Abstract
Plants possess a variety of extracellular leucine-rich repeats receptor-like kinases (LRR-RLKs) to coordinate developmental programs with responses to environmental changes. Out of sixteen families of LRR-RLKs in Arabidopsis, the LRR-RLKII family consists of fourteen individual members, including five Arabidopsis thaliana somatic embryogenesis receptor kinases (AtSERKs). BAK1/AtSERK3 was first identified as a dual co-receptor of BRI1 and FLS2, mediating BR signaling and pathogen-associated molecular pattern (PAMP) triggered immunity (PTI), respectively. Since its identification, many researchers have attempted to elucidate the phosphorylation mechanisms between receptor complexes and identify additional components that interact with receptor complexes to transduce the signaling downstream. Relatively detailed early events in complex formation, phosphorylation sites on the BRI1/BAK1 complex and BAK1-interacting proteins, such as BIK1 and PUB13, have been identified. Small receptor complexes consisting of BAK1 and BIR1 or BAK1 and AtSERK4 regulate cell death during steady state conditions. Moreover, the redundant and distinct functions of AtSERK proteins and other members of the LRR-RLKII family have been revealed. This review focuses on the integration of the information from the most recent studies concerning BAK1 and its homologs.
Keywords: AtSERK, BAK1, BAK1-interacting proteins, BR signaling, cell death, plant immunity
INTRODUCTION
The genes encoding the receptor-like kinases (RLKs) in eukaryotic genomes are greatly expanded in plants. No RLK genes have been found in fungal genomes (Shiu and Bleecker, 2003). The fruit fly (Drosophila melanogaster) contains only one RLK gene, Pelle kinase, which is involved in dorsal-ventral axis determination (Shelton and Wasserman, 1993), and the human genome contains four RLK genes encoding interleukin-1 receptor-associated kinase that functions in immune responses to various pathogens (Janssens and Beyaert, 2003). More than 600 RLK genes encoding receptor-like cytoplasmic kinases without extracellular domains (RLCKs) and receptor-like kinases with extracellular domains (ECD-RLKs) have been identified in the Arabidopsis genome (Shiu and Bleecker, 2001a). More than two-thirds of the RLKs are ECD-RLKs comprising an extracellular domain, a single pass of transmembrane domain and an intracellular cytoplasmic kinase domain. Time-dependent developmental signals and environmental cues are recognized by the ECD and trigger signal transduction mechanisms within the cellular compartments (Shiu and Bleecker, 2001b). Being sessile, plants coordinate developmental programs with responses to external environmental changes in the environment. This need has resulted in exquisite expansion of the genes encoding ECD-RLKs in which the ECDs are variable and the other domains are relatively similar with a minimum cost. Extracellular leucine-rich repeats receptor-like kinases (LRR-RLKs) comprise the largest protein family within the ECD-RLKs. Sixteen families of LRR-RLKs have been reported, which have distinctive protein configurations and contain 4 to 28 LRR domains (Lehti-Shiu et al., 2009).
The functions of several individual LRR-RLKs have been published in the last decade using extensive developmental and biochemical approaches. Twenty-five LRR-containing Brassinosteroid-Insensitive1 (BRI1) protein has been identified, which initiate brassinosteroid (BR) signaling with BRI1-associated kinase1 (BAK1) (Li and Chory, 1997; Li et al., 2002; Nam and Li, 2002), affecting various developmental processes, such as cell elongation and division, vascular development, pollen tube elongation and skotomorphogenesis (Bishop, 2007; Mandava, 1988). In the development of meristematic tissues, CLAVATA1 (CLV1) and ERECTA (ER) maintain the balance between the proliferation and differentiation of the cells at the primordial boundary (Clark et al., 1997; Torri et al., 1996). HAESA is required for the abscission of leaves (Jinn et al., 2000) and EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS (EMS1/EXS) is responsible for anther development (Canales et al., 2002; Zhao et al., 2002). ER also regulates the maintenance of the proper number of the guard cells in a unit area on the leaf (Shpak et al., 2005). Moreover, LRR-RLKs, such as FLAGELLIN SENSITIVE2 (FLS2) and EF-Tu Receptor (EFR), have also been implicated in various responses to biotic stresses for the induction of innate defense programs in Arabidopsis (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006).
Since its identification as a co-receptor of BRI1 that mediates BR signaling (Li et al., 2002; Nam and Li, 2002), BAK1 has been one of the most extensively studied LRR-RLK proteins in Arabidopsis. In addition, the identification of an FLS2/BAK1 complex (Chinchilla et al., 2007) has presented a new paradigm to explain the mechanisms underlying innate immunity and the associated functions of BAK1 as a versatile co-adaptor. Many research studies and reviews have characterized the functional mechanisms involving BAK1 for responses to different pathogenic strains to regulate various developmental aspects (Chinchilla et al., 2009; Clouse, 2011; Kim and Wang, 2010; Postel et al., 2010; Ye et al., 2011). Therefore, this review will focus on the integration of the information from the most recent studies concerning BAK1 and explore various questions for future research studies. Indeed, research focusing on the physiological and developmental functions of each individual LRR-RLK will be accelerated in next decades, resulting in a comprehensive characterization of the interactions and communication between different LRR-RLKs to decipher the regulatory mechanisms that regulate plant life.
Identification of BAK1 in BR signaling
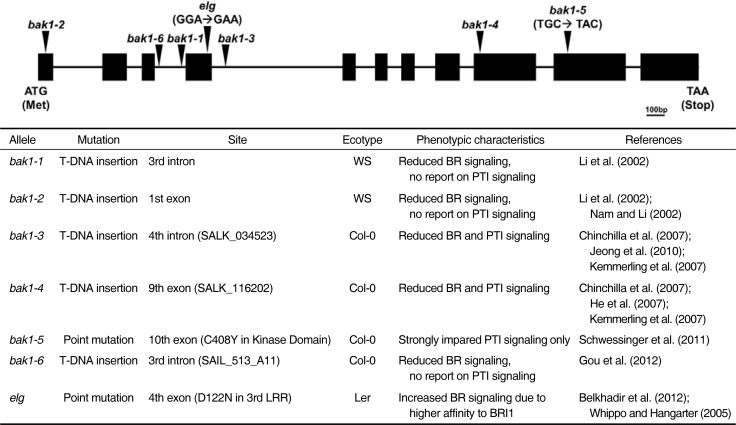
Typical BR biosynthetic mutants, de-etiolated 2 (det2), constitutive photomorphogenesis and dwarfism (cpd), and dwarf4 (dwf4), exhibit dwarfed statures and epinastically curled dark green leaves that are rescued through the exogenous feeding of brassinolide (BL), which is the most bioactive BR (Choe et al., 2000; Li et al., 1996; Szekeres et al., 1996). In contrast, BR insensitive mutant bri1 did not respond to BL, although this mutant exhibited phenotypic alterations similar to the BR biosynthetic mutants and accumulated more BR in the cells (Noguchi et al., 1999). These results indicate that adequate BR signaling and the maintenance of appropriate BR levels are critical for normal development in plants. BRI1 was first identified as a plasma membrane-localized LRR-RLK (Li and Chory, 1997), and thus many studies have attempted to identify novel components that interact with BRI1 directly or are associated with the BR signal initiated through BRI1. BAK1 was identified in two independent studies using different approaches. One study employed the yeast two-hybrid screening of Arabidopsis expression libraries using BRI1 kinase domain as bait to identify direct interacting proteins (Nam and Li, 2002). The overexpression of BAK1 in bri1 partially rescued bri1 mutant phenotypes. The other study implemented the genetic suppressor screening of bri1-5, one of the weak alleles of bri1 (Li et al., 2002). The expression of BAK1 and BRI1 has been localized to the same region in plant tissues. Mutants lacking BAK1 (bak1-1 and bak1-2) exhibited compact rosette phenotypes and reduced sensitivity to BL. So far, seven different mutant alleles of BAK1 have been identified and used in various research studies (Fig. 1).
