Abstract
Chaperonins are involved in protein-folding. The rice genome encodes six plastid chaperonin subunits (Cpn60) - three α and three β. Our study showed that they were differentially expressed during normal plant development. Moreover, five were induced by heat stress (42°C) but not by cold (10°C). The oscpn60α1 mutant had a pale-green phenotype at the seedling stage and development ceased after the fourth leaf appeared. Transiently expressed OsCpn60α1:GFP fusion protein was localized to the chloroplast stroma. Immuno-blot analysis indicated that the level of Rubisco large subunit (rbcL) was severely reduced in the mutant while levels were unchanged for some imported proteins, e.g., stromal heat shock protein 70 (Hsp70) and chlorophyll a/b binding protein 1 (Lhcb1). This demonstrated that OsCpn60α1 is required for the folding of rbcL and that failure of that process is seedling-lethal.
Keywords: chaperonin, Cpn60, plastid, rice, Rubisco
INTRODUCTION
Chaperonins are oligomeric protein complexes that form a double-ring structure. They assist in the folding of newly synthesized or denatured polypeptides in their central cavity through the hydrolysis of ATP (Bukau and Horwich, 1998; Ditzel et al., 1998; Sigler et al., 1998). Two types of chaperonins have been identified. Type I chaperonins are found in bacteria (called GroEL), plastids (Cpn60), and mitochondria (Hsp60), while Type II chaperonins are present in archaea and the eukaryotic cytosol (CCT/TriC). Although both types have a markedly conserved crystal structure (Braig et al., 1994; Ditzel et al., 1998), they also are significantly different. Type I chaperonin is composed of seven subunits per ring while Type II chaperonin has eight or nine subunits. Those in Type I utilize co-chaperonin GroES/cpn10 for substrate encapsulation in the protein-folding process, whereas those in Type II have a built-in “cap” that performs the same function (Hartl, 1996; Horwich et al., 2007; Saibil, 2000). Both types have been identified in plants (Hill and Hemmingsen, 2001).
Type I chaperonins are evolutionarily conserved proteins that are encoded by multiple genes. Many bacteria have at least two GroEL genes. For example, Bradyrhizobium japonicum has seven GroEL homologues and Rhizobium leguminosarum has three (Gould et al., 2007; Kaneko et al., 2002). Likewise, multiple mitochondria and plastid chaperonin homologues are encoded from the nuclear genome in plants (Hill and Hemmingsen, 2001; Peng et al., 2011). In contrast to the Escherichia coli GroEL and mitochondrial Hsp60, which have 14 identical subunits, the plastid Cpn60 has two distinct subunit types, α and β (Hemmingsen and Ellis, 1986; Martel et al., 1990; Nishio et al., 1999). The sequence identity between them is approximately 50%; a similar percentage is found between Cpn60 and GroEL (Hemmingsen et al., 1988; Martel et al., 1990). Although Cpn60 evolved from GroEL-1 in the cyanobacterial ancestor of plastids (Hill and Hemmingsen, 2001; Suzuki et al., 2009), the number of subunits in genomes differs among land plant species. For example, Physcomitrella patens has three α subunits and four β subunits (Suzuki et al., 2009), Brassica napus has three α but only one β (Cloney et al., 1994), while Arabidopsis has two α and four β (Hill and Hemmingsen, 2001).
Bacterial GroEL genes are differentially expressed and regulated by stresses such as high temperature (Glatz et al., 1997; Rodríguez-Quiñones et al., 2005). In Arabidopsis, AtCpn60α1, AtCpn60β1, and AtCpn60β2 are strongly expressed while its remaining Cpn60s are expressed only weakly (Weiss et al., 2009). Other than that, expression patterns for the plastid Cpn60 gene family have not been well studied in most plants.
The structure and mechanisms of chaperonins have been intensively investigated in E. coli by using GroEL (Bukau and Horwich, 1998). At least one GroEL gene has been demonstrated to be essential for viability (Fayet et al., 1989). Approximately 250 of the ∼2,400 cytosolic proteins interact with GroEL (Kerner et al., 2005). In plants, the chloroplast Cpn60 was initially identified as an abundant oligomeric protein that transiently binds nascent Rubisco large subunits (rbcLs) prior to their assembly into the Rubisco holoenzyme (Barraclough and Ellis, 1980). Transfer of newly synthesized rbcL subunits to the holoenzyme is inhibited by the addition of anti-chaperonin antibodies, support that Cpn60 plays a critical role in Rubisco assembly (Cannon et al., 1986; Milos and Roy, 1984). Functional analysis of plastid Cpn60 genes has been conducted in Arabidopsis, where a T-DNA insertion mutant in AtCpn60α1 shows an embryonic-lethal phenotype (Apuya et al., 2001). Deletion of AtCpn60β1 leads to seedling death under short-day conditions (Ishikawa et al., 2003). A mutant lacking AtCpn60β4 is defective in chloroplast NDH activity, indicating that AtCpn60β4 is required for the folding of NdhH, a subunit of the chloroplast NADH dehydrogenase-like complex (Peng et al., 2011). However, involvement of Cpn60 in the folding of rbcL is unclear in these mutants.
Here, we describe how OsCpn60α1 is essential for rice seedling development because it functions in such rbcL-folding.
MATERIALS AND METHODS
Plant materials, growing conditions, and genotyping
T-DNA-tagging lines were generated in Oryza sativa japonica cv. Dongjin and Hwayoung (An et al., 2003; Jeon et al., 2000; Jeong et al., 2006). Their T-DNA flanking sequences were identified via inverse PCR (Kim et al., 2011). In Line 3A-06578, T-DNA was inserted in OsCpn60α1 (Os12g17910). As previously described by Kim et al. (2011), seeds were sterilized in a 0.025% (v/v) prochloraz solution, sown in soil, and placed in an incubator set at a constant 27°C with light from cool-white fluorescent lamps (30 μmol photons m−1s−1). Genotyping was performed with PCR, using two gene-specific primers (GT-F: 5′-AGCGCCTTGGTGCAGACAT-3′ and GT-R: 5′-CTAGGACA GAGATAGTCTGAG-3′) and two gene-specific/T-DNA primers (GT-R and LB1: 5′-TTGGGGATCCTCTAGAGTCGAG -3′).
