Abstract
Store-operated calcium entry (SOCE) channels composed of Stim and Orai proteins play a critical role in diverse biological processes. Upon endoplasmic reticulum (ER)-mediated calcium (Ca2+) depletion, Stim proteins oligomerize with Orai to initiate Ca2+ influx across the plasma membrane. The ubiquitin-like (UBL) and ubiquitin-associated (UBA) domains of ubiquilin 1 are involved in the degradation of presenilin and polyglutamine proteins. Through screening of Orai1 interaction partner(s) that might have an effect on SOCE, ubiquilin 1 was identified as a target of Orai1. However, the UBL and UBA domains of ubiquilin 1 were dispensable for this interaction. Additionally, ubiquilin 1 and Orai1 colocalized in the cytosolic compartment. Ubiquilin 1 increased the ubiquitination of Orai1, resulting in the formation of a high-molecular-weight form. MG132, a proteasome inhibitor, failed to block the degradation of Orai1, whereas bafilomycin A, a lysosome inhibitor, prevented Orai1 degradation. Confocal microscopy studies demonstrated that a fraction of Orai1 colocalized with ubiquilin 1 and the autophagosomal marker LC3. Because Orai1 is a constituent of SOCE, we determined the effect of ubiquilin 1 on Orai1-mediated Ca2+ influx. As we expected, intracellular Ca2+ mobilization, a process normally potentiated by Orai1, was downregulated by ubiquilin 1. Taken together, these findings suggest that ubiquilin 1 downregulates intracellular Ca2+ mobilization and its downstream signaling by promoting the ubiquitination and lysosomal degradation of Orai1.
Keywords: calcium mobilization, Orai1, Stim1, store-operated calcium entry (SOCE), ubiquilin
INTRODUCTION
Calcium ions trigger many biological processes, including contraction, transcription, and apoptosis. Cells maintain a large differential concentration gradient of Ca2+ between the cytoplasm and surrounding compartments to form a calcium battery that enables rapid increases in cytoplasmic Ca2+ by opening ion channels in the plasma membrane or endoplasmic reticulum (ER) (Krapivinsky et al., 2011). Store-operated calcium entry (SOCE) is a widely used plasma membrane Ca2+ entry mechanism that is controlled by the Ca2+ content of the ER (Varnai et al., 2009).
Activation of phospholipase C by cellular signaling cascades hydrolyzes membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) to soluble inositol triphosphate (IP3) (Clapham, 2007). Binding of IP3 to the IP3 receptor (IP3R), a gated Ca2+ channel localized in the ER membrane, increases cytoplasmic Ca2+ within seconds. Minutes later, the Ca2+-depleted ER activates SOCE. SOCE is mediated by the triggered activity of highly selective Orai1 Ca2+ channels known as Ca2+ release-activated Ca2+ (CRAC) channels. Importantly, the activity of Orai1 channels is triggered by decreases in the ER Ca2+ concentration and not by increases in the cytoplasmic Ca2+ concentration. This feature of Orai channels distinguishes it from Ca2+-activated transient receptor potential channels and potassium (K+) channels (Hogan et al., 2010; Kurosaki and Baba, 2010; Wu et al., 2007). In addition to Ca2+, several proteins have been reported to control the S phase of the cell cycle through direct interactions with Stim1 and Orai in various cells (Jeremy et al., 2012; Kawasaki et al., 2010; Lee et al., 2012; Limnander et al., 2011; Pozo-Guisado et al., 2010; Sathish et al., 2012; Srikanth et al., 2012; Walsh et al., 2010). Interestingly, all these proteins transiently activate SOCE through protein-protein interactions. In contrast to SOCE activation, little is known regarding the mechanisms of SOCE downregulation.
Ubiquilins, also known as UBQLNs or proteins linking IAP to cytoskeleton (PLICs), are reported to play a role in protein degradation through interactions with their N-terminal ubiquitin-like (UBL) and C-terminal ubiquitin-associated (UBA) domains. The UBA and UBL domains physically associate with both proteasomes and ubiquitin ligases and thus are thought to functionally link the ubiquitination machinery to the proteasome to affect protein degradation in vivo (Biswas et al., 2011). Ubiquilin 1 regulates the stability of various proteins, including amyloid precursor protein, Trif, presenilin, and polyalanine-induced protein aggregates (Biswas et al., 2011; El et al., 2012; Viswanathan J, 2011; Wang and Monteiro, 2007). Using yeast two-hybrid screening and immunoprecipitation assays, we initially identified ubiquilin 1 as a binding partner of Stim1 and Orai1. In the present study, we determined whether ubiquilin 1 regulates the stability of Orai1 protein and/or activity of SOCE.