Fig. 1.
Seven different mutant alleles of BAK1 have been identified. The T-DNA insertional or missense mutation sites are marked in the schematic of the genomic structure of BAK1. Ecotype and phenotypic characteristics of each mutant allele are described.
Initiation of BR signaling on the plasma membrane through the BRI1/BAK1 complex
The early events in BR signaling on the plasma membrane typically involve phosphorylation-dephosphorylation cascades. LRR-RLKs in plants exhibit serine/threonine kinase activity. However, several reports have clearly demonstrated that BRI1 displays phosphorylation activities on both serine/threonine and tyrosine (Jaillais et al., 2011b; Karlova et al., 2009; Oh et al., 2009). In the absence of BL, BRI1 exists as a homodimer on the plasma membrane (Wang et al., 2005a). BKI1 binds to the C-terminus of BRI1 in the cytoplasm, inhibiting both the activation of BRI1 kinase activity and the association of BAK1 with BRI1 (Wang and Chory, 2006). Once the BR binds to the extracellular domain of BRI1, subsequent conformational changes in BRI1 activate its kinase activity (Kinoshita et al., 2005), leading to the phosphorylation of the tyrosine residue (Y211) of BKI1 (Jaillais et al., 2011b). Phosphorylated BKI1 is released from BRI1 and BRI1 subsequently recruit BAK1 to form a heterotetrameric complex. BL-induced BRI1/BAK1 association requires the functional kinase activity of BRI1, because kinase-inactive BRI1 (K911E) abolished binding with wild type BAK1, while kinase-inactive BAK1 (K317E) could still bind to wild type BRI1 (Wang et al., 2008). The extracellular domains of BRI1 and BAK1 also affect the stable association of the two proteins. The substitution of glutamic acid for leucine (L32E, L46E) in the leucine zipper domain of BAK1 resulted in the reduced binding of BAK1 to BRI1 (Yun et al., 2009). In contrast, the substitution of asparagine for aspartic acid (D122N) in the third LRR of elg mutation resulted in a gain-of-function mutation in BAK1 and the mutant BAK1 exhibited higher affinity for BRI1, even in the presence of low concentrations of BR (Jaillais et al., 2011a; Whippo and Hangarter, 2005). We also observed bri1-113 mutation, in which glycine at position 611 of the 70-amino acid island is changed to glutamic acid (G611E), disrupted the binding of BRI1 with BAK1 (unpublished data). Although BRI1/BAK1 complex has been demonstrated genetically and biochemically in vivo and in vitro using various methods, the crystal structure of this complex has not been solved. Recently, the crystal structure of the extracellular domain of BRI1 has been reported (Hothorn et al., 2011; She et al., 2011). Unlike the horseshoe structure of the LRR of Toll-like receptors (TLRs) in humans, the twenty-five LRR repeats in BRI1 form right-handed super helix structure with seven disulfide bonds at hinge regions. As expected, LRR 21 and LRR 22–25 flank the seventy amino acid island providing a binding pocket for ligands, such as BL or castasterone (CS). Once a ligand binds to that pocket, the BRI1 structure becomes stabilized and subsequently serves as a platform for the binding of co-receptors. These analyses have also shown that the extracellular domain of BRI1 does not exist as a dimer. This indicates that ligand-induced homodimerization or the pre-existing homodimer of BRI1 cannot be realized solely through the extracellular domain of BRI1. The cytoplasmic kinase domain of BAK1, which is fully phosphorylated with ATP analog, has been crystallized (Gao et al., 2012). Although this study additionally confirmed that the threonine residues in the activation loop (T450 and T455) are critical to the functions of BAK1, additional crystal structures of the extracellular domain of BAK1 and/or the extracellular domain complex between BRI1 and BAK1 in the absence or presence of BL will be useful in the future.
BAK1 affects defense resistance through the formation of complexes with various PRRs
In addition to the reduction of BL sensitivity, bak1 mutants (bak1-3 and bak1-4) showed less reduced growth in response to flg22, the critical 22-peptides of flagellin, a PAMP. ROS production in bak1 mutants after flg22 or elf26 treatment was diminished compared with that in the wild type (Chinchilla et al., 2007). The reduction of ROS production was also observed in BAK1-silenced N. benthamiana treated with the CSP22 peptide derived from the bacterial cold-shock protein or INF1, an oomycete elicitor (Heese et al., 2007). These results indicated that BAK1 might be involved in the initial events of PTI in response to various PAMPs in plants.
In addition to the interaction with BRI1, BAK1 also interacts with FLS2 and EFR in Arabidopsis (Chinchilla et al., 2007; Schulze et al., 2010; Schwessinger et al., 2011). FLS2 and EFR are typical pattern recognition receptors (PRR) that directly bind to their ligands, flg22 and elf18 or elf26, respectively, to induce innate immunity in plants (Nekrasov et al., 2009; Postel et al., 2010; Zipfel et al., 2004). The results of kinetic analyses have revealed that the heterodimerization of FLS2 and BAK1 occurs within 15 seconds following flg22 treatment in Arabidopsis culture cells using anti-FLS2 and anti-BAK1 antibodies (Schulze et al., 2010). The formation of the EFR/BAK1 complex occurred within 5 min after stimulation with elf18 (Schwessinger et al., 2011). Therefore, the ligand-induced heterodimerization of FSL2 or EFR and BAK1 is the earliest event for the induction of ROS production and the activation of the MAPK cascade in response to PAMPs/MAMPs. In contrast to the BRI1/BAK1 complex, the ligand-induced formation of the FLS2 (or EFR)/BAK1 complex is independent of the kinase activities of both proteins, at least in vitro (Schwessinger et al., 2011).
Compared with bak1-3 or bak1-4, a new bak1 mutant allele, bak1-5 exhibited normal BR signaling capacity and cell death. As EFR-and FLS2-dependent PTI signaling are strongly impaired in the bak1-5 mutation, bak1-5 mutant can be used for the elucidation of specific aspects of BAK1 in PTI (Schwessinger et al., 2011). The double mutant of efr-1fls2 is hypersusceptible to various infection agents. Subsequent to infection with the virulent hemibiotropic pathogen PtoDC3000, bak1-5 was more susceptible than efr-1fls2. The spraying of the non-adapted Pseudomonas syringae pv. tabaci 6605 induced the hypersusceptible phenotype in both bak1-5 and efr-1fls2. Infection with the virulent isolates of the obligate biotropic oomycete Hyaloperonospora arabidopsidis also led to more sporulation on bak1-5 compared with wild type (Roux et al., 2011). Upon infection with adapted and non-adapted Pseudomonas or oomycete, the BAK1-silenced N. benthamiana exhibited more susceptible characteristics than control plant (Heese et al., 2007). These results suggest that BAK1 is a critical component for the basal defense to various hemibiotropic and biotropic pathogens.
