Abstract
Interstitial cells of Cajal (ICC) are the pacemaker cells that generate the rhythmic oscillation responsible for the production of slow waves in gastrointestinal smooth muscle. Spingolipids are known to present in digestive system and are responsible for multiple important physiological and pathological processes. In this study, we are interested in the action of sphingosine 1-phosphate (S1P) on ICC. S1P depolarized the membrane and increased tonic inward pacemaker currents. FTY720 phosphate (FTY720P, an S1P1,3,4,5 agonist) and SEW 2871 (an S1P1 agonist) had no effects on pacemaker activity. Suramin (an S1P3 antagonist) did not block the S1P-induced action on pacemaker currents. However, JTE-013 (an S1P2 antagonist) blocked the S1P-induced action. RT-PCR revealed the presence of the S1P2 in ICC. Calphostin C (a protein kinase C inhibitor), NS-398 (a cyclooxygenase-2 inhibitor), PD 98059 (a p42/44 inhibitor), or SB 203580 (a p38 inhibitor) had no effects on S1P-induced action. However, c-jun NH2-terminal kinase (JNK) inhibitor II suppressed S1P-induced action. External Ca2+-free solution or thapsigargin (a Ca2+-ATPase inhibitor of endoplasmic reticulum) suppressed action of S1P on ICC. In recording of intracellular Ca2+ ([Ca2+]i) concentration using fluo-4/AM S1P increased intensity of spontaneous [Ca2+]i oscillations in ICC. These results suggest that S1P can modulate pacemaker activity of ICC through S1P2 via regulation of external and internal Ca2+ and mitogen-activated protein kinase activation.
Keywords: interstitial cells of Cajal, intestinal motility, pacemaker activity, sphingosine-1-phosphate
INTRODUCTION
Bioactive sphingolipid metabolites include ceramide, sphingosine, sphingosine-1-phosphate (S1P), and sphingosine phosphorylcholine (Anliker and Chun, 2004). Various biological roles of sphingolipids include stimulation of cell survival and growth, regulation of migration and differentiation, apoptosis, modulation of cardiovascular functions, and smooth muscle contractions (Watterson et al., 2005). As a normal constituent of plasma, S1P is present at concentrations ranging from 0.4 to 1.5 μM (Murata et al., 2000). Growth factors, inflammatory cytokines, and G-protein-coupled receptor agonists, such as muscarinic and purinergic agonists, can stimulate an increase in the concentration of S1P (Alemany et al., 2000; Alvarez et al., 2007; Pfaff et al., 2005; van Koppen et al., 2001). S1P is generated from sphingosine as a result of phosphorylation by sphingosine kinase; its action as an intracellular second messenger occurs through mobilization of intracellular calcium and an extracellular ligand via binding of G-protein coupled receptors. Five of these receptors have been identified; S1P1, S1P2, S1P3, S1P4, and S1P5 (Sanchez and Hla, 2004; Young and Nahorski, 2002). Presence of S1P receptors has been detected in the neuronal system, pulmonary system, endocrine organs, reproductive system, immune system, cardiovascular system, and gastrointestinal system, indicating their involvement in physiological regulation of many organ systems (Hla, 2004). In the gastrointestinal tract, S1P induces contraction of gastric, esophageal smooth muscle and is involved in postoperative intestinal dysmotility (Dragusin et al., 2006; Song et al., 2006; Zhou and Murthy, 2004), suggesting its ability to modulate gastrointestinal motility.
Interstitial cells of Cajal (ICC), which function as pacemaker cells of the gastrointestinal tract, serve as regulators of gastrointestinal motility by generation of slow waves in smooth muscle cells and by translation of enteric neuronal signals (Huizinga et al., 1995; Sanders, 1996; Ward et al., 1994). Disruption of ICC has been reported to evoke abnormal patterns of motility in several pathological conditions, including achalasia, obstructive intestinal disorders, pyloric stenosis, constipation, and diarrhea (Mostafa et al., 2010). As a result of these findings, ICC are now considered therapeutic targets for treatment of gastrointestinal motility disorders.
No physiological data describing the effects of S1P on pacemaker activity of ICC is currently available; therefore, we conducted this study in order to examine the effects of S1P on pacemaker currents and its signal transduction mechanisms in cultured ICC from mouse small intestine.
MATERIALS AND METHODS
Preparation of cells and tissues
All animals received ethical treatment, in accordance with the guiding principles for care and use of animals in the field of physiologic sciences, as approved by the Institutional Animal Use and Care Committee at Chosun University College of Medicine (approval number, CIACUC2012-A0002). For anesthetization, Balb/C mice (3–7 days old) of either sex received diethylether, and were then sacrificed by cervical dislocation. Following its excision 1 cm below the pyloric ring to the cecum, the small intestine was opened along the mesenteric border. Krebs-Ringer bicarbonate solution was used to wash away luminal contents. Isolated tissue was pinned to the base of a Slygard dish, and sharp dissection was performed for removal of mucosa. Small strips of intestinal muscle were equilibrated in calcium-free Hank’s solution containing the following constituents (in mM): KCl, 5.36; NaCl, 125; NaOH, 0.336; Na2HCO3, 0.44; glucose, 10; sucrose, 2.9; and HEPES, 11. Using Tris buffer, pH was adjusted to 7.4 for 30 min. To disperse the cells, they were incubated in an enzymatic solution containing collagenase (1.3 mg/ml; Worthington Biochemicals, USA), bovine serum albumin (2 mg/ml; Sigma, USA), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml) for 15 min at 37°C. After the cells had been finely chopped they were placed on sterile glass coverslips coated with poly-L-lysine in 35-mm culture dishes and incubated in smooth muscle growth medium (SMGM; Cambrex Bio Science, USA) supplemented with 2% antibiotics/ antimycotics (Gibco, USA) and murine stem cell factor (SCF, 5 ng/ml; Sigma) at 37°C in a 95% O2 −5% CO2 incubator.