MATERIALS AND METHODS
Plasmids
Human ubiquilin 1 and Orai1 cDNAs were amplified by PCR using gene-specific primers and cloned into the CMV promoter-derived mammalian expression vectors pCS4-3Flag and pCS4-3HA, respectively. Ubiquilin 1 UBL deletion (Ubqln1 ΔUBL) and UBA deletion (Ubqln1 ΔUBA) constructs were amplified by PCR using specific primers for each domain before cloning into the pCS4-3Flag vector. Orai1 was subcloned into the pCS4-3myc vector. For confocal microscopy experiments, ubiquilin 1 and Orai1 were amplified by PCR using gene-specific primers and cloned into Cerulean-C1 and pmCherry-N1 vectors, respectively. The DNA sequences of the resultant constructs were verified.
Cell culture and generation of an Orai1 stable cell line
HEK 293 and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (P/S) antibiotics. Cells were serum-starved prior to transfection. After 12 h, the growth medium was discarded, the cells were washed once with 1× phosphate-buffered saline (PBS), and then the cells were replenished with serum-free DMEM containing P/S antibiotics for the indicated time points. A stable Orai1-expressing cell line was established by transfecting HeLa cells with pCDNA 3.1 Hygro containing full-length Orai1 and selecting for hygromycin B-resistant cells.
Reagents
The following primary antibodies were used: c-Myc (9E10; Santa Cruz Biotechnology), HA epitope (12CA5; Roche Applied Science), FLAG (M2; Sigma), and actin (SC-1616; Santa Cruz Biotechnology), A secondary antibody against the HA epitope (goat anti-mouse Alexa Fluor 633; Invitrogen) was used. Cells were cultured in the presence of hygromycin B (A.G. Scientific) at a final working concentration of 300 μg/ml for 10 days to select for antibiotic-resistant cells. Stably transfected cells were maintained in 100 μg/ml hygromycin B.
Transfection, Western blotting, and immunoprecipitation
HEK 293 cells were transfected as described previously (Kim et al., 2008). Briefly, HEK 293 cells were seeded into 6-well plates the day before transfection. Transfection was performed using polyethylenimine (Sigma). The details of the protocols used for Western blotting and immunoprecipitation are described else-where (Lee et al., 2011).
Confocal microscopy
Transfected cells (5 × 103/well) were washed with 1× PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 10 min, washed three times with PBS, and blocked with 10% FBS. To detect expression of Orai1 and ubiquilin 1, cells were stained with anti-FLAG and anti-c-Myc primary antibodies, respectively, for 60 min, washed with PBS, incubated for 30 min with the Goat anti-mouse Alexa fluor 633 rhodamine-conjugated secondary antibody, mounted, and analyzed using a laser scanning confocal microscope (Zeiss LSM710).
Proteasome and lysosome inhibitors
Stock solutions (1000×) of MG132 (Sigma), a proteasome inhibitor, and bafilomycin A1 (Sigma), a lysosome inhibitor, were prepared in DMSO. Stock solutions were diluted in DMEM supplemented with 10% FBS to achieve final concentrations of MG132 and bafilomycin A1 at 10 μM and 200 nM, respectively. DMSO was added to control wells as a vehicle control. Cells were incubated with MG132 and bafilomycin A1 for 4 and 9 h, respectively. Cells were then lysed, and Western blotting using ubiquilin 1 and Orai1 antibodies was performed as described above.
Ca2+ mobilization assay
The Orai1 stable cell line was transiently transfected with either ubiquilin 1 (cerulean-C1) alone, Stim1 (pmCherry-N1) alone, or both proteins. After 12 h, cells were incubated with 1 μM Fluo-4 (Invitrogen), a Ca2+ indicator fluorescent dye, for 30 min, washed once with Krebs-Ringer-HEPES buffer (125 mM NaCl, 2.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 5.5 mM glucose, 10 mM HEPES), and replenished with Krebs-Ringer-HEPES high K+-Ca2+ buffer (40.6 mM NaCl, 87 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 5.5 mM glucose, 10 mM HEPES, 1.5 mM CaCl2). EGTA (1.25 mM), thapsigargin (TG; 1 μM), and CaCl2 (2 mM) were then added sequentially into the media, and fluctuations in intracellular Ca2+ mobilization were imaged using a laser scanning confocal microscope (Zeiss LSM710).