Quantitative real-time RT-PCR
Seedlings grown for 14 days at 27°C were then transferred to either heat (42°C) or cold (10°C) conditions. After these stress treatments, total RNAs were prepared from their third leaves, using RNAiso Plus (Takara, Japan). cDNAs were synthesized with M-MLV reverse transcriptase (Promega, USA) and oligo (dT)15 as described previously (Lee et al., 2012). Using these cDNAs as template, we conducted quantitative real-time RT-PCR in a Rotor-Gene 6000 (Qiagen, Germany). The gene-specific primers were 5′-ACTCTCCGAAACTGATTCGAT-3′ (a1-F) and 5′-TCAAGTTCTCATACTTGTCGTT-3′ (a1-R) for OsCpn 60α1, 5′-CTTTCTCAGACAGACTCAGC-3′ and 5′-AAGTTCT CGTGTCTGTCAGC-3’ for OsCpn60α2, 5′-GGATCTTGTAG ATGACTCTGA-3′ and 5′-CCTTGCATCATGGACCTTTC-3′ for OsCpn60α3, 5′-TGCTGCAACTGGACAGTAC-3′ and 5’-TGAC AGCCGTTCCAGTTACA-3′ for OsCpn60β1, 5′-CTGAAATCG TAAGGAAGTCCT-3’ and 5’-CGTCTCAGATGCAACTGTGA-3′ for OsCpn60β2, and 5′-AACGGCAGCTTCGTCATAGA-3′ and 5′-TGAGTATCAGTGCTGGGGAA-3′ for OsCpn60β3. Cycling conditions included denaturing at 95°C for 10 s, annealing at 55°C for 15 s, and extension at 72°C for 25 s. Rice UBQ5 was used to normalize the cDNA quantity (Jain et al., 2006), and transcript levels relative to that gene were calculated by the ΔΔCt method. All experiments were conducted with three samples at each time point.
Chlorophyll quantification
The third leaves from seedlings were homogenized with liquid nitrogen and suspended in 95% ethanol. Residual plant debris was precipitated by centrifugation (13,000 × g for 10 min). The supernatant was used for measuring absorbance at 664.1 and 648.6 nm with a spectrophotometer (Shimadzu, Japan). Chlorophyll contents (a and b) were calculated by the equation of Lichtenthaler and Buschmann (2001).
Transmission electron microscopy
Leaf tissues were fixed in a solution containing 0.1 M sodium phosphate (pH 7.2), 3% glutaraldehyde, and 0.2% Brij35, then post-fixed for 2 h in a solution containing 0.1 M sodium phosphate, 2% osmium tetroxide, and 0.2% Brij35. The samples were stained with 0.5% (w/v) uranyl acetate. Following dehydration, the specimens were embedded in Spurr’s resin. After ultra-thin sectioning, the grids containing specimens were stained with 4% uranyl acetate and examined with a JEOL 1200 transmission electron microscope (JEOL Ltd, Japan).
Generation of OsCpn60α1 overexpression transgenic plants
The full-length cDNA clone (AK120503) of OsCpn60α1 was obtained from the Rice Genome Resource Center (RGRC; http://www.rgrc.dna.affrc.go.jp/index.html.en). The plasmid was digested with SfiI and the fragment encoding OsCpn60α1 was ligated into pGA3593, a sub-cloning vector for rice full-length cDNA (Kim et al., 2009). The fragment isolated with BsrGI/SpeI was inserted between the maize Ubiquitin1 promoter and the NOS terminator in binary vector pGA3426 (Kim et al., 2009). Embryonic calli induced from ‘Dongjin’ wild-type (WT) seeds were co-cultured with Agrobacterium tumefaciens strain LBA4404 harboring the binary plasmid. Transgenic plants were generated as described previously (Lee et al., 1999).
Complementation of the oscpn60α1 mutant
The OsCpn60α1 overexpression (OX) construct was introduced into the oscpn60α1 mutant by crossing the OsCpn60α1 OX plant with OsCpn60α1/oscpn60α1 heterozygote plants. The F1 offspring having both the OX construct and the T-DNA-inserted OsCpn60α1 allele were selected and selfed to produce F2 seedlings that carried the OX construct in an oscpn60α1 homozygous background.
Sub-cellular localization
The protein coding region of OsCpn60α1 without the stop codon was amplified by PCR using a pair of primers (forward: 5′-GTTAACCTTATCCTCAGCTCTCAG-3′ and reverse: 5′-ACTAGTGACGGTAAGGGTTCCCTCT-3′). Restriction enzyme sites for cloning are underlined. The fragment was inserted into multiple cloning sites located between the CaMV 35S promoter and green fluorescent protein (GFP) coding region in the modified pGA3452 vector (Kim et al., 2009). We constructed the p35S-AtTic21:red fluorescent protein (RFP) vector based on the method of Teng et al. (2006). Particle bombardment was performed with onion epidermis cells, using a biolistic particle delivery system (PDS-1000; Bio-Rad, USA). To improve DNA adsorption into the gold particles, we used an alkaline buffer containing 50 mM Tris-HCl (pH 8.8) and 1 mM EDTA (Yoshimitsu et al., 2009). The other parameters were as described previously (Kim et al., 2009). Preparation of mesophyll protoplasts from rice seedling leaves and this transformation procedure were previously reported (Han et al., 2006). After the fusion proteins were transiently expressed, fluorescence signals were observed under a confocal laser scanning microscope (LSM 510 META; Zeiss, Germany).
Protein blot analysis
The third leaves were harvested from 7-d-old seedlings (oscpn60α1 and WT) grown on half-strength MS media at a constant 27°C and under cool-white fluorescent light. Samples were ground under liquid nitrogen and suspended in a protein extraction buffer containing 250 mM sucrose, 25 mM KCl, 50 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 0.5% (v/v) β-mercaptoethanol, 0.5% (v/v) Triton X-100, and a protease inhibitor cocktail (Roche, Switzerland), as described by Armstrong et al. (1995). After any debris was precipitated by centrifugation (13,000 × g for 10 min at 3°C), the supernatant was analyzed. Protein concentrations were determined by Bradford assays. For blotting analysis, 15 μg of total proteins was separated on 10% SDS-polyacrylamide gels. The blots were incubated with the primary anti-chlorophyll a/b binding protein 1 (Lhcb1), anti-stromal Heat shock protein 70 (Hsp70), or anti-rbcL antibodies (Agrisera, Sweden), then processed with an ECL kit (GE Healthcare, USA). To normalize quantities, we used the anti-Histone3 antibody (Santa Cruz Biotechnology, USA). Total proteins were visualized by Coomassie Brilliant Blue (CBB)-staining after SDS-PAGE. Images of the immunoblots were obtained with an LAS4000 image capture system (GE Healthcare).
