Abstract
Synthetic biology is an emerging discipline for designing and synthesizing predictable, measurable, controllable, and transformable biological systems. These newly designed biological systems have great potential for the development of cheaper drugs, green fuels, biodegradable plastics, and targeted cancer therapies over the coming years. Fortunately, our ability to quickly and accurately engineer biological systems that behave predictably has been dramatically expanded by significant advances in DNA-sequencing, DNA-synthesis, and DNA-editing technologies. Here, we review emerging technologies and methodologies in the field of building designed biological systems, and we discuss their future perspectives.
Keywords: genome, genome editing, next-generation sequencing, synthetic biology
INTRODUCTION
With the remarkable development of sequencing technology, the genomes of diverse species from bacteria to human have been completely sequenced. The identification of unknown organisms via genome-sequencing analyses has also been rapidly performed as soon as the species are discovered (Pagani et al., 2012). In addition, sequencing speed has been greatly accelerated by the recent advent of next-generation sequencing (NGS) methods, which appeared in the last decade following the conventional, capillary-based sequencing methods. NGS methods analyze various sizes and types of DNA libraries more accurately, rapidly, and cheaply in a high-throughput fashion (Mardis, 2011; Metzker, 2010; Pareek et al., 2011; Shendure and Lieberman Aiden, 2012; Soon et al., 2013; Zhang et al., 2011). NGS provides powerful tools, not only to discover fundamental biological information, such as whole-genome sequences, variations, and DNA-protein interactions, but also to apply to diagnostics and clinical uses (Encode Project Consortium et al., 2012). For instance, the first human genome projects required 10 years and 3 years for sequencing and analysis, respectively; whereas current NGS technology allows the sequencing of multiple human genomes in a single run, requiring only a single week and reagent cost of less than $5,000 per genome (Lander et al., 2001; Soon et al., 2013; Venter et al., 2001).
The genome sequences contributed tremendously to all bioscience-related studies across biochemistry, agriculture, bioengineering, and medicine, as well as to the bioindustrial production of value-added products such as platform chemicals (Du et al., 2013; Esvelt and Wang, 2013; Heitzer et al., 2013; Reyes et al., 2012; Shokralla et al., 2012). However, the genome sequences did not merely make contributions to the deeper understanding of the molecular mechanisms of cellular function; they also provided engineering targets for various genome-manipulation technologies such as zinc-finger nuclease (ZFN) and transcription activator-like effector nucleases (TALENs) (Bedell et al., 2012a; Cong et al., 2013; Jiang et al., 2013; Kim et al., 2012a; Mali et al., 2013; Wang et al., 2012a). These recent breakthroughs in genome-engineering technologies are now offering new opportunities to produce rationally designed biological functions, which are critical issues that should be addressed by synthetic biology.
A major objective of synthetic biology is the design and engineering of biologically based parts, novel devices, and systems, as well as the redesign of existing biological systems (Endy, 2005; Nandagopal and Elowitz, 2011; Pleiss, 2006). A wide range of biological systems can be engineered to add new functions or to edit existing functions according to a user’s purpose in a modular, reliable, and predictable way. This is possible because biological functions are inherently expressed through proteins and RNAs that are primarily encoded in DNA sequences. In addition, biological regulatory elements such as logic gates, feedback systems, and oscillators can be defined (Khalil and Collins, 2010). Thus, synthetic biology has great potential to deliver important new applications and improve existing industrial processes across many sectors including energy, pharmaceuticals, and materials (Martin et al., 2009; Medema et al., 2011; 2012). Here, we review the emerging tools currently available for synthetic biology that can be integrated to design and build novel biological systems. The future perspective of this new, emerging field is also discussed.
Genome analysis
Next-generation sequencing
DNA sequencing, which has been widely used over 30 years, has made great advances in molecular biology, genetics, diagnosis, and bioinformatics (Sanger et al., 1977). It had inevitable limitations, however, to generate whole-genome sequences by an individual researcher because of its speed, cost, and resolution. The advent of new technology for high-throughput DNA sequencing, NGS, overcame these barriers and caused a revolution in genome science.
Currently, three representative sequencers with different platform technologies are commercially available. The first commercial NGS platform was the Roche 454 System based on ‘pyrosequencing’. Instead of sequencing in multiple tubes or microplate wells, the DNA library is amplified by emulsion polymerase chain reaction (PCR) on the surfaces of hundreds of thousands of beads (Dressman et al., 2003; Margulies et al., 2005). The current GS FLX+ system can sequence about 500 Mb of raw sequence in 23 h with a consensus accuracy of 99.99% and an average read length of 1,000 bp. Compared with other sequencing platforms, the Roche 454 system has an advantage in mapping repetitive regions because of its longer read length; but it has the disadvantage of an increasing error rate when sequencing a homopolymer: a row of repeats of the same short sequence (Rothberg and Leamon, 2008). The second commercial platform, the Illumina Genome Analyzer, is based on sequencing by synthesis and emerged in 2006. Different from the method using micro-beads mentioned above, the adaptor-linked DNA library binds to complementary adaptors immobilized in a flow cell and forms clusters via bridge amplification (Adessi et al., 2000). The clustered library produces a signal by incorporating four differentially labeled fluorescent dNTPs, and the sequencing progresses by repeating the process. The HiSeq 2,500 system currently produces a maximum read length of two 100 bp paired-end reads in high-output mode, which results in about 600 Gb of output within 11 days. The MiSeq system, a bench-top sequencer, produces a read length of two 250 bp paired-end reads and a maximum of 8.5 Gb of data per run. The third commercial system, the Sequencing by Oligonucleotide Ligation and Detection (SOLiD) sequencer using the Polonator technology, was commercially released in 2007 (Shendure et al., 2005). The open-source sequencer uses emulsion PCR, to immobilize the DNA library onto a solid support, and cyclic sequencing-by-ligation chemistry. A recent version, the 5,500 Series Genetic Analysis Systems, can sequence a maximum of 20 Gb of DNA per day. Personal sequencers, such as the MiSeq and Ion torrent™, of small size are efficient for relatively fast analyses. The difference of the Ion torrent™ system compared with other sequencing techniques is the use of a semiconductor-based sequencing technique rather than signal detection methods, such as the use of fluorescent dyes. In this system, when a dNTP is incorporated into DNA, it produces a pyrophosphate and a proton. The DNA library is immobilized in microwells, and when a dNTP is incorporated into the DNA by DNA polymerase, the change in pH caused by the protons can be detected as a voltage change. Sequencing data can be obtained rapidly and at low cost, because the Ion torrent™ system does not use expensive dNTPs labeled with fluorescent dyes and an optical detection device. The Ion PGM sequencer produces a maximum read length of 400 bp and generates between 1 and 2 Gb of data within several hours. The maximum read length and the total output of each platform is continuously increasing.
Unlike the NGS platforms that depend on DNA-library amplification, third-generation sequencing platforms determine DNA sequences directly from a single DNA molecule (Pareek et al., 2011; Schadt et al., 2010). This single-molecule sequencing can overcome sequencing errors and biases, caused by the favor of certain sequences during the amplification step, that potentially distort the abundances of various DNA fragments in the sample. These novel methods rely on sequencing by synthesis, sequencing by degradation, or sequencing by direct physical inspection of the DNA molecule via a specially engineered DNA polymerase, various artificial nanopores, or advanced microscopy techniques (Clarke et al., 2009; Zhang et al., 2011). Although most third-generation sequencing platforms are currently still in development, they will offer advantages over current technologies, such as higher throughput, faster turnaround time, longer read lengths, higher accuracy, smaller amounts of starting material, and lower sequencing costs (Schadt et al., 2010).
