Abstract
Mammalian spermatogenesis is a complex process involving an intrinsic genetic program of germ cell-specific and-predominant genes. In the present study, we analyzed the Ly-1 reactive clone (Lyar) gene in the mouse. Lyar, which is known to be expressed abundantly in the testis, encodes a nucleolar protein that contains a LYAR-type C2HC zinc finger motif and three nuclear localization signals. We herein confirmed that Lyar is expressed predominantly in the testis, and further showed that this expression is specific to germ cells. Protein analyses with an anti-LYAR antibody demonstrated that the LYAR protein is present in spermatocytes and spermatids, but not in sperm. To assess the functional role of LYAR in vivo, we used a gene-trap mutagenesis approach to establish a LYAR-null mouse model. Lyar mutant mice were born live and developed normally. Male mutant mice lacking LYAR were fully fertile and showed intact spermatogenesis. Taken together, our results demonstrate that LYAR is strongly preferred in male germ cells, but has a dispensable role in spermatogenesis and fertility.
Keywords: gene-trap, LYAR, nucleolar protein, spermatogenesis, testis
INTRODUCTION
Spermatogenesis (i.e., male germ cell development) involves the continuous mitotic proliferation of spermatogonial stem cells, the meiotic division of spermatocytes, and dramatic morphological changes from haploid spermatids to highly specialized sperm (spermiogenesis). The tightly regulated nature of mitotic progression, meiotic progression and spermiogenesis suggests the presence of a highly organized network of genes specifically expressed in germ cells during spermatogenesis. The regulation of gene expression during spermatogenesis occurs at three levels: intrinsic, interactive, and extrinsic (Eddy, 2002). The intrinsic program determines which genes are utilized and when the genes are expressed in germ cells. The interactive process between germ cells and somatic cells is necessary for germ cell proliferation and progression. The extrinsic program regulates the interactive process via external influences. Notably, the intrinsic program involves germ cell-and stage-specific gene expression patterns that constitute the unique features of male reproduction.
The nucleolus, which is a noticeable compartment of the nucleus, plays a crucial role in ribosome biogenesis, which includes the processing of precursor rRNA, the transcription of ribosomal DNA, and pre-ribosome assembly (Ginisty et al., 1999; Lo et al., 2006; Melese and Xue, 1995; Shaw and Jordan, 1995). The rate of rRNA synthesis correlates with cell proliferation and varies depending upon the proliferative status of the cell. Nucleolar proteins have been implicated in the control of cell proliferation and growth (Chen et al., 1991; Grisendi et al., 2006). The rate of ribosomal RNA synthesis is dramatically altered during spermatogenesis and the generation of various types of germ cells. A previous autoradiographic study found that spermatogonia show very active rRNA synthesis, which peaks at the midpachytene stage in spermatocytes and then decreases in late spermatids, coinciding with changes in chromatin structure (Kierszenbaum and Tres, 1978). Therefore, the regulation of nucleolar functions in the testis could be important for the proper production of male germ cells.
During the course of our studies into unknown or unexplored genes with testis-specific or-predominant expression, we investigated the Ly-1 antibody reactive clone (Lyar) gene in mice. Lyar encodes a nucleolar protein that consists of 388 amino acid residues and has a LYAR-type C2HC zinc finger motif along with three nuclear localization signals. Lyar was first identified from a mouse T-cell leukemia line, and was shown to be induced during oncogenic transformation, suggesting that it may function as a novel cell growth-regulating nucleolar oncoprotein (Su et al., 1993). A microarray study showed that Lyar is highly upregulated in undifferentiated human embryonic stem cells (ESCs), and its expression is dramatically downregulated upon differentiation (Cai et al., 2006). In addition, Lyar was found to be overexpressed in human medulloblastoma (MB), the most frequent childhood brain tumor (Swartling et al., 2010). Recently LYAR was known to regulate the self-renewal and differentiation of ESCs (Li et al., 2009).
Previous studies have indicated that Lyar is abundantly expressed in the testis (Su et al., 1993). Considering the known functions of LYAR in various cell types, this suggests that it might be involved in male germ cell proliferation and differentiation. However, the expression patterns and functions of Lyar in germ cells are unknown. In the present study, we report our investigation of Lyar in mice, providing comprehensive information on its expression in male germ cells and its function in reproduction. In particular, we generated mice carrying a gene-trap mutation in Lyar and found that the loss of LYAR in the testis did not affect spermatogenesis or fertility.
MATERIALS AND METHODS
Reverse transcription polymerase chain reaction
The testis-specific expression of the Lyar gene was examined by reverse transcription-polymerase chain reaction (RT-PCR) analysis of cDNAs from nine different mouse tissues (testis, ovary, brain, heart, kidney, liver, lung, spleen and ES cells), as well as germ cell-lacking testes from W/Wv (c-kit) mutant mice. Total RNA was extracted using the TRIzol reagent (Molecular Research Center, USA) according to the manufacturer’s protocol, and cDNA was synthesized using Omniscript reverse transcriptase (Qiagen, Netherlands). A specific region of the Lyar transcript was amplified with the primers [5′-GTG GGA TCC CCG GAG GCA AAG GCT AT-3′ (forward) and 5′-CGG GAA TTC TCA GGC CTC GGT TTG CTC TT-3′ (reverse)]. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), which was used as a control, was amplified using the primers [5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ (forward) and 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′ (reverse)]. Stage-specific expression during spermatogenesis was examined using total RNA obtained from the testes of prepubertal and adult male mice (ages: 8, 10, 12, 14, 16, 20, 30, and 84 days).