RESULTS
Expression analysis of the rice Cpn60 family
Rice has three α subunit genes and three β subunit genes of Cpn60 (Peng et al., 2011). Here, we named them OsCpn60α1 (Os12g17910), OsCpn60α2 (Os03g64210), OsCpn60α3 (Os 09g38980), OsCpn60β1 (Os06g02380), OsCpn60β2 (Os02g 01280), and OsCpn60β3 (ChrSy.fgenesh.gene.28). Sequencing of the cDNAs revealed that the public annotation of OsCpn60β3 is incorrect because it misses 93 nucleotides (Supplementary Fig. S1). The primary structures share 60 to 86% identity among α subunits and 61 to 84% identity among β subunits (Supplementary Fig. S2A).
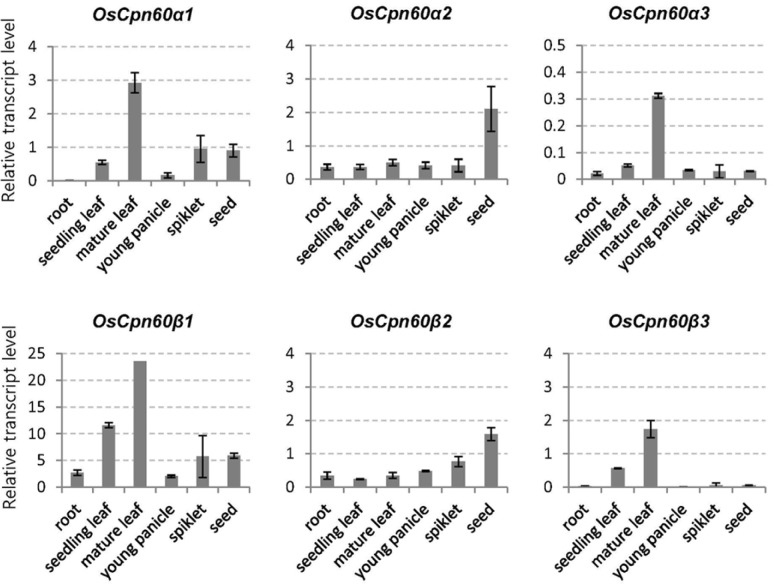
Expression patterns for these genes were studied in seedling roots and leaves, flag leaves, young panicles, spikelets, and developing seeds. Expression was strongest for OsCpn60β1, moderate for OsCpn60α1, OsCpn60α2, OsCpn60β2, and OsCpn60β3, and low for OsCpn60α3 (Fig. 1). Whereas OsCpn60α2 and OsCpn60β2 were dominantly expressed in developing seeds, transcript levels for the other genes were higher in the flag leaves. Our results are consistent with public microarray data from the Rice Oligonucleotide Array database (http://www.ricearray.org/) (Supplementary Fig. S3).
Fig. 1.
Temporal-spatial expression patterns of plastid Cpn60 genes using quantitative real-time RT-PCR. Roots and leaves were sampled from 14-d-old seedlings grown in soil at constant 27°C/light. Flag leaves and spikelets at heading stage, young panicles (2–5 cm long), and developing seeds at 10 d after pollination were sampled from paddy field. UBQ5 was internal control. Error bars indicate standard deviations; n = 3.
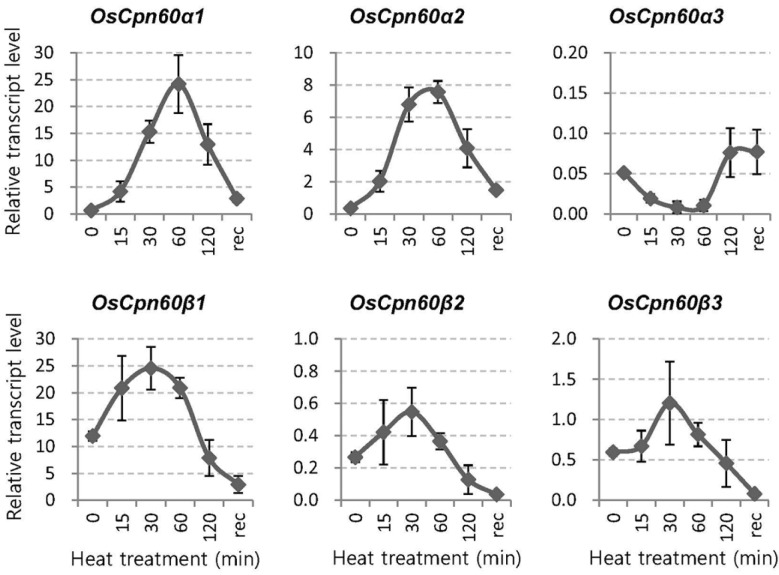
To examine whether these genes are responsive to heat or cold stress, we subjected seedlings first grown at 27°C to either 42°C or 10°C. Within 1 h of heat exposure, mRNA levels were significantly increased for α1 and α2 genes but not for the α3 gene (Fig. 2). By comparison, transcription levels were elevated by approximately two-fold for all three β genes after such treatment. This indicated that the α1 and α2 proteins have dominant functions under heat stress. In contrast, cold conditions did not affect the expression of any of these six genes (Supplementary Fig. S4).
Fig. 2.
Expression of plastid Cpn60 genes in response to heat stress. Seedlings (14-d-old) first grown in soil at constant 27°C were subjected to 42°C. Their third leaves were sampled during treatment (15, 30, 60, and 120 min) and in recovery (2 h after end of exposure period, when returned to 27°C). UBQ5 was internal control. Error bars indicate standard deviations; n = 3.