Solutions and drugs
To bathe the cells, they were placed in a standard solution containing the following (in mM): KCl, 5; NaCl, 135; CaCl2, 2; glucose, 10; MgCl2, 1.2; and HEPES, 10. Using Tris buffer, the standard solution was adjusted to pH 7.4. The pipette solution contained the following (in mM): K-aspartate, 120; KCl, 20; MgCl2, 5; K2ATP, 2.7; Na2GTP, 0.1; creatine phosphate disodium, 2.5; EGTA, 0.1; and HEPES, 5. Using Tris buffer, the solution was adjusted to pH 7.4.
Sphingosine 1-phosphate, suramin, SEW2871, JTE-013, calphostin C, PD 98059, SB 203580, NS-398, and thapsigargin were purchased from Sigma (USA). FTY720 phosphate was purchased from Cayman Chemicals (USA). JNK inhibitor II (SP600125) was purchased from Calbiochem Co. (USA). Drugs used in this study were dissolved in an appropriate solvent, according to the product information.
Patch clamp experiments
The whole cell patch-clamp technique was used in recording of membrane currents (voltage clamp) and membrane potentials (current clamp). The Axopatch 1-D (Axon Instruments, USA) was used for amplification of currents or potentials. An IBM-compatible personal computer and pClamp software (version 9.2; Axon Instruments) were used for application of command pulse. Data were filtered at 5 KHz. All experiments were performed at 30°C.
ICC picking and RT-PCR
After 2 days of culture, an even distribution of single cells was observed. The distinct morphology of ICC was easily identified among the mixture of other cells. Patch clamp microelectrodes with a slightly larger pore size were used for collection of single cells. Following placement of the microelectrode near the single isolated cell, slight negative pressure was applied, resulting in entry of the cell into the electrode. Each cell collected was then transferred to a tube containing PBS and maintained in a chilled condition. A total of 5–10 cells were added to a single tube.
Following the manufacturer’s protocol, total RNA were extracted from the isolated ICC using Trizol reagent (Generay Biotech). During each step, care was taken to avoid loss of RNA. SuperScript one-step RT-PCR with Platinum Taq (Invitrogen, USA) was used for reverse transcription polymerase chain reaction (RT-PCR) and cDNA amplification of isolated RNA. For amplification, sense and antisense primers were selected according to the mouse sequence for indicated genes: S1P1 (NM_007901), 5′- ACC TTT GGC ACT TTT ATT GA -3′ and 5′- TAA AGT AAT GCT TTT GGG GA -3′ (227 bp); S1P2 (NM_010333), 5′- CCG TCA TCT TAC TGG CTA TC -3′ and 5′- TTG AGC AGT GAG TTA AGG GT -3′ (255 bp); S1P3 (NM_010101), 5′- AAA CCA GTG ACA CCA GAG AC -3′ and 5′- GAT TGG GCA TCA AAT GTA GT -3′ (245 bp); S1P4 (NM_010102), 5′- TTT CCT GTT ATG CTC AAG GT -3′ and 5′- ATA CAG TTG GAA CAG TTG GG -3′ (259 bp); S1P5 (NM_053190), 5′- CCA ACC CCA ATA AAT AAA CA -3′ and 5′- AGA GCT GTG GAC AAA GGT AA -3′ (247 bp); c-Kit (AY536430), 5′- GCA CAG AAG GAG GCA CTT ATA CCT -3′ and 5′-TGA GAC AGG AGT GGT ACA CCT TTG-3′ (215 bp); Myosin (NM_013607), 5′- AGG CAG ACC TCA TGC AGC TCC AAG A -3′ and 5′- CCT CAT TCT GTT CAT CCC GAG CCT G -3′ (340 bp) and PGP 9.5 (NM_011670), 5′-GCC AAC AAC CAA GAC AAG CTG GAA -3′ and 5′-GCC GTC CAC GTT GTT GAA CAG AAT -3′ (213 bp). Using these primer pairs, reverse transcription PCR was performed at 45°C for 30 min on cDNA templates derived from mRNA isolated from the separated ICC. Cycling parameters included: initial denaturation at 95°C for 5 min, then 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 35 cycles followed by a 10-min final extension period at 72°C; 2 μl of product resulting from the procedure described above was used for re-amplification for another 30 cycles, followed by electrophoresis of 5 μl of this product on a 2% agarose gel and staining with ethidium bromide for visualization of DNA fragments.