Antibodies and immunoblot analysis
The hydrophilic region of LYAR (amino acids 190–286) was PCR amplified, digested, and ligated into the pGEX-5X-2 vector (GE Healthcare, UK). Then, anti-LYAR antibody was generated according to previously described protocols (Lee et al., 2011). Anti-GAPDH, monoclonal anti-α-tubulin, anti-mouse ADAM2, and anti-nucleolin were purchased from AbFrontier (Korea), Sigma-Aldrich (USA), Millipore (USA), and Abcam (UK), respectively. Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse IgG secondary antibodies (Jackson ImmunoResearch, USA) were used for immuno-blot analyses. The immunoblot analysis was carried out as described in Oh et al. (2009). The bound primary IgG was then detected with HRP-conjugated anti-rabbit antibodies.
Preparation of testicular cells, testicular sperm, and epididymal sperm
All animal investigations were performed according to the guidelines for animal care and use of Gwangju Institute of Science and Technology. Testicular (spermatogenic) cells and testicular sperm were separated as described in (Phelps et al., 1990). Mature sperm were collected according to previously described protocols (Lee et al., 2011).
Cell culture and expression of EGFP-Lyar
HEK293T cells were obtained from the American Type Culture Collection (ATCC, USA) and cultured with 5% CO2 in Dulbecco’s modified Eagle’s medium (Gibco, USA) supplemented with 10% fetal bovine serum, 100 U/ml of penicillin and 100 μg/ml of streptomycin at 37°C. The open reading frame of the Lyar gene was amplified from mouse testis cDNA using the appropriate primers [5′-TTC GAA TTC GCA AGA GTT CAA AC-3′ (forward) and 5′-GGT GGA TCC CCT TCA GAA GCT TTA C-3′ (reverse)] and inserted into the pEGFP-N2 vector (BD Clontech, USA). HEK293T cells were transiently transfected with the Lyar-EGFP construct using the Lipofectamine LTX reagent (Invitrogen, USA) ac-cording to the manufacturer’s instructions. At 24 h after transfection, cells were fixed with formaldehyde, stained with Hoechst 33342 (Sigma-Aldrich), and analyzed under a fluorescent microscope.
Generation of mutant mice
An RRG292 embryonic stem (ES) cell line carrying a Lyar gene-trap allele was purchased from BayGenomics. The ES cells (which were derived from 129-strain mice) were injected into C57/BL6 blastocysts using standard procedures, and chimeric mice were generated. Chimeric males were mated with C57/BL6 females and germ-line transmission in pups was confirmed. The Lyargt/gt line was obtained by crossing heterozygotes. Mice were genotyped to discriminate between wild-type and mutant alleles using the following primers: 5′-CAC AAG ATC TGA CGC-3′ (forward) and 5′-GTC ATG CTC AAC TGG-3′ (reverse) for the wild-type allele; and 5′-GGTCGG CTG ACA-3′ (forward) and 5′-AGT ATC GGC CTC AGG AAG ATC G-3′ (reverse) for the gene-trap allele.
Phenotypic analyses of mutant mice
To test the fertility of Lyar-mutant males, Lyar-mutant and wild-type (WT) males (8-wk-old) were mated with C57BL/6 females and the fertility rate was calculated as described in (Lee et al., 2011). To determine sperm counts, sperm samples were collected from the cauda epididymis and vas deferens of 8-wk-old Lyar-mutant and WT males, and sperm cells were determined using a hemocytometer under a light microscope. To analyze testicular integrity, testes from WT and Lyar-mutant mice were fixed by immersion in Bouin’s fixative for 24 h, embedded in paraffin, and sectioned using standard protocols. After being deparaffinized, the sections were stained with hematoxylin and eosin and the testicular morphologies were observed under a light microscope.
Statistics
Results are presented as means ± standard errors of the mean (SEMs) or standard deviations (SDs). The statistical significance of between-mean differences was determined using a two-tailed Student’s t-test.