Genome sequencing
With its high accuracy, fast speed, and low cost, NGS is widely used to determine the genome sequences of various organisms and the variations between genome sequences (Pushkarev et al., 2009; Wheeler et al., 2008). As of June 2012, a total of 3920 bacterial genomes and 854 eukaryotic genomes have been completely sequenced (Pagani et al., 2012). In addition to routine de novo genome sequencing, NGS can be also used for the diagnosis and monitoring of pathogens including viruses, bacteria, fungi, and parasites. NGS can produce full information about the genotype of a pathogen within several hours and help to formulate a drug for an unknown pathogen as soon as possible for public health purposes. NGS could replace complicated and time-consuming techniques used in routine clinical microbiology practices with a simple, more efficient workflow (Didelot et al., 2012; Shendure and Lieberman Aiden, 2012; Soon et al., 2013).
The obvious applications of NGS are de novo genome sequencing, whole-genome resequencing, and targeted resequencing. The first application of NGS to genome sequencing was to elucidate Acinetobacter baumannii pathogenesis using 454 sequencing technology (Smith et al., 2007). Despite the sequencing power of NGS, the short reads obtained from NGS draw a setback in terms of genome assembly and mapping applications (Wold and Myers, 2008). For example, repetitive sequences are widely distributed across the entire human genome, therefore the short reads are placed equally at multiple chromosomal locations. Efforts in bioinformatics have been made to overcome the limitation; but many challenges still exist to either efficiently assemble short reads de novo or else align them to a reference genome. The currently available bioinformatics tools for short-read alignment and de novo and reference-guided assembly have been reported elsewhere (Martin and Wang, 2011; Zhang et al., 2011).
Nevertheless, NGS allows for very deep genomic coverage. Whole-genome resequencing is therefore being widely performed for the correct identification of SNPs and structural variations such as insertions, deletions, copy number variations, and rearrangements. For example, a large number of human genomic variations have been determined by multi-institute consortia projects (1000 Genomes Project Consortium et al., 2010; Ball et al., 2012; Encode Project Consortitum, 2012; Hugo Pan-Asian SNP Consortium et al., 2009). To date, more than 30 million SNPs have been discovered by human genome sequencing projects. Copy number variation has also been shown to be associated with various diseases including glomerulone-phritis (Aitman et al., 2006) and Crohn’s disease (McCarroll et al., 2008). Resequencing can also be applied to sequence an entire bacterial genome to identify acquired mutations. For instance, accumulated mutations in microbes adaptively evolved in the laboratory have been detected by comparing polymorphisms between ancestral and evolved genomes (Araya et al., 2010; Atsumi et al., 2010; Charusanti et al., 2010; Conrad et al., 2009; Kishimoto et al., 2010).
Resequencing is not about analyzing the whole genome but instead sequencing subsets of genes or specific parts of genome. Targeted resequencing analyzes the interesting parts and concentrates studies on specific targets. Comparing the specific sites between different samples may assist the discovery of novel biological mechanisms (Qi et al., 2013a; Ram et al., 2011). Exome sequencing is one resequencing method that identifies protein-coding regions. The method is valuable for the diagnosis of genetic diseases, such as human pancreatic cancer (Wang et al., 2012b) and Mendelian disorders (Ku et al., 2011), caused by mutations in coding regions.
Transcriptome sequencing
The physiological properties of cells are programmed by a set of RNA molecules defined as the transcriptome. This means that distinct sets of genes are simultaneously activated or repressed by diverse regulators in accordance with changing environments. Thus, it is important to measure the levels of RNA transcripts to elucidate physiologically relevant biological processes. To readily analyze genome-wide cellular transcription, microarrays based on hybridization have been widely available (Schena et al., 1995; Selinger et al., 2000). However, the array-based approach is limited by a high rate of noise due to cross-hybridization, the dynamic range of detection due to signal saturation, and the inability to detect transcripts with low copy numbers per cell. In addition, array-based approaches require extensive normalization based on complicated statistical calculations to compare expression data from different experiments (Pinto et al., 2011).
NGS addressed the limitations of the array-based approaches with the introduction of RNA-seq, which was first invented in both yeast and mammalian cells (Cloonan et al., 2008; Mortazavi et al., 2008; Nagalakshmi et al., 2008; Wilhelm et al., 2008). Unlike the array-based approaches, which depend on hybridization, RNA-seq allows the unambiguous mapping of transcripts to unique regions of the genome with single-base resolution. Hence, there is lower background noise (Soon et al., 2013). In addition to the accurate quantification of the transcriptome of known genes, RNA-seq allows the determination of correct gene annotation, expressed single nucleotide polymorphisms (SNPs), novel genes, and RNAs with high levels of reproducibility. Moreover, the strand-specific RNA sequencing principle is based on analyzing primary mRNA transcripts. Therefore, it allows the identification of more reliable and more accurate genome architectures (Cho et al., 2009; Kim et al., 2012b; Levin et al., 2010; Perkins et al., 2009; Qiu et al., 2010; Seo et al., 2012; Sharma et al., 2010).
Even without a reference genome, de novo transcriptome assembly can be done by using short reads from RNA-seq. This method has facilitated the reconstruction of the entire transcriptome (Grabherr et al., 2011; Martin and Wang, 2011). With the reconstructed transcriptome, BLAST or other gene prediction tools are used to identify functional annotation. Many assemblers, such as Multiple-k (Surget-Groba and Montoya-Burgos, 2010), Rnnotator (Martin et al., 2010), Trans-ABySS (Robertson et al., 2010), Oases (Schulz et al., 2012), and Trinity (Grabherr et al., 2011), have been developed. An example of de novo transcriptome assembly is the identification of novel genes related to useful biosynthetic pathways. Capsaicinoid from chili pepper, for example, is a practical compound that has various medical applications. Because the capsaicinoid biosynthetic pathway was not fully identified, de novo assembly was performed, and three novel structural genes in the biosynthetic pathway were discovered (Liu et al., 2013). Similarly, insecticide-resistance genes were identified in species of insect pests (Hsu et al., 2012). De novo transcriptome assembly can be more beneficial than genome sequencing alone, because RNA-seq not only reconstructs entire transcriptome, but it also measures the expression level of the target genes without genome sequences. Moreover, de novo transcriptome assembly with RNA-seq has the advantage of detecting additional transcription information, like alternative splicing sites in eukaryotes.
Interactome sequencing
To design and construct new synthetic circuits or pathways within a producer cell, synthetic biology demands the availability of standardized regulatory parts including promoters, ribosome-binding sites, terminators, DNA-binding proteins, and corresponding protein-binding sites (Cheng and Lu, 2012; Khalil and Collins, 2010; Nandagopal and Elowitz, 2011; Wang et al., 2013a). In this regard, NGS can be used to obtain the library of synthetic biology parts, enabling the development of novel devices and networks. The methods to identify the interactions between DNA and proteins (e.g., transcription factors) are chromatin immunoprecipitation (ChIP)-based techniques such as ChIP-seq and ChIP-exo (Johnson et al., 2007; Rhee and Pugh, 2011; Robertson et al., 2007). For ChIP-seq, DNA-protein complexes are specifically isolated by an antibody against the target protein. Purified DNA obtained from the immunoprecipitated DNA-protein complexes is ligated with sequencing adaptors, amplified by PCR, and massively sequenced. Unlike electrophoretic mobility shift assay, the sequencing results verify in vivo interactions between proteins and their corresponding binding sites. Although microarrays have been frequently used to map DNA-protein or RNA-protein interactions at the genome scale, NGS technologies are quickly replacing the use of microarrays. Compared with previous microarray-based results, ChIP-seq has superior resolution, requires less input DNA, produces less background noises, and has a better detection limit (Furey, 2012; Park, 2009; Valouev et al., 2008). In addition, NGS can be used to determine the interaction between RNA-binding proteins and RNA via a technique called ultraviolet (UV) crosslinking and immunoprecipitation (CLIP). After the irradiation of cells with UV 254 nm (HITS-CLIP) (Yeo et al., 2009) or UV 365 nm (PAR-CLIP) (Hafner et al., 2010), the cells are lysed, and RNA-protein complexes are pulled down using antibody-immobilized beads. Like ChIP-seq, protein-binding sites on RNAs are collected from the sequencing library.