Measurement of intracellular Ca2+ concentration
Fluo-4/AM, which was initially dissolved in dimethyl sulfoxide and stored at −20°C, was used for monitoring of changes in the intracellular Ca2+ ([Ca2+]i) concentration. Cultured ICC on coverslips (25 mm) were rinsed twice using a bath solution, followed by incubation in bath solution containing 1 μM fluo-4/AM with 5% CO2 at 37°C for 5 min, and rinsed 2 more times with the bath solution. They were then mounted on a perfusion chamber, and scanned every 0.4 s using a Nikon Eclipse TE200 inverted microscope equipped with a Perkin-Elmer Ultraview confocal scanner and a Hamamatsu Orca ER 12-bit CCD camera (× 200). Fluorescence excitation occurred at a wavelength of 488 nm, and emitted light was observed at 515 nm. The temperature of the perfusion chamber containing the cultured ICC was maintained at 30°C during scanning of Ca2+ imaging. Variations of [Ca2+]i fluorescence emission intensity were expressed as F1/F0 where F0 is the intensity of the first imaging.
Statistical analysis
Data were expressed as means ± standard errors. The Student’s t and one way ANOVA followed by Dunnett’s test were applied for evaluation of differences. P values of < 0.05 were considered statistically significant. N values reported in the text refer to the number of cells used in patch-clamp experiments.
RESULTS
Effect of S1P on pacemaker activity generated by ICC
Cultures of cells contained single cells and networks of cells that had gross morphological properties similar to ICC in situ, including fusiform cell bodies, large, prominent nuclei with little perinuclear cytoplasm, and multiple, thin processes extending from the nuclear region that were often interconnected with processes of neighboring cells. Recordings were made from ICC with the patch clamp technique as soon as the network-like structures. Recordings were made from cells within networks that had morphologies similar to the cells that were immunopositive for c-Kit.
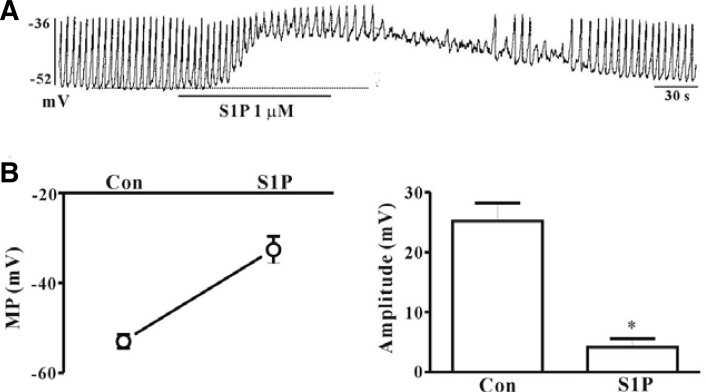
Under current-clamp mode (I = 0), spontaneous depolarization (pacemaker potentials) of ICC was observed. The resting membrane potential was −53 ± 1.5 mV and the amplitude of pacemaker potential was 25.2 ± 3 mV. Treatment of ICC with S1P (1 μM) resulted in membrane depolarization and decreased amplitude of pacemaker potentials (Fig. 1A). In the presence of S1P, depolarization of the membrane to −32.6 ± 3 mV (n = 4, Fig. 1B) and a decrease in the amplitude of pacemaker potentials to 4.2 ± 1.4 mV were observed (n = 4, Fig. 1C).
Fig. 1.
Effects of S1P on pacemaker potentials in cultured ICC from mouse small intestine. (A) Pacemaker potentials from ICC exposed to S1P (1 μM) in current clamp mode (I = 0). (B, C) Summary of the effects of S1P on pacemaker potentials in ICC. Bars represent means ± SE (n = 4). *Asterisks indicate a significant difference from the control (p < 0.05). Dotted lines indicate basal membrane potential levels. Con, control; MP, membrane potentials.
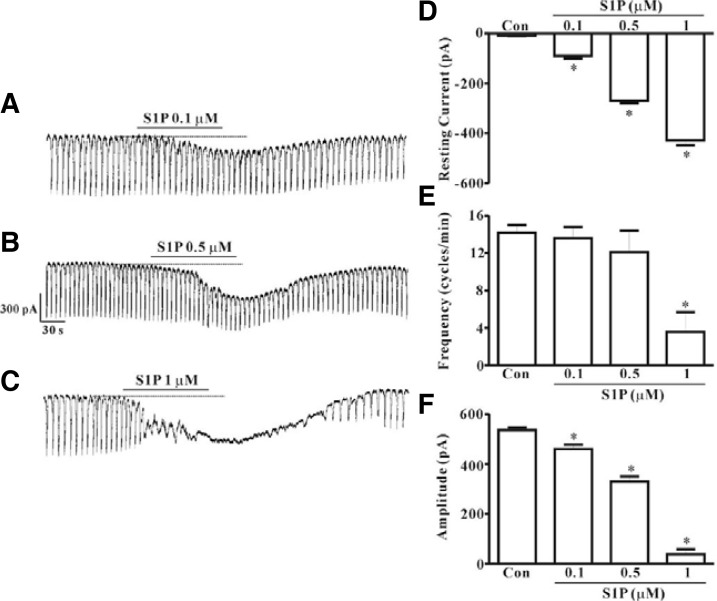
Under voltage clamp at a holding potential of −70 mV, spontaneous inward pacemaker currents were generated in ICC. Treatment with S1P (0.1, 0.5, or 1 μM) resulted in concentration-dependent production of tonic inward currents and decreased frequency and amplitude of pacemaker currents (Figs. 2A–2C). A summary of the values and a bar graph on the effects of S1P are shown in Figs. 2D–2F (n = 5).