RESULTS
LYAR expression
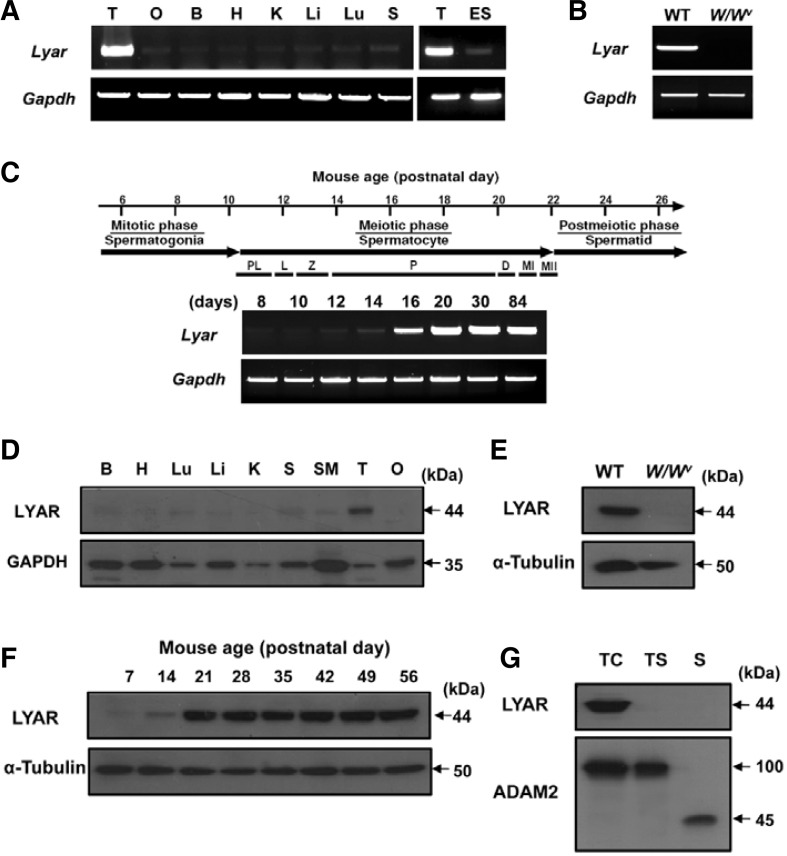
We first analyzed the expression profiles of LYAR at the transcriptional and protein levels. The tissue distribution of Lyar transcripts was examined by RT-PCR using cDNAs from different adult mouse tissues. We found that Lyar was expressed strongly in the testis and very weakly in the other tissues (Fig. 1A). Since Lyar is known to be expressed in undifferentiated ES cells (Li et al., 2009), we also compared Lyar expression between the testis and undifferentiated ES cells. Consistent with the prior report, undifferentiated ES cells were found to express Lyar; however, the expression level was greatly lower than that of the testis. To investigate which cells in the testis transcribed Lyar, we performed RT-PCR with cDNA from the testes of W/Wv (c-kit) mutant mice, which lack germ cells. We found that Lyar transcripts were absent from the testes of the mutant mice (Fig. 1B), suggesting that the Lyar mRNA is expressed in germ cells. RT-PCR was additionally performed to investigate the expression of Lyar during testicular development. We prepared testes from WT mice on various postnatal days (8, 10, 12, 14, 16, 20, 30, and 84 days). If a particular gene is transcribed in germ cells during spermatogenesis, the transcript will appear in the testis at a certain post-partum time point corresponding to a specific stage of spermatogenesis. Transcription of Lyar was first detected at the early stage of spermatogenesis (postnatal day 12) and increased considerably at postnatal day 16, corresponding to the development of pachytene spermatocytes (Fig. 1C).
Fig. 1.
The expression pattern of Lyar. transcripts and proteins. (A) The expression pattern of Lyar in various mouse tissues was determined by RT-PCR. Gapdh was included as a loading control. Abbreviations: T, testis; O, ovary; B, brain; H, heart; K, kidney; Li, liver; Lu, lung; S, spleen; ES, embryonic stem cells; Gapdh, glyceraldehyde-3-phosphate dehydrogenase. (B) Germ cell-specific expression of Lyar. RT-PCR was performed using testes from wild-type (WT) and germ cell-lacking W/Wv. mice. (C) Developmental expression pattern of Lyar. Juvenile spermatogenesis consists of the mitotic, meiotic, and postmeiotic phases. Stage-specific expression of Lyar was determined from mouse testes on different postnatal days (8, 10, 12, 14, 16, 20, 30, and 84 days). Abbreviations: PL, preleptotene; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; MI, meiotic division I; MII, meiotic division II. (D) Tissue distribution of the LYAR protein. An anti-GAPDH antibody was used as a control. B, brain; H, heart; Lu, lung; Li, liver; K, kidney; S, spleen; SM, smooth muscle; T, testis; O, ovary. (E) The LYAR protein in WT and W/Wv testes. Total testicular lysates from WT and germ cell-lacking W/Wv mutant mice were immunoblotted with the anti-LYAR antibody. An anti-α-tubulin antibody was used as a loading control. (F) Developmental expression of LYAR during spermatogenesis was determined by immunoblotting using total testis lysates obtained from prepubertal and adult male mice of various postnatal ages (7, 14, 21, 28, 35, 42, 49, and 56 days). (G) Stage-specific expression of LYAR. Protein samples from testicular cells (TC), testicular sperm (TS) and epididymal mature sperm (S) were immunoblotted with the anti-LYAR antibody. ADAM2 (a disintegrin and metalloprotease 2) protein, showing the 100 kDa-precursor and 45 kDa-processed forms, was included as a reference protein.
