Abstract
A highly oxidative stress-tolerant japonica rice line was isolated by T-DNA insertion mutation followed by screening in the presence of 50 mM H2O2. The T-DNA insertion was mapped to locus Os09g0547500, the gene product of which was annotated as lysine decarboxylase-like protein (GenBank accession No. AK062595). We termed this gene OsLDC-like 1, for Oryza sativa lysine decarboxylase-like 1. The insertion site was in the second exon and resulted in a 27 amino acid N-terminal deletion. Despite this defect in OsLDC-like 1, the mutant line exhibited enhanced accumulation of the polyamines (PAs) putrescine, spermidine, and spermine under conditions of oxidative stress. The generation of reactive oxygen species (ROS) in the mutant line was assessed by qRT-PCR analysis of NADPH oxidase (RbohD and RbohF), and by DCFH-DA staining. Cellular levels of ROS in osldc-like 1 leaves were significantly lower than those in the wild-type (WT) rice after exposure to oxidative, high salt and acid stresses. Exogenously-applied PAs such as spermidine and spermine significantly inhibited the stress-induced accumulation of ROS and cell damage in WT leaves. Additionally, the activities of ROS-detoxifying enzymes were increased in the homozygous mutant line in the presence or absence of H2O2. Thus, mutation of OsLDC-like 1 conferred an oxidative stress-tolerant phenotype. These results suggest that increased cellular PA levels have a physiological role in preventing stress-induced ROS and ethylene accumulation and the resultant cell damage.
Keywords: lysine decarboxylase-like 1, polyamine, rice, stress, tolerance
INTRODUCTION
Plants are continuously exposed to a variety of environmental stresses, including pathogens, drought, extremes of temperature (low or high), strong light and high salinity. These stresses are responsible for substantial losses in crop production. Survival under stress conditions is dependent on the ability of plants to perceive these threats and to respond in a timely manner with the appropriate physiological, developmental and biochemical changes.
In plants, reactive oxygen species (ROS) such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide are produced continuously as by-products of aerobic metabolism. Under physiological steady-state conditions, ROS are scavenged by a number of antioxidant defense factors, and there is a balance between ROS production and scavenging (Dat et al., 2003). The antioxidant system in plant cells consists of various radical-scavenging molecules, including low molecular weight antioxidants such as ascorbate, glutathione and carotenoids, as well as antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione S-transferase (GST) and glutathione reductase. This equilibrium may be perturbed by a number of adverse environmental factors that generate ROS by activating oxidases and peroxidases, or that lead to the rapid accumulation of ROS, a phenomenon known as an oxidative burst (Apostol et al., 1989).
Although plants have a number of mechanisms to counteract the detrimental effects of increased ROS levels during abiotic stress conditions, in some circumstances, ROS per se also serve as important signaling molecules. ROS are key components of several regulatory processes in plants, including stomatal closure and gravitropism, as well as plant responses to abiotic stresses and pathogen challenge (Desikan et al., 2005). As a result, considerable research has focused not only on the mechanisms of ROS generation and removal in plants, but also their functions during development (Kim et al., 2007), as well as during biotic or abiotic stress (Apel et al., 2004). It was also reported that ROS scavenger protein and its expression could enhance acquired tolerance of oxidative stress through induction of various cell rescue proteins (Kim et al., 2012).
Polyamines (PAs) are ubiquitous low molecular weight aliphatic cations that are present in all organisms from bacteria to plants and animals. The major PAs in plants are putrescine, spermidine, and spermine, and to a lesser extent, cadaverine. Putrescine is formed directly from ornithine by ornithine decarboxylase (ODC) or indirectly from arginine by arginine decarboxylase (ADC). Spermine and spermidine are synthesized from putrescine by the addition of aminopropyl groups transferred from decarboxylated S-adenosylmethionine (SAM). SAM is also an intermediate of ethylene biosynthesis, suggesting a potential relationship between PA and ethylene biosynthesis (Faust and Wang, 1993). Cadaverine is synthesized from lysine by lysine decarboxylase (LDC) (Bagni and Tassoni, 2001; Flores et al., 1989). PAs localize to the cytoplasm as well as organelles such as vacuoles, mitochondria, and chloroplasts (Kusano et al., 2008).
In plants, the major PAs putrescine, spermidine, and spermine have been shown to be involved in development, senescence and stress responses (Alcázar et al., 2006). Cadaverine, which is relatively less abundant, is present in various bacteria and in several higher plant families such as Gramineae, Poaceae, Solanaceae, and in particular, Leguminosae (Ohe et al., 2009). The long-standing observation that putrescine levels increase in response to potassium deficiency has led investigators to examine changes in PA levels following exposure to single or combinations of stress (Groppa and Benavides, 2008; Kusano et al., 2008). In many cases, stress leads to an accumulation of free or conjugated PAs, which indicates that PA biosynthesis may be an integral component of plant stress responses. Whether the increase in PAs under conditions of stress is due to de novo synthesis or reduced degradation remains a matter of debate.
In PA-deficient Saccharomyces cerevisiae, there is excess accumulation of ROS and the cells eventually acquire a typical apoptotic phenotype (Chattopadhyay et al., 2006). If, however, these cells are grown in the presence of adequate levels of spermidine, excess ROS production is abrogated. Although cadaverine has no clear functional relationship with major polyamines, it has been shown to play a role in the induction of cell division in dormant tubers of Helianthus tuberosus (Bagni et al., 1993). Cadaverine also appears to act as a superoxide radical scavenger and is essential for the neutralization of external pH, thus helping to protect cells from acid stress (Kim et al., 2006). PAs can directly react with oxygen radicals in vitro (Ha et al., 1998), but this scavenging capacity has not been demonstrated in vivo (Chattopadhyay et al., 2006). Thus, the precise mechanism by which PAs decrease ROS levels is not known. For example, it is unclear whether they act indirectly, at the level of the enzymes involved in the synthesis or degradation of ROS, or by interacting directly with ROS.
In the current study, we investigated the generation and accumulation of ROS and the activities of ROS-detoxifying enzymes in a mutant rice strain lacking lysine decarboxylase-like 1 (OsLDC-like 1) following exposure to abiotic stresses. The mutant produced much higher levels of PAs compared to the wild-type (WT) strain and exhibited increased tolerance to oxidative stress. Our results suggest that PAs mediate tolerance to abiotic stresses through their ability to decrease ROS generation and enhance ROS degradation.
MATERIALS AND METHODS
Plant material and identification of stress-tolerant T-DNA mutant lines
Rice (Oryza sativa ssp. japonica cv. Dongjin) seeds from a library of T-DNA insertion mutants were surface-sterilized and germinated. Plants were cultured hydroponically in a growth chamber (29°C/21°C) with a 16 h photoperiod (Koh et al., 2007). To screen for oxidative stress-tolerant mutants, leaf strips of two-month-old plants grown in soil were treated with H+-2-[N-morpholino]-ethanesulfonic acid (MES) buffer containing 50 mM H2O2 for 18 h, at which point the extent of necrotic and chlorotic damage was determined. Tolerant lines were isolated and the T-DNA insertion sites were determined by inverse PCR (An et al., 2003). Heterozygous (HT) or homozygous (HM) progeny were identified by genotyping of the seedlings using two gene-specific primers targeting the coding region and one primer that targeted the T-DNA insert.
Determination of chlorophyll content
Freeze-dried powder of whole leaves was extracted with 85% acetone and chlorophyll content was determined by spectroscopy at 648 nm and 663 nm to quantify chlorophyll a and b, respectively, as previously described (Wi and Park, 2002).
Determination of ion leakage
Leaf segments were treated with 50 mM H2O2 and then the conductivity of the bathing solution was measured with a Consort conductivity meter (CYBERSCAN CON1500; Eutech; Singapore). This value was referred to as value A. Segments were then incubated in bathing solution in sealed tubes at 95°C for 10 min. After cooling to room temperature, the conductivity of the bathing solution again was measured; this value was referred to as value B. For each sample, ion leakage (expressed as a percentage) was calculated as value A/value B) × 100.
Determination of PA biosynthesis
PA levels were measured as described by Wi and Park (2002). Briefly, supernatants of leaf extracts (0.2 g) were mixed with saturated sodium carbonate (0.2 ml) and dimethylaminonaphthalene-1-sulfonyl chloride (0.4 ml). The mixtures were incubated at 25°C for one day and then the dansylated products were extracted with benzene and separated by thin layer chromatography in chloroform:triethylamine (25:2, v/v). The separated PAs were quantified by spectrofluorimetry against commercial standards (excitation/emission wavelengths: 350/495 nm; RF-1501, Shimadzu, Japan). LDC activity was measured according to the procedure of Park and Lee (1994). Crude leaf tissue extract served as the source of enzyme. Briefly, crude extract (200 μl) was incubated with 0.1 μCi of L-[1-14C] Lys in reaction buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.1 mM DTT, and 0.1 mM PLP) in a total volume of 500 μl at 37°C for 60 min. 14CO2 that was adsorbed during the reaction onto a piece of filter paper was measured using a liquid scintillation counter.