Fig. 2.
Effects of S1P on pacemaker currents in cultured ICC from mouse small intestine. (A, B, and C) Pacemaker currents from ICC exposed to S1P (0.1, 0.5, and 1 μM) at a holding potential of −70 mV. Responses to S1P are summarized in (D, E, and F) (n = 5). Bars represent mean values ± SE. *(P < 0.05) Significantly different from the untreated control. Dotted lines indicate the zero current levels. Con, control.
Identification of receptor subtypes of S1P
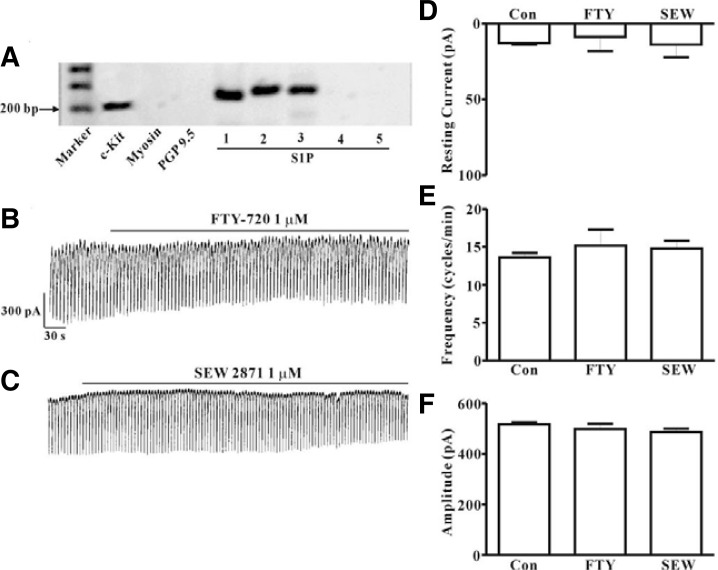
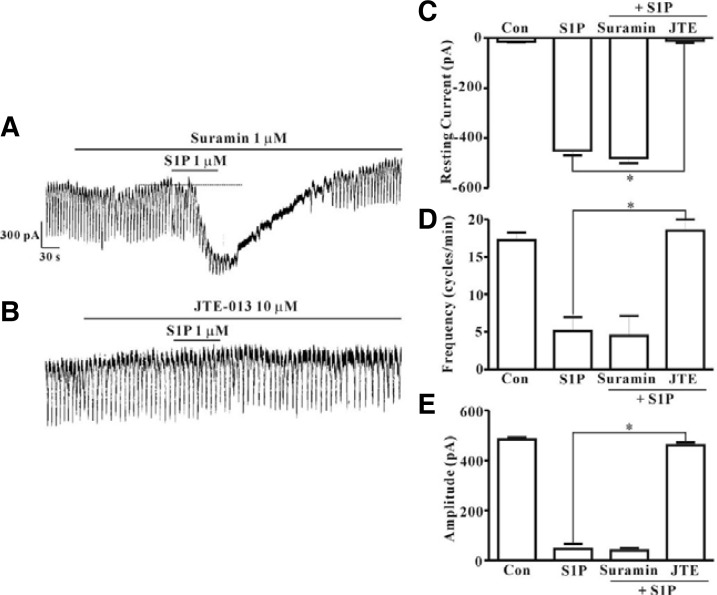
RT-PCR with c-Kit positive cells and pharmacological study using various S1P receptor agonists or antagonists for identification of the receptor subtypes of S1P in ICC were performed. Prior to performance of the RT-PCR assay, we first collected ICC that showed distinct morphology in the culture system (approximately 5-10 cells). In order to determine whether or not the collected cells contained muscle cells and neurons, we also performed RT–PCR for myosin, a smooth muscle cell marker, and PGP9.5, a neuron marker. As shown in Fig. 3A, lane 3, no band for myosin or PGP9.5 was observed, indicating that muscle cells and neurons were not present in our collected sample. PCR assays of ICC using S1P1, S1P2, and S1P3 primers yielded a product of the appropriate size. Results showed that products from PCR using S1P1, S1P2, and S1P3 were produced from c-Kit positive cells; however, amplification of S1P4 and S1P5 was not observed (Fig. 3A). Next, in order to determine which type of receptor is involved in S1P-induced action on pacemaker currents in ICC, we examined the effects of FTY720P (1 μM), an S1P1,3,4,5 agonist, and SEW 2871 (1 μM), an S1P1 agonist. Both drugs had no effects on pacemaker currents (Figs. 3B and 3C). A summary of the values and a bar graph on the effects of S1P receptor subtype agonists are shown in Figs. 3D, 3E, and 3F (n = 6). We also examined the effects of suramin, an S1P3 antagonist. S1P (1 μM) still generated tonic inward currents in the presence of suramin (10 μM) (Fig. 4A). However, we observed the blocking effect of JTE-013, an S1P2 antagonist (10 μM), on S1P-induced tonic inward currents (Fig. 4B). The effects of S1P receptor subtype antagonists on S1P-induced tonic inward currents are summarized in Figs. 4C–4E (n = 4).