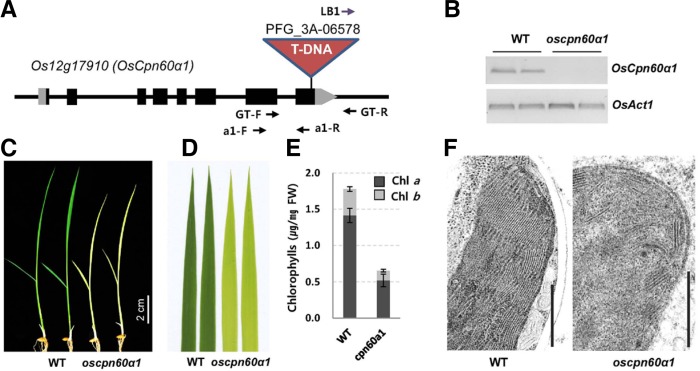
The oscpn60α1 mutant showed pale-green and seedling-lethal phenotypes
We obtained a T-DNA insertion mutant in the eighth exon of OsCpn60α1 (Fig. 3A). Its transcripts were barely detectable in the T-DNA homozygous line (Fig. 3B), suggesting that oscpn60α1 is a null allele. Homozygous mutant seedlings were pale green (Figs. 3C and 3D). In progeny of the OsCpn60α1/oscpn60α1 heterozygote, that phenotype was segregated in an approximately 3:1 ratio (WT:oscpn60α1); all pale-green seedlings were oscpn60α1 homozygous plants. This indicated that the mutant phenotype was recessive and was caused by a defect in a single genomic locus. When leaf chlorophyll contents were measured, we found that both chlorophyll a and b wer e severely decreased in the mutant, to approximately 37% of that recorded from the WT siblings (Fig. 3E). That is, after the fourth leaf emerged from an oscpn60α1 plant, no further leaves were produced, resulting in a lethal character. TEM analyses showed that chloroplast development was impaired in the mutant (Fig. 3F).
Fig. 3.
Phenotypes of oscpn60α1 mutant. (A) Schematic diagram of OsCpn60α1 genomic structure. Black box, exon; gray box, UTR region. T-DNA insertion, genotyping primers (GT-F, GT-R, and LB1), and RT-PCR primers (a1-F and a1-R) are depicted. (B) RT-PCR analysis of OsCpn60α1 expression in segregant WT and oscpn60α1 mutants. OsActin1 was internal control. (C) Phenotypes of oscpn60α1 mutant; 11-d-old seedlings were photographed. (D) Enlarged image of 3rd leaves in C. (E) Quantification of chlorophyll after being extracted from 3rd leaves of 10-d-old seedlings grown at constant 27°C. Error bars indicate standard deviations; n = 5. (F) TEM image of chloroplasts. Scale bar = 0.5 μm.
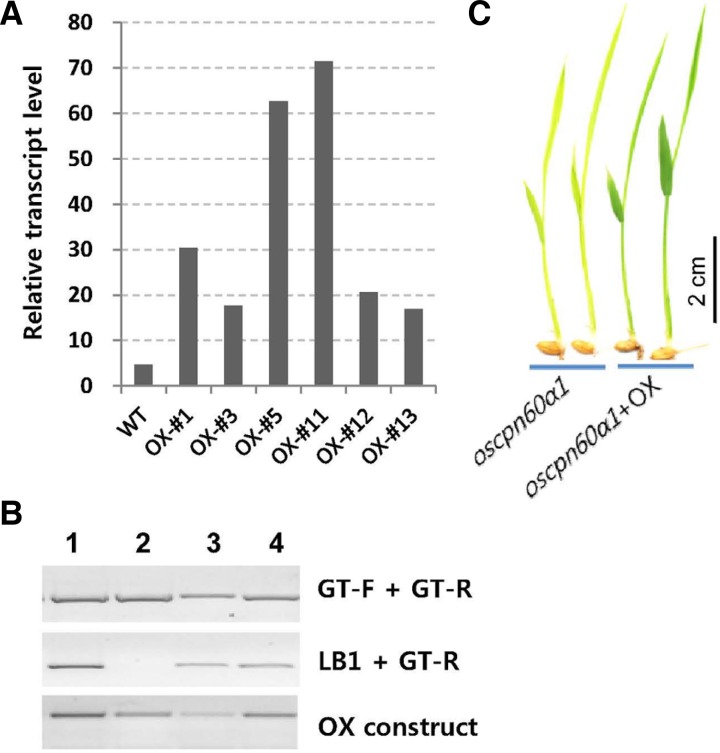
To test their heat tolerance, we generated transgenic plants that over-express OsCpn60α1. Its protein coding region was laid between the maize Ubiquitin1 promoter and the NOS transcription terminator. From 20 transgenics, we selected six that strongly over-expressed that gene (Fig. 4A). After they were exposed to a constant 40°C, we found that they developed chlorotic leaves to the same extent as similarly treated WT plants, thus indicating that OsCpn60α1overexpression did not confer heat tolerance.
Fig. 4.
Complementation of oscpn60α1 mutant by introducing OsCpn60α1 overexpression construct. (A) Relative expression level of overexpressing (OX) lines. For genetic complementation, OX-#3 line was used as female donor for crossing with OsCpn60α1/oscpn60α1 line. (B) PCR analysis of complementation. Among 4 F1 progeny, plants 1, 3, and 4 carried OX construct in OsCpn60α1/oscpn60α1 heterozygous background. Genotyping primers are described in Fig. 1A. (C) Phenotypes of F2 plants of oscpn60α (left) and oscpn60α carrying OsCpn60α1-OX construct.
To verify that the mutant phenotypes were due to a lack of OsCpn60α1 functioning, we introduced the OsCpn60α1 OX construct into the mutant. The oscpn60α1 mutants carrying the OsCpn60α1 transgene developed normal green leaves (Fig. 4C), thereby demonstrating that the mutant phenotype was due to an absence of functional OsCpn60α1.
