress. (A) After soybean seedlings were treated with 30 μM SB202190 for 60 min, they were treated with 300 mM NaCl for 5 min. Total protein extracted from these seedlings was subjected to an in-gel kinase assay. (B) SB202190-treated soybean seedlings were submerged in solution containing 300 mM NaCl and XTT for the indicated times. Absorbance of the solutions was measured at the time points. Mean ± SD of 4 samples. (C) A constitutively activated soybean MAPKK, GMKK1, was cloned into a JJ2053 vector and transfected into the soybean protoplast using PEG transfection. After protoplasts were incubated for 10 h for gene expression and then further incubated with 0.5 mM XTT for 2 h, XTT-formazan was detected using microscopy. (D) XTT-formazan was also measured at A470 after 10 h incubation. B.F. indicates bright field. Mean ± SD of 2 samples.
DISCUSSION
Salt stress is a major abiotic factor that causes reduced seed germination and plant growth development. MAPK activation during salt stress has been well-studied. However, the regulation mechanism of MAPK activity has not been examined in detail.
Our previous data suggest that PA signaling regulates GMK1 activity at early stages during salt stress, but this activity is sustained for up to 30 min. Therefore, a second activator may regulate GMK1 activity during salt stress. ROS was a good candidate as a regulator, because it is generated during many types of stresses and is a well-known MAPK activator (Yamamizo et al., 2006; Zhang et al., 2006).
To test this possibility, we carried out following experiments. First, we investigated ROS generation using DCFDA and XTT for H2O2 and O2−, respectively. Normal soybean roots color green when observed using the FITC channel of a fluorescent microscope because of their high flavonoid content. However, DCFDA-treated soybean roots color red upon contact with H2O2 when observed using the FITC channel. Superoxide was detected using XTT, which is converted to formazan when in contact with O2− and the concentration was determined using a spectrophotometer. As shown in Fig. 1, ROS generation increased in a time-dependent manner. This result is similar to the previous reports using soybean. H2O2 is generated in soybean leaves following 150 mM NaCl treatment (Balestrasse et al., 2008) and in drought conditions (Lee et al., 2010). Moreover, it was also reported that ROS is generated by NADPH-oxidase during salt stress (An et al., 2007; Levine et al., 2007). NADPH-oxidase, which is located in the plasma membrane, generates a reactive oxygen intermediate (ROI) under biotic and abiotic stress (Torres and Dangl, 2005; Yang et al., 2007). ROI, like O2−, is directly converted to H2O2 by superoxide dismutase (SOD). We suggest that NADPH-oxidase is responsible for this phenomenon because DPI-treated soybeans produced significantly less H2O2 than control cells (Supplementary Fig. S1) and SOD activity was not changed during salt stress (Supplementary Fig. S2).
Next, we examined whether GMK1 is activated by ROS by treating seedlings with 1 mM H2O2. As shown in Figs. 2A and 2B, GMK1 was activated as early as 3 min after treatment and its activity was sustained for up to 60 min. Moreover, GMK1 activity was not reduced by PA generation inhibitors (Fig. 2C), suggesting that H2O2 regulates GMK1 activity independently of PA generated by PLD and PLC.
From these results, ROS was thought to be a late stage activator for GMK1 under salt stress. To test this hypothesis, we treated soybean seedlings with DPI followed by treatment with 300 mM NaCl at different time points. In ROS generation-blocked seedlings, GMK1 activity was rapidly decreased beginning 10 min after treatment, while relatively strong activity was observed at 5 min (Fig. 3A). These data suggest that ROS is involved in sustained GMK1 activity from 10 min after salt treatment.
We also examined the effect of inhibitors against PA generation. Neomycin and n-butanol significantly diminished GMK1 activity 3 and 5 min after 300 mM NaCl treatment, respectively (Figs. 3C and 3D). Moreover, PA levels peaked at 5 min of 300 mM NaCl treatment (Im et al., 2012). These data also strongly support the hypothesis. Thus, salt stress-activated GMK1 is dually regulated by two signal molecules, PA and H2O2, at different time points. PLC activation occurred within several seconds, whereas PLD activation required longer time (minutes) than PLC in mammalian cells (Nishizuka, 1995). This study also supports our data.
Many mammalian MAPKs are translocated into the nucleus as a part of a signal transduction pathway to regulate gene expression (Plotnikov et al., 2011). However, the translocation mechanism has not been well-studied in plant. To examine GMK1 translocation during salt stress, soybean seedlings were treated with 300 mM NaCl for various time; 10, 30, and 60 min. The GMK1 signal did not correlated with the DAPI signal up to 30 min after treatment (data not shown). However, after 60 min, the signal corresponded to that of DAPI (Fig. 4B). Moreover, GMK1 was only detected in the nuclei of root cells treated with NaCl for 60 min (Figs. 4C and 4D). These results suggest that GMK1 is translocated to the nucleus during salt stress. Localization of MAPK to the nucleus has been previously reported in several different plant species (Coronado et al., 2002; Liu et al., 2011; Samaj et al., 2002). However, translocation of MAPK under salt stress has not been reported yet.