Fig. 3.
Agarose gel of the RT-PCR products of the S1P receptors using c-Kit positive cells and effects of FTY720P or SEW 2871 on pacemaker activity in cultured ICC from mouse small intestine. (A) This representative 1.2% agarose gel was loaded with 5 μl of PCR product and stained with ethidium bromide. (B) Pacemaker currents from ICC exposed to FTY720P (1 μM) at a holding potential of −70 mV. (C) Pacemaker currents from ICC exposed to SEW 2871 (1 μM) at a holding potential of −70 mV. Responses to FTY720P or SEW 2871 are summarized in (D, E, and F) (n = 6). Bars represent mean values ± SE. Con, control; FTY, FTY720 phosphate; SEW, SEW 2871.
Fig. 4.
Effects of suramin or JTE-013 on S1P-induced actions in cultured ICC from mouse small intestine. (A) Pre-treatment with suramin (10 μM) did not result in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. (B) Pre-treatment with JTE-013 (10 μM) resulted in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. Effects of suramin or JTE-013 on S1P-induced actions are summarized in (C, D, and E) (n = 4). Bars represent mean values ± SE. *(P < 0.05) Significantly different from S1P alone. Dotted lines indicate the zero current levels. Con, control; JTE, JTE-013.
Effect of protein kinase C inhibitor, cyclooxygenase-2 inhibitor, or Ca2+-ATPase inhibitor of endoplasmic reticulum on S1P-induced action in ICC
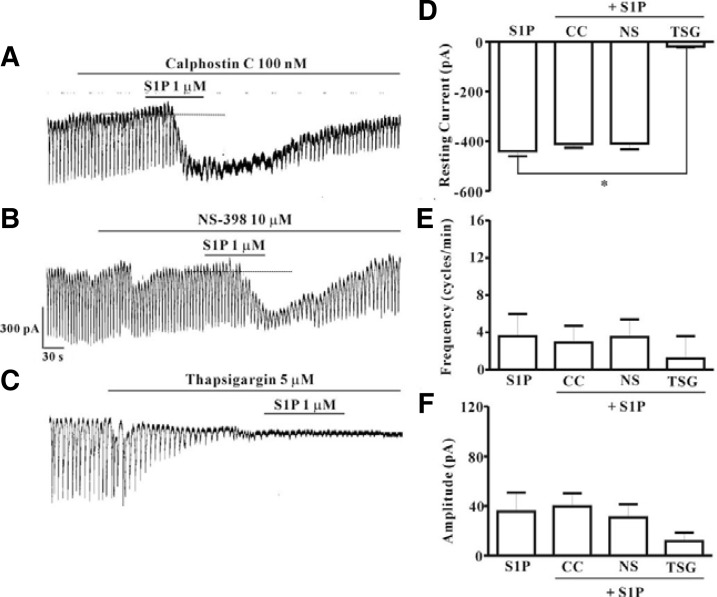
In order to determine the signaling mechanism of S1P-induced effects on ICC, we examined the effects of calphostin C, an inhibitor of protein kinase C, or NS-398, a specific cyclooxygenase-2 inhibitor, or thapsigargin, a Ca2+-ATPase inhibitor of endoplasmic reticulum. Neither calphostin C (100 nM) nor NS-398 (10 μM) had an effect on modulation of tonic inward currents by S1P (Figs. 5A and 5B) and the value did not differ significantly when compared with that obtained from treatment with S1P in the absence of calphostin C or NS-398 (n = 5, Figs. 5D–5F). However, treatment with thapsigargin (5 μM) resulted in inhibition of pacemaker currents and blockade of S1P-induced tonic inward currents (Fig. 5C). A summary of the inhibitory effect of thapsigargin on S1P-induced tonic inward currents is shown in Figs. 5D–5F (n = 4).
Fig. 5.
Effects of calphostin C, NS-398, or thapsigargin on S1P-induced actions in cultured ICC from mouse small intestine. (A) Pre-treatment with calphostin C (100 nM) did not result in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. (B) Pre-treatment with NS-398 (10 μM) did not result in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. (C) Pre-treatment with thapsigargin (5 μM) resulted in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. Responses to treatment with calphostin C, NS-398, or thapsigargin on S1P-induced actions are summarized in (D, E, and F) (n = 4). Bars represent mean values ± SE. *(P < 0.05) Significantly different from S1P alone. Dotted lines indicate the zero current levels. Con, control; CC, calphostin C; NS, NS-398; TSG, thapsigargin.