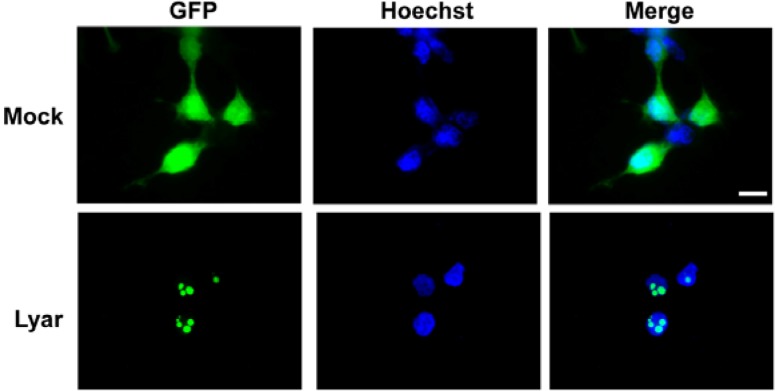
To determine the protein expression pattern of LYAR, we generated a polyclonal antibody against LYAR using a GST recombinant protein (residues 190–286). Initially, the tissue distribution of LYAR was investigated by immunoblot analysis using lysates from various tissues. As shown in Fig. 1D, a specific band with the expected molecular size of 44 kDa was predominantly detected in the testis. We also performed immunoblot analysis on testes from WT and W/Wv mutant mice and found the expression pattern similar to those observed in the RT-PCR analysis (Figs. 1B and 1E). Further immunoblot analyses were carried out using mouse testes obtained on different postnatal days, and the results showed a developmentally regulated expression pattern similar to that found in the RT-PCR analysis (Figs. 1C and 1F). In addition, the expression pattern of LYAR during spermatogenesis was analyzed in cells from different stages of sperm development, including testicular spermatogenic cells, testicular sperm, and mature sperm from the epididymis. We found that LYAR was present in testicular cells corresponding to spermatogonia, spermatocytes and round spermatids, but not in testicular sperm and mature sperm (Fig. 1G). Finally, we investigated the subcellular localization of LYAR. The anti-LYAR antibody did not work for immunocytochemistry, so we transiently transfected HEK293T cells with vectors encoding the GFP-tagged full-length gene sequence. We observed GFP signals in the nucleoli (Fig. 2). Taken together, our results demonstrate that LYAR is a nucleolar protein that is highly expressed in the testis, where it exhibits spermatogenic cell-specific and developmentally regulated expression patterns.
Fig. 2.
Subcellular localization of Lyar. HEK293T cells were transfected with the Lyar-EGFP construct and visualized under fluorescent microscopy. The pEGFP-N2 vector was used as a positive control (Mock). The Hoechst 33342 dye was used for nuclear staining. Scale bar = 20 μm.
Generation of Lyar-mutant mice
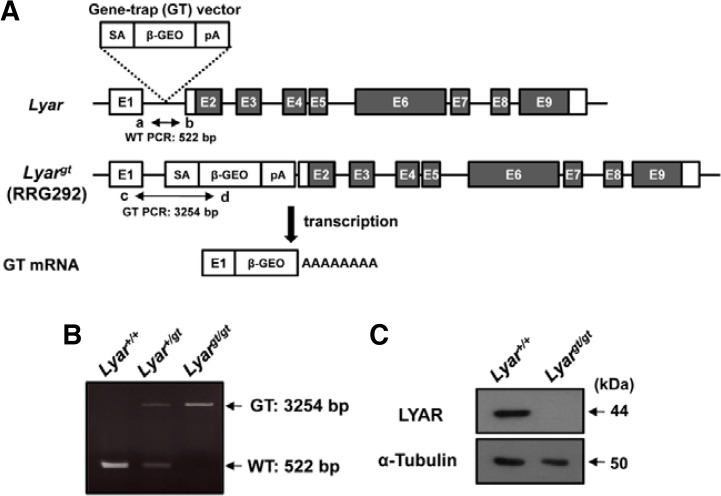
To establish the in vivo role of Lyar during spermatogenesis, we generated Lyar mutant mice by gene-trap mutagenesis. Gene trapping is a high-throughput method that randomly generates loss-of-function mutations by introducing insertional mutations across the mammalian genome (Araki et al., 2009). We screened a public gene-trap database to obtain an ES cell line that contained an insertion in the Lyar gene and expressed an abnormal fusion transcript, and mapped the precise location of the genomic insertion by DNA sequencing analysis. The integration of the gene-trap vector occurred in a position of intron 1, producing a fusion transcript lacking exons 2–9 (Fig. 3A). The ES cell clones were injected into C57BL/6J blastocysts and chimeric mice and heterozygous mice carrying the gene-trap allele (Lyar+/gt) were generated. Finally, homozygous (Lyargt/gt) males were obtained by intercrossing with heterozygous mice. Lyar+/+, Lyar+/gt and Lyargt/gt mice were validated by PCR genotyping based on sequence information (Fig. 3B). Lyargt/gt mice were born live with the predicted Mendelian ratio, indicating that Lyar is not essential for embryonic development. The weights and growth rates of Lyargt/gt newborn pups were indistinguishable from those of their WT (Lyar+/+) littermates. To confirm that gene trapping of Lyar generated a null mutation, we performed immunoblot analyses on testes using the anti-LYAR antibody described above. This analysis revealed that LYAR was absent from the testes of Lyargt/gt mice (Fig. 3C).