Translocation of MAPKs is mediated by phosphorylation of their active sites (Farooq and Zhou, 2004). Two signaling molecules, PA and H2O2, activated GMK1 during salt stress. Therefore, they may regulate translocation of GMK1 to nucleus. However, PA increased for only 5 min during NaCl treatment, whereas H2O2 increased continually during treatment. Therefore, H2O2 may regulate translocation of GMK1 during salt stress.
Although our data clearly showed that both PA and H2O2 regulate GMK1 activity, the relationship between PA/GMK1 and H2O2 production has not been well established. In tobacco, MAPK signaling regulates the NO- and NADPH-oxidase dependent oxidative burst (Asai et al., 2008), and positive feedback regulation between MAPK and ROS have been reported (Yamamizo et al., 2006; Zhang et al., 2006). However, AtMPK9 negatively regulates ROS accumulation during mechanical wounding (Takahashi et al., 2011). This information suggests that regulation of ROS generation by MAPK is different depending on the conditions. Therefore, we also investigated the relationship between GMK1 and ROS generation. The MAPK inhibitor SB202190 reduced ROS generation as well as GMK1 activity in NaCl treatment (Figs. 5A and 5B). Moreover, O2− generation in protoplasts containing a constitutively activated form of GMKK1 was increased compared to the vector control (Figs. 5C and 5D). These results may suggest that GMK1 also regulates ROS generation.
However, the time points of regulation in ROS generation by PA and GMK1 differ. ROS generation was reduced 30 min after NaCl treatment in n-butanol- or neomycin-treated soybeans in which PA generation was inhibited (Supplementary Fig. S3). However, ROS significantly increased even after 30 min of 300 mM NaCl treatment (Fig. 1A). Therefore, PA can directly regulate ROS generation during salt stress. Previous studies also have shown that PA binds directly to NADPH-oxidase (Zhang et al., 2009). However, in SB202190-treated soybeans in which activation of GMK1 was inhibited, ROS generation was reduced compared to the control only 120 min after NaCl treatment (Fig. 5B). To further examine these seemingly contradictory data, we introduced TESD-GMKK1 to soybean protoplasts. After 10 h of gene expression, we treated protoplast with XTT for 2 h and acquired images under a bright field microscope to observe the conversion of XTT to a red color by O2−. As shown Fig. 5C, more O2− was generated than in the vector control, but not significantly. However, after an additional 8 h of incubation with XTT, ROS generation increased by more than 3-fold (Fig. 5D), suggesting that GMK1 indirectly regulates ROS generation during salt stress.
Two types of NADPH-oxidases, StrbohA and StrbohB regulate ROS generation with different time points of elicitor treatment in potato (Yoshioka et al., 2001). StrbohA is involved in ROS generation within 1 h of elicitor treatment, while elicitor-induced StrbohB is involved after 6 h of the treatment. Multiple NADPH-oxidase-like genes were identified in soybean genome (www.phytozome.net) and GMK1 is translcoated to nucleus by NaCl treatment. Moreover, conditionally activated MAPK induces oxidative burst in tobacco (Yoshioka et al., 2009). Therefore, GMK1 could regulate gene expression of other type of NADPH-oxidase in soybean.
Based on the results of this study and our previous study, we propose a signaling pathway involving GMK1 during salt stress. Salt stress increases PA for 5 min in the presence of PLD and PLC; PA induces activity of GMK1 and NADPH-oxidase. Next, O2− generated from NADPH-oxidase is directly converted to H2O2 by SOD, and H2O2 maintains the activity of GMK1 at later times. Activated GMK1 is translocated to the nucleus and may induce downstream reactions including gene expression of NADPH-oxidase.