Effect of mitogen-activated protein kinase inhibitors on S1P-induced action in ICC
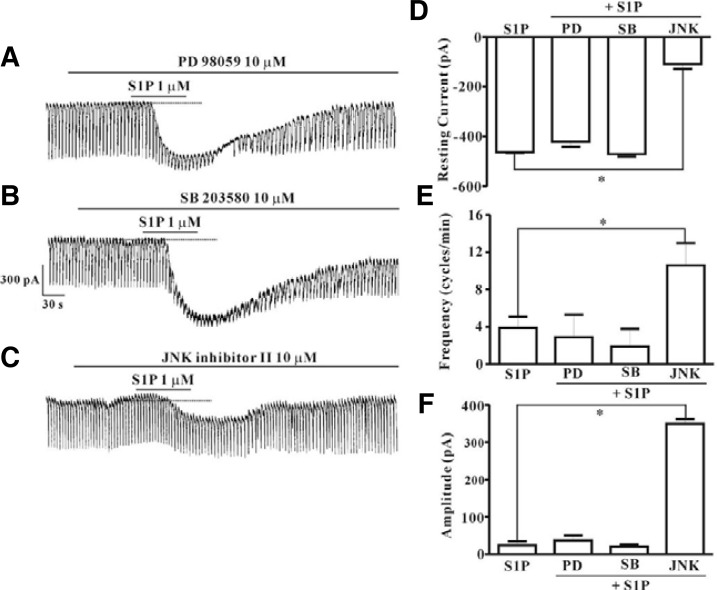
ICC were pretreated with selective MAPK inhibitors to evaluate the involvement of mitogen-activated protein kinases (MAPKs) in S1P-induced effects on pacemaker activity in ICC. Neither PD 98059, a selective p42/44 inhibitor (10 μM), nor SB 203580, a selective p38 inhibitor (10 μM), had an effect on the S1P-induced tonic inward currents (Figs. 6A and 6B), and the value did not differ significantly when compared with that obtained from treatment with S1P in the absence of PD 98059 or SB 203580 (n = 4, Figs. 6D–6F). However, treatment of ICC with JNK, c-jun NH2-terminal kinase, inhibitor II (10 μM) resulted in inhibition of S1P (1 μM)-induced tonic inward currents (Fig. 6C). A summary of the inhibitory effect of JNK inhibitor II on S1P-induced tonic inward currents is shown in Figs. 6D–6F (n = 4).
Fig. 6.
Effects of PD 98059, SB 203580, or JNK inhibitor II on S1P-induced actions in cultured ICC from mouse small intestine. (A) Pre-treatment with PD 98059 (10 μM) did not result in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. (B) Pre-treatment with SB 203580 (10 μM) did not result in blockade of S1P (1 μM)-induced actions at a holding potential of −70 mV. (C) Pre-treatment with JNK inhibitor II (10 μM) resulted in suppression of S1P (1 μM)-induced actions at a holding potential of −70 mV. Responses to PD 98059, SB 203580, or JNK inhibitor II on S1P-induced actions are summarized in (D), (E), and (F) (n = 4). Bars represent mean values ± SE. *(P < 0.05) Significantly different from S1P alone. Dotted lines indicate the zero current levels. Con, control; PD, PD 98059; SB, SB 203580; JNK, JNK inhibitor II.
The roles of external Ca2+ or internal Ca2+ in S1P-induced action in ICC
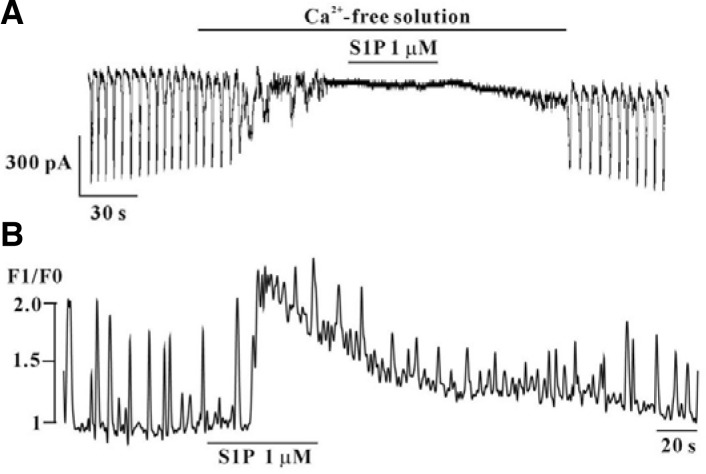
To investigate the role of external Ca2+ or internal Ca2+, testing of S1P was conducted under external Ca2+-free conditions, and performed intracellular Ca2+ ([Ca2+]i) analysis using a Ca2+ indicator. Application of external Ca2+-free solution resulted in complete inhibition of pacemaker currents in voltage clamp mode at a holding potential of −70 mV; under this condition, blockade of the effects of S1P-induced effects on pacemaker currents was observed (n = 4, Fig. 7A). We also measured spontaneous [Ca2+]i change of ICC, which is associated with cell clusters. Spontaneous [Ca2+]i oscillations observed in ICC loaded with fluo-4/AM and data on the time series showed regular spontaneous [Ca2+]i oscillations. Intensity of [Ca2+]i was increased in the presence of S1P (n = 4, Fig. 7B).
Fig. 7.
Effects of S1P on external Ca2+-free condition and on intracellular Ca2+ oscillation in cultured ICC from mouse small intestine. (A) Pre-treatment with Ca2+-free solution resulted in inhibition of S1P (1 μM)-induced actions at a holding potential of −70 mV. (B) Fluo-4/AM was used for monitoring changes in the intracellular Ca2+ concentration.
DISCUSSION
Findings from the present study demonstrated modulation of pacemaker currents of ICC by S1P, which is mediated by S1P2 via regulation of external and internal Ca2+ and a MAPK-dependent pathway.