Measurement of ROS with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA)
Epidermal strips were peeled from intact leaves and incubated for 1 h in 20 mM phosphate buffer (pH 6.0) to remove any ROS that may have been generated by stripping. The samples were then exposed to stressors such as 200 mM NaCl, 10 mM potassium phosphate buffer (pH 3.0), or 50 mM H2O2 for 30 min, as indicated. After stress treatment, the epidermal strips were floated on a solution of 50 μM DCFH-DA (Sigma Chemicals, USA) for 10 min and then observed by fluorescence microscopy (excitation: 450 ± 490 nm; barrier 520 ± 560 nm) equipped with a cooled CCD camera (OLYMPUS, FV500, Japan).
For ROS quantification (Joo et al., 2005), approximately 300 mg of frozen leaf tissue was ground in liquid nitrogen and total protein was extracted in 1 ml of Tris(hydroxymethyl)amino-methane hydroxychloride buffer (pH 7.2), followed by centrifugation at 13,000 × g. Approximately 800 μl of extract was mixed with 1 mM DCFH-DA and incubated in the dark for 10 min at room temperature. Fluorescence was measured by spectrofluoroscopy (excitation/emission wavelengths: 485/525 nm; Shimadzu, RF-1501, Japan). Total protein was quantified by the Bradford method. Average fluorescence from three independent measurements was normalized to protein content and the results were expressed as relative fluorescence units (RFU) per milligram of protein.
Determination of ethylene production
Ethylene production in leaf segments was measured as described by Wi and Park (2002). Tissue was sealed in 20 ml vials for 1 h and then ethylene content was determined in 1 ml of headspace gas by gas chromatography using an activated alumina column at 250°C and a flame ionization detector (Hewlett Packard 5890 Series II, USA).
Semiquantitative reverse transcriptase (RT)-PCR and quantitative real-time RT-PCR
Total RNA was isolated as previously described (Wi and Park, 2002). Specific primers for RT-PCR were designed based on GenBank database sequences (Supplementary Table 1). For semiquantitative RT-PCR analysis of LDC-like 1 and LDC (GenBank accession No. AK065415), first-strand cDNA was generated from different tissues and then 1 μl of the RT reaction was used as the template. Amplification was carried out over 20–25 cycles. To analyze the relative abundance of transcripts by quantitative real-time RT-PCR (qRT-PCR), 1 μg of total RNA from leaf segments was reverse-transcribed for 30 min at 42°C in a 20 μl volume using the High Fidelity Prime-Script™ RT-PCR kit (Takara, Japan), according to the manufacturer’s instructions. Gene-specific PCR primers were designed according to the following criteria: a predicted melting temperature of 60°C ± 5°C, primer length of 20 to 24 nucleotides, a guanine-cytosine content of 50% to 60%, and a predicted PCR amplicon length of 100 to 250 basepairs (bp). qRT-PCR was performed in optical 96-well plates with a Chromo 4™ Continuous Fluorescence Detector (Bio-Rad, USA). Reactions were performed in a final volume of 20 μl containing 10 μl of 2× SYBR Green Master Mix, 0.5 μM of each primer, and 10 ng of cDNA. PCR conditions were as follows: 95°C for 15 min followed by 45 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s, and then 72°C for 10 min. Fluorescence threshold cycle (Ct) data were analyzed using the MJ Opticon Monitor Software version 3.1 (Bio-Rad, USA) and then exported to Microsoft Excel for further analysis. Relative expression levels in each cDNA sample were normalized to a β-actin reference gene. PCR efficiencies (90% to 95%) for all primers were determined by serial dilution of cDNA from RNA samples.
Activity measurements of ROS-detoxifying enzymes
SOD activity was determined by spectroscopy using the xanthine oxidase/cytochrome c method (McCord and Fridovich, 1969). The amount of SOD required to inhibit the reduction of cytochrome c by 50% was defined as 1 unit of activity. For the APX assay, leaf discs (about 2 cm2) were homogenized in 50 mM potassium phosphate buffer (pH 7.0) with 1 mM ascorbate. APX activity was measured as the decrease in A290 (extinction coefficient, 2.8 mM−1 cm−1) over 2 min, as described by Nakano and Asada (1987). One unit of APX activity was defined as the amount of enzyme required for the oxidation of 1 μmol ascorbate per 1 min. For the CAT assay, leaf discs (about 2 cm2) were frozen in liquid nitrogen and then ground in a microcentrifuge tube fitted with a pestle in 0.75 ml of ice cold 50 mM potassium phosphate buffer, pH 7.4, containing 1.13 mg of DTT. Enzyme activity was based on the amount of decomposition of H2O2 and was measured by a change in A240. GST activity was assayed using 1-chloro-2,4-dinotrobenzene (CDNB) as a substrate (Habig et al., 1974). The reaction was followed for 5 min at 340 nm (extinction coefficient, 9.6 mM−1 cm−1). Soluble protein was quantified using the Bradford method (Bradford, 1976) and bovine serum albumin as the standard.
RESULTS
Isolation of a rice osldc-like 1 mutant
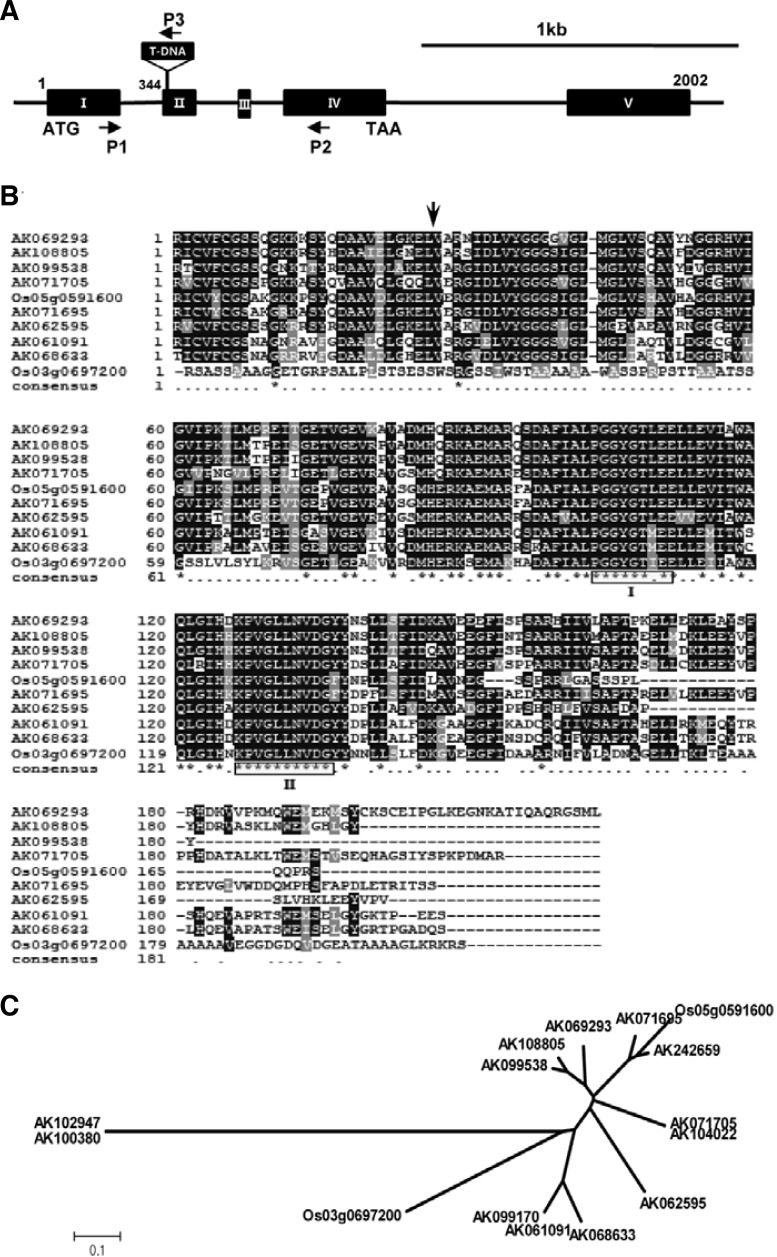
A library of japonica rice T-DNA insertion mutants was screened under oxidative stress conditions in the presence of 50 mM H2O2 to identify putative abiotic stress tolerance genes. Several oxidative stress-tolerant lines were isolated and the T-DNA insertion sites were mapped to putative antioxidative stress-responsive genes, including genes encoding an endo-1,4-beta-glucanase, a regulatory subunit of protein phosphatase, UV-B and ozone similarly-regulated protein (UOS-1), a peroxisomal Ca-dependent solute carrier, a ferredoxin-dependent glutamate synthase, and several unknown genes. Line 1C-117-18 was identified as the most tolerant to oxidative stress among all of the mutants. Sequencing using inverse PCR revealed that the insertion site was located in the Os09g0547500 locus. The gene product was annotated as a LDC-like protein (GenBank accession No. AK062595) by the National Center for Biotechnology Information (NCBI) database and the Rice Annotated Project Database (RAP-DB, http://rapdb.lab.nig.ac.jp/); therefore, we named the gene OsLDC-like 1, for Oryza sativa lysine decarboxylase-like gene 1. OsLDC-like 1 was located on Chromosome 9 and composed of five exons. It encoded a putative 1.1 kb cDNA, which translated into a 227 amino acid protein with a predicted molecular weight of 24.3 kDa. Inverse PCR analysis showed that the T-DNA insertion site was in the second exon of OsLDC-like 1, resulting in 27 amino acid N-terminal deletion (Fig. 1A).