In addition to the PAMPs/MAMPs derived from outside sources, plants also need to survive endogenous elicitors [now known as danger-associated molecular patterns (DAMPs)] derived from potential changes inside the cell (Boller and Felix, 2009). PEPR1 in Arabidopsis interacts with AtPEP1, an endogenous peptide induced in response to cell wall degradation, wounding and JA or ethylene treatment (Huffaker et al., 2006; Krol et al., 2010). Recently, BAK1 was also reported to interact with PEPR1 and its homolog PEPR2 in yeast and in vivo (Roux et al., 2011). Further studies are needed to determine whether AtPEP1-induced cellular responses, such as increase in cytosolic calcium levels and the activation of chloride channels leading to membrane depolarization, are dependent on the formation of PEPR1/BAK1 complex in vivo.
Regulation of the kinase activities of BAK1 complexes that mediate BR signaling or plant immunity
The initial phosphorylation of BAK1 through BRI1, followed by heterotetramerization was reported to activate BAK1 kinase activity, resulting in the subsequent transphosphorylation of both proteins (Wang et al., 2008). Extensive analyses of many recombinant proteins containing site-specific mutations using quadruple Time-of-Flight (Q-TOF) liquid chromatography-mass spectrometry (LC/MS/MS) analyses and transgenic approaches in vivo, have been performed to identify the phosphorylation sites of BRI1 and BAK1. Fifteen confirmed and putative autophosphorylation sites (Y831, T842, T872, T880, S887, Y956, T982, S1044, T1045, T1049, Y1052, Y1057, Y1072, S1162, and S1168) in BRI1 were reported throughout the cytoplasmic domain, including the juxtamembrane region, and five transphosphorylation sites (S838, T846, S858, S1166, and T1180) were clustered in juxtamembrane and C-terminal region of BRI1 (Kim and Wang, 2010; Wang et al., 2005a; 2005b; 2008). In BAK1, four autophosphorylation sites (S286, S604, Y610, and S612) and six transphosphorylation sites (S290, T312, T446, T449, T450, and T455) have been identified (Karlova et al., 2009; Wang et al., 2008; Yun et al., 2009). Of note, four out of six transphosphorylation sites are located in the activation loop of BAK1. Based on these findings, a potential scenario involving early stage of BR signaling near the plasma membrane has been proposed. The binding of BR to BRI1 activates the basal kinase activity of BRI1. Activated BRI1 becomes autophosphorylated at the C-terminus, and subsequent conformational changes result in the release of BKI1 from BRI1. Activated BRI1 also transphosphorylates threonine residues in the activation loop of BAK1, resulting in the activation of BAK1. Activated BAK1 becomes autophosphorylated and phosphorylates BRI1 in the juxtamembrane and C-terminal regions to fully activate BRI1 (Wang et al., 2008). The phosphorylated sites of BRI1 and BAK1 do not always contribute to positive BR signaling. The phosphorylation of T832 in BRI1 and S286 in BAK1 reduced interactions between BAK1 and BRI1, respectively (Karlova et al., 2009). The inhibition of autophosphorylation at Y831 in BRI1 through a substitution with phenylalanine rescued the defective growth in bri1-5 more than wild type BRI1 did (Oh et al., 2011).
It remains unknown whether all the putative phosphorylation sites need to be phosphorylated to regulate the BR response. If complete phosphorylation is required, then what is the sequential priority of phosphorylation at these sites? If complete phosphorylation is not required, is it possible for the phosphorylation at different phosphorylation sites to regulate certain specific aspects of BR responses in different temporal and spatial spectrums?
Compared with BRI1/BAK1 complex, the identification of phosphorylation sites on FLS2 and EFR and the elucidation of the mechanisms involving phosphorylation events between these immune response receptors and BAK1 have not been thoroughly studied. Several phosphorylation sites (T867, T1040, and T1072) of FLS2 were identified through targeted mutagenesis. Mutations at these sites abolished FLS2 activity and the induction of downstream PTI events (Robatzek et al., 2006). However, there is no information concerning whether these sites are autophosphorylated or transphosphorylated through BAK1 in a ligand-induced manner. Recently, BAK1 was shown to transphosphorylate kinase-dead EFR, but EFR was not able to transphosphorylate kinase-dead BAK1 (Schwessinger et al., 2011). This suggested that the transphosphorylation of EFR by BAK1 occurs prior to the activation of EFR activity, and this feature is different from the BRI1/BAK1 complex, in which kinase-dead BRI1 and kinase-dead BAK1 can be transphosphorylated through the wild type BAK1 and BRI1 proteins, respectively, in vitro (Li et al., 2002; Schwessinger et al., 2011). Further mapping of the phosphorylation sites and characterization of the mechanisms in the early stages of the activation of immune receptor complexes should be performed in the near future.
BAK1 and its homolog BKK1/AtSERK4/BAK7 control cell death
In addition to being a co-receptor of ligand binding LRR-RLKs, distinct functions of BAK1 in cell death control have been reported When infected with the virulent bacteria PtoDC3000, bak1-3 and bak1-4 mutants showed necrotic phenotypes in the leaves compared with wild type plant. Necrotropic fungal infection has also resulted in more active fungal growth in bak1 mutants than in wild type (Kemmerling et al., 2007). These results indicated that uncontrolled cell death occurred in bak1 when specifically infected with virulent or necrotropic pathogens. These cell death phenotypes were more dramatic in bak1bkk1 double mutant (He et al., 2007). AtSERK4 is one of the members of AtSERK family, which are orthologs of DcSERK1 (Hecht et al., 2001). BAK1 was identified as an AtSERK3; therefore, each member of the AtSERK family has been examined for potential redundant functions with BAK1 in BR signaling. Because the overexpression of AtSERK4 resulted in the phenotypic rescue of bri1-5, AtSERK4 was renamed BAK1-LIKE 1 (BKK1) (He et al., 2007). The bak1bkk1 double mutant displayed reduced growth in the early stages of growth and seedling lethality due to excessive ROS production and the constitutive activation of PR genes. Similar phenotypes were observed in transgenic plants containing the BAK7 RNA-interference (RNAi) construct, in which BAK1 and BAK7 expressions were simultaneously downregulated (Jeong et al., 2010). BAK7 corresponds to AtSERK4. BAK7RNAi transgenic plants narrowly escaped from seedling lethality and exhibited reduced growth and early senescence with the increased expression of senescence-induced genes. Although a reduced growth rate is a typical phenomenon in bri1 mutants, cell death and early senescence have not been associated with the lack of BR signaling. Therefore, the control of cell death has been proposed as an independent function of BAK1 and its closest homologs. It is not known whether the BAK1 and AtSERK4/BKK1/BAK7 or any other AtSERKs would form receptor complexes in vivo.