However, several points on GMK1 signaling pathway under salt stress need to be elucidated. First, OXI1 is a serine/threonine protein kinase activated by H2O2 and PA in Arabidopsis. The OXI1 null mutant is unable to activate AtMPK3 and AtMPK6 following H2O2 treatment (Rentel et al., 2004), suggesting that OXI1 functions downstream of ROS but upstream of the MAPK module (Pitzschke and Hirt, 2006). However, OMTK1 is a MAPKKK of alfalfa that is directly activated by H2O2 (Nakagami et al., 2004) and PA generated from phospholipase Dα directly activates AtMPK6 during salt stress (Yu et al., 2010). Further studies are necessary to understand the functions of the upstream elements in the GMK1 signaling pathway. Second, SB202190 has been widely used as a p38 MAPK-specific inhibitor but, it cannot inhibit extracellular signal-related kinase (ERK) activity in animal cells. Plant MAPKs include one type of ERK; GMK1 also belongs to this family. However, SB202190 reduces the activity of a Bradyrhizobia-activated ortholog of SIMK (Fernandez-Pascual et al., 2006), and our data also showed same result (Fig. 5C). It may differently regulate MAPK activity in plant. Third, the ROS generation level increased up to 60 min after NaCl treatment, but GMK1 activity decreased after 30 min according to our previous study (Im et al., 2012). There are two plausible explanations for this observation. First, a negative regulator of GMK1 may be present. In Arabidopsis, AtKP1 down-regulates AtMPK6 activity (Ulm et al., 2002) and AtDsPTP1 inactivates AtMAPK3/6 (Gupta et al., 1998). Second, GMK1 may be physically isolated from upstream activators such as GMKK1 because GMK1 is translocated from the nearby plasma membrane to the nucleus during salt stress.
Supplementary Material
Acknowledgments
This work was supported by Brain Korea 21 Research Fellowships from the Korean Ministry of Education and Human Resource Development (awarded to J.H.I.) and partially by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0076694). We thank Dr. Goon-Bo Kim of Sogang University for his kindly assistance in protoplast isolation.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Ahlfors R., Macioszek V., Rudd J., Brosche M., Schlichting R., Scheel D., Kangasjarvi J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40:512–522. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- Alvarez M.E., Pennell R.I., Meijer P.J., Ishikawa A., Dixon R.A., Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- An L.Z., Yang Y.L., Xua S.J., Chen N.L. NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol. 2007;164:1429–1435. doi: 10.1016/j.jplph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Asai S., Ohta K., Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrasse K.B., Zilli C.G., Tomaro M.L. Signal transduction pathways and haem oxygenase induction in soybean leaves subjected to salt stress. Redox Rep. 2008;13:255–262. doi: 10.1179/135100008X308966. [DOI] [PubMed] [Google Scholar]
- Broughton W.J., Dilworth M.J. Control of leghaemo-globin synthesis in snake beans. Biochem. J. 1971;125:1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado M.J., Gonzalez-Melendi P., Segui J.M., Ramirez C., Barany I., Testillano P.S., Risueno M.C. MAPKs entry into the nucleus at specific interchromatin domains in plant differentiation and proliferation processes. J. Struct. Biol. 2002;140:200–213. doi: 10.1016/s1047-8477(02)00542-7. [DOI] [PubMed] [Google Scholar]
- Dat J.F., Lopez-Delgado H., Foyer C.H., Scott I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M., Zeier J., Marocco A., Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq A., Zhou M.M. Structure and regulation of MAPK phosphatases. Cell. Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pascual M., Lucas M.M., de Felipe M.R., Bosca L., Hirt H., Golvano M.P. Involvement of mitogen-activated protein kinases in the symbiosis Bradyrhizobium-Lupinus. J. Exp. Bot. 2006;57:2735–2742. doi: 10.1093/jxb/erl038. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Huang Y., Kieber J., Luan S. Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. Plant J. 1998;16:581–589. doi: 10.1046/j.1365-313x.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Im J.H., Lee H., Kim J., Kim H.B., Kim S., Kim B.M., An C.S. A salt stress-activated mitogen-activated protein kinase in soybean is regulated by phosphatidic acid in early stages of the stress response. J. Plant Biol. 2012;55:303–309. [Google Scholar]
- Jiang M.Y., Zhang A.Y., Zhang J.H., Tan M.P., Hu X.L. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006;141:475–487. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuhara M., Otsuka T., Ezaki B. Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Sci. 2005;169:369–373. [Google Scholar]
- Lee S., Hirt H., Lee Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001;26:479–486. doi: 10.1046/j.1365-313x.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- Lee J., Rudd J.J., Macioszek V.K., Scheel D. Dynamic changes in the localization of MAPK cascade components controlling pathogenesis-related (PR) gene expression during innate immunity in parsley. J. Biol. Chem. 2004;279:22440–22448. doi: 10.1074/jbc.M401099200. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim J., Im J.H., Kim H.B., Oh C.J., An C.S. Mitogen-activated protein kinase is involved in the symbiotic interaction between Bradyrhizobium japonicum USDA110 and soybean. J. Plant Biol. 2008;51:291–296. [Google Scholar]