Fig. 1.
T-DNA insertion site, structure of OsLDC-like 1, and phylogenetic analysis of OsLDC-like 1 genes. (A) Schematic diagram of OsLDC-like 1 from rice line 1C-117-18. Black boxes with roman numerals represent exons; lines between boxes are introns. The T-DNA insertion site and its orientation are shown. Small arrows indicate the gene-specific primers used for RT-PCR analysis and genotyping of OsLDC-like 1. ATG and TAA indicate start and stop codons, respectively. Scale bar = 1 kb. (B) Alignment of OsLDC-like 1 with related proteins. The deduced amino acid sequence of OsLDC-like 1 was aligned with LDC-like protein sequences obtained from the GenBank database. Alignment was performed using the Clustal X program. OsLDC-like 1 is annotated with GenBank accession No. AK062595. Box I indicates the PG-GXGTXXE motif, and Box II indicates a highly conserved region of unknown function. A vertical arrow shows the T-DNA insertion site in osldc-like 1. (C) Phylogenetic analysis of 15 OsLDC-like genes in rice. The dendrogram was generated by MEGA4 software using the neighbor-joining method. The scale bar corresponds to 0.1 amino acid substitutions per residue.
In the Oryza sativa ssp. japonica genome, at least 10 hypothetical proteins have been annotated as LDC-like proteins. The amino acid sequence encoded by OsLDC-like 1 was 29–67% identical to other putative OsLDC-like proteins and contained a PFAM03641 domain, a peptide sequence that defines members of the c100695 superfamily of lysine decarboxylases (http://www.ncbi.nlm.nih.gov/Structure/cdd), although at present there is no biochemical evidence to support this annotation (Häkkinnen et al., 2007). The members of this superfamily share a highly conserved motif, PGGXGTXXE (where X indicates any amino acid), which has been suggested to form part of the catalytic site (Fig. 1B). A phylogenetic tree of the 15 japonica LDC-like genes revealed three distinct subgroups (Fig. 1C).
Using primers specific for OsLDC-like 1 (Supplementary Table 1), we performed semiquantitative RT-PCR analysis of OsLDC-like 1 expression in plants. In untreated WT rice plants, OsLDC-like 1 was strongly expressed in stems, whereas minimal expression was detected in leaves and roots (Fig. 2). Expression was dramatically induced by oxidative stress (H2O2) in these organs in WT plants. OsLDC-like 1 was not expressed in the untreated- or H2O2-treated knock-out mutant line, which indicated that the T-DNA insertion completely abolished expression in the osldc-like 1 mutant. Thus, deletion of OsLDC-like 1 appeared to confer oxidative stress tolerance.
Fig. 2.
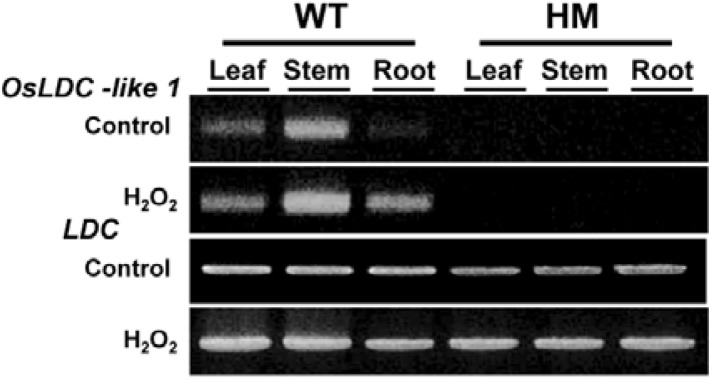
Semiquantitative RT-PCR analysis of OsLDC-like 1 transcripts in organs of WT and osldc-like 1 (HM) progeny. Stress-induced expression of OsLDC-like 1 and LDC was analyzed after treatment with 50 mM H2O2 for 18 h.
Stress tolerance in osldc-like 1 plants: determination of chlorophyll content, ion leakage and ethylene production following exposure to H2O2
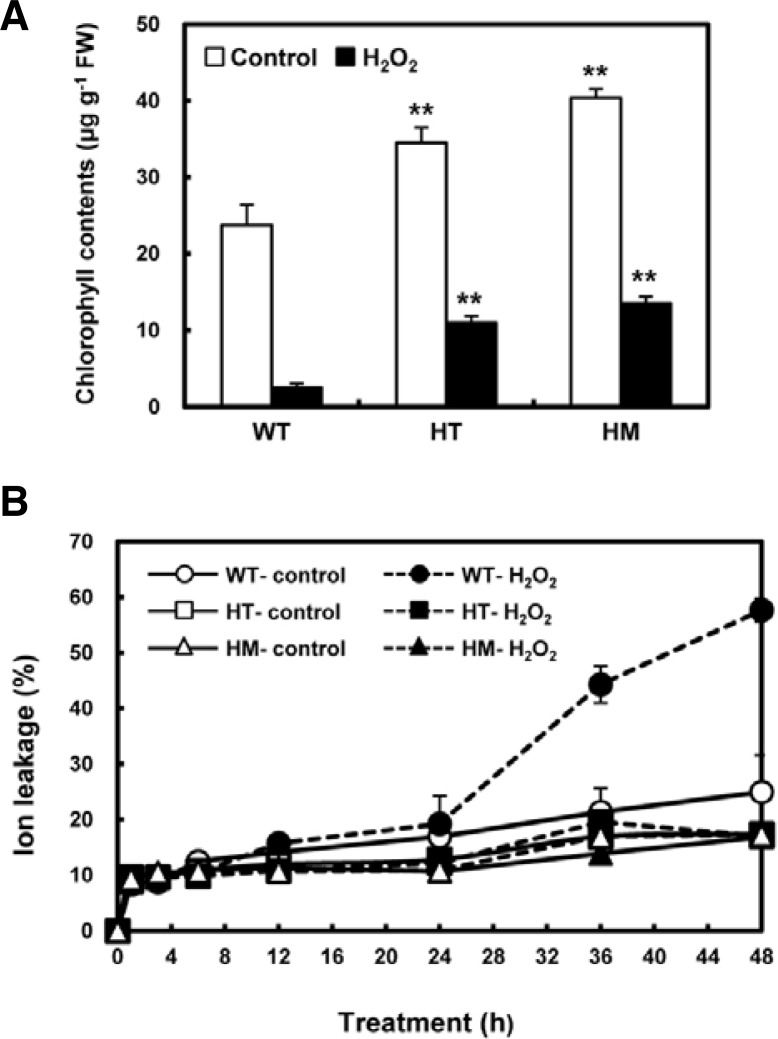
To characterize the oxidative stress-tolerant phenotype of the osldc-like 1 line in more detail, we monitored changes in chlorophyll fluorescence emission, a necrosis indicator in plants exposed to biotic or abiotic stresses (Chaerle et al., 2004). Chlorophyll content in WT leaves was lower than HT or HM mutant leaves in the presence or absence of H2O2. After exposure to 50 mM H2O2 for 18 h to induce oxidative stress, total chlorophyll content was significantly reduced in WT leaves (by 93%), HT mutant leaves (by 75%), and HM mutant leaves (by 66%) (Fig. 3A). The reduction of chlorophyll B was more significant in the two osldc-like 1 mutants compared to chlorophyll A (data not shown). Although there was a marked reduction in total chlorophyll content in both WT and mutant plants after oxidative stress, chlorophyll levels remained 3 times higher in the HT mutant compared to WT plants. The changes in chlorophyll content correlated with subsequent symptoms of necrotic damage, which were apparent to the eye. These results indicated that OsLDC-like 1 may confer susceptibility to oxidative stress.
Fig. 3.