BAK1 interacting proteins are involved in plant defense and cell death
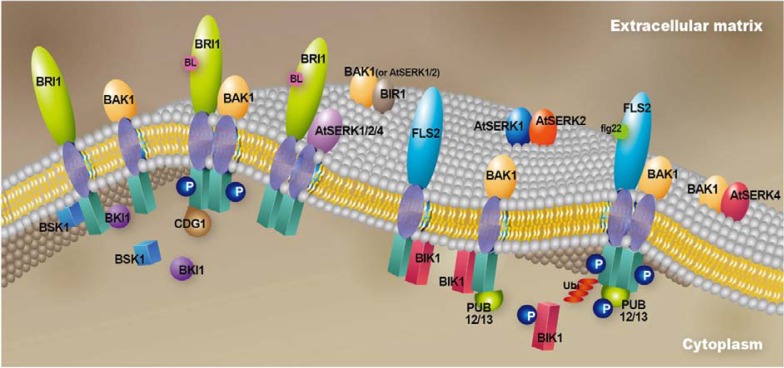
Various receptor complexes containing BAK1 or AtSERK proteins exist in the vicinity of plasma membrane (Fig. 2). Following the full activation of receptor complexes BRI1/BAK1, the sequential downstream events mediating BR signaling in the cytoplasm have been established relatively well through the identification of several critical components. BSK1, a member of the RLCKXII subfamily, was reported to bind to BRI1 (Tang et al., 2008a). BRI1 activates BSK1 through phosphorylation at a serine residue (S230), and activated BSK1 translocates to the cytoplasm to transduce positive BR signaling (Tang et al., 2008b). Recently, another RLCK, CDG1, was reported to interact with BRI1 in yeast. BRI1 phosphorylates several serine residues of CDG1. CDG1 also interacts with the protein phosphatase 1 BSU1 and phosphorylates serine residue S764 of BSU1. Subsequently, the activated BSU1 binds to the cytoplasmic kinase BIN2 acting negatively in BR signaling to dephosphorylate the tyrosine residue Y200 of BIN2. This event eventually diminishes the activity of BIN2, activating BR signaling (Kim et al., 2011). Kim et al. (2011) also showed that CDG1 did not bind to BAK1 in yeast. Thus far, there have been no reports concerning BAK1 interacting proteins in BRI1/BAK1 complex for BR signaling.
Fig. 2.
Various receptor complexes containing BAK1 or AtSERK proteins exist in the vicinity of plasma membrane. Binding of BL to BRI1 triggers the release of BKI1 from BRI1 and recruits BAK1 to form a complex. The subsequent sequential phosphorylation fully activates the kinase activities of BRI1 and BAK1, and BRI1 phosphorylates BSK1 and CDG1. BRI1 forms complexes with AtSERK1, AtSERK2 and At-SERK4. Cytoplasmic kinase BIK1 interacts with both FLS2 and BAK1 separately on the plasma membrane under steady-state conditions. PUB12 or PUB13 are bound to BAK1. Flagellin pulls the FLS2/BIK1 and BAK1/BIK1 complexes together to form FLS2/BAK1/BIK1/PUB13. Transphosphorylation between these proteins results in the release of BIK1 from FLS2 and the polyubiquitination of FLS2 through PUB13. Another complex consisting of BAK1 and BIR1 together with cytoplasmic BON1 or BON3 is also localized on the plasma membrane. BIR1 also interacts with AtSERK1 and AtSERK2. These complexes are involved with the repression of ETI in the absence of pathogen effectors. Complex formation between the AtSERK1 proteins, AtSERK1 and AtSERK2 in male reproductive organ development, and an association of the AtSERK3/AtSERK4 complex with cell death control have been assumed, although direct evidence has not been reported.
However, a few proteins have been identified that interact with BAK1 in plant immunity. Recently, Two independent studies have shown that BIK1, an RLCK, previously known to be required for resistance to Botrytis cyneria (Veronese et al., 2006), is an important component for integrating PTI and the effector-triggered immunity (ETI). An assessment of the flg22-induced expression of RLCKs revealed that BIK1 is a binding protein of both FLS2 and BAK1 (Lu et al., 2010). The phosphorylation of BIK1 occurred in a minute in response to flg22 and was dependent on FLS2 and BAK1, indicating that BIK1 would be a component for the transduction of the flg22 signal downstream of the FLS2/BAK1 complex. Co-immunoprecipitation of BIK1 with FLS2 in bak1 mutant and co-immunoprecipitation of BIK1 with BAK1 in fls2 mutant indicated that the FLS2/BIK1 or BAK1/BIK1 complex formation was an independent of BAK1 or FLS2, respectively. The transphosphorylation of BAK1 and BIK1 is bi-directional, and BIK1 was able to transphosphorylate FLS2, although the transphosphorylation of BIK1 through FLS2 was not detected (Lu et al., 2010). The results of an independent study characterized BIK1 as one of the substrates for AvrPphB, an effector with cysteine protease activity (Zhang et al., 2010). It was shown that flg22 induced BIK1 phosphorylation, and that BIK1 was able to interact with various PRRs, such as FLS2, EFR and chitin elicitor receptor kinase 1 (CERK1), although it is not known whether the flg22-induced phosphorylation of BIK1 resulted from the direct activity of FLS2. Interestingly, in their system, the interaction of BIK1 and BAK1 occurred only when the ligand-binding receptor, FLS2 or EFR was co-expressed (Zhang et al., 2010). This is somewhat different from the results of Lu et al. (2010), showing that BIK1 and BAK1 interact independently of other PRR receptors. These authors also demonstrated that the FLS2/BIK1 interaction was reduced following flg22 treatment, implying that flg22-induced phosphorylated BIK1 is dissociated from FLS2 in a similar manner as BSK1. BRI1-activated BSK1 was released to the cytoplasm in response to BR (Tang et al., 2008b). However, there has been no report whether the BSK1 also bind to BAK1. Taken together, these results and the plasma membrane localization of BIK1 through its myristoylation motif (Veronese et al., 2006) suggest that in the absence of ligand, heterodimers of FLS2/BIK1 and BAK1/BIK1 could form on the plasma membrane of the cells. Once the cells are exposed to flg22, the ligand-induced complex formation between FLS2 and BAK1 facilitates the transient heterotetramer formation, followed by the dissociation of BIK1 from FLS2. The detailed phosphorylation events and sequential order of phosphorylation among FLS2, BAK1 and BIK1 remain unknown.
Two typical plant U-box (PUB) proteins, PUB12 and PUB13, were revealed as interactors with BAK1 in a yeast two-hybrid screen using the BAK1 cytoplasmic kinase domain as bait. The flagellin-induced PUB13 phosphorylation through BAK1 activated the E3 ligase activity of PUB13, leading to the polyubiqutination of FLS2 (Lu et al., 2011). This finding suggests that the intensity of PTI can be regulated through FLS2 degradation in the FLS2/BAK1 complex, although the underlying subtle mechanisms are still unknown.