Sub-cellular localization of OsCpn60α1
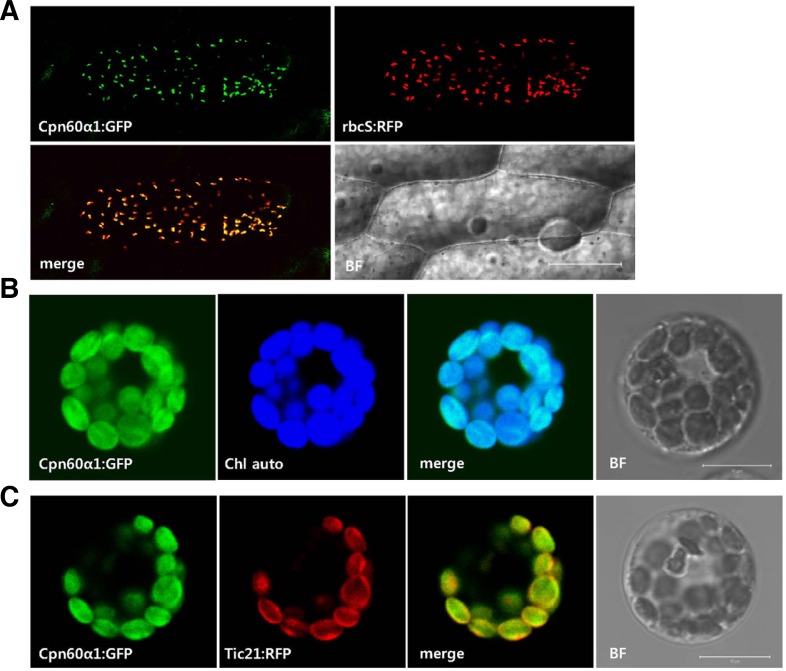
We performed transient expression analysis to examine the sub-cellular localization of OsCpn60α1. Full-length cDNA was fused to the GFP coding region in-frame and placed under the CaMV 35S promoter. The construct was co-introduced with the plastid marker plasmid p35S-rubisco small subunit (rbcS):RFP into onion epidermal cells by particle bombdardment. From this, we observed that the GFP signal was merged with RFP (Fig. 5A), indicating that OsCpn60α1 was localized to the plastids. To confirm this, we used protoplasts isolated from rice seedlings and noted that the OsCpn60α1:GFP signal was overlaid with chlorophyll autofluorescence (Fig. 5B).
Fig. 5.
Subcellular localizations of OsCpn60α1. (A) p35S-OsCpn60α1: GFP construct was co-transfected with plasmid marker p35S-rbcS: RFP into onion epidermis cells by particle bombdardment. Scale bar = 100 μm. (B) OsCpn60α1:GFP fusion protein was expressed alone in rice mesophyll protoplasts. GFP signal was merged with chlorophyll auto-fluorescence (Chl auto). (C) p35S-OsCpn60α1:GFP construct was co-electroporated with p35SAtTic21: RFP plasmid (a chloroplast inner-membrane marker) into rice mesophyll protoplasts. BF, bright field; Scale bar = 10 μm.
Cpn60 is a major protein immuno-precipitated by Tic110, a component of the inner-membrane import apparatus (Kessler and Blobel, 1996). There, it is thought to assist in the re-folding of proteins imported into the chloroplasts (Li and Chiu, 2010). To test whether OsCpn60α1 is co-localized with the Tic complex, we used rice mesophyll protoplasts to co-electroporate the Cpn60 construct with the p35S-AtTic21:RFP vector, which encodes a subunit of the Tic complex (Teng et al., 2006). This experiment showed that the OsCpn60α1:GFP fusion protein was co-localized with AtTic21::RFP, which was located mainly in the envelope. However, the Cpn60 protein was also found in the stroma (Fig. 5C). These results indicated that OsCpn60α1 occurs predominantly in the stromal region but also has a minor association with the chloroplast inner membrane.
The rbcL protein level was dramatically reduced in the oscpn60α1 mutant
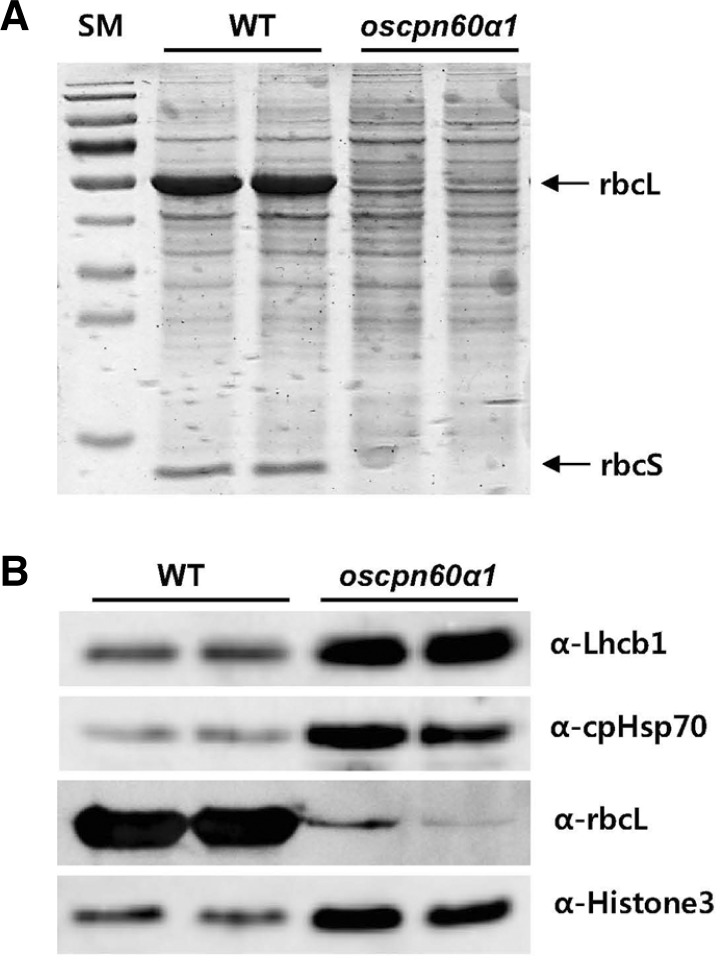
We analyzed chloroplast proteins using immuno blot analysis. Total proteins were extracted from seedling leaves. Visualization by CBB-staining revealed two major bands that we de-duced as rbcL encoded from the plastid genome and a Rubisco small subunit (rbcS) encoded from the nuclear genome. Both were severely diminished in the mutant (Fig. 6A). To confirm that one of those lost bands was rbcL, we conducted Western blotting with the anti-rbcL antibody and found that the rbcL level was dramatically reduced in the mutant (Fig. 6B).
Fig. 6.