RESULTS
Interaction between ubiquilin 1 and Orai1
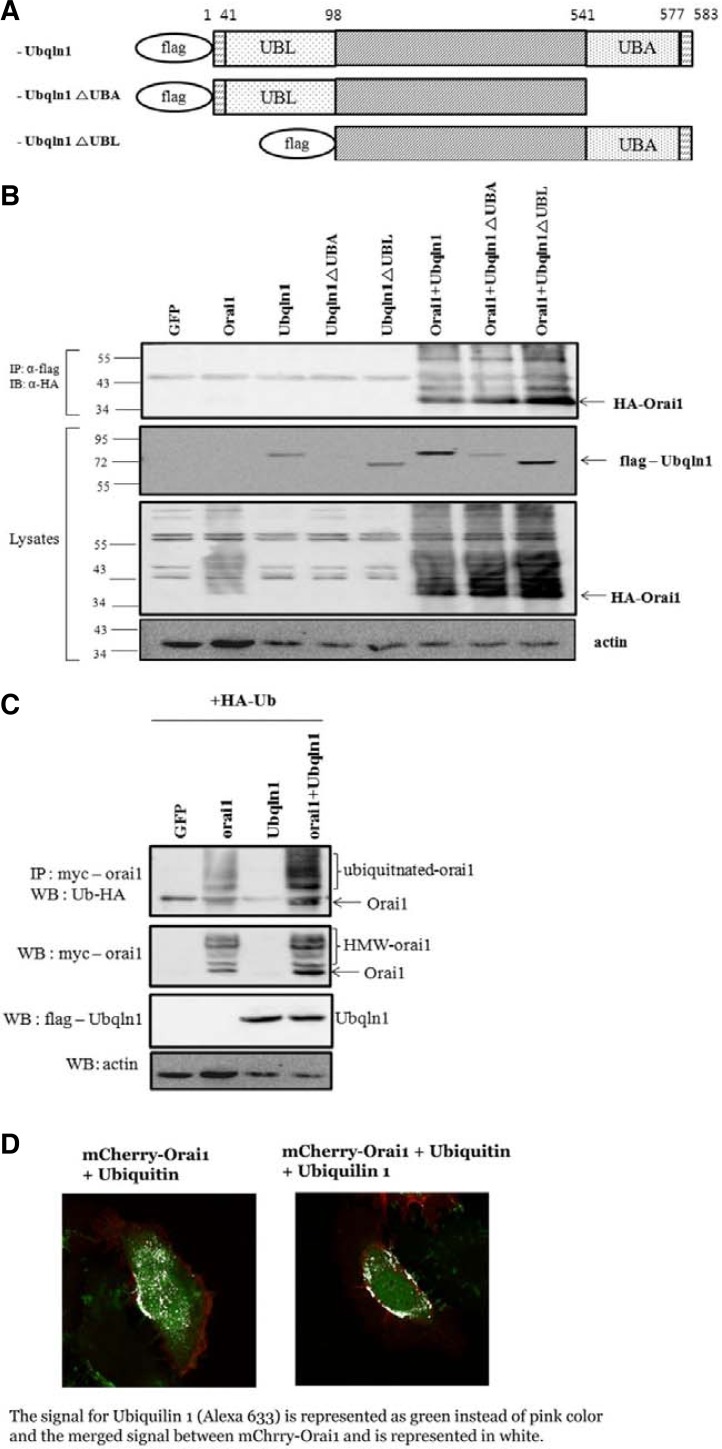
We previously identified ubiquilin 1 as a binding partner of Stim1 through yeast two-hybrid screening. However, subsequent biochemical studies have revealed that Orai1, which together with Stim1 constitutes the SOCE channel, possesses a greater affinity for ubiquilin 1 than Stim1. Therefore, we determined the biological significance of the ubiquilin and Orai1 relationship. To accomplish this, wild-type and mutant forms of the ubiquilin 1 UBA and UBL domains were constructed as shown in Fig. 1A. Contrary to our expectations, no difference in binding was observed between either mutant ubiquilin or wild-type ubiquilin. Interestingly, coexpression of wild-type and mutant forms of ubiquilin 1 increased Orai1 stability (Fig. 1B). When expressed alone, Orai1, like many other multi-transmembrane proteins, migrated as ladders and smeared upon Western blot analysis; however, the additional expression of ubiquilin 1 increased the overall signal intensity of Orai1. The ubiquitin ligase Nedd4-2 was shown to inhibit SOCE activity by targeting Orai1 (Eylenstein et al., 2011). However, it is unknown whether Nedd4-2 ubiquitinates Orai1 or if it is an Orai1 binding partner. In the present study, expression of Orai1 alone in the presence of ubiquitin induced ubiquitination of Orai1 in HEK 293 cells. Importantly, coexpression of Orai1 and ubiquilin 1 markedly enhanced Orai1 ubiquitination, whereas ubiquilin expression was unaffected (Fig. 1C). Based on previous reports of an association between ubiquilin 1 and ubiquitin, we extended our investigation to determine whether ubiquilin 1 regulates Orai1 