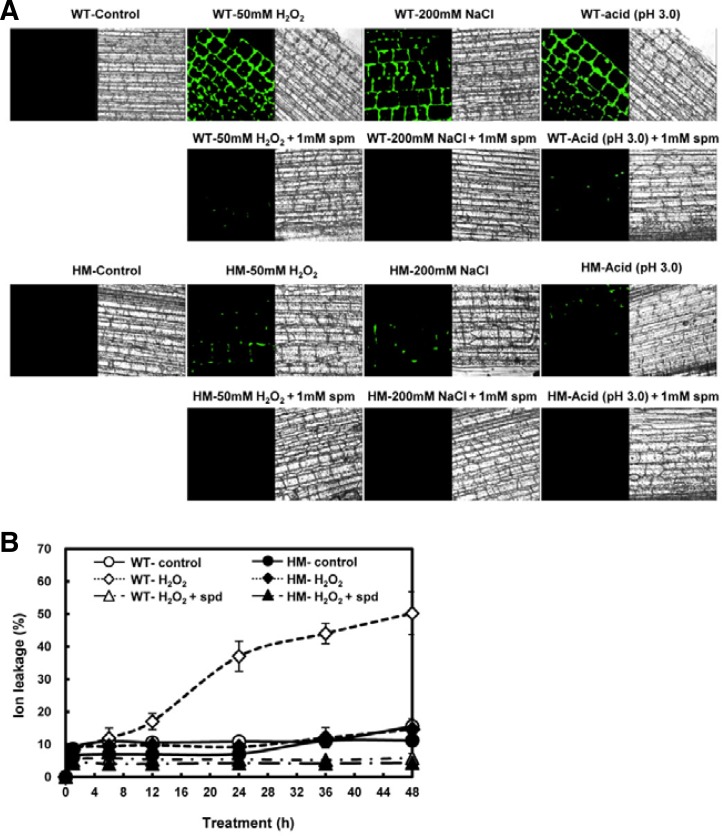
Chlorophyll content and ion leakage in H2O2-treated WT and osldc-like 1 rice plants. (A) Eight-week-old rice leaf segments were treated with H2O2 for 18 h and then chlorophyll content was measured. (B) Ion leakage in H2O2-treated WT and osldc-like 1 rice plants. Leaves were incubated in the presence (closed symbols) of 50 mM H2O2 for 48 h. Untreated controls (open symbols) were incubated in 20 mM MES buffer. Data represent means WT (○, •); HT mutant, (□, ▪); HM mutant, (▵, ▴). Data represents means. Differences between H2O2-treated osldc-like 1 and WT rice plants based on the two-tailed Student’s t-test are indicated by two asterisks (P < 0.01).
To confirm the stress-tolerant phenotype of the osldc-like 1 mutants, we also assessed cell damage, as measured by ion leakage, an indicator of membrane injury, in leaf discs following exposure to oxidative stress. Ion leakage was measured over a period of 48 h. In both WT and mutant plants, ion leakage rapidly increased within 1 h of treatment with 50 mM H2O2. After this initial rise, there was no difference between untreated and H2O2-treated HT and HM leaf discs up to 48 h (Fig. 3B). On the other hand, ion leakage in WT plants steadily increased over 24 h of exposure to oxidative stress and then increased more dramatically up to 48 h. These results suggested that stress tolerance in the osldc-like 1 HT and HM mutants was due to decreased sensitivity to membrane damage.
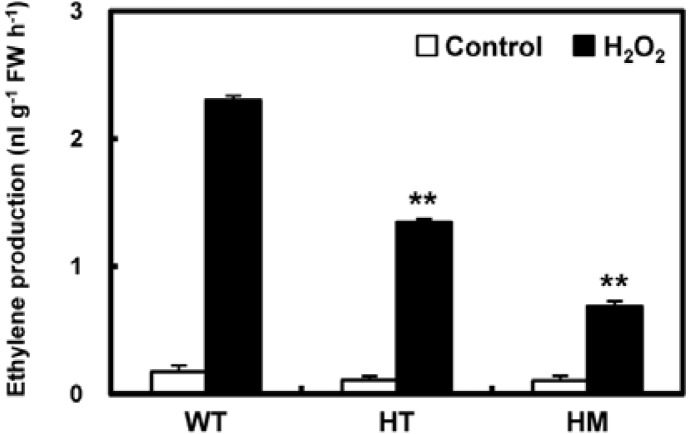
We also determined the level of stress-induced ethylene production in WT and mutant plants after 1 h of treatment with H2O2. In the absence of treatment and following incubation in MES buffer, ethylene biosynthesis was at similar basal levels in WT and knock-out mutant leaves (Fig. 4). One hour after stress treatment, ethylene levels in WT plants were significantly upregulated, approximately 12.8-fold. Ethylene production in the HM mutant also increased but to a lesser extent (by 6.3-fold) following H2O2 treatment and never reached more than 30% of that seen in H2O2-treated WT plants. Thus, the stress tolerance observed as a result of the loss of OsLDC-like 1 appeared to be due in part to reduced ethylene production.
Fig. 4.
Analysis of stress-induced ethylene production in WT and osldc-like 1 mutants. Ethylene was measured by floating leaf segments on 20 mM MES buffer without (controls, open bars) or with 50 mM H2O2 (final concentration; filled bars) and then incubating the leaves in air-tight glass vials for 1 h under illumination (photon flux density of 50 bar−1s−1). Significant differences between H2O2-treated osldc-like 1 and WT plants based on the two-tailed Student’s t-test are indicated by two asterisks (P < 0.01).
Increased PA biosynthesis in osldc-like 1 mutants
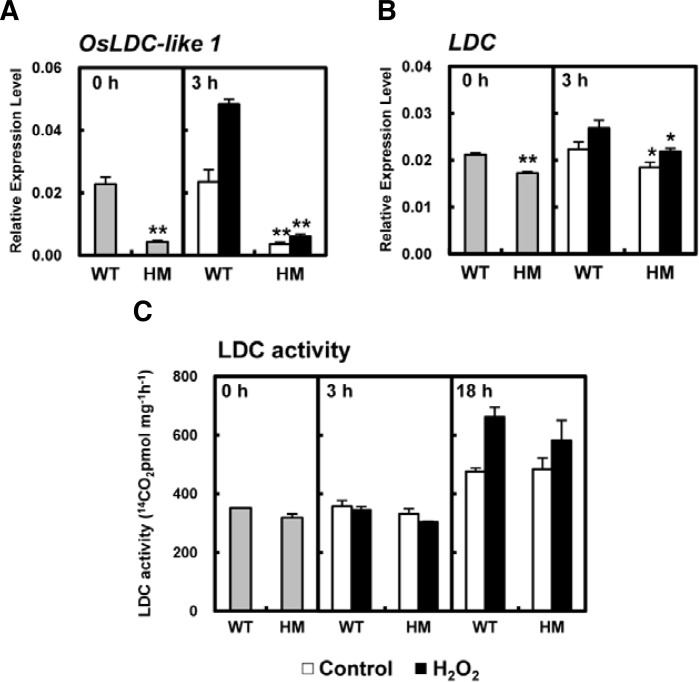
To investigate the role of OsLDC-like 1 in the plant stress response, we measured changes in the transcript levels of OsLDC-like 1 and LDC in the leaves of WT and osldc-like 1 plants before and after exposure to oxidative stress. Interestingly, 3 h after H2O2 treatment, the levels of OsLDC-like 1 transcript were effectively increased 2.1-fold in WT leaves compared to untreated WT controls (Fig. 5A). In contrast, OsLDC-like 1 transcription was decreased to 10% of that seen in WT plants in H2O2-treated HM leaves, most likely due to the osldc-like 1 mutation. LDC transcription was slightly decreased in the osldc-like 1 HM mutant relative to WT leaves with or without H2O2 treatment (Fig. 5B); however, LDC enzymatic activity was similar in WT and osldc-like 1 HM leaves (Fig. 5C).
Fig. 5.
Quantitative real-time RT-PCR analysis of OsLDC-like 1 and LDC transcripts and measurement of LDC activity. Expression levels of OsLDC-like 1 (A) and LDC (B), normalized to the reference gene β-actin, were calculated after qRT-PCR using gene-specific primers. At time 0, leaf discs were treated with 50 mM H2O2 in 20 mM MES buffer for 3 h. Relative expression is represented by the means ± SDs. (C) LDC activity. At time 0, leaf discs were treated with 50 mM H2O2 in 20 mM MES buffer for 18 h. Enzyme activity is represented by the means ± SDs. Significant differences between H2O2-treated osldc-like 1 HM and WT rice plants based on the two-tailed Student’s t-test are indicated by one (P < 0.05) or two asterisks (P < 0.01).