Fig. 3.
Generation of Lyar mutant mice. (A) Schematic diagram of the Lyar gene and gene-trap vector insertion. The numbers in the boxes represent exons and the filled boxes indicate the coding regions of LYAR. The gene-trap construct contains a splicing acceptor sequence (SA), β-galactosidase-neomycin resistance fusion gene (β-geo), and polyadenylation signal (pA). Arrows show the primers (a, b, c, and d) used in (B). Abbreviations: WT, PCR from wild-type allele; GT, PCR from allele with gene-trap mutation. (B) PCR genotyping analysis using the allele-specific primers shown in (A). Amplification with primers a and b (WT allele) yielded a 522-bp PCR product, and amplification with primers c and d (GT allele) yielded a 3254-bp PCR product. (C) Deficiency of LYAR expression in Lyar mutant testes. Testicular samples from WT (Lyar+/+) and Lyar mutant mice (Lyargt/gt) were immunoblotted with an antibody against LYAR. An anti-α-tubulin antibody was included as a control for sample loading.
Lyargt/gt mice are fertile
To determine whether the lack of LYAR affected reproductive functions, we carried out fertility tests. Adult WT and Lyargt/gt males were mated with WT females for 3 months and the numbers of pups were counted. The average litter size of pups produced over the breeding period was not significantly altered in Lyargt/gt males compared with WT males (Table 1). Adult Lyargt/gt female mice also exhibited normal fertility (data not shown).
Table 1.
Effect of LYAR deficiency on male fertility
| Parameter | Genotype of males | |
|---|---|---|
|
| ||
| Lyar+/+ | Lyargt/gt | |
| Number of males mated | 9 | 6 |
| Number of females mated | 9 | 6 |
| Males producing plugs (%) | 100 | 100 |
| Pregnant females (%) | 100 | 100 |
| Average litter size | 8.22 | 8.83 |
| Fertility rate* | 100 | 107.42 |
Defined as a percentage of the average WT litter size.
Phenotypic analyses of Lyar-mutant mice
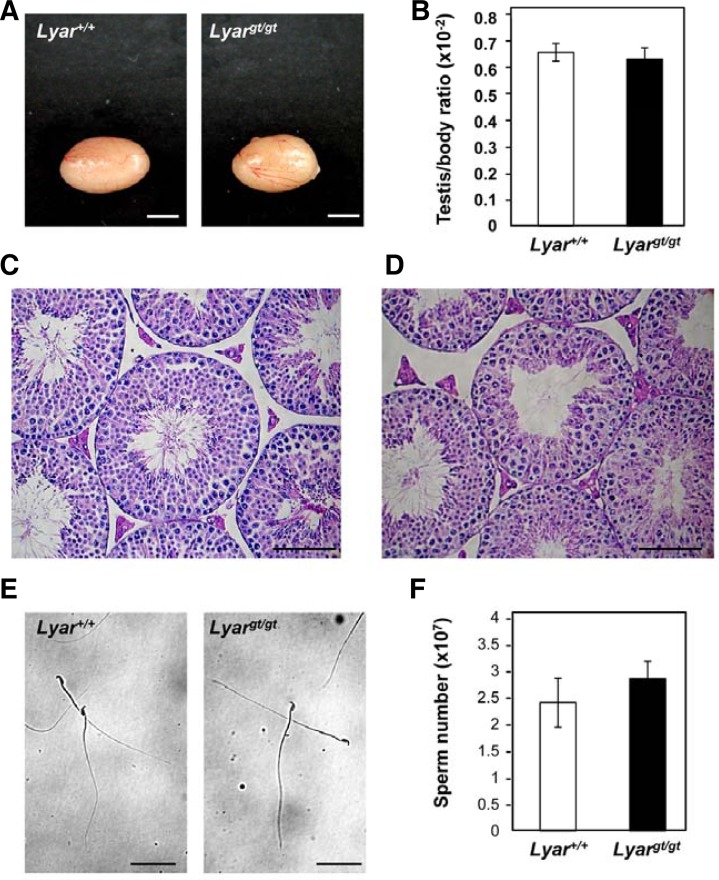
To determine whether there were any testicular changes in Lyargt/gt mice, we observed testes from adult mutant and WT mice. In size and morphology, testes from mutant males were similar to those from WT littermates (Fig. 4A), and the testis-to-body-weight ratio was not altered in Lyargt/gt mice compared to WT mice (Fig. 4B). To investigate whether Lyargt/gt testes had any defects in spermatogenesis, we performed histological analyses. The testicular cross-sections from Lyargt/gt mice did not show any abnormalities, and germ cells from all stages of spermatogenesis were present in the seminiferous tubules (Figs. 4C and 4D). To determine whether the lack of LYAR affected sperm production, we evaluated mature sperm collected from the cauda epididymis and vas deferens of 2 month-old Lyargt/gt mice and their WT littermates. No morphological abnormalities were detected in the sperm of Lyargt/gt mice (Fig. 4E), and the sperm were motile (date not shown). In addition, the sperm number was not significantly altered in Lyargt/gt mice, suggesting that the lack of LYAR did not affect the completion of spermatogenesis (Fig. 4F). These results collectively demonstrate that the lack of LYAR did not affect testicular integrity or spermatogenesis, and that LYAR-null mice had normal fertility.