ubiquitination. To this end, HeLa cells were transfected with Orai1 (pmCherry-N1), ubiquitin (HA tag), and ubiquilin 1 (Myc tag), and the colocalization of these proteins was analyzed. The presence of ubiquilin 1 (Fig. 2B, right) increased the colocalization (shown as a white signal) of Orai1 and ubiquitin compared with the absence of ubiquilin (Fig. 2B, left).
Fig. 1.
Ubiquilin 1 interacts with Orai1. (A) Full-length ubiquilin 1 and deletion mutants of the UBL and UBA domains of ubiquilin 1 (FLAG N-terminal tag) were expressed with Orai1 (HA N-terminal tag) in HEK 293 cells. (B) To determine the effect of ubiquilin on the stability of Orai1, transfected cells were subjected to immunoprecipitation using anti-FLAG antibody (for ubiquilin) and Western blotting using anti-HA antibody (for ubiquitin). Ubiquitination of Orai1 was determined by transfecting HEK 293 cells with Orai1 (Myc tag), ubiquilin 1 (Flag tag), and ubiquitin (HA tag). Cell lysates were obtained and subjected to immunoprecipitation with anti-c-Myc antibody (for Orai1) and Western blotting with anti-HA antibody. (C) To identify the nature of the high-molecular-weight form of Orai1 shown in Fig. 1B, Orai1 and/or ubiquilin 1 were expressed with ubiquitin. Cell lysates were obtained and subjected to immunoprecipitation with anti-c-Myc antibody and Western blotting with anti-HA antibody. (D) To determine the level of Orai1 ubiquitination, ubiquitin alone or in combination with ubiquilin 1 were transiently expressed in the Orai1 stable HeLa cell line, and confocal imaging was performed using anti-HA primary and anti-HA Alexa Fluor 633 goat anti-mouse secondary antibodies.
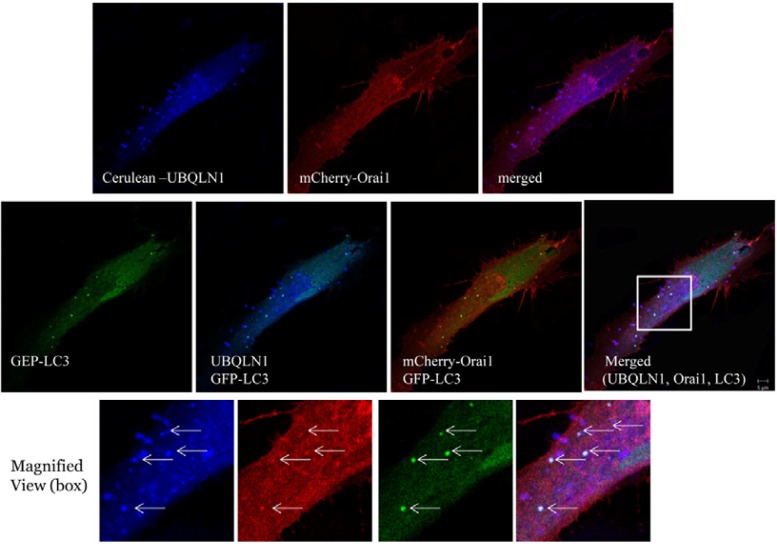
Fig. 2.