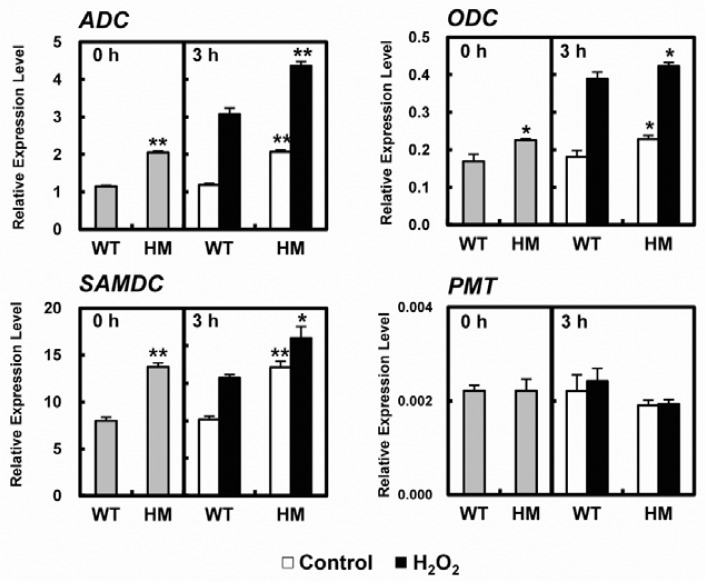
PA levels in the cell are tightly regulated by enzymes that catalyze the various polyamine biosynthetic pathways, including ADC, ODC, and SAM decarboxylase (SAMDC) (Wi and Park, 2002). We next investigated changes in the relative expression of PA biosynthetic genes in WT and osldc-like 1 HM mutants in response to H2O2 treatment. The expression of PA biosynthesis genes was measured by real-time qRT-PCR (Fig. 6). Basal levels of ADC, ODC, and SAMDC transcripts were higher in osldc-like 1 HM mutant leaves compared to WT leaves under normal (unstressed) conditions (Fig. 6). The stress-induced levels of ADC and SAMDC transcripts were significantly higher in the osldc-like 1 HM mutant compared to WT plants. By contrast, the mRNA levels of putrescine N-methyl-transferase (PMT), which catalyzes the SAM-dependent methylation of putrescine in the nicotine biosynthesis pathway (Biastoff et al., 2009), were similar in WT and osldc-like 1 HM mutant leaves in the presence or absence of oxidative stress. These results suggested that increased cellular PA levels in the osldc-like 1 mutant are due to up-regulation of enzymes that are directly involved in PA biosynthesis.
Fig. 6.
Quantitative real-time RT-PCR analysis of ADC, ODC, SAMDC and PMT transcripts. Expression levels, normalized to the reference gene β-actin, were calculated after qRT-PCR using gene-specific primers. At time 0, leaf discs were treated with 50 mM H2O2 in 20 mM MES buffer for 3 h. Relative expression is reported as the means ± SDs. Significant differences between H2O2-treated osldc-like 1 HM and WT rice plants based on the two-tailed Student’s t-test are indicated by one (P < 0.05) or two asterisks (P < 0.01).
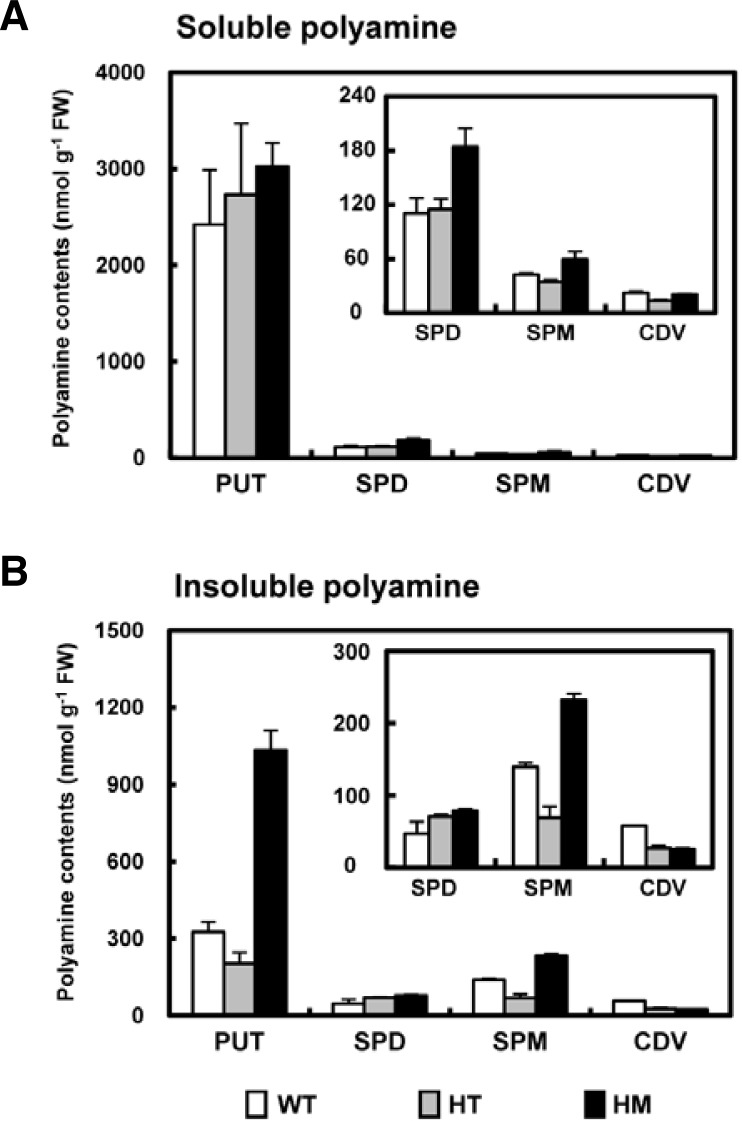
We also measured the levels of putrescine, cadaverine, spermidine and spermine in untreated leaves of 8-week-old rice plants. Even in knock-out mutants in which OsLDC-like 1 expression was completely absent, soluble and insoluble forms of cadaverine were detectable, albeit at much lower levels compared to WT plants (Fig. 7A). However, in the HM mutant, the level of insoluble putrescine was significantly higher (3.17-fold) compared to WT plants. Similarly, levels of spermidine and spermine, both the soluble (Fig. 7A) and insoluble forms (Fig. 7B), were much higher in the HM mutant. As a result, total soluble PA content, expressed as the combined value of the four soluble PAs, was higher in knock-out plants compared to WT. This increased PA level could be due to the increased activity of the PA-synthesizing enzymes ADC, ODC, and SAMDC observed in osldc-like 1 plants (see Fig. 6). There is strong evidence that stress-induced changes in free amino acid and PA content play an important role in plant stress responses, and that these alterations are responsible for stress tolerance (Livia et al., 2002). The current results suggested that increased levels of endogenous PAs in both the basal and stresssed state may underlie the stress-tolerant phenotype of rice lacking OsLDC like-1.
Fig. 7.
Changes in PA levels in WT and osldc-like 1 plants. Soluble PAs (A) and insoluble PAs (B) were measured in leaf segments (0.2 g) of 8-week-old rice plants exposed to 50 mM H2O2 for 18 h. Insoluble and soluble PA levels are shown as the means ± SD. PUT, putrescine; SPD, spermidine; SPM, spermine; CDV, cadaverine.
Inhibition of ROS accumulation in the osldc-like 1 mutant after abiotic stress
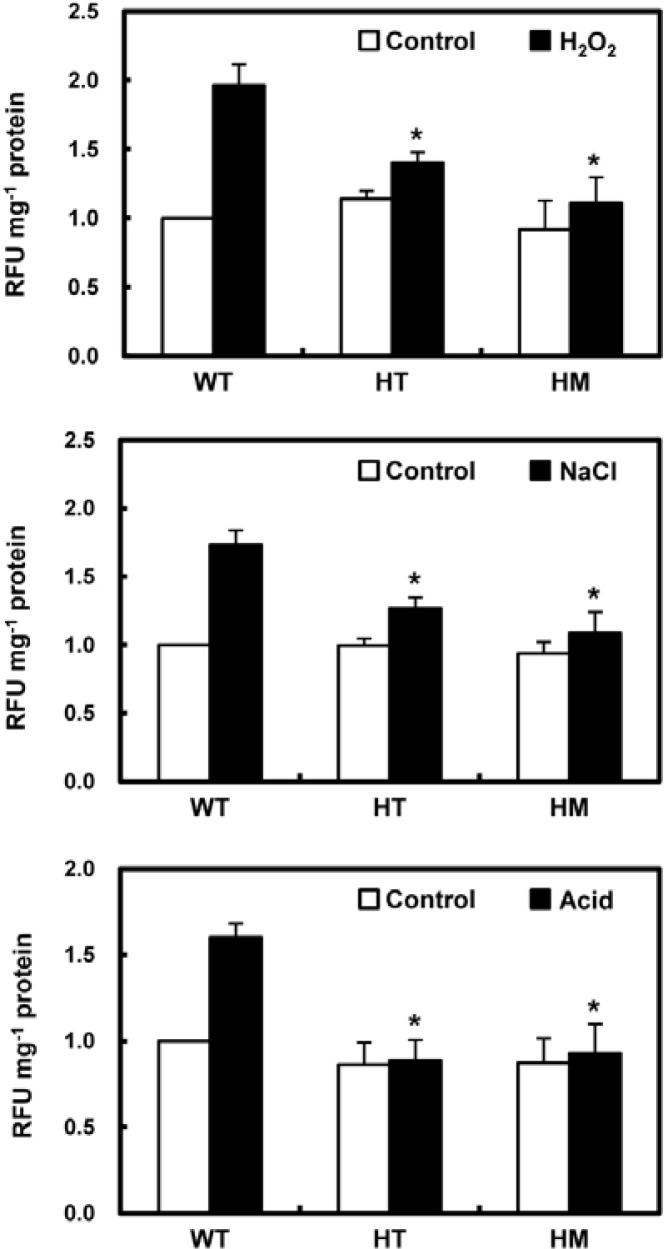
To investigate the role of OsLDC like-1 in ROS generation and subsequent ROS-mediated stress signaling and/or damage induction, we measured ROS levels in WT and mutant plants during the first 30 min (ROS burst) after exposure to several stresses. ROS accumulation was quantified by the conversion of exogenously-supplied DCFH-DA to the highly fluorescent DCF. ROS levels were significantly increased in WT rice leaves 30 min after exposure to oxidative stress (50 mM H2O2), salt stress (200 mM NaCl), and acid stress (10 mM potassium phosphate buffer, pH 3.0) (Fig. 8). However, similar exposure of osldc-like 1 HM and HT mutants had a relatively weak effect on ROS accumulation.