Fig. 4.
Phenotypic analyses of Lyar-mutant mice. (A) Macroscopic appearance of adult testes from a Lyargt/gt mouse and a WT littermate (Lyar+/+). Scale bar = 3 mm. (B) Comparison of the testis-weight-to-body-weight ratio in Lyar+/+ (n = 13) and Lyargt/gt mice (n = 14). Testes were trimmed of fat and weighed. Values are means ± SEMs. The average testis-weight-to-body-weight ratio for WT and Lyargt/gt males was 0.67 ± 0.03 and 0.64 ± 0.04%, respectively. Histological analyses of testes from adult Lyar+/+ mice (C) and Lyargt/gt mice (D). Histological sections of seminiferous tubules obtained from the testes were stained with hematoxylin and eosin. Scale bar = 100 μm. (E) Morphologies of Lyar+/+ and Lyargt/gt sperm. Sperm were prepared from the cauda epididymis and vas deferens and observed under a light microscope. Scale bar = 100 μm. (F) Number of mature sperm from Lyar+/+ (n = 9) and Lyargt/gt mice (n = 10). Values are means ± SEMs. The average number of mature sperm in WT and Lyargt/gt males was 2.40 ± 0.46 × 107 and 2.86 ± 0.32 × 107, respectively.
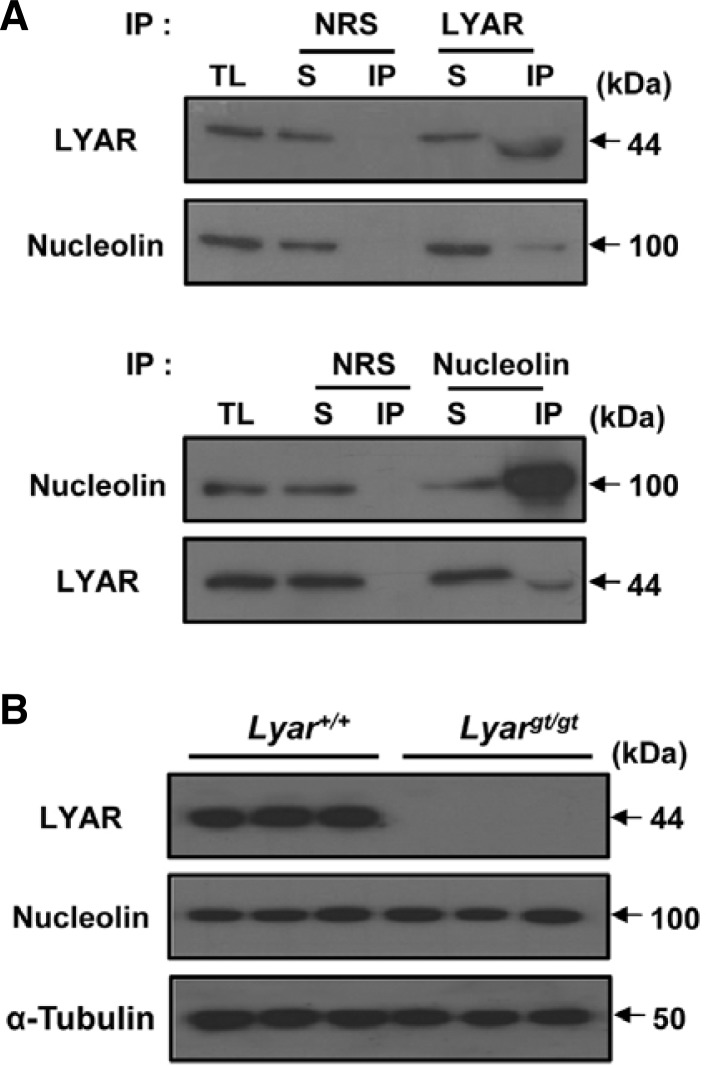
Characterization of a LYAR/nucleolin complex in the testis
LYAR was previously shown to interact with nucleolin and act as a putative proteolytic inhibitor of nucleolin in ESCs (Li et al., 2009). To assess the possible in vivo interaction between LYAR and nucleolin in the testis, we performed immunoprecipitation analyses with total lysates from mouse testes. As shown in Fig. 5A, the anti-LYAR antibody, but not normal rabbit control IgG, was able to immunoprecipitate nucleolin from testicular lysates. The anti-nucleolin antibody also was capable of immunoprecipitating LYAR. These results indicate that an endogenous LYAR-nucleolin complex forms in the testis. Comparison between the levels of precipitated proteins and the proteins remaining in the supernatant revealed that not all of the LYAR and nucleolin proteins formed complexes. To further investigate whether LYAR regulates the steady-state level of nucleolin in the testis, we performed immunoblot analyses using testes from Lyargt/gt and WT mice. We found that LYAR deficiency did not cause any detectable change in the level of nucleolin in the Lyargt/gt testes (Fig. 5B). Thus, our results indicate that although LYAR and nucleolin interact, LYAR does not affect the integrity of nucleolin in the testis.