Immuno-blot analysis of chloroplast proteins. Equal amounts of total protein extracted from 3rd leaves of segregant WT and oscpn60α1 seedlings were separated by SDS-PAGE, visualized with CBB-staining (A), and immuno-detected by antibodies against chloroplast proteins Lhcb1, chloroplast Hsp70, and rbcL (B). Histone3 was loading control.
Nuclear-encoded chloroplast proteins were also tested. Neither the chloroplast-localized Hsp70 (cpHsp70) nor the light-harvesting chlorophyll a/b binding protein 1 (Lhcb1), a subunit of the major light-harvesting antenna complex II (LHCII) in the thylakoid membrane, was reduced in the mutant (Fig. 6B). This suggested that folding of those proteins was not dependent on OsCpn60α1.
DISCUSSION
Rice plastid Cpn60 family
Subunit types for plant plastid Cpn60s are assumed to have arisen from an ancient gene duplication event (Archibald et al., 2000; Wastl et al., 1999). The numbers of subunits vary among land plant species (Cloney et al., 1994; Suzuki et al., 2009). Rice contains three α and three β subunits. Our study showed that expression of the rice Cpn60 genes is developmentally regulated. For example, OsCpn60α1 was preferentially functional in vegetative organs, especially the flag leaves, whereas OsCpn60α2 was expressed primarily in developing seeds. Among the β subunit genes, OsCpn60β1 expression was much higher than that of the other two, suggesting that it functions dominantly as a molecular chaperone.
In contrast to bacterial GroEL and mitochondrial Hsp60s that are composed of identical subunits, the plastid Cpn60 chaperonins from several plant species have roughly equal amounts of two distinct subunits, α and β (Bonk et al., 1996; Musgrove et al., 1987; Nishio et al., 1999; Viitanen et al., 1995). Through expression analysis we observed similar trends between the α and β subunit genes, with patterns for OsCpn60α1 and OsCpn60α3 resembling those for OsCpn60β1 and OsCpn60β3. Expression was high in flag leaves and low in roots and young panicles. Similar patterns were found between OsCpn60α2 and OsCpn 60β2, which were preferentially expressed in the seeds. These results implied that the co-expressed α and β subunits form a functional hetero-oligomer. For example, α2 and β2 act as a chaperone that preferentially functions in seeds.
In a reconstitution assay of Cpn60 oligomers that used pea α and β monomers expressed in E. coli, Dickson et al. (2000) showed that the β subunit monomers or α/β mixed monomers, but not the α subunit monomers, form functional tetradecamers in the presence of chaperonin 10 (Cpn10). Moreover, Arabidopsis β homo-oligomers function in vitro with authentic chloroplast Cpn10s (Vitlin et al., 2011). Among our six rice Cpn60 genes, OsCpn60β1 expression was the strongest, presenting the possibility that it creates a functional β1 homo-oligomer in planta.
An Arabidopsis mutant lacking AtCpn60β1 is more sensitive to heat stress when compared with WT plants (Ishikawa et al., 2003). Transcription of OsCpn60α1 and OsCpn60α2 was strongly induced by exposure to a high temperature while all of the β subunit genes were only slightly induced. This result is consistent with the heat transcriptome analyses (Jung and An, 2012; Jung et al., 2012). Therefore, these expression data suggest that the α1 and α2 subunits function dominantly under heat stress.
OsCpn60α1 is an essential subunit
In the early seedling stage, oscpn60α1 null plants developed a pale-green phenotype and did not produce additional leaves after the fourth one emerged, resulting in lethality. This outcome demonstrated that OsCpn60α1 is essential for seedling growth. Similarly, an Arabidopsis mutant lacking AtCpn60α1 has white cotyledons and fails to develop further (Apuya et al., 2001). However, the oscpn60α1 phenotypes are milder than those of that Atcpn60α1 mutant. Whereas the Arabidopsis mutants do not develop any leaves and their cotyledons do not accumulate chlorophyll, our rice mutants produced four leaves with reduced levels of chlorophyll. This was probably due to the presence of two genes in rice that are homologous to the Arabidopsis α1 gene (Supplementary Fig. S2B). Those rice genes were expressed at similar levels in the seedling leaves. In Arabidopsis, a single mutant of AtCpn60β1 or AtCpn60β2 can complete its life cycle, but plants of the double mutant will be albino (Suzuki et al., 2009). Because our oscpn60α1 mutant did not survive beyond the four-leaf stage, we could conclude that other Cpn60 subunits cannot fully complement the α1 function and that substrates of OsCpn60α1 differ from those of other subunits.
OsCpn60α1 is a key factor for folding rbcL
The Arabidopsis chloroplast contains approximately 3000 nucleus-encoded proteins (Leister, 2003) and 88 plastid genome-encoded proteins (Li and Chiu, 2010). To gain functional activity these proteins should be folded correctly. In E. coli, approximately 250 different proteins interact with GroEL chaperonin; however, obligate GroEL-dependence is limited to only ∼85 substrates, including 13 essential proteins (Kerner et al., 2005).
In our oscpn60α1 plants, only a trace amount of rbcL was observed but levels of Lhcb1 and stromal Hsp70 proteins were not altered. This indicated that OsCpn60α1 is a key factor for rbcL-folding in the rice chloroplast. Association of a newly synthesized rbcL with Cpn60 has been reported in higher plants (Barraclough and Ellis, 1980; Cannon et al., 1986; Gatenby and Ellis, 1990; Gutteridge and Gatenby, 1995; Roy, 1989; Roy and Cannon, 1988). The rbcL-Cpn60 binary complex is considered an obligatory intermediate before assembly into the Rubisco holoenzyme (Gutteridge and Gatenby, 1995; Liu et al., 2010). However, Cpn60 has not been shown to bind with newly synthesized rbcS subunits during or after their import from the cytosol (Ellis and Hemmingsen, 1989). The amount of rbcL subunit is unchanged in four mutants of Arabidopsis: β1 (Ishikawa et al., 2003; Suzuki et al., 2009), β2 (Suzuki et al., 2009), β1/β2 double (Suzuki et al., 2009), and β4 (Peng et al., 2011). Thus, our results provide the first functional identification of a Cpn60 subunit that is involved in the folding of rbcL.