Fig. 8.
Analysis of intracellular ROS levels in leaf segments of WT and osldc-like 1 rice plants in response to abiotic stresses. Rice leaves were subjected to oxidative stress (top panel) with 50 mM H2O2 for 30 min, salt stress (middle panel) with 200 mM NaCl, or acid stress (bottom panel) with 10 mM potassium phosphate buffer (pH 3.0). ROS were measured by DCFH-DA fluorescence. Values were normalized to total protein content in leaf extracts and relative fluorescence units (RFU) were calculated relative to the value obtained for control treatment of WT plants. RFUs are reported as the means ± SD. Significant differences between stress-treated osldc-like 1 and WT rice plants based on the two-tailed Student’s t-test are indicated by one asterisk (P < 0.05).
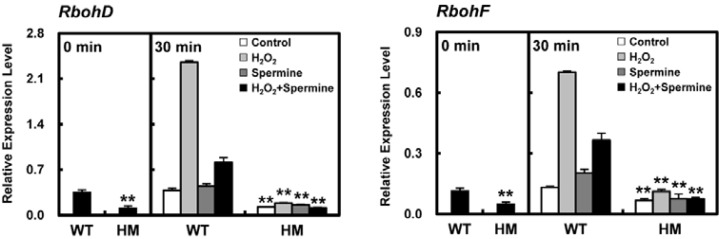
The ability to generate ROS is determined by the expression of RbohD and of RbohF, which encode NADPH oxidase. Expression of both genes was dramatically increased in WT rice 30 min after H2O2 treatment (Fig. 9). In contrast, there was slight, if any, induction of RbohD and RbohF expression in response to H2O2 treatment in the osldc-like 1 mutant. The low expression of RbohD and RbohF and near absence of an ROS burst following oxidative stress in osldc-like 1 mutants may represent a protective mechanism against further cellular damage in these plants.
Fig. 9.
Quantitative RT-PCR analysis of RbohD and RbohF transcripts. Expression levels, normalized to the reference gene β-actin, were calculated after qRT-PCR using gene-specific primers. At time 0, leaf discs were treated with 50 mM H2O2 in 20 mM MES buffer with or without 1 mM spermine for 30 min. Relative expression is reported as the means ± SDs. Significant differences between H2O2-treated osldc-like 1 HM and WT rice plants based on the two-tailed Student’s t-test are indicated by one (P < 0.05) or two asterisks (P < 0.01).
To test the hypothesis that increased cellular PA levels in the osldc-like 1 mutant block ROS production, WT plants exposed to oxidative stress were treated with spermine and the effect on RbohD and RbohF expression was assessed. Spermine treatment resulted in a dramatic inhibition of the H2O2-induced upregulation of the RbohD and RbohF transcription in WT plants (Fig. 9), which suggested that the decrease in ROS accumulation in the osldc like-1 mutant was due to increased levels of endogenous PA. Additionally, the expression level of RbohD was relative higher than RbohF, which indicated that RbohD expression is specifically involved in stress-induced ROS generation.
ROS accumulation was also assessed using a histochemical assay using DCFH-DA. The plant epidermis was exposed to various stresses for 30 min and ROS generation was evaluated by confocal microscopy. The levels of DCF fluorescence were much higher in WT leaves compared to the osldc-like 1 HM mutant, and spermine (1 mM) prevented ROS accumulation in response to all three abiotic stresses (Fig. 10A). The addition of spermidine (1 mM) also dramatically inhibited H2O2-induced ion leakage (Fig. 10B). These results suggested that, in the osldc-like 1 mutant, in which endogenous PA content was considerably elevated, increased stress tolerance is due to a greatly reduced ROS burst and then reduction of subsequent cell damage. As such, high levels of endogenous PAs in the osldc-like 1 mutant appear to function as free radical scavengers.
Fig. 10.
Histochemical analysis of intracellular ROS accumulation and ion leakage in leaves. (A) WT and osldc-like 1 HM leaves were incubated with H2O2 for 30 min. Leaf disc images were obtained by confocal microscopy after incubation with DCFH-DA for 10 min. In the case of treatment with spermine, leaf discs were co-treated with spermine in MES buffer containing 50 mM H2O2, 200 mM NaCl, or 10 mM potassium phosphate buffer (pH 3.0). (B) Ion leakage in H2O2-treated WT and osldc-like 1 rice plants. Ion leakage was also determined in H2O2-treated rice leaves co-treated with 1 mM spermidine. The leaves were incubated in the presence of 50 mM H2O2 for 48 h. Data are reported as the means ± SD.
Antioxidant enzymes in the osldc-like 1 mutant exhibit higher activities after exposure to abiotic stresses
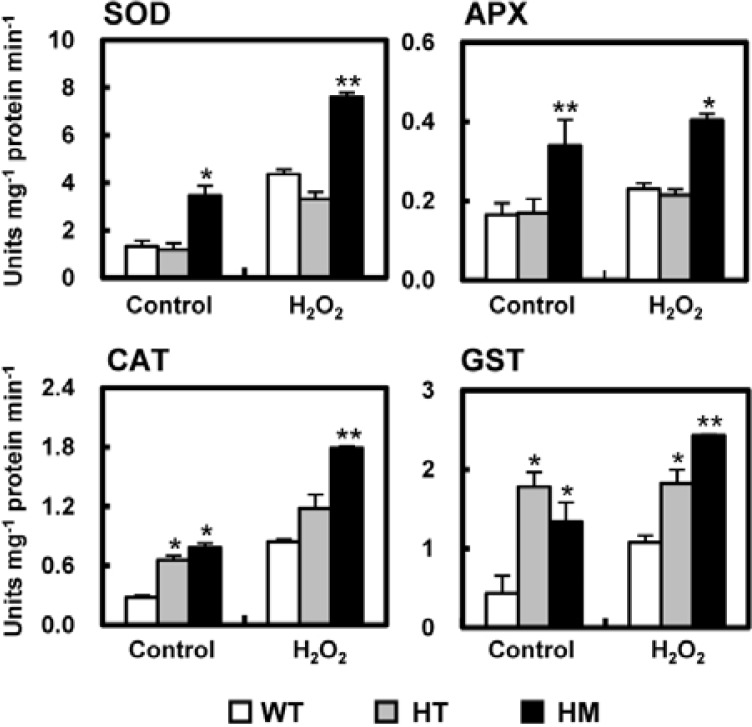
One way that plants resist the damage induced by environmental stresses is through manipulation of cellular antioxidant systems. The increased expression of ROS-detoxifying genes appears to be a general response to abiotic stress and has been observed in several studies of oxidative challenge. Since antioxidative proteins play an important role in the adaptation process, it was of interest to determine the capacity of the stress-tolerant osldc-like 1 mutant for ROS detoxification.
The basal activity level of several ROS-detoxifying enzymes (SOD, APX, CAT, and GST) was higher in untreated knock-out mutants, particularly the HM mutant, compared to WT rice (Fig. 11). After H2O2 treatment for 3 h, the activities of these enzymes were significantly increased in osldc-like1 plants compared to WT plants. The HM line exhibited the most prominent increase in ROS-detoxifying enzyme activity, with or without stress treatment, compared to WT plants. These results suggested that, in addition to the decreased induction of expression of ROS-synthesizing enzymes, lower ROS accumulation in the osldc-like1 mutants may also be due, at least in part, to an enhanced induction of expression of ROS-detoxification enzymes by PAs.
Fig. 11.
Activities of the ROS-detoxifying enzymes SOD, APX, CAT and GST. At time 0, leaf discs were treated with 50 mM H2O2 in 20 mM MES buffer for 3 h. Enzyme activity is reported as means ± SD. Significant differences in stress-treated osldc-like 1 and WT rice plants based on the two-tailed Student’s t-test are indicated by one (P < 0.05) or two asterisks (P < 0.01).