Fig. 5.
Association of LYAR with nucleolin. (A) Interaction between endogenous LYAR and nucleolin in the testis. Immunoprecipitation of LYAR and nucleolin was performed in testicular lysates, and coprecipitated proteins were subjected to immunoblotting with the anti-LYAR and anti-nucleolin antibodies. IP with normal rabbit serum (NRS) was performed as a control. Abbreviations: TL, total lysates; S, supernatant; IP, immunoprecipitant. (B) Nucleolin level in Lyargt/gt mice. Immunoblotting of whole cell lysates of Lyar+/+ and Lyargt/gt testes was performed with anti-LYAR and anti-nucleolin antibodies. An anti-α-tubulin antibody was used to control sample loading.
DISCUSSION
Here, we provide new information on the testis-predominant nucleolar protein, LYAR, at the transcriptional, protein, and functional levels in mice. RT-PCR analyses of Lyar showed very strong expression in the testis compared to other tissues. In the testis, Lyar expression seemed to be specific to germ cells. Analysis of developmental expression patterns demonstrated that Lyar expression starts at an early stage of spermatogenesis and dramatically increases at postnatal day 16, corresponding to the development of pachytene spermatocytes. Protein analyses demonstrated that LYAR is developmentally regulated during spermatogenesis, and that its expression is significantly increased during the postmeiotic phase. Further immunoblot analyses showed the presence of LYAR in testicular spermatogenic cells, including spermatogonia, spermatocytes and round spermatids. Thus, LYAR is strongly expressed in germ cells and may play a role in spermatogenesis.
To determine the in vivo role of Lyar in the testis, we generated Lyar mutant mice. Western blot analysis in total lysates from adult testis showed the absence of LYAR in the Lyargt/gt mice. Although LYAR was previously reported to function in the self-renewal and differentiation of embryonic stem cells (Li et al., 2009), Lyar mutant mice were born live and our fertility tests demonstrated that Lyargt/gt males are fertile. We were unable to observe any abnormalities in the testicular morphology and spermatogenesis of LYAR-null males, or in the morphology and number of their mature sperm. These results are somewhat unexpected. Since LYAR is abundant in spermatogenic cells and has been suggested to function as a putative regulator of cell proliferation in ESCs (Li et al., 2009), we had hypothesized that the lack of LYAR would affect germ cell proliferation and differentiation. However, Lyar mutant testes exhibited normal spermatogenesis and produced sperm with normal fertility. Thus, our results indicate that LYAR is functionally redundant for spermatogenesis.
In ESCs, LYAR was previously shown to form a complex with nucleolin, which is one of the most abundant proteins in the nucleolus (Bugler et al., 1982). Nucleolin has been implicated in the processes of ribosome biogenesis, including rRNA transcription and nucleo-cytoplasmic transport, and has been reported to act as a nucleic acid helicase (Tuteja and Tuteja, 1998). The level of nucleolin was found to positively correlate with cell proliferation, and decreased expression of nucleolin significantly reduced the cell growth rate of ESCs (Derenzini et al., 1995; Li et al., 2009; Roussel and Hernandez-Verdun, 1994). In non-proliferating cells, nucleolin activity is regulated by autocatalysis, which can be inhibited by nuclear extracts from proliferative cells (Chen et al., 1991; Fang and Yeh, 1993). LYAR was shown to prevent this self-cleavage in vitro, making it a putative proteolytic inhibitor of nucleolin (Li et al., 2009). In the present study, our immunoprecipitation analysis confirmed the presence of an endogenous LYAR-nucleolin complex in the testis. However, nucleolin did not undergo autocatalysis in LYAR-null testes. Thus, our results indicate that LYAR does not regulate the autocleavage of nucleolin in vivo.
During the preparation of our manuscript, another study on Lyar-mutant mice generated by gene-trap insertion was reported (Wang et al., 2012). Similar phenotypes were observed in the mice with reduced LYAR expression, in that the mutant mice were viable and fertile. Further genetic analyses showed that Lyar and p53 double-mutant female mice exhibited embryonic lethality, suggesting that there is a genetic interaction between the two genes during embryogenesis (Wang et al., 2012). Thus, it is possible that the alterations in molecular or cellular processes caused by Lyar deficiency could be limited by p53 or a p53-like factor during spermatogenesis.