Ubiquilin1 colocalizes with Orai1 and LC3. The colocalization of Orai1, ubiquilin 1, and LC3 was determined in HEK 293 cells expressing Orai1 (mCherry tagged), ubiquilin 1 (Cerulean tagged), and LC3 (GFP tagged) using confocal imaging. Colocalization of ubiquilin 1 and Orai1 is shown in the top row, and colocalization of ubiquilin 1, Orai1, and LC3 is shown in the middle row. The bottom row indicates a 2.5-fold magnified image of the boxed image in the middle row.
Colocalization of Orai1 and ubiquilin 1 in autophagosomes
Ubiquilin 1 is localized in the cytoplasm and nucleus in both punctate and diffuse patterns, whereas Orai1 is predominantly localized in the plasma membrane in a punctate pattern (Ficklin et al., 2005). We determined the colocalization patterns of ubiquilin and Orai1 in HEK 293 cells using confocal microscopy. Ubiquilin 1 and Orai1 were colocalized in the plasma membrane and cytoplasm in punctate patterns, yielding an approximate colocalization level of 90% (data not shown). For subsequent confocal microscopy experiments, ubiquilin 1 and Orai1 were expressed in HeLa cells as chimeric proteins with N-terminal Cerulean and mCherry tags, respectively. Ubiquilin 1 localized with Orai1 predominantly in a punctate pattern in the cytosol (Fig. 2). LC3, an autophagosomal marker, partially colocalized with ubiquilin 1 and Orai1 in the cytosol.
Lysosome-mediated degradation of Orai1 by ubiquilin 1
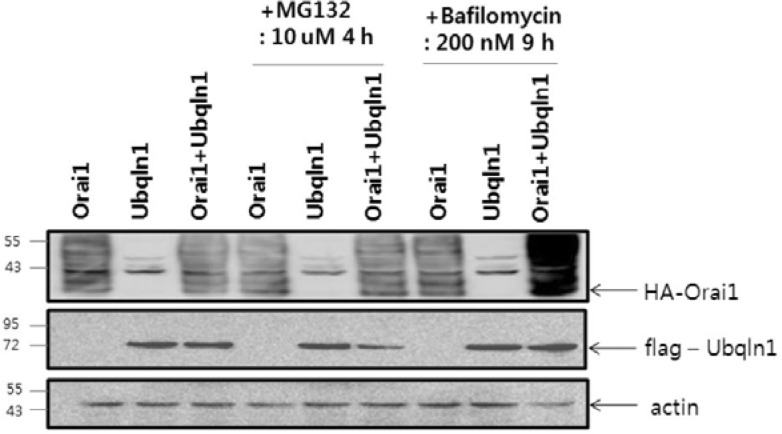
To determine the effect of ubiquilin on Orai1 degradation, cells in which Orai1 was expressed alone or with ubiquilin 1 were treated with the proteasome inhibitor MG132 or the lysosome inhibitor bafilomycin A. MG132 failed to block Orai1 degradation, whereas bafilomycin A prevented Orai1 degradation. The expression of ubiquilin 1 was unaffected by the chemical inhibitors. These data suggest that ubiquilin 1 promotes lysosomal degradation of Orai1 in the autophagosome (Fig. 3).
Fig. 3.
Lysosomal degradation of Orai1 is mediated by ubiquilin 1. Orai1 and ubiquilin 1 were coexpressed in HEK 293 cells and treated with either the proteasome inhibitor MG132 (10 μM) for 4 h or the lysosome inhibitor bafilomycin A (200 nM) for 9 h. Cell lysates were obtained, and Western blotting was performed using Orai1 and ubiquilin 1 antibodies. Actin was used to ensure equal loading of the protein samples. The results were confirmed twice in subsequent experiments.