Supplementary Material
Acknowledgments
We thank Kyungsook An for seed management, Inhwan Hwang for providing the p35S-rbcS::RFP vector, and Priscilla Licht for English editing. This work was supported by grants from the Next-Generation BioGreen 21 Program (No. PJ008215), Rural Development Administration, Republic of Korea; the National Research Foundation of Korea (NRF) grant, the Ministry of Education, Science and Technolgy (MEST), Republic of Korea (2012055219); and Kyung Hee University (20110269).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- An S, Park S, Jeong DH, Lee DY, Kang HG, Yu JH, Hur J, Kim SR, Kim YH, Lee M, et al. Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant Physiol. 2003;133:2040–2047. doi: 10.1104/pp.103.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apuya NR, Yadegari R, Fischer RL, Harada JJ, Zimmerman JL, Goldberg RB. The Arabidopsis embryo mutant schlepperless has a defect in the chaperonin-60α gene. Plant Physiol. 2001;126:717–730. doi: 10.1104/pp.126.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald JM, Logsdon JM, Jr, Doolittle WF. Origin and evolution of eukaryotic chaperonins: phylogenetic evidence for ancient duplications in CCT genes. Mol Biol Evol. 2000;17:1456–1466. doi: 10.1093/oxfordjournals.molbev.a026246. [DOI] [PubMed] [Google Scholar]
- Armstrong GA, Runge S, Frick G, Sperling U, Apel K. Identification of NADPH: protochlorophyllide oxidoreductases A and B: a branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol. 1995;108:1505–1517. doi: 10.1104/pp.108.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough R, Ellis RJ. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated pea chloroplasts. Biochim. Biophys. Acta. 1980;608:19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Bonk M, Tadros M, Vandekerckhove J, Al-Babili S, Beyer P. Purification and characterization of chaperonin 60 and heat-shock protein 70 from chromoplasts of Narcissus pseudonarcissus. Plant Physiol. 1996;111:931–939. doi: 10.1104/pp.111.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. The crystal structure of the bacterial chaperonin GroEL at 2.8 Á. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Cannon S, Wang P, Roy H. Inhibition of ribulose bisphosphate carboxylase assembly by antibody to a binding protein. J Cell Biol. 1986;103:1327–1335. doi: 10.1083/jcb.103.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloney LP, Bekkaoui DR, Feist GL, Lane WS, Hemmingsen SM. Brassica napus plastid and mitochondrial chaperonin-60 proteins contain multiple distinct polypeptides. Plant Physiol. 1994;105:233–241. doi: 10.1104/pp.105.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R, Weiss C, Howard RJ, Alldrick SP, Ellis RJ, Lorimer G, Azem A, Viitanen PV. Reconstitution of higher plant chloroplast chaperonin 60 tetradecamers active in protein folding. J Biol Chem. 2000;275:11829–11835. doi: 10.1074/jbc.275.16.11829. [DOI] [PubMed] [Google Scholar]
- Ditzel L, Löwe J, Stock D, Stetter KO, Huber H, Huber R, Steinbacher S. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell. 1998;93:125–138. doi: 10.1016/s0092-8674(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hemmingsen SM. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989;14:339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby AA, Ellis RJ. Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol. 1990;6:125–149. doi: 10.1146/annurev.cb.06.110190.001013. [DOI] [PubMed] [Google Scholar]
- Glatz A, Horváth I, Varvasovszki V, Kovács E, Török Z, Vigh L. Chaperonin genes of the Synechocystis PCC 6803 are differentially regulated under light-dark transition during heat stress. Biochem Biophys Res Commun. 1997;239:291–297. doi: 10.1006/bbrc.1997.7463. [DOI] [PubMed] [Google Scholar]
- Gould PS, Burgar HR, Lund PA. Homologous cpn60 genes in Rhizobium leguminosarum are not functionally equivalent. Cell Stress Chaper. 2007;12:123–131. doi: 10.1379/CSC-227R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S, Gatenby AA. Rubisco synthesis, assembly, mechanism and regulation. Plant Cell. 1995;7:809–819. doi: 10.1105/tpc.7.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MJ, Jung KH, Yi G, Lee DY, An G. Rice Immature Pollen 1 (RIP1) is a regulator of late pollen development. Plant Cell Physiol. 2006;47:1457–1472. doi: 10.1093/pcp/pcl013. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen SM, Ellis RJ. Purification and properties of ribulose bisphosphate carboxylase large subunit binding protein. Plant Physiol. 1986;80:269–276. doi: 10.1104/pp.80.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, Hendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hill JE, Hemmingsen SM. Arabidopsis thaliana type I and II chaperonins. Cell Stress Chaper. 2001;6:190–200. doi: 10.1379/1466-1268(2001)006<0190:attiai>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Tanaka H, Nakai M, Asahi T. Deletion of a chaperonin 60β gene leads to cell death in the Arabidopsis lesion initiation 1 mutant. Plant Cell Physiol. 2003;44:255–261. doi: 10.1093/pcp/pcg031. [DOI] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;45:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000;22:561–570. doi: 10.1046/j.1365-313x.2000.00767.x. [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Park S, Kang HG, Park GG, Kim SR, Sim J, Kim YO, Kim MK, Kim SR, et al. Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J. 2006;45:123–132. doi: 10.1111/j.1365-313X.2005.02610.x. [DOI] [PubMed] [Google Scholar]
- Jung KH, An G. Application of MapMan and RiceNet drives systematic analyses of the early heat stress transcriptome in rice seedlings. J Plant Biol. 2012;55:436–449. [Google Scholar]
- Jung KH, Gho HJ, Kim SR, Ronald PC, An G. Genome-wide identification and analysis of early heat stress responsive genes in rice. J Plant Biol. 2012;55:458–468. [Google Scholar]
- Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, Sasamoto S, Watanabe A, Idesawa K, Iriguchi M, Kawashima K, et al. Complete genomic sequence of nitrogenfixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002;9:225–256. doi: 10.1093/dnares/9.6.225. [DOI] [PubMed] [Google Scholar]