ChIP-seq determines the targets of transcription factors in bacteria and eukaryotes. For instance, GlxR is one of the key transcriptional regulators in Corynebacterium glutamicum, an amino acid-producing bacterium. The investigation of C. glutamicum’s regulatory network using ChIP-seq revealed 21 novel binding sites and concluded that GlxR is responsible for carbon-source metabolism and energy conversion (Jungwirth et al., 2013). Taken together, this approach will greatly expand our understanding of genome-wide associations between proteins and DNA. It will also grow the library of synthetic biology parts, thus enhancing our ability to model cellular behavior.
Genome design
Minimal genome as a programmable biological chassis
Approximately 20% of the 4,000 genes found in E. coli have not been functionally annotated (Keseler et al., 2013). In addition, numerous other cellular components with unknown genes are the most significant barriers to rational genome design. The genome-reduction approach provides a chassis for the construction of synthetic genomes with improved metabolic efficiency by minimizing redundant genes and regulatory circuits. Four criteria can guide the design of a useful biological chassis: growth rate, fermentation ability, genetic manipulation, and additional gene deletion. For example, the deletion of large blocks of nonessential genes that are not needed for the metabolic pathways of interest can reduce the production of unwanted by-products, increase the genome stability, and simplify the metabolism without physiological compromise (Forster and Church, 2006; Giga-Hama et al., 2007; Glass et al., 2006; Morimoto et al., 2008; Posfai et al., 2006; Yu et al., 2002).
A method for creating random and repeated genomic deletions was developed to create deletions in Escherichia coli MG1655 using a Tn5 derivative: essentially, a rearranged composite transposon that promotes the use of the internal transposon ends (Goryshin et al., 2003). The method was used to minimize the E. coli MG1655 genome down to an average of 200 kb (averaging 10 kb per deletion) through 20 cycles of random integration and deletion steps. The deletion locations were confirmed by microarray-hybridization experiments. Lee et al. reengineered the E. coli MDS42 chromosome to be 14.3% smaller than that of its parental E. coli strain, MG1655 (Lee et al., 2009). The genome reduction increased the production of L-threonine in MDS42 by overexpressing a feedback-resistant threonine operon (thrA*BC), deleting the genes that encode threonine dehydrogenase (tdh) and threonine transporters (tdcC and sstT), and introducing a mutant threonine exporter (rhtA23) (Lee et al., 2009). The resulting strain, MDS-205, showed an ∼83% increase in L-threonine production compared with a wildtype E. coli strain. Westers et al. (2003) deleted 0.53 Mb (7.7% of the genome) from Bacillus subtilis, whose wild-type genome size is 4.2 Mb. Another B. subtilis strain, MGB874, was created by step-by-step deletions of 28 regions (20.7% of the genome) in which single deletions do not affect cell growth (Morimoto et al., 2008). When plasmids encoding extracellular cellulase and protease enzymes were transformed into MGB874, their productivities were enhanced up to 1.7- and 2.5-fold, respectively, relative to the wild-type strain. Genome reduction in Saccharomyces cerevisiae was also attempted to create a mutant genome in which 531.5 kb of the wild-type genome was deleted (Murakami et al., 2007). This mutant increased its ethanol and glycerol production by 1.8- and 2-fold, respectively; and its resistance to external stresses such as temperature, pH, salt, and changes of growth media was equivalent to that of the wild type. The deletion of a large genomic region in the fission yeast Saccharomyces pombe to find an efficient mutant for heterologous protein production was also achieved using homologous recombination between the chromosome and a fragment of linear DNA (Giga-Hama et al., 2007; Hirashima et al., 2006).
The genome-reduction approach has been focused on the deletion of unnecessary genes. Because the goal of synthetic biology is to implement in a cell artificial regulatory circuits that can be predictable, measurable, and controllable, the vast number of endogenous regulatory interactions should reduced and replaced by well-defined orthogonal equivalents.
Genome synthesis and assembly
The first synthetic genome was that of poliovirus (Cello et al., 2002). Subsets of the full-length viral genome were first synthesized and cloned separately into plasmids that were subsequently used to transform living cells. Recently, Gibson et al. (2010) achieved whole-genome design, synthesis, and assembly. They synthesized the 1.08 Mb Mycoplasma mycoides JCVI-syn1.0 genome starting from digitized genome-sequence information. Furthermore, they transplanted the chemically synthesized genome into a Mycoplasma capricolum recipient cell to make variants of M. mycoides that are controlled only by the synthetic chromosome. Thus, genome engineering is being greatly advanced by the improvement of DNA synthesis techniques in rapid time at an affordable price. The synthesis capacity has broadened from producing small primers to de novo synthesis of genes with any length and any complexity. Genescale synthesis is also becoming highly commercialized. It is now inexpensive: near $0.29 per bp. The chemical synthesis of up to 4.5 Mb in a month is now feasible. The high-quality error-correction methods save time and labor at a reasonable price, frequently requiring less time and labor than conventional molecular cloning and sequencing (Richmond et al., 2004; Tian et al., 2004).
Although the accuracy of the traditional DNA synthesis method is very high, the quantity of each reaction batch limits the synthesis of whole genomes. To overcome this problem, attempts have been made to synthesize massive numbers of oligonucleotides on solid supports, like beads and microarrays, in a high-throughput fashion (Tian et al., 2004). In 2010, the Church group introduced a new technology using a combinatorial method of microarray and Roche 454 to synthesize a target DNA library. First, microarray-derived oligonucleotide pools were amplified and sequenced using Roche 454. After the sequencing, the correctly synthesized oligonucleotide was extracted, and the DNA was re-amplified. Compared with the microarray library, the finalized library-error rate was reduced by 500 fold (Matzas et al., 2010). Many studies on using microarray-to-gene synthesis and expanding the length of the oligonucleotide have been attempted. The microarray-derived oligonucleotides were selectively amplified by specific short primers. Using the amplified oligonucleotide pool, the target genes were assembled, producing up to a total of 35 kb (Kosuri et al., 2010). In addition, a 0.5 to 1 kb oligonucleotide was individually synthesized on subarrays and endonucleased by the mismatch-specific endonuclease. This approach reduced the error rate to 0.19 errors per kb and assembled the target gene (Quan et al., 2011).