S1P receptors are G-protein coupled receptors with five subtypes of S1P1–5. Wide expression of S1P1, S1P2, and S1P3 has been observed, whereas S1P4 is confined to lymphoid, hematopoietic cells, and lung cells. High expression of S1P5 in glial cells and spleen has been reported (Watterson et al., 2005). Coupled with various G-proteins, these S1P receptors mediate diverse biological actions. S1P induces contraction of rabbit gastric smooth muscle through S1P1 and S1P2 (Zhou and Murthy, 2004), contraction of cat esophageal smooth muscle cells through S1P2, contraction of cerebral arteries of mice through the S1P3 (Salomone et al., 2008), and contraction of airway smooth muscles through the S1P2 (Rosenfeldt et al., 2003). In the present study, treatment of ICC with S1P resulted in depolarization of the membrane and increased induction of tonic inward pacemaker currents, suggesting that S1P may mediate excitatory action on intestinal motility. RT-PCR analysis detected transcripts for the S1P1, S1P2 and S1P3 in ICC. Results from pharmacological study showed that suramin, an S1P3 antagonist, did not result in blockade of S1P-induced effects on pacemaker currents. In addition, SEW 2871, an S1P1 agonist, and FTY720P, an S1P1,3,4 and 5 agonist, had no effect on pacemaker currents. However, JTE-013, an S1P2 antagonist, blocked the effects of S1P on pacemaker currents. Therefore, it appears that S1P1, 2, and 3 are present in ICC and that S1P2 activation can result in modulation of intestinal motility through regulation of pacemaker activity of ICC.
S1P2 activation leads to mediation of various intracellular signaling pathways, including activation of phospholipase C, mitogen-activated protein kinase (MAPK), Rho kinase, protein kinase C, and COX-2 (Nodai et al., 2007; Sanchez and Hla, 2004; Takuwa et al., 2001). Expression of protein kinase C and COX-2 in intestinal ICC and PLC-dependent pacemaking activity of ICC have been reported (Furness et al., 2006; Porcher et al., 2002; Ward et al., 2000). In addition, COX-2 and MAPK-dependent pathways were found to mediate the inhibitory action of H2O2 on pacemaker currents of ICC (Choi et al., 2010), thereby confirming the presence of various operating signal pathways in ICC. In the present study, S1P-induced tonic inward currents were inhibited by JNK inhibitor but not by PD98059, an ERK inhibitor, and not by SB202190, a p38 MAPK inhibitor. Treatment with protein kinase C inhibitor (calphostin C) or COX-2 inhibitor (NS-398) did not result in blockade of S1P-induced effects on pacemaker currents. These data suggested JNK may involve in S1P-induced effects on pacemaker currents. Contraction of cat esophageal smooth muscle cells by activation of S1P2, which is mediated by phospholipase C and protein kinase C, leads to activation of p42/p44 MAPK (Song et al., 2006). S1P has been reported to induce G-protein dependent cyclooxygenase-2 expression in association with activation of ERK and p38 MAPKs in rat intestinal smooth muscle cells (Dragusin et al., 2006).
The actions of many G-protein coupled receptors are mediated by release of PLC-dependent IP3-gated Ca2+ from the ER (Fukata et al., 2001). S1P receptors also have a role in mobilization of intracellular Ca2+ in various cell types (Bischoff et al., 2000; Spiegel and Merrill, 1996; Young and Nahorski, 2002). The pacemaking mechanisms of intestinal ICC are driven by activation of Ca2+-dependent pacemaker channels, such as TRP or Ca2+-activated Cl− channels, which are coupled with release of IP3-gated Ca2+ from the endoplasmic reticulum (Huizinga et al., 2002; Kim et al., 2005; Nakayama et al., 2007; Ward et al., 2000). S1P stimulation of phosphoinositide hydrolysis and [Ca2+]i increase in vascular smooth muscle, gastric smooth muscle, and HEK cells has been reported (Blom et al., 2005; Dragusin et al., 2006; Hemmings, 2006). In this study, treatment with external free Ca2+-solution resulted in blockade of S1P-induced tonic inward currents. Treatment with thapsigargin, a Ca2+-uptake inhibitor of ER, also resulted in blockade of S1P-induced tonic inward currents, and S1P increased [Ca2+]i concentration of ICC. These results suggest that S1P-induced effects on ICC are external and internal Ca2+-dependent.
The findings from our study demonstrate the presence of functional S1P receptors in intestinal ICC. Treatment of ICC with S1P resulted in depolarization of the membrane and increased tonic inward currents, which were activated by release of Ca2+ from internal stores induced by activation of S1P2. Together with MAPK involve in S1P-induced effects. Thus, the S1P receptor can modulate intestinal motility through its action on ICC.
Acknowledgments
The present study was supported by grants from the Clinical Medicine Research Institute at Chosun University Hospital (2011).