DISCUSSION
We screened a library of approximately 40,000 rice T-DNA insertion mutants in the presence of H2O2 to induce oxidative stress and isolated several stress-resistant lines. One of the most highly stress-resistant lines carried an insertion in OsLDC-like 1, a gene that was rapidly expressed in WT rice in response to oxidative stress (Fig. 2). This line was chosen for further analysis. In the osldc like-1 mutant line, there was an increased accumulation of the PAs putrescine, spermidine, and spermine, and this was accompanied by increased resistance to oxidative stress compared to WT plants, based on analysis of chlorophyll content (Fig. 3A) and ion leakage (Fig. 3B) following exposure to H2O2. The idea that constitutively elevated PA content in the osldc-like 1 mutant plays a role in the increased tolerance to oxidative, high salt, and acid stress was strengthened by the finding that spermine treatment almost completely prevented the accumulation of ROS in the leaves of stressed WT rice (Fig. 10). This would be similar to the observed oxidant resistance of tomato (Ormrod and Beckerson, 1986) and tobacco (Bors et al., 1989), in which putrescine, spermidine, and spermine provide considerable protection against ozone injury. Stress-induced ROS accumulation can act synergistically to induce ethylene production (Wi et al., 2012). Interestingly, ethylene production was significantly lower in the osldc-like 1 mutant after H2O2 treatment (Fig. 4), suggesting that the decreased levels of ROS in these plants was not sufficient to induce ethylene production.
PAs share a common precursor with ethylene (i.e., SAM) and have been implicated in the regulation of gene expression, chromatin stabilization, and protection against DNA damage (Ha et al., 1998). We previously reported that increased PA biosynthesis in transgenic plants can result in broad tolerance to various stresses (Wi et al., 2006). Putrescine biosynthesis in plants is induced by abiotic stresses such as ozone (Scalet et al., 1995) and osmotic pressure (Flores and Galston, 1984). Furthermore, application of exogenous putrescine or spermidine can partially counteract stress-induced membrane damage and attenuate salt injury in barley seedlings (Zhao and Qin, 2004). Consistent with these previous reports, we found that stress-induced ROS generation and accumulation in WT leaves was effectively abrogated by the addition of spermine (Figs. 9 and 10).
PAs have been shown to directly scavenge oxygen radicals in vitro (Ha et al., 1998) and can protect cells from the toxic effects of ROS such as H2O2, singlet oxygen, and oxygen radicals (Chattopadhyay et al., 2003; Tkachenko et al., 2001). PAs have also been described as membrane protectors (Alcázar et al., 2006; Kusano et al., 2008), a property that is consistent with the observation that the addition of spermine dramatically inhibited H2O2-induced ion leakage in WT plants (Fig. 10B). PA-pretreated plants may become more tolerant to oxidative stress due to increased expression and/or activity of antioxidative enzymes and antioxidants (Durmus and Kadioglu, 2005), an idea that is strengthened by the results of our current study.
LDC-like 1 gene disruption, which resulted in a non-functional protein, was associated with considerably elevated endogenous PA content (with the exception of cadaverine). The increase in insoluble putrescine was particularly prominent. The crystal structures of two putative LDC-like proteins, encoded by At5g11950 and At2g37210 (23.8 kDa and 23.6 kDa, respectively), have been determined (Jeon et al., 2006). In each structure, two monomers occupy an asymmetric unit, and the monomers adopt an α/β fold structure consisting of eight α-helices and seven β-strands. OsLDC-like 1 shares 53% and 61% amino acid similarity with the proteins encoded by At5g11950 and At2g37210, respectively.
Although the LDC-like proteins do not exhibit lysine decarboxylase activity, genes homologous to OsLDC-like 1 are found in a wide range of organisms. A search of the database yields at least 11 homologues annotated in the Arabidopsis genome (Jeon et al., 2006) and 10 in rice (Kurakawa et al., 2007). These genes are differently expressed depending on the plant organ, plant growth stage and stress conditions (Ohe et al., 2009; Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/). We hypothesize that the biochemical role of the LDC-like proteins may differ from that of LDC proteins. Under conditions of salinity stress during rice vegetative growth, LDC-like gene 1 was expressed at higher levels in strain IR29, which carries a salt-sensitive genotype, vs the salt-tolerant strain FL478, implying a negative regulatory role of LDC-like 1 in stress adaptation. The current results help clarify the potential role of this rice protein in the plant response to abiotic stresses.
Several studies have provided clues as to the biochemical function of LDC-like 1 in plant cells. Goossens and Rischer (2007), studying pyridine alkaloid metabolism in tobacco, isolated an LDC-like gene, MC126, as a putative catalyst of pyrimidine alkaloid biosynthesis, including synthesis of nicotine, nornicotine, anabasine, and anatabine. Overexpression of MC126 in tobacco resulted in a 3-fold increase in nicotine accumulation (Goossens and Rischer, 2007). It is likely that OsLDC like-1 does not function in the conversion of putrescine to nicotine, given that the level of PMT transcription was unaffected in the osldc-like I mutant (Fig. 6). Another homologue of OsLDC-like 1, LONELY GUY (LOG) in rice, is required for the maintenance of meristem activity, and loss of function LOG mutants exhibit premature termination of the shoot meristem (Kurakawa et al., 2007). It has been suggested that LOG encodes a novel cytokinin-activating enzyme, a plant cytokinin riboside 5′-monophosphate phosphoribohydrolase-related protein, which directly converts inactive cytokinin nucleotides to their free-base, biologically active forms (Kuroha et al., 2009). LDC-like was down-regulated in rice leaves after treatment with the cytokinin trans-zeatin (Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/), implying that there is a type of feedback inhibition at play. The application of putrescine or spermidine resulted in increased endogenous levels of cytokinin and vice versa (Wang et al., 2009).
In the osldc-like 1 mutant, PA content was significantly increased in both the soluble and insoluble fractions, although the level of cadaverine was decreased slightly (Fig. 7A). Above a certain threshold level, PAs can be harmful to plant cells and can induce cell death. Because of this, intracellular free PA concentrations are tightly regulated at various steps, including de novo synthesis, conjugation, degradation, and transport (Kusano et al., 2008). In the osldc-like 1 HM mutant, free putrescine was increased by only 25%, whereas there was a 3.18-fold increase in conjugated putrescine (Figs. 7A and 7B). In plants, PAs are present as free bases or conjugated to small molecules, mainly hydroxycinnamic acids, and to various macromolecules such as proteins (Alcázar et al., 2006). It was recently suggested that there is a reverse PA pathway in plants from spermine to putrescine (Kusano et al., 2008). The biosynthetic pathway that converts putrescine into spermidine and spermine is also important in conferring plant stress tolerance (Capell et al., 2004).
The precise role of PAs in plant-pathogen interactions has been elusive, although dramatic changes in PA metabolism have been reported in response to pathogen exposure. Increased PA levels have been reported to enhance plant resistance to pathogens; on the other hand, spermidine-mediated susceptibility was enhanced by the reduction of ethylene production (Nambeesan et al., 2012). It is very likely that the physiological functions of PAs in stress tolerance are modulated by other hormones. For example, in the osldc-like 1 mutant, decreased levels of cytokinin might enhance PA-mediated stress tolerance, in which case PAs serve as effective substitutes for cytokinin in the growth of the shoot meristem.
In counterbalance to the ROS scavenging properties of the PAs, PA oxidation results in the generation of H2O2, a ROS (Groppa and Benavides, 2008). In some host-pathogen interactions, the accumulation of H2O2 as a result of PA catabolism may play a key role in triggering the hypersensitive response; in particular, increased levels of apoplast-accumulated spermine could trigger pathogenesis-related proteins or caspases, which in turn promote the hypersensitive response (Yamakawa et al., 1998). In the current study, we examined the localization of H2O2 in situ using DCFH-DA, oxidation of which by cytosolic H2O2 yields the highly fluorescent DCF (Allan et al., 2001). Exogenous treatment with spermidine and spermine, which are degraded by PA oxidation, almost completely blocked stress-induced ROS accumulation, and this was due to reduced ROS generation and stress-induced ion leakage.
The detailed mechanisms by which PAs confer oxidative stress resistance remain unclear. For example, they may function as signaling molecules per se in endogenous defense pathways induced in response to stress. There are a number of possibilities, which are not mutually exclusive and could, in fact, function additively. As an important component of the plant stress response, PAs are currently an active area of research. Although their precise roles and mechanisms of action in plant stress responses have long been a matter of debate, the data increasingly support an important role for PAs in regulating stress responses. The results of the current study suggest that PAs mediate abiotic stress tolerance through their ability to function as antioxidants, resulting in decreased intracellular ROS generation and ethylene production. These reductions help protect the cell against oxidative damage and enhance viability.