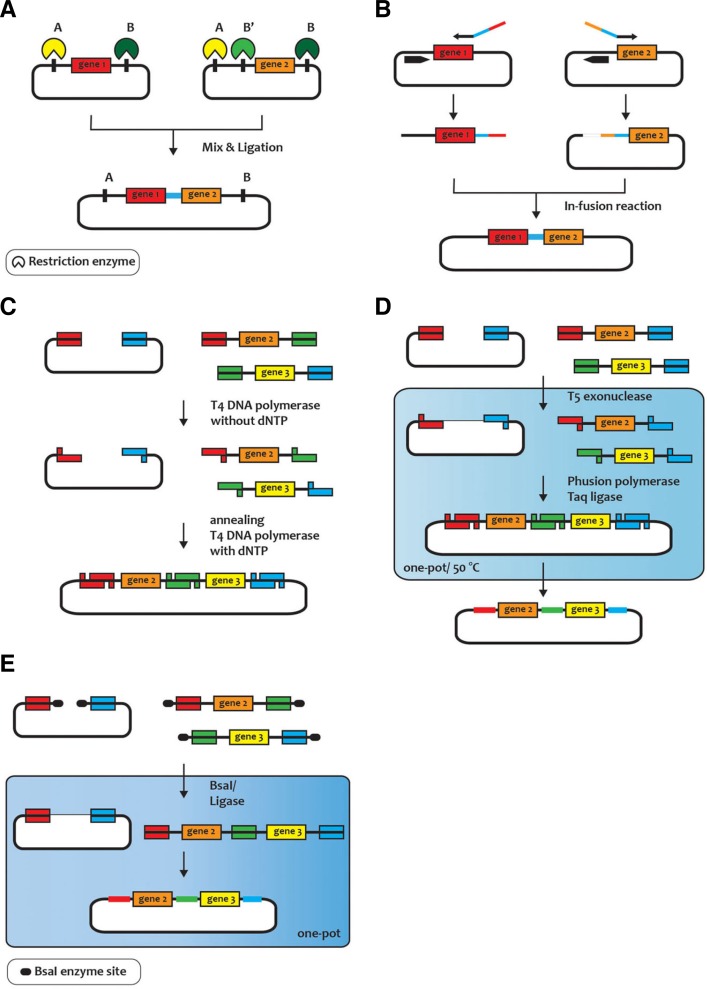
Gene assembly methods have been used to build targeted genetic circuits. For example, the BioBricks library contains various promoters, ribosome-binding sites (RBSs), coding sequences, and transcriptional terminators (Canton et al., 2008). The standard assembly, as shown in Fig. 2A, uses restriction enzymes. Using restriction enzyme and ligase, the target genes and the destination vector are given the same two sticky ends and ligated to form a new plasmid. However, this method requires different numbers of restriction-enzyme sites, depending on the number of target genes. After the ligation, a scar sequence may remain because of the effects of the restriction sites. The digested products also need time-consuming purification steps. An in-fusion BioBrick assembly (Fig. 2B) was newly developed to avoid the purification steps and the scar sequence (Sleight et al., 2010). The sequence and ligase-independent cloning (Fig. 2C) method was developed to avoid the use of restriction and ligation enzymes (Li and Elledge, 2007). Using PCR, the target gene with 25-bp sequence homologous to the linearized destination vector is amplified. Without dNTPs, the 3′ exonuclease activity of T4 DNA polymerase makes 5′ overhang. After the exonuclease activity, the annealing and adding of dNTPs trigger the homologous recombination of the target gene to form a new vector. Gibson assembly (Fig. 2D) uses a linearized destination vector and PCR products and the 5′ exonuclease activity of T5 exonuclease (Gibson et al., 2009). Golden Gate assembly (Fig. 2E), another method of DNA assembly (Engler et al., 2008) that uses type IIS endonuclease, has different recognition and cleavage sites that produce insert-DNA and a vector. This simple procedure helps multiple target assemblies to be worked simultaneously. The circular polymerase-extension cloning method, which uses double-stranded DNA and a linearized destination vector, was developed to assemble more efficiently (Quan and Tian, 2009; Zhang et al., 2012). The uracil excision-based cloning method solved the difficulty in controlling the exonuclease activity (Bitinaite et al., 2007) and the DNA-fragment assembly by introducing nicking endonuclease (Wang et al., 2013b).
Fig. 2.
Genome assembly methods. (A) Standard assembly with restriction enzymes, (B) In-fusion assembly, (C) Sequence and ligase independent cloning (SLIC), (D) Gibson assembly, and (E) Golden Gate assembly.
Genome editing tools
An in-depth understanding of the complex genetic functions of complete genome sequences requires extensive genetic, biochemical, cytological, and physiological analyses. Likewise, the design of new biological systems demands the precise manipulation of genome sequences. In this context, genome editing is a powerful technology that introduces predetermined sequence changes to specific genomic loci (Perez-Pinera et al., 2012). This has been facilitated by the development of engineered nucleases with versatile and programmable DNA-binding domains, such as zinc-finger proteins and transcription activator-like effectors that are fused to the catalytic domain of a restriction endonuclease (Esvelt and Wang, 2013; Perez-Pinera et al., 2012). These artificially engineered nucleases are used to create specific double-stranded breaks (DSBs) at desired locations in the genome, which are subsequently repaired by endogenous homology-directed repair (HDR) pathways or nonhomologous end-joining (NHEJ) (Brugmans et al., 2007; Kanaar et al., 1998; Sung and Klein, 2006). HDR is an error-free DSB repair system that uses homologous DNA as a template. NHEJ often induces small insertions and deletions at breakpoint junctions, because it uses non-homologous template DNA (Gaj et al., 2012). Currently, two families of engineered nucleases are commercially available: ZFN and TALENs.
Zinc finger nucleases
ZFNs are chimeric endonucleases generated by the fusion of the nonspecific cleavage domain of the type IIS FokI restriction endonuclease with custom-designed Cys2-His2-zinc-finger proteins (Kim and Chandrasegaran, 1994). ZFNs have been used as a powerful genome-editing tools to generate targeted mutagenesis (Bibikova et al., 2002; Maeder et al., 2008; Urnov et al., 2005) and chromosomal rearrangements (Brunet et al., 2009; Lee et al., 2010) in numerous species including Caenorhabditis elegans (Morton et al., 2006), Drosophila melanogaster (Bibikova et al., 2002), silkworms (Takasu et al., 2010), monarch butterflies (Merlin et al., 2012), zebrafish (Doyon et al., 2008; Meng et al., 2008), sea urchins (Ochiai et al., 2010), Arabidopsis thaliana (Osakabe et al., 2010; Zhang et al., 2010), tobacco (Cai et al., 2009; Maeder et al., 2008; Townsend et al., 2009), corn (Shukla et al., 2009), mice (Carbery et al., 2010; Meyer et al., 2010), rats (Geurts et al., 2009), and humans (Hockemeyer et al., 2009; Holt et al., 2010; Zou et al., 2009).
A single-stranded breaks system, a modification of the tools that use ZFNs to induce DSBs, was also developed by fusing DNA-nicking enzymes (nickases) with zinc-finger proteins. It allowed accurate, site-specific genome modifications at only the on-target site (Brunet et al., 2009; Kim et al., 2012a). In addition, a ZFNickase that stimulates HDR but not NHEJ was developed by the mutation of a critical residue for FokI cleavage activity in one monomer of the ZFN heterodimer. It allowed the ZFNs to heterodimerize on DNA, but it restricted the cleavage to a single DNA strand. Consequently, ZFNickase induced gene addition at an endogenous CCR5 locus without the significant increase caused by the error-prone NHEJ repair pathway (Wang et al., 2012a).
Transcription activator-like effector nucleases
Transcription activator-like (TAL) effectors expressed by bacterial plant pathogens of the genus Xanthomona Xanthomonas consist of the translocation domain at the N-terminus, the repeated domain for DNA binding, nuclear localization, and the transcriptional activation domain at the C-terminus. The TAL effector repeats are highly conserved domains consisting of 33 to 35 amino acids, except for two internal positions known as repeat variable di-residues that display specificity to a target gene (Boch et al., 2009; Moscou and Bogdanove, 2009). By means of this specific protein, a TAL effector was engineered to produce a TALEN (Bogdanove and Voytas, 2011; Carlson et al., 2012) that associates with the FokI nuclease, which has an N-terminal DNA-binding domain and a non-specific DNA cleavage domain at the C-terminus (Wah et al., 1997). To function as a dimer, the FokI domain requires two constructs of unique DNA-binding domains with proper orientation and spacing in the target genome. Therefore, TALENs can be used to generate site-specific DSBs that can be repaired by joining, either by NHEJ or by homology-directed repair without donor DNAs, resulting in gene insertion or correction as well as the disruption of the gene of interest in various species such as yeast (Li et al., 2011), Xenopus embryos (Lei et al., 2012), plants (Cermak et al., 2011), nematodes (Wood et al., 2011), zebrafish (Huang et al., 2011; Sander et al., 2011), rats (Tesson et al., 2011), and human somatic (Cermak et al., 2011; Matsumura et al., 2010; Miller et al., 2011) and pluripotent stem cells (Hockemeyer et al., 2011).