Downregulation of Orai1-mediated Ca2+ mobilization and SOCE activity by ubiquilin 1
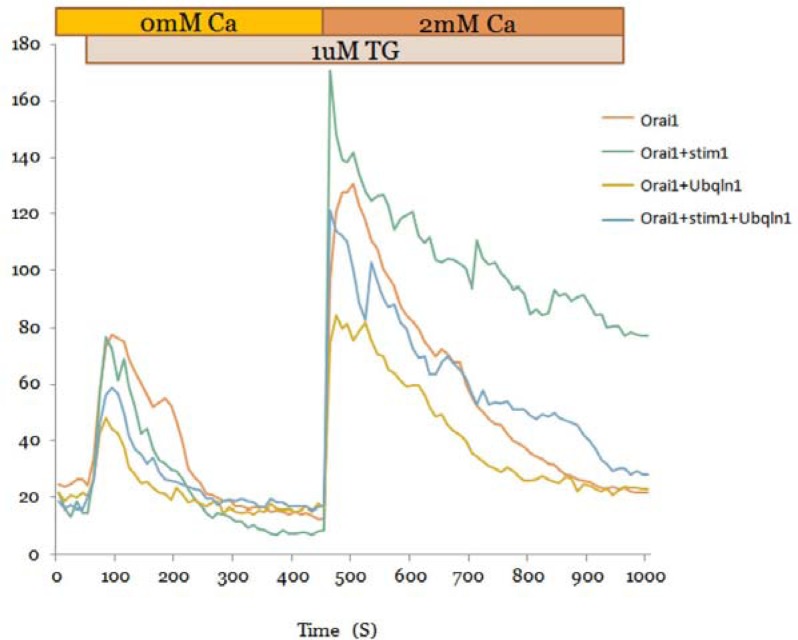
Because Orai1 is intimately linked to SOCE activity, we determined the effect of ubiquilin 1 on Ca2+ homeostasis. To accomplish this, HeLa cells expressing Orai1, Orai1/Stim1, Orai1/ubiquilin1, and Orai1/Stim1/ubiquilin1 were stimulated with TG to induce ER Ca2+ depletion and activation of Orai1-mediated SOCE. Cells expressing Orai1/Stim1 demonstrated the strongest response to TG treatment, followed by Orai1, Orai1/Stim1/ubiquilin 1, and Orai1/ubiquilin 1. These results suggest that ubiquilin 1 downregulates Orai1-mediated Ca2+ mobilization and SOCE activity (Fig. 4).
Fig. 4.
Ubiquilin 1 downregulates Orai1-mediated Ca2+ mobilization. A HeLa cell line stably expressing Orai1 was established and transiently transfected with Stim1 (mCherry) alone, ubiquilin 1 (Cerulean) alone, or both Stim1 and ubiquilin 1. Ca2+ mobilization was determined on average in five cells of each group. Data shown are representative of three independent experiments.
DISCUSSION
Orai1, a multi-transmembrane protein, functions as a Ca2+ channel in the plasma membrane to maintain intracellular Ca2+ homeostasis. Ca2+ homeostasis is a complex and dynamic process that involves Ca2+ entry across the cell membrane, buffering within the cytoplasm, removal by active transport systems, and storage and release from intracellular compartments (Augustine et al., 2003; Berridge et al., 2000; Schubert et al., 2006). Improper function of Orai1 is associated with dysregulation of SOCE, intracellular Ca2+ homeostasis and sensitivity to cell death (Flourakis et al., 2010).
Ubiquilin 1 is well known to function not only in neurodegenerative diseases, including Alzheimer’s disease, which involves abnormal protein aggregation [β-amyloid (Aβ) peptides derived from presenilin], but also in neurotransmission (nicotinic acetylcholine receptors), ER stress (protein-disulfide isomerase), and the immune response (Trif) (Biswas et al., 2011). In the present study, we found that ubiquilin 1 binds to Orai1. Surprisingly, the UBL and UBA domains of ubiquilin 1 were dispensable for the Orai1 and ubiquilin 1 interaction. Additionally, further study did not reveal an essential domain of ubiquilin 1 that was involved in this interaction (data not shown). Together, these findings suggest that Orai1 may interact with multiple regions of ubiquilin 1. Coexpression of ubiquilin 1 and Orai1 increased Orai1 expression but not that of ubiquilin 1. Ubiquilin 1 increased both the monomeric and high-molecular-weight (ubiquitinated) forms of Orai1.
Confocal analyses have indicated that most Orai1 and ubiquilin 1 colocalize predominantly in the cytosol. Previous studies have demonstrated that Orai1 and ubiquilin 1 colocalize as aggregates or in oligomeric forms (Rothenberg et al., 2010; Zhou et al., 2010). However, in the present study, Orai1 and ubiquilin 1 colocalized as cytosolic punctate forms. Ubiquilin 1 has recently been reported to function in autophagy (Rothenberg et al., 2010). We found that ubiquilin 1, Orai1, and the autophagosome protein LC3 were colocalized in the cytosol. Thus, it is possible that Orai1 may be targeted to the autophagosome by ubiquilin 1 for degradation. It is unclear whether this mode of Orai1 clearance is triggered by ER stress mediated by the autophagosome or is a means to avoid cell death induced by overactive Orai1-mediated SOCE activity.