A BAK1-interacting receptor-like kinase1 (BIR1) is another BAK1-interacting protein that belongs to RLKX group with five LRRs (Gao et al., 2009). BIR1 expression was induced after infection with Pseudomonas syringae pv. maculicola ES4326 for 48 h. One of the mutant alleles of BIR1, bir1-1, showed seedling lethality at 22°C, and escaped from early death under 28°C growth conditions. The activated cell death phenotypes in bir1-1 were similar to those observed in the bak1bkk1 double mutant. In this cell death process, the salicylic acid (SA) level or signaling capacity are involved because the bir1-1 mutant accumulates 20-fold more SA than wild type plants and the double mutants of bir1-1, and any single mutant defective in SA signaling or SA synthesis showed partially suppressed temperature-sensitive bir1-1 phenotypes. These results were consistent with the previous report that the overexpression of the NahG gene in bak1bkk1 also repressed the cell death phenotype of the double mutant (He et al., 2007). Further analyses revealed that BIR1 could directly bind to BAK1 in the transgenic bir1-1 plant containing flag-tagged BIR1 using the anti-flag antibody and mass spectrometric analysis of the peptides that co-immunoprecipitated with BIR1 (Gao et al., 2009). Suppressor screening of bir1-1 identified sobir1-1 (suppressor of bir1-1). Double mutants for bir1-1sobir1-1 showed more growth than bir1-1, and the cell death-related phenotypes in bir1-1 became much milder in the double mutants. SOBIR1 encodes an RLK with 4 LRRs. Therefore, it is reasonable to propose that SOBIR1 could be a component for a large complex involved in cell death control. However, BIR1 did not directly bind to SOBIR1 based on the results from bimolecular fluorescence complementation (BiFC) and yeast two-hybrid analyses, and the interaction with BAK1 and SOBIR1 has not been tested yet (Gao et al., 2009). Recently, in addition to BAK1, a calcium-dependent phospholipid-binding protein BON1 was shown to interact with BIR1 in yeast two-hybrid screening using the C-terminal A domain of BON1 as bait (Wang et al., 2011). From this study, BIR1 was reported to interact with BON3 and BON1, and their interaction was dependent on the kinase activity of BIR1. The bir1bon1 double mutant displayed more severe growth arrest and seedling lethality at an earlier stage compared with the bon1 or bir1 single mutants. BON1 and BON3 repress a number of R genes in the absence of pathogen effectors (Li et al., 2009). These findings suggest that BON1 acts as a negative regulator of the R gene-triggered pathway, resulting in the regulation of growth, defense and eventual cell death. BON1 also interacted with BAK1. Furthermore, BAK1 could phosphorylate both BON1 and BIR1 in vitro (Wang et al., 2011). Although there have been no reports of this phosphorylation event in vivo, and it has not been determined whether the phosphorylation of BON1 and BIR1 would affect the activity of each protein, BAK1 might control cell death and the interwoven connection between PTI and ETI depending on the presence of pathogenic factors. These suggest that BAK1 and FLS2 participate in a receptor complex for initiating PTI and BAK1 also forms a complex with BON1 that represses ETI in the absence of effectors
The coexistence of BR signaling and PTI responses is regulated through BR homeostasis
BAK1 was identified as a dual co-receptor for the ligand-binding receptors BRI1 and FLS2; however, it remains unknown whether the two different pathways for BR signaling and innate plant defenses could be reconciled using the same component. It has been proposed that the up-regulation of BR signaling leads to the sequestration of BAK1, which would otherwise form a complex with the FLS2 in the plasma membrane, resulting in the down-regulation of the first line of defense in response to subsequent PAMP signalings. The opposite case might also be possible. However, this mechanism does not seem to be so simple.
Recently, two reports provided evidences for the complexity of the two concomitant signaling. Albrecht et al. (2012) reported that the activation of BAK1 through simultaneous treatment with epiBL did not alter the immune response, and the activation of BAK1 through flg22 did not affect BR responses. Even the pretreatment of epiBL did not affect the amount of the BAK1/FLS2 complex. These results suggested that BAK1 is not a limiting factor in these two processes. However, when BAK1 formed more complexes with BRI1 through the overexpression of BRI1, these transgenic plants showed insensitivity to flg22, elf18 and peptidoglycans (PGNs), similar to the bak1 or fls2 mutants (Belkhadir et al., 2012). This result might support the idea that increased BR signaling suppresses the initiation of PTI. Compared with the transgenic plants that show an increase of BR signaling capacity through the accumulation of signaling components, Belkhadir et al (2012) also observed that a gain-of-function mutant of BRI1, sud1, exhibited the increased suppression of the bacterial growth of PtoDc3000 hrcC when infected with flg22, and the basal phosphorylation level of FLS2 in sud1 was higher than that in the wild type. Moreover, these authors also demonstrated that sud1 alone could not activate flg22-induced responses and WRKY gene expression without BAK1. As sud1 exhibits intrinsically enhanced BR signaling, these results suggested that increased BAK1-mediated BR signaling also mediates the activation of defense signaling in plants. Both groups demonstrated that, in addition to BAK1, BR itself could affect plant defense. The pretreatment of epiBL was shown to suppress flg22-induced ROS burst and the expression of PTI marker genes (Albrecht et al., 2012). The reduction of flg22-induced responses was also observed in DWF4-overexpressing transgenic plants, in which more BR biosynthesis was expected (Belkhadir et al., 2012). The effects of exogenously treated BR on the resistance to the biotic stresses in various species of plants have not been conclusive with contradictory results in many cases. BR has been known to activate the genes encoding pathogenesis-related (PR) proteins. BR-biosynthetic mutant cpd showed the reduced expression of PR1, PR2 and PR5 (Szekeres et al., 1996). Consistently, 24-epiBL induced PR1 gene expression (Divi et al., 2010). The application of BL has been reported to increase resistance to bacterial and fungal pathogens in rice and tobacco, although in this case, BL itself did not induce PR gene expression (Nakashita et al., 2003). In contrast, the BR-induced promotion of mycelial growth and spore formation was observed in potato tuber, indicating the immunoinhibiting effect of BR (Bajguz and Hayat, 2009). Taken together, these results suggest that the simple aliquot of BAK1 for ligand-binding receptors at any time might not occur in vivo. Further mechanistic studies concerning the effective amount of complexes to exert specific roles and different stabilities of the complexes of BRI1/BAK1 and FLS2/BAK1 should be pursued.