The TALENs are broadly applied to custom-designed genome-editing tools in a high-throughput manner (Miller et al., 2011; Reyon et al., 2012). Reyon et al. designed the fast ligation-based automatable solid-phase high-throughput (FLASH) system, which is fast and economical for the large-scale assembly of TALENs (Reyon et al., 2012). Recently, Bedell VM et al. provided an advanced TALENs approach for targeted zebrafish genome editing, which was a bottleneck in the genome modification field (Bedell et al., 2012b).
RNA-guided CRISPR nucleases
Clustered regularly interspaced short palindromic repeats (CRISPRs) are short, repetitive genomic sequences that function as an immune system in 90% of reported archaea and 40% of known bacteria (Jansen et al., 2002; Makarova et al., 2011; Sorek et al., 2008). The CRISPR-associated system cleaves viral and plasmid DNAs into short fragments, which are integrated into the host chromosome at one end of the repeated region of the CRISPR locus. When the foreign DNA is transferred into the host cell, the records of prior integration events are transcribed and used to produce a library of short CRISPR-derived RNAs (crRNAs) by a CRISPR-associated or RNase III family nuclease. Each crRNA is complementary to a foreign target DNA and mediates the sequence-specific cleavage of the foreign DNA by the endonuclease function of Cas proteins (Makarova et al., 2011; Sorek et al., 2008; Wiedenheft et al., 2012). For example, the Type II CRISPR system from Streptococcus pyogenes consists of a single gene encoding the Cas9 protein, a mature crRNA, and a partially complementary trans-acting RNA (tracrRNA) (Jinek et al., 2012). In this system, a single CRISPR-associated protein (Cas9) in complex with the crRNA and the tracrRNA catalyzes the cleavage of complementary sequences, called proto-spacers, present within the target DNA. Upon recognizing the 20-nucleotide sequence, guided by the crRNA, Cas9 generates blunt DSBs at the site 3 bp upstream of the conserved proto-spacer-adjacent motif (PAM) (Jinek et al., 2012; Mali et al., 2013). Subsequently, the DSBs stimulate NHEJ repair in genomic DNA. Although the PAMs are short DNA sequences (NGG, NGGNG, NNAGAAW, and NAAR), they are necessary, in addition to at least 12 bp of perfect homology, for CRISPR endonuclease activity (Cong et al., 2013; Deltcheva et al., 2011; Deveau et al., 2008; Esvelt and Wang, 2013; Horvath et al., 2008; Jinek et al., 2012; Mali et al., 2013; van der Ploeg, 2009). The requirement of tracrRNA, which plays an important role in the maturation of crRNA (Deltcheva et al., 2011; Horvath et al., 2008), can be engineered to a designed, hairpin-structured single-guide RNA (sgRNA) that mimics the tracrRNA-crRNA complex (Jinek et al., 2012). Thus, the engineered CRISPR system requires the Cas9 protein and the sgRNA, and therefore represents a facile strategy for targeted genome editing. Recently, it has been demonstrated that the engineered CRISPR system can be applied to genome editing in humans (Cho et al., 2013; Cong et al., 2013; Jinek et al., 2013; Mali et al., 2013), zebrafish embryos (Hwang et al., 2013), S. cerevisiae (Dicarlo et al., 2013), and bacterial cells (Jiang et al., 2013; Jinek et al., 2012), as well as to the RNA-guided programming of gene expression (Hale et al., 2012; Qi et al., 2012; 2013b).
CONCLUSION
The ultimate goal of synthetic biology is to build novel biological systems that have new functions or to engineer existing biological systems to have better efficiency. For this, it is useful to consider the four core technical areas of synthetic biology: indexing biological parts, DNA synthesis and assembly, sequencing, and genome editing. Indeed, NGS platforms have addressed the technical barriers to securing enormous numbers of biological parts, such as promoters, RBSs, terminators, and regulatory protein-binding sites, as well as new genes including enzymes and regulatory proteins. Because the functions of biological parts are host-specific in many cases, some may not function as expected in their new context. Thus, host-specific libraries of biological parts, such as the strength of RBSs or regulatory sequences, will be useful to build new biological systems. In addition, the desired biological parts are often not single genes but are instead complete operons, such as a biosynthetic pathway consisting of several genes. Furthermore, the candidate parts need to be combined into transcriptional units with a well-designed regulatory circuitry in the context of genome-scale metabolic and regulatory networks. Manually curated, genome-scale biochemical reconstructions of around 40 different bacteria and the community-driven, global reconstruction of the human metabolism will be useful to predict the suitability of the transcriptional unit (Feist et al., 2009; Thiele et al., 2013).
Although the decrease in the cost of DNA synthesis has dramatically accelerated the construction of thousands of variants of DNA parts, codon optimization is also important for the efficient heterologous expression of the desired biological parts. For this, several computational algorithms are currently available. In addition, the fast and efficient formation of new gene clusters requires the simultaneous assembly of biological parts in a robust and flexible manner. New genome editing methods are also bringing down the number of errors in the genomes synthesized with the current sequencing capabilities. In addition to these experimental efforts, it is also crucial to develop computational algorithms for the de novo design of metabolic pathways with regulatory circuitry. With the many challenges to the understanding of natural biological systems, the rapid progress of emerging tools for synthetic biology has begun to provide genomes for applications in the areas of energy, health care, biochemicals, and the environment.
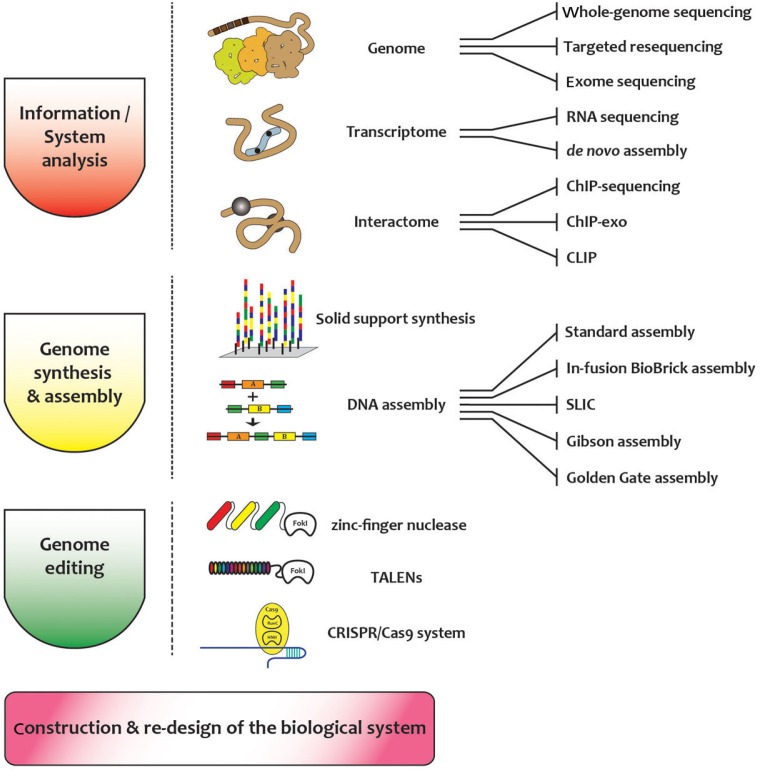
Fig. 1.
Overall scheme and related synthetic biology tools for building new biological systems.