In conclusion, our work provides comprehensive information on LYAR in male reproduction through in vitro and in vivo analyses. In particular, we report for the first time that LYAR is a nucleolar protein abundantly expressed in spermatocytes and spermatids, but not sperm. Our inclusive analysis of Lyar mutant mice revealed that LYAR does not have an essential function in spermatogenesis and is dispensable for male reproduction.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (2012R1 A1A2039947) and Gwangju Institute of Science and Technology Systems Biology Infrastructure Establishment Grant.
REFERENCES
- Araki M., Araki K., Yamamura K. International Gene Trap Project: towards gene-driven saturation mutagenesis in mice. Curr. Pharm. Biotechnol. 2009;10:221–229. doi: 10.2174/138920109787315006. [DOI] [PubMed] [Google Scholar]
- Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur. J. Biochem. 1982;128:475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- Cai J., Chen J., Liu Y., Miura T., Luo Y., Loring J.F., Freed W.J., Rao M.S., Zeng X. Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem Cells. 2006;24:516–530. doi: 10.1634/stemcells.2005-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.M., Chiang S.Y., Yeh N.H. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J. Biol. Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- Derenzini M., Sirri V., Trere D., Ochs R.L. The quantity of nucleolar proteins nucleolin and protein B23 is related to cell doubling time in human cancer cells. Lab. Invest. 1995;73:497–502. [PubMed] [Google Scholar]
- Eddy E.M. Male germ cell gene expression. Recent Prog. Horm. Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- Fang S.H., Yeh N.H. The self-cleaving activity of nucleolin determines its molecular dynamics in relation to cell proliferation. Exp. Cell Res. 1993;208:48–53. doi: 10.1006/excr.1993.1221. [DOI] [PubMed] [Google Scholar]
- Ginisty H., Sicard H., Roger B., Bouvet P. Structure and functions of nucleolin. J. Cell Sci. 1999;112(Pt 6):761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- Grisendi S., Mecucci C., Falini B., Pandolfi P.P. Nucleophosmin and cancer. Nat. Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A.L., Tres L.L. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed. Proc. 1978;37:2512–2516. [PubMed] [Google Scholar]
- Lee B., Park I., Jin S., Choi H., Kwon J.T., Kim J., Jeong J., Cho B.N., Eddy E.M., Cho C. Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J. Biol. Chem. 2011;286:29108–29117. doi: 10.1074/jbc.M111.244905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang B., Yang A., Lu R., Wang W., Zhou Y., Shi G., Kwon S.W., Zhao Y., Jin Y. Ly-1 antibody reactive clone is an important nucleolar protein for control of self-renewal and differentiation in embryonic stem cells. Stem Cells. 2009;27:1244–1254. doi: 10.1002/stem.55. [DOI] [PubMed] [Google Scholar]
- Lo S.J., Lee C.C., Lai H.J. The nucleolus: reviewing oldies to have new understandings. Cell Res. 2006;16:530–538. doi: 10.1038/sj.cr.7310070. [DOI] [PubMed] [Google Scholar]
- Melese T., Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr. Opin. Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- Oh J. S., Han C., Cho C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol Cells. 2009;28:441–446. doi: 10.1007/s10059-009-0140-x. [DOI] [PubMed] [Google Scholar]
- Phelps B.M., Koppel D.E., Primakoff P., Myles D.G. Evidence that proteolysis of the surface is an initial step in the mechanism of formation of sperm cell surface domains. J. Cell Biol. 1990;111:1839–1847. doi: 10.1083/jcb.111.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P., Hernandez-Verdun D. Identification of Ag-NOR proteins, markers of proliferation related to ribosomal gene activity. Exp. Cell Res. 1994;214:465–472. doi: 10.1006/excr.1994.1283. [DOI] [PubMed] [Google Scholar]
- Shaw P.J., Jordan E.G. The nucleolus. Annu. Rev. Cell Dev. Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Su L., Hershberger R.J., Weissman I.L. LYAR, a novel nucleolar protein with zinc finger DNA-binding motifs, is involved in cell growth regulation. Genes Dev. 1993;7:735–748. doi: 10.1101/gad.7.5.735. [DOI] [PubMed] [Google Scholar]
- Swartling F.J., Grimmer M.R., Hackett C.S., Northcott P.A., Fan Q.W., Goldenberg D.D., Lau J., Masic S., Nguyen K., Yakovenko S., et al. Pleiotropic role for MYCN in medulloblastoma. Genes Dev. 2010;24:1059–1072. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja R., Tuteja N. Nucleolin: a multifunctional major nucleolar phosphoprotein. Crit. Rev. Biochem. Mol. Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- Wang G., Fulkerson C.M., Malek R., Ghassemifar S., Snyder P.W., Mendrysa S.M. Mutations in Lyar and p53 are synergistically lethal in female mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2012;94:729–737. doi: 10.1002/bdra.23048. [DOI] [PubMed] [Google Scholar]