Although Orai1 colocalized with the autophagosomal marker LC3, the predominant pathway of ubiquilin-mediated Orai1 degradation was not conclusive in the present study (the proteasome inhibitor MG132 and the lysosome inhibitor bafilomycin A were used to address this question). Our data suggest that the lysosomal pathway was the predominant pathway of ubiquilin-mediated Orai1 degradation. However, we could not exclude the potential involvement of the proteasome pathway in ubiquilin-mediated Orai1 degradation. Because ubiquilin 1 can be targeted to lysosomes through the autophagosome and chaperone-mediated pathways (Rothenberg et al., 2010) and contains a motif (KTDRQ) similar to the consensus lysosomal targeting sequence (KFERQ)], further investigation of Orai1 degradation pathways and the role of ubiquilin 1 in these pathways is warranted.
SOCE and its components Orai1 and Stim1 regulate Ca2+ influx upon ER Ca2+ depletion (Liou et al., 2005; Zhang et al., 2005). Along this line, we sought to study the effect of ubiquilin 1 on the SOCE process. Transient transfection of ubiquilin1 and/or Stim1 in the Orai1 stable cell line and SOCE assay using Fluo-4 revealed that ubiquilin 1 downregulated the SOCE activity of Stim1 and Orai1. Recently, intracellular Ca2+ concentrations have been shown to regulate autophagy by affecting various components, including Vps34 (Decuypere et al., 2011). Thus, the relationship between Ca2+ influx and ubiquilin 1 activity is an important area of investigation.
Our study demonstrated that ubiquilin 1 interacts with Orai1 independent of its UBL and UBA domains and colocalizes with Orai1 and LC3 in a punctate pattern in the cytosol. Furthermore, ubiquilin 1 increases lysosomal degradation of Orai1 and inhibited Orai1-mediated SOCE activity.
Acknowledgments
This work was by a grant from the Next-Generation BioGreen 21 Program to JK Choi (No. PJ008127) and to HK Lee (No. PJ008196), Rural Development Administration, Republic of Korea, and was also supported by a grant of the National Research Foundation of Korea (2012-0005747) to Eung-Gook Kim.
REFERENCES
- Augustine G.J., Santamaria F., Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. Signal transduction. The calcium entry pas de deux. Science. 2000;287:1604–1605. doi: 10.1126/science.287.5458.1604. [DOI] [PubMed] [Google Scholar]
- Biswas N., Liu S., Ronni T., Aussenberg S.E., Liu W., Fujita T., Wang T. The ubiquitin-like protein PLIC-1 or ubiquilin 1 inhibits TLR3-Trif signaling. PLoS One. 2011;6:e21153. doi: 10.1371/journal.pone.0021153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Decuypere J.P., Bultynck G., Parys J.B. A dual role for Ca2+ in autophagy regulation. Cell Calcium. 2011;50:242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- El A.A., Stieren E.S., Barral J.M., Boehning D. Ubiquilin-1 regulates amyloid precursor protein maturation and degradation by stimulating K63-linked polyubiquitination of lysine 688. Proc. Natl. Acad. Sci USA. 2012;109:13416–13421. doi: 10.1073/pnas.1206786109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylenstein A., Gehring E.M., Heise N., Shumilina E., Schmidt S., Szteyn K., Munzer P., Nurbaeva M.K., Eichenmuller M., Tyan L., et al. Stimulation of Ca2+-channel Orai1/STIM1 by serum-and glucocorticoid-inducible kinase 1 (SGK1) FASEB J. 2011;25:2012–2021. doi: 10.1096/fj.10-178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficklin M.B., Zhao S.l., Feng G.P. Ubiquilin-1 regulates nicotine-induced up-regulation of neuronal nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:34088–34095. doi: 10.1074/jbc.M506781200. [DOI] [PubMed] [Google Scholar]