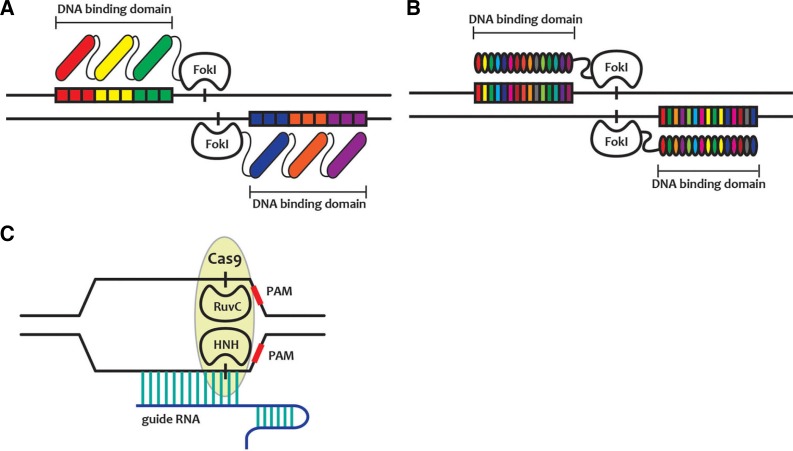
Fig. 3.
Schematic diagram of genome editing methods. (A) Zinc-finger nuclease (ZFNs), which is recognized and bound with three nucleotides of the genome; (B) Transcription activator-like effector nuclease (TALENs), in which each amino acid in the DNA-binding domain recognizes one nucleotide; and (C) Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 system, which uses guide RNAs for recognizing target DNA.
Acknowledgments
This work was supported by the Intelligent Synthetic Biology Center of Global Frontier Project (2011-0031957-0032011-0031962), the Converging Research Center Program (2012K001400), and the Basic Core Technology Development Program for the Oceans and the Polar Regions (2011-0021053) funded by the Ministry of Education, Science and Technology.
REFERENCES
- 1000 Genomes Project Consortium. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adessi C, Matton G, Ayala G, Turcatti G, Mermod JJ, Mayer P, Kawashima E. Solid phase DNA amplification: characterisation of primer attachment and amplification mechanisms. Nucleic Acids Res. 2000;28:E87. doi: 10.1093/nar/28.20.e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, et al. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- Araya CL, Payen C, Dunham MJ, Fields S. Whole-genome sequencing of a laboratory-evolved yeast strain. BMC Genomics. 2010;11:88. doi: 10.1186/1471-2164-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S, Wu TY, Machado IM, Huang WC, Chen PY, Pellegrini M, Liao JC. Evolution, genomic analysis, and reconstruction of isobutanol tolerance in Escherichia coli. Mol Syst Biol. 2010;6:449. doi: 10.1038/msb.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MP, Thakuria JV, Zaranek AW, Clegg T, Rosenbaum AM, Wu X, Angrist M, Bhak J, Bobe J, Callow MJ, et al. A public resource facilitating clinical use of genomes. Proc Natl Acad Sci USA. 2012;109:11920–11927. doi: 10.1073/pnas.1201904109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012a;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Person AD, Larson JD, McLoon A, Balciunas D, Clark KJ, Neff KI, Nelson KE, Bill BR, Schimmenti LA, et al. The lineage-specific gene ponzr1 is essential for zebrafish pronephric and pharyngeal arch development. Development. 2012b;139:793–804. doi: 10.1242/dev.071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitinaite J, Rubino M, Varma KH, Schildkraut I, Vaisvila R, Vaiskunaite R. USER friendly DNA engineering and cloning method by uracil excision. Nucleic Acids Res. 2007;35:1992–2002. doi: 10.1093/nar/gkm041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- Brugmans L, Kanaar R, Essers J. Analysis of DNA double-strand break repair pathways in mice. Mut Res. 2007;614:95–108. doi: 10.1016/j.mrfmmm.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Brunet E, Simsek D, Tomishima M, DeKelver R, Choi VM, Gregory P, Urnov F, Weinstock DM, Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proc Natl Acad Sci USA. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CQ, Doyon Y, Ainley WM, Miller JC, Dekelver RC, Moehle EA, Rock JM, Lee YL, Garrison R, Schulenberg L, et al. Targeted transgene integration in plant cells using designed zinc finger nucleases. Plant Mol Biol. 2009;69:699–709. doi: 10.1007/s11103-008-9449-7. [DOI] [PubMed] [Google Scholar]
- Canton B, Labno A, Endy D. Refinement and standardization of synthetic biological parts and devices. Nat Biotechnol. 2008;26:787–793. doi: 10.1038/nbt1413. [DOI] [PubMed] [Google Scholar]
- Carbery ID, Ji D, Harrington A, Brown V, Weinstein EJ, Liaw L, Cui X. Targeted genome modification in mice using zinc-finger nucleases. Genetics. 2010;186:451–459. doi: 10.1534/genetics.110.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DF, Fahrenkrug SC, Hackett PB. Targeting DNA with fingers and TALENs. Mol Ther Nucleic Acids. 2012;1:e3. doi: 10.1038/mtna.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cello J, Paul AV, Wimmer E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science. 2002;297:1016–1018. doi: 10.1126/science.1072266. [DOI] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charusanti P, Conrad TM, Knight EM, Palsson B. Genetic Basis of Growth adaptation of E. coli after deletion of pgi, a major metabolic gene. PLoS Genet. 2010;6:e1001186. doi: 10.1371/journal.pgen.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AA, Lu TK. Synthetic biology: an emerging engineering discipline. Annu Rev Biomed Eng. 2012;14:155–178. doi: 10.1146/annurev-bioeng-071811-150118. [DOI] [PubMed] [Google Scholar]
- Cho BK, Zengler K, Qiu Y, Park YS, Knight EM, Barrett CL, Gao Y, Palsson BO. The transcription unit architecture of the Escherichia coli genome. Nat Biotechnol. 2009;27:1043–1049. doi: 10.1038/nbt.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Clarke J, Wu HC, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol. 2009;4:265–270. doi: 10.1038/nnano.2009.12. [DOI] [PubMed] [Google Scholar]
- Cloonan N, Forrest AR, Kolle G, Gardiner BB, Faulkner GJ, Brown MK, Taylor DF, Steptoe AL, Wani S, Bethel G, et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Methods. 2008;5:613–619. doi: 10.1038/nmeth.1223. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, NY) 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad TM, Joyce AR, Applebee MK, Barrett CL, Xie B, Gao Y, Palsson BO. Whole-genome resequencing of Escherichia coli K-12 MG1655 undergoing short-term laboratory evolution in lactate minimal media reveals flexible selection of adaptive mutations. Genome Biol. 2009;10:R118. doi: 10.1186/gb-2009-10-10-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau H, Barrangou R, Garneau JE, Labonte J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. Phage response to CRISPR-encoded resistance in Strep-tococcus thermophilus. J Bacteriol. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA. 2003;100:8817–8822. doi: 10.1073/pnas.1133470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Mercante D, Fang Z. An artificial functional family filter in homolog searching in next-generation sequencing metagenomics. PLoS One. 2013;8:e58669. doi: 10.1371/journal.pone.0058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encode Project Consortium. Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Doyle F, Epstein CB, Frietze S, Harrow J, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy D. Foundations for engineering biology. Nature. 2005;438:449–453. doi: 10.1038/nature04342. [DOI] [PubMed] [Google Scholar]
- Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt KM, Wang HH. Genome-scale engineering for systems and synthetic biology. Mol Syst Biol. 2013;9:641. doi: 10.1038/msb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist AM, Herrgard MJ, Thiele I, Reed JL, Palsson BO. Reconstruction of biochemical networks in microorganisms. Nat Rev Microbiol. 2009;7:129–143. doi: 10.1038/nrmicro1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster AC, Church GM. Towards synthesis of a minimal cell. Mol Syst Biol. 2006;2:45. doi: 10.1038/msb4100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey TS. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet. 2012;13:840–852. doi: 10.1038/nrg3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Guo J, Kato Y, Sirk SJ, Barbas CF., 3rd Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat Methods. 2012;9:805–807. doi: 10.1038/nmeth.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- Giga-Hama Y, Tohda H, Takegawa K, Kumagai H. Schizosaccharomyces pombe minimum genome factory. Biotechnol Appl Biochem. 2007;46:147–155. doi: 10.1042/BA20060106. [DOI] [PubMed] [Google Scholar]
- Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA, 3rd, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proc Natl Acad Sci USA. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryshin IY, Naumann TA, Apodaca J, Reznikoff WS. Chromosomal deletion formation system based on Tn5 double transposition: use for making minimal genomes and essential gene analysis. Genome Res. 2003;13:644–653. doi: 10.1101/gr.611403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr, Jungkamp AC, Munschauer M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, Resch AM, Glover CV, 3rd, Graveley BR, Terns RM, et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol Cell. 2012;45:292–302. doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl-Geigl J, Mauermann O, Lackner C, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4140. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hirashima K, Iwaki T, Takegawa K, Giga-Hama Y, Tohda H. A simple and effective chromosome modification method for large-scale deletion of genome sequences and identification of essential genes in fission yeast. Nucleic Acids Res. 2006;34:e11. doi: 10.1093/nar/gnj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Romero DA, Coute-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyaval P, Fremaux C, Barrangou R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J Bacteriol. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, Chien TY, Hu CC, Chen MJ, Wu WJ, Feng HT, Haymer DS, Chen CY. Discovery of genes related to insecticide resistance in Bactrocera dorsalis by functional genomic analysis of a de novo assembled transcriptome. PLoS One. 2012;7:e40950. doi: 10.1371/journal.pone.0040950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011;29:699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- Hugo Pan-Asian SNP Consortium. Abdulla MA, Ahmed I, Assawamakin A, Bhak J, Brahmachari SK, Calacal GC, Chaurasia A, Chen CH, Chen J, et al. Mapping human genetic diversity in Asia. Science (New York, N.Y.) 2009;326:1541–1545. doi: 10.1126/science.1177074. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. ELife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- Jungwirth B, Sala C, Kohl TA, Uplekar S, Baumbach J, Cole ST, Puhler A, Tauch A. High-resolution detection of DNA binding sites of the global transcriptional regulator GlxR in Corynebacterium glutamicum. Microbiology. 2013;159:12–22. doi: 10.1099/mic.0.062059-0. [DOI] [PubMed] [Google Scholar]
- Kanaar R, Hoeijmakers JH, van Gent DC. Molecular mechanisms of DNA double strand break repair. Trends Cell Biol. 1998;8:483–489. doi: 10.1016/s0962-8924(98)01383-x. [DOI] [PubMed] [Google Scholar]
- Keseler IM, Mackie A, Peralta-Gil M, Santos-Zavaleta A, Gama-Castro S, Bonavides-Martinez C, Fulcher C, Huerta AM, Kothari A, Krummenacker M, et al. EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res. 2013;41:D605–612. doi: 10.1093/nar/gks1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Chandrasegaran S. Chimeric restriction endonuclease. Proc Natl Acad Sci USA. 1994;91:883–887. doi: 10.1073/pnas.91.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Kim S, Kim DH, Choi BS, Choi IY, Kim JS. Precision genome engineering with programmable DNA-nicking enzymes. Genome Res. 2012a;22:1327–1333. doi: 10.1101/gr.138792.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Hong JS, Qiu Y, Nagarajan H, Seo JH, Cho BK, Tsai SF, Palsson BO. Comparative analysis of regulatory elements between Escherichia coli and Klebsiella pneumoniae by genome-wide transcription start site profiling. PLoS Genet. 2012b;8:e1002867. doi: 10.1371/journal.pgen.1002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Iijima L, Tatsumi M, Ono N, Oyake A, Hashimoto T, Matsuo M, Okubo M, Suzuki S, Mori K, et al. Transition from positive to neutral in mutation fixation along with continuing rising fitness in thermal adaptive evolution. PLoS Genet. 2010;6:e1001164. doi: 10.1371/journal.pgen.1001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuri S, Eroshenko N, Leproust EM, Super M, Way J, Li JB, Church GM. Scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nat Biotechnol. 2010;28:1295–1299. doi: 10.1038/nbt.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CS, Naidoo N, Pawitan Y. Revisiting Mendelian disorders through exome sequencing. Hum Genet. 2011;129:351–370. doi: 10.1007/s00439-011-0964-2. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lee JH, Sung BH, Kim MS, Blattner FR, Yoon BH, Kim JH, Kim SC. Metabolic engineering of a reduced-genome strain of Escherichia coli for L-threonine production. Microb Cell Fact. 2009;8:2. doi: 10.1186/1475-2859-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim E, Kim JS. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 2010;20:81–89. doi: 10.1101/gr.099747.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CH, Dawid IB, Chen Y, Zhao H. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs) Proc Natl Acad Sci USA. 2012;109:17484–17489. doi: 10.1073/pnas.1215421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JZ, Yassour M, Adiconis X, Nusbaum C, Thompson DA, Friedman N, Gnirke A, Regev A. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods. 2010;7:709–715. doi: 10.1038/nmeth.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods. 2007;4:251–256. doi: 10.1038/nmeth1010. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, Weeks DP, Yang B. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 2011;39:6315–6325. doi: 10.1093/nar/gkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li W, Wu Y, Chen C, Lei J. De novo transcriptome assembly in chili pepper (Capsicum frutescens) to identify genes involved in the biosynthesis of capsaicinoids. PLoS One. 2013;8:e48156. doi: 10.1371/journal.pone.0048156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER. A decade’s perspective on DNA sequencing technology. Nature. 2011;470:198–203. doi: 10.1038/nature09796. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Wang Z. Next-generation transcriptome assembly. Nat Rev Genet. 2011;12:671–682. doi: 10.1038/nrg3068. [DOI] [PubMed] [Google Scholar]
- Martin CH, Nielsen DR, Solomon KV, Prather KL. Synthetic metabolism: engineering biology at the protein and pathway scales. Chem Biol. 2009;16:277–286. doi: 10.1016/j.chembiol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Martin J, Bruno VM, Fang Z, Meng X, Blow M, Zhang T, Sherlock G, Snyder M, Wang Z. Rnnotator: an automated de novo transcriptome assembly pipeline from stranded RNA-Seq reads. BMC Genomics. 2010;11:663. doi: 10.1186/1471-2164-11-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Takasu N, Nagata M, Nakamura K, Kawai M, Yoshino S. Effect of ultrafine zinc oxide (ZnO) nano-particles on induction of oral tolerance in mice. J Immunotoxicol. 2010;7:232–237. doi: 10.3109/1547691X.2010.487879. [DOI] [PubMed] [Google Scholar]
- Matzas M, Stahler PF, Kefer N, Siebelt N, Boisguerin V, Leonard JT, Keller A, Stahler CF, Haberle P, Gharizadeh B, et al. High-fidelity gene synthesis by retrieval of sequence-verified DNA identified using high-throughput pyrosequencing. Nat Biotechnol. 2010;28:1291–1294. doi: 10.1038/nbt.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, Goyette P, Zody MC, Hall JL, Brant SR, Cho JH, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, Breitling R, Bovenberg R, Takano E. Exploiting plug-and-play synthetic biology for drug discovery and production in microorganisms. Nat Rev Microbiol. 2011;9:131–137. doi: 10.1038/nrmicro2478. [DOI] [PubMed] [Google Scholar]