- Lee B.H., Alam I., Sharmin S.A., Kim K.H., Yang J.K., Choi M.S. Proteome analysis of soybean roots subjected to short-term drought stress. Plant Soil. 2010;333:491–505. [Google Scholar]
- Levine A., Leshem Y., Seri L. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- Liu J.Z., Horstman H.D., Braun E., Graham M.A., Zhang C.Q., Navarre D., Qiu W.L., Lee Y., Nettleton D., Hill J.H., et al. Soybean homologs of MPK4 negatively regulate defense responses and positively regulate growth and development. Plant Physiol. 2011;157:1363–1378. doi: 10.1104/pp.111.185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. Superoxide-dismutase and peroxidase - positive activity stain applicable to polyacrylamidegel electropherograms. Arch. Biochem. Biophys. 1977;183:511–515. doi: 10.1016/0003-9861(77)90386-1. [DOI] [PubMed] [Google Scholar]
- Nakagami H., Kiegerl S., Hirt H. OMTK1, a novel MAPKKK, channels oxidative stress signaling through direct MAPK interaction. J. Biol. Chem. 2004;279:26959–26966. doi: 10.1074/jbc.M312662200. [DOI] [PubMed] [Google Scholar]
- Neill S., Desikan R., Hancock J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Ogasawara Y., Kaya H., Hiraoka G., Yumoto F., Kimura S., Kadota Y., Hishinuma H., Senzaki E., Yamagoe S., Nagata K., et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- Pitzschke A., Hirt H. Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzschke A., Hirt H. Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol. 2009;149:606–615. doi: 10.1104/pp.108.131557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. BBA-Mol. Cell Res. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Raho N., Ramirez L., Lanteri M.L., Gonorazky G., Lamattina L., ten Have A., Laxalt A.M. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J. Plant Physiol. 2011;168:534–539. doi: 10.1016/j.jplph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Reichheld J.P., Vernoux T., Lardon F., Van Montagu M., Inze D. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J. 1999;17:647–656. [Google Scholar]
- Rentel M.C., Lecourieux D., Ouaked F., Usher S.L., Petersen L., Okamoto H., Knight H., Peck S.C., Grierson C.S., Hirt H., et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- Samaj J., Ovecka M., Hlavacka A., Lecourieux F., Meskiene I., Lichtscheidl I., Lenart P., Salaj J., Volkmann D., Bogre L., et al. Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth. EMBO J. 2002;21:3296–3306. doi: 10.1093/emboj/cdf349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F., Mizoguchi T., Yoshida R., Ichimura K., Shinozaki K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell. 2011;41:649–660. doi: 10.1016/j.molcel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Tester M., Davenport R. Na(+) tolerance and Na(+) transport in higher plants. Ann. Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Ulm R., Ichimura K., Mizoguchi T., Peck S.C., Zhu T., Wang X., Shinozaki K., Paszkowski J. Distinct regulation of salinity and genotoxic stress responses by Arabidopsis MAP kinase phosphatase 1. EMBO J. 2002;21:6483–6493. doi: 10.1093/emboj/cdf646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.J., Wang Y.J., Zhou Y.H., Tao Y., Mao W.H., Shi K., Asami T., Chen Z.X., Yu J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009;150:801–814. doi: 10.1104/pp.109.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizo C., Kuchimura K., Kobayashi A., Katou S., Kawakita K., Jones J.D.G., Doke N., Yoshioka H. Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol. 2006;140:681–692. doi: 10.1104/pp.105.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Xua S.J., An L.Z., Chen N.L. NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol. 2007;164:1429–1435. doi: 10.1016/j.jplph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Yang K.Y., Liu Y.D., Zhang S.Q. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci USA. 2001;98:741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Asai S., Yoshioka M., Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells. 2009;28:321–329. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Sugie K., Park H.J., Maeda H., Tsuda N., Kawakita K., Doke N. Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant Microbe Interact. 2001;14:725–736. doi: 10.1094/MPMI.2001.14.6.725. [DOI] [PubMed] [Google Scholar]
- Yu L., Nie J., Cao C., Jin Y., Yan M., Wang F., Liu J., Xiao Y., Liang Y., Zhang W. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 2010;188:762–773. doi: 10.1111/j.1469-8137.2010.03422.x. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Wang C.X., Qin C.B., Wood T., Olafsdottir G., Welti R., Wang X.M. The oleate-stimulated phospholipase D, PLD delta, and phosphatidic acid decrease H2O2-induced cell death in arabidopsis. Plant Cell. 2003;15:2285–2295. doi: 10.1105/tpc.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.Y., Jiang M.Y., Zhang J.H., Tan M.P., Hu X.L. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006;141:475–487. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhu H., Zhang Q., Li M., Yan M., Wang R., Wang L., Welti R., Zhang W., Wang X. Phospholipase D alpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.