Overlapping and non-overlapping physiological roles of the proteins in the LRR-RLKII family
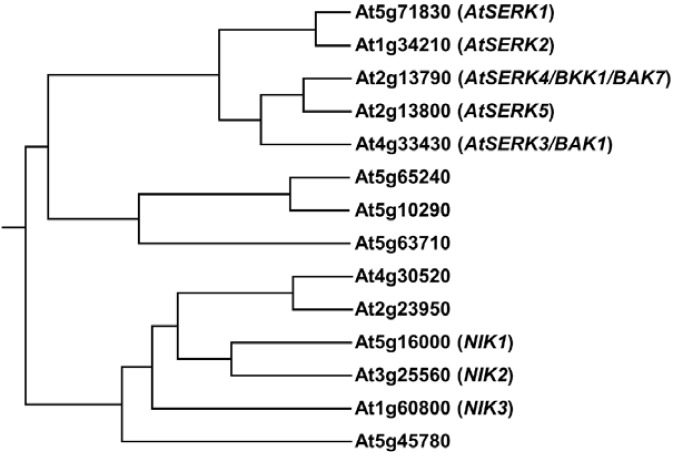
The LRR-RLKII family consists of fourteen individual members including five AtSERKs (Fig. 3). All fourteen genes have similar genomic structures containing five LRRs, except At5g63710 which lacks the fourth LRR, in their ECD, single pass transmembrane domain a cytoplasmic kinase domain (Zhang et al., 2006). BAK1 corresponds to AtSERK3, a member of the At-SERK family. Since the identification of DcSERK1 in the carrot, somatic embryogenesis-associated SERK proteins have been reported in many species, such as Dactylis glomerata, Medicago truncatula, Helianthus annuus, Ocotea catharinensi, Citrus unshiu and Arabidopsis (Hecht et al., 2001; Nolan et al., 2003; Schmidt et al., 1997; Shimada et al., 2005; Thomas et al., 2004). Five Arabidopsis SERK proteins were identified through sequence homology to DcSERK1. AtSERK1, the closest homolog of DcSERK1, is expressed in developing ovules and early embryo, confirming the competence for somatic embryogenesis in tissue culture (Hecht et al., 2001). Therefore, other AtSERK1 homologs had been assumed to possess related functions to somatic embryogenesis. However, the BAK1/AtSERK3 was identified as a co-receptor of BRI1; therefore, the plausible functions of the proteins in the AtSERK family have been expanded to include pleiotropic roles in plant development. Partial cDNA encoding AtSERK2 was implemented in a yeast twohybrid assay using the BRI1 kinase domain as bait (data not shown). Immunoprecipitation against an anti-GFP antibody, followed by LC/MS using total protein from the AtSERK1-CFP transgenic plants revealed that AtSERK1 was involved in a large complex containing BRI1 (Karlova et al., 2006). AtSERK4, the closest homolog of BAK1/AtSERK3, for-med a complex with BRI1 in fluorescence resonance energy transfer (FRET) analyses (Jeong et al., 2010). The short petiole and compact rosette phenotypes of bri1-5 or bri1-301 could be rescued through the overexpression of AtSERK1, AtSERK2, BAK1/AtSERK3, or AtSERK4 (Albrecht et al., 2008; Jeong et al., 2010; Li et al., 2002; Nam and Li, 2002). Recently, Gou et al. (2012) produced transgenic bri1-5 overexpressing each of fourteen members of the LRR-RLKII family and clearly showed that only the overexpression of SERK1, SERK2, BAK1 and BKK1 rescued bri1-5 phenotypes. Therefore, at least four members of AtSERKs (AtSERK1-4) have been considered to have redundant functions in BR signaling. However, the degree of participation in BR signaling is not equal. Except for the bak1, no detectable BR-related phenotypes have been reported from any single mutant of other AtSERKs. Moreover, only the double mutants of serk1serk3 showed reduced sensitivity to exogenously treated BL than each single mutant (Albrecht et al., 2008). The serk1bak1bkk1 triple mutant showed similar phenotypes to those of the null bri1 mutant (Gou et al., 2012).
Fig. 3.
Individual members of LRR-RLK in the LRR-RLKII family. The phylogenetic analysis of LRR-RLK in the LRR-RLKII family in Arabidopsis was performed based on CLUSTAL 2.1 multiple sequence alignment. The phylogenetic trees were generated using PHYML and MEGA.
One interesting feature in transgenic plants overexpressing AtSERKs is that the restoration of rosette shape did not guarantee the promotion of hypocotyl and root growth of bri1 mutant. The overexpression of AtSERK1, AtSERK2, or AtSERK4 did not enhance the hypocotyl elongation of bri1-301 compared with that of the non-transformant (Albrecht et al., 2008). The severe inhibition of root growth in the serk1bak1bkk1 triple mutant was a phenotype specific to this mutant because this phenotype has not been observed in the bri1 mutant (Gou et al., 2012). The overexpression of AtSERK4/BAK7 did not change the sensitivity to BRZ in hypocotyl growth (Jeong et al., 2010). These results implied that separate BR-independent regulation mechanisms for hypocotyl and root growth have been developed in plant growth. Furthermore, the growth of rosette leaves might not always reflect the BR effect. The dwarfed rosette phenotypes shown in serk3serk4 double mutant were not rescued upon the overexpression of bes1-D, indicating that a subset of AtSERKs could exert specific functions that are not mediated through BR signaling (Albrecht et al., 2008). This notion was supported through the results of several studies, as the spontaneous cell death phenotypes shown in the bak1bkk1 double mutant were distinct because none of the bri1 mutants exhibited such phenotypes (He et al., 2007). BIR1, one of the cell death regulators, was shown to interact with AtSERK1 and AtSERK2 as well as with BAK1, in a BiFC analysis (Gao et al., 2009). As BIR1 did not interact with BRI1 or FLS2 in the same assay, BIR1 was considered as a specific interacting partner with AtSERKs in controlling cell death. The production of the proper pollen through anther development was abolished in the serk1serk2 double mutant, and the overexpression of bes1-D did not rescue the defective anther phenotype of this double mutant, suggesting that anther development is a specific process that requires both AtSERK1 and AtSERK2 (Albrecht et al., 2005; Colcombet et al., 2005). Recently, severely retarded root growth accompanied with a lack of starch granule accumulation and reduced PIN1 expression was observed in the serk1bak1bkk1 triple mutant. As these defective root phenotypes were not observed in the cpd or bri1 mutants, it was speculated that a subset of the AtSERK family are involved in BR-independent root development and the subsequent regulation of gravitropic responses (Du et al., 2012).
Except for five AtSERKs, little is known about the functions of the other nine members. A few studies have reported that three proteins, NIK1, NIK2, and NIK3, are interacting partners of geminivirus nuclear shuttle proteins and showed higher susceptibility to geminivirus infection in knockout mutants of these genes (Fontes et al., 2004). NIK1 was subsequently shown to be required for the nuclear localization of the ribosomal protein (Carvalho et al., 2008). These results indicated that another subset of LRR-RLKII subfamily, to which AtSERKs do not belong, might be involved in specific processes induced through viral infections.
CONCLUSION
Based on numerous studies, it is highly likely that the members of the LRR-RLKII family proteins, including BAK1, play multiple functions as versatile co-receptors of other families of LRR-RLKs. Therefore, the identification of corresponding partners of LRR-RLKs that form complexes on the plasma membrane and the elucidation of phosphorylation mechanisms specific to each complex in response to specific stimuli using fine phosphosite mapping combined with molecular genetic confirmation have great potential to integrate our understanding of the regulatory web of developmental processes.
Acknowledgments
This work was supported by a grants from the Next-Generation BioGreen 21 Program (SSAC, grant #: PJ008003), Rural Development Administration, Republic of Korea, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0010-058).