- Flourakis M., Lehen’kyi V., Beck B., Raphael M., Vandenberghe M., Abeele F.V., Roudbaraki M., Lepage G., Mauroy B., Romanin C., et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan P.G., Lewis R.S., Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy T.S., Amber M.B, Shilan W., James W.P., Nasser M.R. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr. Biol. 2012;22:1487–1493. doi: 10.1016/j.cub.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ueyama T., Lange I., Feske S., Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J. Biol. Chem. 2010;285:25720–25730. doi: 10.1074/jbc.M109.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.R., Oh B.C., Choi J.K., Bae S.C. Pim-1 kinase phosphorylates and stabilizes RUNX3 and alters its subcellular localization. J. Cell Biochem. 2008;105:1048–1058. doi: 10.1002/jcb.21906. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Krapivinsky L., Stotz S.C., Manasian Y., Clapham D.E. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc. Natl. Acad. Sci USA. 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Baba Y. Ca2+ signaling and STIM1. Prog. Biophys. Mol. Biol. 2010;103:51–58. doi: 10.1016/j.pbiomolbio.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Lee J.W., Nam H.J., Shim J.H., Song Y.S., Kang K.W. PI3-kinase/p38 kinase-dependent E2F1 activation is critical for Pin1 induction in Tamoxifen-resistant breast cancer cells. Mol Cells. 2011;32:107–111. doi: 10.1007/s10059-011-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Bae G.U., Leem Y.E., Choi H.K., Kang T.M., Cho H., Kim S.T., Kang J.S. Phosphorylation of Stim1 at serine 575 via netrin-2/Cdo-activated ERK1/2 is critical for the promyogenic function of Stim1. Mol. Biol Cell. 2012;23:1376–1387. doi: 10.1091/mbc.E11-07-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A., Depeille P., Freedman T.S., Liou J., Leitges M., Kurosaki T., Roose J.P., Weiss A. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat. Immunol. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J., Kim M.L., Heo W.D., Jones J.T., Myers J.W., Ferrell J.E., Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo G.E., Campbell D.G., Deak M., varez-Barrientos A., Morrice N.A., Alvarez I.S., Alessi D.R., Martin-Romero F.J. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J. Cell Sci. 2010;123:3084–3093. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- Rothenberg C., Srinivasan D., Mah L., Kaushik S., Peterhoff C. M., Ugolino J., Fang S., Maria C.A., Nixon R.A., Monteiro M.J. Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy. Hum. Mol. Genet. 2010;19:3219–3232. doi: 10.1093/hmg/ddq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V., Abcejo A.J., Thompson M.A., Sieck G.C., Prakash Y.S., Pabelick C.M. Caveolin-1 regulation of store-operated Ca2+ influx in human airway smooth muscle. Eur. Respir. J. 2012;40:470–478. doi: 10.1183/09031936.00090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T., Weiler R., Feigenspan A. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J. Neurophysiol. 2006;96:1278–1292. doi: 10.1152/jn.00191.2006. [DOI] [PubMed] [Google Scholar]
- Srikanth S., Jew M., Kim K.D., Yee M.K., Abramson J., Gwack Y. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1) Proc. Natl. Acad. Sci USA. 2012;109:8682–8687. doi: 10.1073/pnas.1200667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P., Hunyady L., Balla T. STIM and Orai: the long-awaited constituents of store-operated calcium entry. Trends Pharmacol. Sci. 2009;30:118–128. doi: 10.1016/j.tips.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan J., Haapasalo A., Bottcher C., Miettinen R., Kurkinen K.M., Lu A., Thomas A., Maynard C.J., Romano D., Hyman B.T., et al. Alzheimer's disease-associated ubiquilin-1 regulates presenilin-1 accumulation and aggresome formation. Traffic. 2011;12:330–348. doi: 10.1111/j.1600-0854.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Doherty M.K., Tepikin A.V., Burgoyne R.D. Evidence for an interaction between Golli and STIM1 in store-operated calcium entry. Biochem. J. 2010;430:453–460. doi: 10.1042/BJ20100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Monteiro M.J. Ubiquilin overexpression reduces GFP-polyalaninez-induced protein aggregates and toxicity. Exp. Cell Res. 2007;313:2810–2820. doi: 10.1016/j.yexcr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.M., Luik R.M., Lewis R.S. Some assembly required: constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–172. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.L., Yu Y., Roos J., Kozak J.A., Deerinck T.J., Ellisman M.H., Stauderman K.A., Cahalan M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.B., Ramachandran S., Oh-Hora M., Rao A., Hogan P.G. Pore architecture of the ORAI1 store-operated calcium channel. Proc. Natl. Acad. Sci USA. 2010;107:4896–4901. doi: 10.1073/pnas.1001169107. [DOI] [PMC free article] [PubMed] [Google Scholar]