Supplementary Material
Acknowledgments
This work was supported in part by the Biogreen 21 Program (grant no. 20050301-034-483-006-05-00) from Rural Development Administration. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A1A3015731).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Alcázar R., Marco F., Cuevas J.C., Patron M., Ferrando A., Carrasco P., Tiburcio A.F., Altabella T., Chattopadhyay M.K., Tabor C.W., et al. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006;28:1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- Allan A.C., Lapidot M., Culver J.N., Fluhr R. An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein. Plant Physiol. 2001;126:97–108. doi: 10.1104/pp.126.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S., Park S., Jeong D.H., Lee D.Y., Kang H.G., Yu J.H., Hur J., Kim S.R., Kim Y.H., Lee M., et al. Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant Physiol. 2003;133:2040–2047. doi: 10.1104/pp.103.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Apostol I., Heinstein P.F., Low P.S. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni N., Tassoni A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids. 2001;20:301–317. doi: 10.1007/s007260170046. [DOI] [PubMed] [Google Scholar]
- Bagni N., Altamura M.M., Biondi S., Mengoli P., Torrigiani P. Polyamines and morphogenesis in normal and transgenic plant cultures. In: Roubelakis-Angelakis K.A., Tran Than Van K., editors. Morphogenesis in Plants, Molecular Approaches. Plenum Press; New York, NY: 1993. pp. 89–111. [Google Scholar]
- Biastoff S., Brandt W., Dräger B. Putrescine N-methyltransferase – the start for alkaloids. Phytochemistry. 2009;70:1708–1718. doi: 10.1016/j.phytochem.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Bors W., Langebartels C., Michel C., Sandermann H.J. Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry. 1989;28:1589–1595. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for quantitation of microgram quantities of protein using the principle of protein-dye protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capell T., Bassie L., Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci USA. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaerle L., Hagenbeek D., De Bruyne E., Valcke R., Van Der Straeten D. Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 2004;45:887–896. doi: 10.1093/pcp/pch097. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M.K., Tabor C.W., Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci USA. 2003;100:2261–226. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M.K., Tabor C.W., Tabor H. Polyamine deficiency leads to accumulation of reactive oxygen species in a spe2 Δ mutant of Saccharomyces cerevisiae. Yeast. 2006;23:751–761. doi: 10.1002/yea.1393. [DOI] [PubMed] [Google Scholar]
- Dat J.F., Pellinen R., Beeckman T., Van De Cotte B., Langebartels C., Kangasjärvi J., Inzé D., Van Breusegem F. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 2003;33:621–632. doi: 10.1046/j.1365-313x.2003.01655.x. [DOI] [PubMed] [Google Scholar]
- Desikan R., Hancock J.T., Bright J., Harrison J., Weir I., Hooley R., Neill S.J. A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol. 2005;137:831–834. doi: 10.1104/pp.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus N., Kadioglu A. Spermine and putrescine enhance oxidative stress tolerance in maize leaves. Acta Physiol. Plant. 2005;27:515–522. [Google Scholar]
- Faust M., Wang S.Y. Polyamines in horticuturally important plants. Hortic. Rev. 1993;14:333–356. [Google Scholar]
- Flores H.E., Galston A.W. Osmotic stress-induced polyamine accumulation in cereal leaves. Physiological parameters of the response. Plant Physiol. 1984;75:102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H.E., Protacio C.M., Signs M.W. Primary and secondary metabolism of polyamines in plants. In: Newman K. H., Barz W., Reinhard E., editors. Primary and Secondary Metabolism of Plant Cell Cultures. Plenum Press; New York, NY: 1989. pp. 329–393. [Google Scholar]
- Goossens A., Rischer H. Implementation of functional genomics for gene discovery in alkaloid producing plants. Phytochem. Rev. 2007;6:35–49. [Google Scholar]
- Groppa M.D., Benavides M.P. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- Ha H.C., Sirisoma N.S., Kuppusamy P., Zweier J.L., Woster P.M., Casero R.A., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;25:7130–7139. [PubMed] [Google Scholar]
- Häkkinen S.T., Tilleman S., Swiatek A., De Sutter V., Rischer H., Vanhoutte I., Van Onckelen H., Hilson P., Inze D., Oksman-Caldentey K., et al. Functional characterization of genes involved in pyridine alkaloid biosynthesis in tobacco. Phytochemistry. 2007;68:2773–2785. doi: 10.1016/j.phytochem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Jeon W.B., Allard S.T., Bingman C.A., Bitto E., Han B.W., Wesenberg G.E., Phillips G.N., Jr X-ray crystal structures of the conserved hypothetical protein from Arabidopsis thaliana gene loci At5g11950 and At5g37210. Proteins. 2006;65:1051–1054. doi: 10.1002/prot.21166. [DOI] [PubMed] [Google Scholar]
- Joo J.H., Wang S.Y., Chen J.G., Jones A.M., Fedoroff N.V. Different signaling and cell death roles of geterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17:975–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Choi S.H., Lee J.K. Lysine decarboxylase expression by Vibrio vulnificus is induced by SoxR in response to superoxide stress. J. Bacteriol. 2006;188:8586–8592. doi: 10.1128/JB.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Kim H.S., Kim H.N., Kim Y.S., Baek K.H., Park Y.I., Joung H., Jeon J.H. Growth and tuberization of transgenic potato plants expressing sense and antisense sequences of Cu/Zn superoxide dismutase from lily chloroplast. J. Plant Biol. 2007;50:490–495. [Google Scholar]
- Kim I.S., Kim Y.S., Yoon H.S. Rice ASR1 protein with reactive oxygen species scavenging and chaperone-like activities enhances acquired tolerance to abiotic stresses in Saccharomyces cerevisiae. Mol Cells. 2012;33:285–293. doi: 10.1007/s10059-012-2253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., Lee S.C., Kim M.K., Koh J.H., Lee S., An G., Choe S., Kim S.R. T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 2007;65:453–466. doi: 10.1007/s11103-007-9213-4. [DOI] [PubMed] [Google Scholar]
- Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- Kuroha T., Tokunaga H., Kojima M., Ueda N., Ishida T., Nagawa S., Fukuda H., Sugimoto K., Sakakibara H. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Berberich T., Tateda C., Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- Livia S.S., Gábor K., Zoltán S. Effect of salt stress on free amino acid and polyamine content in cereals. Acta Biol. Szeged. 2002;46:73–75. [Google Scholar]
- McCord J.M., Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Nakano Y., Asada K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Nambeesan S., AbuQamar S., Laluk K., Mattoo A.K., Mickelbart M.V., Ferruzzi M.G., Mengiste T., Handa A.K. Polyamines attenuate ethylene-mediated defense responses to abrogate resistance to Botrytis cinerea in tomato. Plant Physiol. 2012;158:1034–1045. doi: 10.1104/pp.111.188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohe M., Scoccianti V., Bagni N., Tassoni A., Matsuzaki S. Putative occurrence of lysine decarboxylase isoforms in soybean (Glycine man) seedlings. Amino Acids. 2009;36:65–70. doi: 10.1007/s00726-008-0029-6. [DOI] [PubMed] [Google Scholar]
- Ormrod D.P., Beckerson D.W. Polyamines as antiozonants for tomato. HortScience. 1986;21:1070–1071. [Google Scholar]
- Park K.Y., Lee S.H. Effects of ethylene and auxin on polyamine levels in suspension-cultured tobacco cells. Physiol. Plant. 1994;90:382–390. [Google Scholar]
- Scalet M., Federico R., Guido M.C., Manes F. Peroxidase activity and polyamine changes in response to ozone and simulated acid rain in Aleppo pine needles. Environ. Exp. Bot. 1995;35:417–425. [Google Scholar]
- Tkachenko A., Nesterova L., Pshenichnov M. The role of the natural polyamine putrescine in defense against oxidative stress in Escherichia coli. Arch Microbiol. 2001;176:155–157. doi: 10.1007/s002030100301. [DOI] [PubMed] [Google Scholar]
- Wang Y., Luo J.P., Wu H.Q., Jin H. Conversion of protocorm-like bodies of Dendrobium huoshanense to shoots: the role of polyamines in relation to the ratio of total cytokinins and indole-3-acetic acid. J. Plant Physiol. 2009;166:2013–2022. doi: 10.1016/j.jplph.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Wi S.J., Park K.Y. Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells. 2002;13:209–220. [PubMed] [Google Scholar]
- Wi S.J., Kim W.T., Park K.Y. Overexpression of carnation S-adenosyl-methionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006;25:1111–1121. doi: 10.1007/s00299-006-0160-3. [DOI] [PubMed] [Google Scholar]
- Wi S.J., Ji N.R., Park K.Y. Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol. 2012;159:251–265. doi: 10.1104/pp.112.194654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H., Kamada H., Satoh M., Ohashi Y. Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 1998;118:1213–1222. doi: 10.1104/pp.118.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.G., Qin P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regul. 2004;42:97–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.