REFERENCES
- Albrecht C., Russivano E., Hecht V., Baaijens E., de Vries S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell. 2005;17:3337–3349. doi: 10.1105/tpc.105.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C., Russinova E., Kemmerling B., Kwaaitaal M., de Vries S.C. Arabidopsis somatic embryogenesis receptor kinase proteins serve brassinosteroid-dependent and- independent signaling pathways. Plant Physiol. 2008;148:611–619. doi: 10.1104/pp.108.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C., Boutrot F., Segonzac C., Schwessinger B., Gimenez-Ibanez S., Chinchilla D., Rathjen J.P., de Vries S.C., Zipfel C. Brassinosteroids inhibit pathogen-associated molecular pattern–triggered immune signaling independent of the receptor kinase BAK1. Proc. Natl. Acad. Sci USA. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz A., Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009;47:1–8. doi: 10.1016/j.plaphy.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y., Jaillais Y., Epple P., Balsemão-Pires E., Dangl J.L., Chory J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc. Natl. Acad. Sci USA. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.J. Refining the plant steroid hormone biosynthesis pathway. Trends Plant Sci. 2007;12:377–380. doi: 10.1016/j.tplants.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Boller T., Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Canales C., Bhatt A.M., Scott R., Dickinson H. EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr. Biol. 2002;12:1718–1727. doi: 10.1016/s0960-9822(02)01151-x. [DOI] [PubMed] [Google Scholar]
- Carvalho C.M., Santos A.A., Pires S.R., Rocha C.S., Saraiva D. I., Machado J.P.B., Mattos E.C., Fietto L.G., Fontes E.P.B. Regulated nuclear trafficking of rpL10A mediated by NIK1 represents a defense strategy of plant cells against virus. PLoS Pathog. 2008;4:e1000247. doi: 10.1371/journal.ppat.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D.G., Felix G., Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defense. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Chinchilla D., Shan L., He P., de Vries S., Kemmerling B. One for all: the receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–541. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S., Tanaka A., Noguchi T., Fujioka S., Takatsuto S., Ross A.S., Tax F.E., Yoshida S., Feldmann K.A. Lesions in the sterol delta reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000;21:431–443. doi: 10.1046/j.1365-313x.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- Clark S.E., Williams R.W., Meyerowitz E.M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;59:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Clouse S.D. Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell. 2011;23:1219–1230. doi: 10.1105/tpc.111.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet J., Boisson-Dernier A., Ros-Palau R., Vera C.E., Schroeder J.I. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–3361. doi: 10.1105/tpc.105.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divi U.K., Rahman T., Krishna P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:151. doi: 10.1186/1471-2229-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Yin H., Zhang S., Wei Z., Zhao B., Zhang J., Gou X., Lin H., Li J. Somatic embryogenesis receptor kinases control root development mainly via brassinosteroid-independent actions in Arabidopsis thaliana. J. Integ. Plant Biol. 2012;54:388–399. doi: 10.1111/j.1744-7909.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- Fontes E.P.B., Santos A.A., Luz D.F., Waclawovsky A.J., Chory J. The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev. 2004;18:2545–2556. doi: 10.1101/gad.1245904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang X., Wang D., Xu F., Ding X., Zhang Z., Bi D., Cheng Y.T., Chen S., Li X., et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe. 2009;6:34–44. doi: 10.1016/j.chom.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Gao J., Ma Y., Sun Y., Zhao H., Hong D., Yana L., Loua Z. Crystallization and preliminary crystallographic analysis of Arabidopsis thaliana BRI1-associated kinase 1 (BAK1) cytoplasmic domain. Acta Cryst. 2012;F68:340–342. doi: 10.1107/S1744309112004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. FLS2: an LRR receptor-like kinase involved in the perception of bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- Gou X., Yin H., Du J., Yi J., Xu S., Lin H., Clouse S.D., Li J. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet. 2012;8:e1002452. doi: 10.1371/journal.pgen.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Gou X., Yuan T., Lin H., Asami T., Yoshida S., Russle S.D., Li J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Hecht V., Veille-Calzada J.P., Hartog M.V., Schmidt E.D., Boutilier K., Grossniklaus U., de Vries S.C. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- Heese A., Hann D.R., Glimenez-Ibanez S., Jones A.M.E., He K., Li J., Schroeder J.I., Peck S.C., Rathjen J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn M., Belkhadir Y., Dreux M., Dabi T., Noel J.P., Wilson I.A., Chory J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature. 2011;474:467–471. doi: 10.1038/nature10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A., Pearce G., Ryan C.A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci USA. 2006;103:10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Belkhadir Y., Balsemão-Pires E., Dangl J.L., Chory C. Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation. Proc. Natl. Acad. Sci USA. 2011a;108:8503–8507. doi: 10.1073/pnas.1103556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Hothorn M., Belkhadir Y., Dabi T., Nimchuk Z.L., Meyerowitz E.M., Chory C. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011b;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Beyaert R. Functional diversity and regulation of different interlukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–392. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Jeong Y.J., Shang Y., Kim B.H., Kim S.Y., Song J.H., Lee J.S., Lee M.M., Li J., Nam K.H. BAK7 displays unequal genetic redundancy with BAK1 in brassinosteroid signaling and early senescence in Arabidopsis. Mol Cells. 2010;29:259–266. doi: 10.1007/s10059-010-0024-0. [DOI] [PubMed] [Google Scholar]
- Jinn T.L., Stone J.M., Walker J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000;14:108–117. [PMC free article] [PubMed] [Google Scholar]
- Karlova R., Boeren S., Russinova E., Aker J., Vervoort J., de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:625–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R., Boeren S., van Dongen W., Kwaaitaal M., Aker J., Vervoort J., de Vries S. Identification of in vitro phosphorylation sites in the Arabidopsis thaliana somatic embryogenesis receptor-like kinases. Proteomics. 2009;9:368–379. doi: 10.1002/pmic.200701059. [DOI] [PubMed] [Google Scholar]
- Kemmerling B., Schwedt A., Rodriguez P., Mazzotta S., Frank M., Qamar S.A., Mengiste T., Betsuyaku S., Parker J.E., Müssig C., et al. The BRI1-aossociated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr. Biol. 2007;17:1116–1122. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Wang Z.Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Guan S., Burlingame A.L., Wang Z.Y. The CDG1 kinase mediate brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Caňo-Delgado A., Seto H., Hiranuma S., Fujuika S., Chory J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- Krol E., Mentzel T., Chinchilla D., Boller T., Felix G., Kemmerling B., Postel S., Arents M., Jeworutzki E., Al-Rasheid K.A.S., et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 2010;285:13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti-Shiu M.D., Zou C., Hanada K., Shiu S.H. Evolutionary history and stress regulation of plant receptor-like kinase/Pelle genes. Plant Physiol. 2009;150:12–26. doi: 10.1104/pp.108.134353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chory J. A putative leucine-rich repeat receptor kinase involve in brassinosteroid signal transduction. Cell. 1997;90:927–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Li J., Wen J., Lease K.A., Dorke J.T., Tax F.E., Walker J.C. BAK1, an Arabidopsis LRR receptor-like kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Li Y., Pennington B.O., Hua J. Multiple R-like genes are negatively regulated by BON1 and BON3 in Arabidopsis. Mol. Plant Microbe Interact. 2009;22:840–848. doi: 10.1094/MPMI-22-7-0840. [DOI] [PubMed] [Google Scholar]