REFERENCES
- Alemany R., Sichelschmidt B., Zu Heringdorf D.M., Lass H., Van Koppen C.J., Jakobs K.H. Stimulation of sphingosine-1-phosphate formation by the P2Y2 receptor in HL-60 cells: Ca2+ requirement and implication in receptor-mediated Ca2+ mobilization, but not MAP kinase activation. Mol. Pharmacol. 2000;58:491–497. doi: 10.1124/mol.58.3.491. [DOI] [PubMed] [Google Scholar]
- Alvarez S.E., Milstien S., Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrin. Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Anliker B., Chun J. Lysophospholipid G protein-coupled receptors. J. Biol. Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- Bischoff A., Czyborra P., Fetscher C., Meyer Zu, Heringdorf D., Jakobs K.H., Michel M.C. Sphingosine 1 phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom T., Slotte J.P., Pitson S.M., Törnquist K. Enhancement of intracellular sphingosine-1-phosphate production by inositol 1, 4, 5-trisphosphate-evoked calcium mobilisation in HEK-293 cells: endogenous sphingosine-1-phosphate as a modulator of the calcium response. Cell. Signal. 2005;17:827–836. doi: 10.1016/j.cellsig.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Choi S., Yeum C.H., Kim Y.D., Park C.G., Kim M.Y., Park J.S., Jeong H.S., Kim B.J., So I., Kim K.W. Receptor tyrosine and MAP kinase are involved in effects of H2O2 on interstitial cells of Cajal in murine intestine. J. Cell. Mol. Med. 2010;14:257–266. doi: 10.1111/j.1582-4934.2008.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragusin M., Wehner S., Kelly S., Wang E., Merrill A.H., Jr., Kalff J.C., Van Echten-Deckert G. Effects of sphingosine-1-phosphate and ceramide-1-phosphate on rat intestinal smooth muscle cells: implications for postoperative ileus. FASEB J. 2006;20:1930–1932. doi: 10.1096/fj.05-5518fje. [DOI] [PubMed] [Google Scholar]
- Fukata Y., Kaibuchi K., Amano M. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Furness J.B., Hind A.J., Ngui K., Robbins H.L., Clerc N., Merrot T., Tjandra J.J., Poole D.P. The distribution of PKC isoforms in enteric neurons, muscle and interstitial cells of the human intestine. Histochem. Cell Biol. 2006;126:537–548. doi: 10.1007/s00418-006-0190-5. [DOI] [PubMed] [Google Scholar]
- Hemmings D.G. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch. Pharmacol. 2006;373:18–29. doi: 10.1007/s00210-006-0046-5. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 2004:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H.B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huizinga J.D., Zhu Y., Ye J., Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S., So I., Stanfield P.R., Kim K.W. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Mostafa R.M., Moustafa Y.M., Hamdy H. Interstitial cells of Cajal, the Maestro in health and disease. World J. Gastroenterol. 2010;16:3239. doi: 10.3748/wjg.v16.i26.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Kajioka S., Goto K., Takaki M., Liu H.N. Calcium associated mechanisms in gut pacemaker activity. J. Cell. Mol. Med. 2007;11:958–968. doi: 10.1111/j.1582-4934.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodai A., Machida T., Izumi S., Hamaya Y., Kohno T., Igarashi Y., Iizuka K., Minami M., Hirafuji M. Sphingosine 1-phosphate induces cyclooxygenase-2 via Ca2+-dependent, but MAPK-independent mechanism in rat vascular smooth muscle cells. Life Sci. 2007;80:1768–1776. doi: 10.1016/j.lfs.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Powaga N., Akinci S., Schütz W., Banno Y., Wiegand S., Kummer W., Wess J., Haberberger R.V. Activation of the SPHK/S1P signalling pathway is coupled to muscarinic receptor-dependent regulation of peripheral airways. Respir. Res. 2005;6:48. doi: 10.1186/1465-9921-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C., Horowitz B., Bayguinov O., Ward S.M., Sanders K.M. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology. 2002;122:1442–1454. doi: 10.1053/gast.2002.33065. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H.M., Amrani Y., Watterson K.R., Murthy K.S., Panettieri R.A., Jr., Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- Salomone S., Potts E., Tyndall S., Ip P., Chun J., Brinkmann V., Waeber C. Analysis of sphingosine 1 phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br. J. Pharmacol. 2008;153:140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T., Hla T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Sanders K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Song H.J., Choi T.S., Chung F.Y., Park S.Y., Ryu J.S., Woo J.G., Min Y.S., Shin C.Y., Sohn U.D. Sphingosine 1-phosphate-induced signal transduction in cat esophagus smooth muscle cells. Mol Cells. 2006;21:42–51. [PubMed] [Google Scholar]
- Spiegel S., Merrill A., Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Okamoto H., Takuwa N., Gonda K., Sugimoto N., Sakurada S. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol. Cell. Endocrinol. 2001;177:3–11. doi: 10.1016/s0303-7207(01)00441-5. [DOI] [PubMed] [Google Scholar]
- Van Koppen C.J., Meyer zu Heringdorf D., Alemany R., Jakobs K.H. Sphingosine kinase-mediated calcium signaling by muscarinic acetylcholine receptors. Life Sci. 2001;68:2535–2540. doi: 10.1016/s0024-3205(01)01049-9. [DOI] [PubMed] [Google Scholar]
- Ward S.M., Burns A.J., Torihashi S., Sanders K.M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Ordög T., Koh S., Baker S.A., Jun J., Amberg G., Monaghan K., Sanders K.M. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson K.R., Ratz P.H., Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell. Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Young K., Nahorski S. Sphingosine 1-phosphate: a Ca2+ release mediator in the balance. Cell Calcium. 2002;32:335–341. doi: 10.1016/s0143416002001835. [DOI] [PubMed] [Google Scholar]
- Zhou H., Murthy K.S. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am. J. Physiol. Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]