- Kerner MJ, Naylor DJ, Ishihama Y, Maier T, Chang HC, Stines AP, Georgopoulos C, Frishman D, Hayer-Hartl M, Mann M, et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell. 2005;122:209–220. doi: 10.1016/j.cell.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Kessler F, Blobel G. Interaction of the protein import and folding machineries in the chloroplast. Proc. Natl. Acad. Sci. USA. 1996;93:7684–7689. doi: 10.1073/pnas.93.15.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Lee DY, Yang JI, Moon S, An G. Cloning vectors for rice. J Plant Biol. 2009;52:73–78. [Google Scholar]
- Kim SR, Jeon JS, An G. Development of an efficient inverse PCR method for isolating gene tags from T-DNA insertional mutants in rice. Methods Mol Biol. 2011;678:139–146. doi: 10.1007/978-1-60761-682-5_11. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeon JS, Jung KH, An G. Binary vectors for efficient transformation of rice. J Plant Biol. 1999;42:310–316. [Google Scholar]
- Lee S, Kim YS, Jeon US, Kim YK, Schjoerring JK, An G. Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol. Cells. 2012;33:269–275. doi: 10.1007/s10059-012-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D. Chloroplast research in the genomic age. Trends Genet. 2003;19:47–56. doi: 10.1016/s0168-9525(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Li HM, Chiu CC. Protein transport into chloroplasts. Annu Rev Plant Biol. 2010;61:157–180. doi: 10.1146/annurev-arplant-042809-112222. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad R, editor. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc.; 2001. pp. F4.3.1–F4.3.8. [Google Scholar]
- Liu C, Young AL, Starling-Windhof A, Bracher A, Saschenbrecker S, Rao BV, Rao KV, Berninghausen O, Mielke T, Hartl FU, et al. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Nature. 2010;463:197–202. doi: 10.1038/nature08651. [DOI] [PubMed] [Google Scholar]
- Martel R, Cloney LP, Pelcher LE, Hemmingsen SM. Unique composition of plastid chaperonin-60: alpha and beta polypeptide-encoding genes are highly divergent. Gene. 1990;94:181–187. doi: 10.1016/0378-1119(90)90385-5. [DOI] [PubMed] [Google Scholar]
- Milos P, Roy H. ATP-released large subunits participate in the assembly of RuBP carboxylase. J Cell Biochem. 1984;24:153–162. doi: 10.1002/jcb.240240206. [DOI] [PubMed] [Google Scholar]
- Musgrove JE, Johnson RA, Ellis RJ. Dissociation of the ribulose bisphosphate-carboxylase large-subunit binding protein into dissimilar subunits. Euro J Biochem. 1987;163:529–534. doi: 10.1111/j.1432-1033.1987.tb10900.x. [DOI] [PubMed] [Google Scholar]
- Nishio K, Hirohashi T, Nakai M. Chloroplast chaperonins: evidence for heterogeneous assembly of alpha and beta Cpn60 polypeptides into a chaperonin oligomer. Biochem Biophys Res Commun. 1999;266:584–587. doi: 10.1006/bbrc.1999.1868. [DOI] [PubMed] [Google Scholar]
- Peng L, Fukao Y, Myouga F, Motohashi R, Shinozaki K, Shikanai T. A chaperonin subunit with unique structures is essential for folding of a specific substrate. PLoS Biol. 2011;9:e1001040. doi: 10.1371/journal.pbio.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rodríguez-Quiñones F, Maguire M, Wallington EJ, Gould PS, Yerko V, Downie JA, Lund PA. Two of the three groEL homologues in Rhizobium leguminosarum are dispensable for normal growth. Arch Microbiol. 2005;183:253–265. doi: 10.1007/s00203-005-0768-7. [DOI] [PubMed] [Google Scholar]
- Roy H. Rubisco assembly: a model system for studying the mechanism of chaperonin action. Plant Cell. 1989;1:1035–1042. doi: 10.1105/tpc.1.11.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H, Cannon S. Ribulose bisphosphate carboxylase assembly: what is the role of the large subunit binding protein? Trends Biochem Sci. 1988;13:163–165. doi: 10.1016/0968-0004(88)90139-9. [DOI] [PubMed] [Google Scholar]
- Saibil H. Molecular chaperones: containers and surfaces for folding, stabilising or unfolding proteins. Curr Opin Struct Biol. 2000;10:251–258. doi: 10.1016/s0959-440x(00)00074-9. [DOI] [PubMed] [Google Scholar]
- Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem. 1998;67:581–608. doi: 10.1146/annurev.biochem.67.1.581. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakanishi H, Bower J, Yoder DW, Osteryoung KW, Miyagishima SY. Plastid chaperonin proteins Cpn60α and Cpn60β are required for plastid division in Arabidopsis thaliana. BMC Plant Biol. 2009;9:38. doi: 10.1186/1471-2229-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YS, Su YS, Chen LJ, Lee YJ, Hwang I, Li HM. Tic21 is an essential translocon component for protein translocation across the chloroplast inner envelope membrane. Plant Cell. 2006;18:2247–2257. doi: 10.1105/tpc.106.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen PV, Schmidt M, Buchner J, Suzuki T, Vierling E, Dickson R, Lorimer GH, Gatenby A, Soll J. Functional characterization of the higher plant chloroplast chaperonins. J Biol Chem. 1995;270:18158–18164. doi: 10.1074/jbc.270.30.18158. [DOI] [PubMed] [Google Scholar]
- Vitlin A, Weiss C, Demishtein-Zohary K, Rasouly A, Levin D, Pisanty-Farchi O, Breiman A, Azem A. Chloroplast β chaperonins from A. thaliana function with endogenous cpn10 homologs in vitro. Plant Mol Biol. 2011;77:105–115. doi: 10.1007/s11103-011-9797-6. [DOI] [PubMed] [Google Scholar]
- Wastl J, Fraunholz M, Zauner S, Douglas S, Maier UG. Ancient gene duplication and differential gene flow in plastid lineages: the GroEL/Cpn60 example. J Mol Evol. 1999;48:112–117. doi: 10.1007/pl00006438. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bonshtien A, Farchi-Pisanty O, Vitlin A, Azem A. Cpn20: Siamese twins of the chaperonin world. Plant Mol Biol. 2009;69:227–238. doi: 10.1007/s11103-008-9432-3. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu Y, Tanaka K, Tagawa T, Nakamura Y, Matsuo T, Okamoto S. Improvement of DNA/Metal particle adsorption in tungsten-based biolistic bombardment; Alkaline pH is necessary for DNA adsorption and suppression of DNA degradation. J Plant Biol. 2009;52:524–532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.