- Lu D., Wu S., Gao X., Zhang Y., Shan L., He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1438–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava N.B. Plant growth-promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988;39:23–52. [Google Scholar]
- Nakashita H., Yasuda M., Nitta T., Asami T., Fujioka S., Arai Y., Sekimato K., Takatsuto S., Yamaguchi I., Yosida S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33:887–898. doi: 10.1046/j.1365-313x.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Nam K.H., Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Nekrasov V., Li J., Batoux M., Roux M., Chu Z.H., Lacombe S., Rougon A., Bittel P., Kiss-Papp P, Chinchilla D., et al. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J. 2009;28:3428–3438. doi: 10.1038/emboj.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Fusioka S., Choe S., Takatsuto S., Yoshida S., Yuan H., Feldmann K.A., Tax F.E. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulates brassinosteroids. Plant Physiol. 1999;121:743–752. doi: 10.1104/pp.121.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K.E., Irwanto R.R., Rose R.J. Auxin up-regulates MtSERK1 expression in both Medicago truncatula and root-forming and embryogenic cultures. Plant Physiol. 2003;133:218–230. doi: 10.1104/pp.103.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.H., Wang X., Kota U., Goshe M.B., Clouse S.D., Huber S.C. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci USA. 2009;106:658–663. doi: 10.1073/pnas.0810249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.A., Sun J., Oh D.H., Zielinski R.E., Clouse S.D., Huber S.C. Enhancing Arabidopsis leaf growth by engineering the BRASSINOSTEROID INSENSITIVE1 receptor kinase. Plant Physiol. 2011;157:120–131. doi: 10.1104/pp.111.182741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel S., Küfner I., Beuter C., Mazzotta S., Schwedt A., Borlotti A., Halter T., Kemmering B., Nürnberger T. The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur. J. Cell Biol. 2010;89:169–174. doi: 10.1016/j.ejcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Chinchilla D., Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M., Schwessinger B., Albrecht C., Chinchilla D., Jones A., Holton N., Malinovsky F.G., Tör M., de Vries S., Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.D., Guzzo F., Toonen M.A., de Vries S.C. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Schulze B., Mentzel T., Jehle A.K., Mueller K., Beeler S., Boller T., Felix G., Chinchilla D. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Roux M., Kadota Y., Ntoukakis V., Sklenar J., Jones A., Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002640. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J., Han Z., Kim T.W., Wang J., Cheng W., Chang J., Shi S., Wang J., Yang M., Wang Z.Y., et al. Structural insight into brassinosteroid perception by BRI1. Nature. 2011;474:472–488. doi: 10.1038/nature10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton C.A., Wasserman S.A. Pelle encodes a protein kinase required to establish dorsoventral polarity in the Drosophila embryo. Cell. 1993;72:515–525. doi: 10.1016/0092-8674(93)90071-w. [DOI] [PubMed] [Google Scholar]
- Shimada T., Hirabayashi T., Endo T., Fujii H, Kita M., Omura M. Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CitSERK) from Citrus unshiu. Marc. Sci. Hortic. 2005;103:233–238. [Google Scholar]
- Shiu S.H., Bleecker A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci USA. 2001a;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. Plant receptor-like kinases gene family: diversity, function and signaling. Sci. STKE. 2001b;2001:RE22. doi: 10.1126/stke.2001.113.re22. [DOI] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak E.D., McAbee J.M., Pillitteri L.J., Torii K.U. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- Szekeres M., Németh K., Koncz-Kálmán Z., Mathur J., Kauschmann A., Altmann T., Rédei G.P., Nagy F., Schell J., Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Tang W., Deng Z., Oses-Prieto J.A., Suzuki N., Zhu S.W., Zhang X., Burlingame A.L., Wang Z.Y. Proteomic studies of brassinosteroid signal transduction using prefractionation and two-dimensional DIGE. Mol. Cell Proteomics. 2008a;7:728–738. doi: 10.1074/mcp.M700358-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Kim T.W., Oses-Prieto J.A., Sun Y., Deng Z., Zhu Z., Wang R., Burlingame A.L., Wang Z.Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008b;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Meyer D., Himber C., Steinmerz A. A special expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol. Biochem. 2004;42:34–42. doi: 10.1016/j.plaphy.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Torri K.U., Mitsukawa N., Oosumi T., Matsuura Y., Yokoyama R., Whittier R.F., Komeda Y. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese P., Nakagami H., Bluhm S., AbuQumar S., Chen X., Salmeron J., Dietrich R.A., Hirt H., Mengiste T. The Membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell. 2006;18:257–273. doi: 10.1105/tpc.105.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling from a plasma membrane. Science. 2006;131:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- Wang X., Li X., Meisenhelder J., Hunter T., Yoshida S., Asami T., Chory J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005a;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wang X., Goshe M.B., Soderblom E.K., Phinney B.S., Kuchar J.A., Li J., Asami T., Yoshida S., Huber S.C., Clouse S.D. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROIDINSENSITIVE1 receptor kinase. Plant Cell. 2005b;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kota U., He K., Blackburn K., Li J., Goshe M.B., Huber S.C., Clouse S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Wang Z., Meng P., Zhang X., Ren D., Yang S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 2011;67:1081–1093. doi: 10.1111/j.1365-313X.2011.04659.x. [DOI] [PubMed] [Google Scholar]
- Whippo C.W., Hangarter R.P. A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol. 2005;139:448–457. doi: 10.1104/pp.105.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., Li L., Yin Y. Recent advances in the Regulation of brassinosteroid Signaling and biosynthesis pathways. J. Integ. Plant Biol. 2011;53:455–468. doi: 10.1111/j.1744-7909.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Yun H.S., Bae Y.H., Lee Y.J., Chang S.C., Kim S.K., Li J., Nam K.H. Analysis of phosphorylation of the BRI1/BAK1 complex in Arabidopsis reveals amino acid residues critical for receptor formation and activation of BR signaling. Mol Cell. 2009;27:183–190. doi: 10.1007/s10059-009-0023-1. [DOI] [PubMed] [Google Scholar]
- Zhang X.S., Choi J.H., Heinz J., Chetty C.S. Domain-specific positive selection contributes to the evolution of Arabidopsis leucine-rich repeat receptor-like kinase (LRR-RLK) genes. J. Mol. Evol. 2006;63:612–621. doi: 10.1007/s00239-005-0187-z. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li W., Xiang T., Liu Z., Laluk K., Ding X., Zou Y., Gao M., Zhang Z., Chen S., et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zhao D.Z., Wang G.F., Speal B., Ma H. The EXCESS MICROSPOROCYTES 1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Gene Dev. 2002;16:2021–2031. doi: 10.1101/gad.997902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Robatzek S., Navarro L., Oakeley E.J., Jones J.D., Felix G., Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D.G., Boller T., Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]