- Medema MH, van Raaphorst R, Takano E, Breitling R. Computational tools for the synthetic design of biochemical pathways. Nat Rev Microbiol. 2012;10:191–202. doi: 10.1038/nrmicro2717. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin C, Beaver LE, Taylor OR, Wolfe SA, Reppert SM. Efficient targeted mutagenesis in the monarch butterfly using zinc finger nucleases. Genome Res. 2012;23:159–168. doi: 10.1101/gr.145599.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Meyer M, de Angelis MH, Wurst W, Kuhn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci USA. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, et al. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 2008;15:73–81. doi: 10.1093/dnares/dsn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Morton J, Davis MW, Jorgensen EM, Carroll D. Induction and repair of zinc-finger nuclease-targeted double-strand breaks in Caenorhabditis elegans somatic cells. Proc Natl Acad Sci USA. 2006;103:16370–16375. doi: 10.1073/pnas.0605633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501–1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Murakami K, Tao E, Ito Y, Sugiyama M, Kaneko Y, Harashima S, Sumiya T, Nakamura A, Nishizawa M. Large scale deletions in the Saccharomyces cerevisiae genome create strains with altered regulation of carbon metabolism. Appl Microbiol Biotechnol. 2007;75:589–597. doi: 10.1007/s00253-007-0859-2. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandagopal N, Elowitz MB. Synthetic biology: integrated gene circuits. Science. 2011;333:1244–1248. doi: 10.1126/science.1207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H, Fujita K, Suzuki K, Nishikawa M, Shibata T, Sakamoto N, Yamamoto T. Targeted mutagenesis in the sea urchin embryo using zinc-finger nucleases. Genes Cells. 2010;15:875–885. doi: 10.1111/j.1365-2443.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- Osakabe K, Osakabe Y, Toki S. Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA. 2010;107:12034–12039. doi: 10.1073/pnas.1000234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, Markowitz VM, Kyrpides NC. The Genomes OnLine Database (GOLD) v.4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2012;40:D571–579. doi: 10.1093/nar/gkr1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinera P, Ousterout DG, Gersbach CA. Advances in targeted genome editing. Curr Opin Chem Biol. 2012;16:268–277. doi: 10.1016/j.cbpa.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins TT, Kingsley RA, Fookes MC, Gardner PP, James KD, Yu L, Assefa SA, He M, Croucher NJ, Pickard DJ, et al. A strand-specific RNA-Seq analysis of the transcriptome of the typhoid bacillus Salmonella typhi. PLoS Genet. 2009;5:e1000569. doi: 10.1371/journal.pgen.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AC, Melo-Barbosa HP, Miyoshi A, Silva A, Azevedo V. Application of RNA-seq to reveal the transcript profile in bacteria. Genet Mol Res. 2011;10:1707–1718. doi: 10.4238/vol10-3gmr1554. [DOI] [PubMed] [Google Scholar]
- Pleiss J. The promise of synthetic biology. Appl Microbiol Biotechnol. 2006;73:735–739. doi: 10.1007/s00253-006-0664-3. [DOI] [PubMed] [Google Scholar]
- Posfai G, Plunkett G, 3rd, Feher T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, et al. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- Pushkarev D, Neff NF, Quake SR. Single-molecule sequencing of an individual human genome. Nat Biotechnol. 2009;27:847–850. doi: 10.1038/nbt.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Haurwitz RE, Shao W, Doudna JA, Arkin AP. RNA processing enables predictable programming of gene expression. Nat Biotechnol. 2012;30:1002–1006. doi: 10.1038/nbt.2355. [DOI] [PubMed] [Google Scholar]
- Qi XP, Du ZF, Ma JM, Chen XL, Zhang Q, Fei J, Wei XM, Chen D, Ke HP, Liu XZ, et al. Genetic diagnosis of autosomal dominant polycystic kidney disease by targeted capture and next-generation sequencing: utility and limitations. Gene. 2013a;516:93–100. doi: 10.1016/j.gene.2012.12.060. [DOI] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013b;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Cho BK, Park YS, Lovley D, Palsson BO, Zengler K. Structural and operational complexity of the Geobacter sulfurreducens genome. Genome Res. 2010;20:1304–1311. doi: 10.1101/gr.107540.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J, Tian J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS One. 2009;4:e6441. doi: 10.1371/journal.pone.0006441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J, Saaem I, Tang N, Ma S, Negre N, Gong H, White KP, Tian J. Parallel on-chip gene synthesis and application to optimization of protein expression. Nat Biotechnol. 2011;29:449–452. doi: 10.1038/nbt.1847. [DOI] [PubMed] [Google Scholar]
- Ram JL, Karim AS, Sendler ED, Kato I. Strategy for microbiome analysis using 16S rRNA gene sequence analysis on the Illumina sequencing platform. Syst Biol Reprod Med. 2011;57:162–170. doi: 10.3109/19396368.2011.555598. [DOI] [PubMed] [Google Scholar]
- Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol. 2012;10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell. 2011;147:1408–1419. doi: 10.1016/j.cell.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond KE, Li MH, Rodesch MJ, Patel M, Lowe AM, Kim C, Chu LL, Venkataramaian N, Flickinger SF, Kaysen J, et al. Amplification and assembly of chip-eluted DNA (AACED): a method for high-throughput gene synthesis. Nucleic Acids Res. 2004;32:5011–5018. doi: 10.1093/nar/gkh793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, Euskirchen G, Bernier B, Varhol R, Delaney A, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- Robertson G, Schein J, Chiu R, Corbett R, Field M, Jackman SD, Mungall K, Lee S, Okada HM, Qian JQ, et al. De novo assembly and analysis of RNA-seq data. Nat Methods. 2010;7:909–912. doi: 10.1038/nmeth.1517. [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Leamon JH. The development and impact of 454 sequencing. Nat Biotechnol. 2008;26:1117–1124. doi: 10.1038/nbt1485. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Turner S, Kasarskis A. A window into third-generation sequencing. Hum Mol Genet. 2010;19:R227–240. doi: 10.1093/hmg/ddq416. [DOI] [PubMed] [Google Scholar]
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger DW, Cheung KJ, Mei R, Johansson EM, Richmond CS, Blattner FR, Lockhart DJ, Church GM. RNA expression analysis using a 30 base pair resolution Escherichia coli genome array. Nat Biotechnol. 2000;18:1262–1268. doi: 10.1038/82367. [DOI] [PubMed] [Google Scholar]
- Seo JH, Hong JS, Kim D, Cho BK, Huang TW, Tsai SF, Palsson BO, Charusanti P. Multiple-omic data analysis of Klebsiella pneumoniae MGH 78578 reveals its transcriptional architecture and regulatory features. BMC Genomics. 2012;13:679. doi: 10.1186/1471-2164-13-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermuller J, Reinhardt R, et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464:250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- Shendure J, Lieberman Aiden E. The expanding scope of DNA sequencing. Nat Biotechnol. 2012;30:1084–1094. doi: 10.1038/nbt.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, Rosenbaum AM, Wang MD, Zhang K, Mitra RD, Church GM. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–1732. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- Shokralla S, Spall JL, Gibson JF, Hajibabaei M. Next-generation sequencing technologies for environmental DNA research. Mol Ecol. 2012;21:1794–1805. doi: 10.1111/j.1365-294X.2012.05538.x. [DOI] [PubMed] [Google Scholar]
- Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009;459:437–441. doi: 10.1038/nature07992. [DOI] [PubMed] [Google Scholar]
- Sleight SC, Bartley BA, Lieviant JA, Sauro HM. In-Fusion BioBrick assembly and re-engineering. Nucleic Acids Res. 2010;38:2624–2636. doi: 10.1093/nar/gkq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon WW, Hariharan M, Snyder MP. High-throughput sequencing for biology and medicine. Mol Syst Biol. 2013;9:640. doi: 10.1038/msb.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Kunin V, Hugenholtz P. CRISPR--a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol. 2006;7:739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- Surget-Groba Y, Montoya-Burgos JI. Optimization of de novo transcriptome assembly from next-generation sequencing data. Genome Res. 2010;20:1432–1440. doi: 10.1101/gr.103846.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu Y, Kobayashi I, Beumer K, Uchino K, Sezutsu H, Sajwan S, Carroll D, Tamura T, Zurovec M. Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem Mol Biol. 2010;40:759–765. doi: 10.1016/j.ibmb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Tesson L, Usal C, Menoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng X, Zhang L, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, et al. A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 doi: 10.1038/nbt.2488. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Gong H, Sheng N, Zhou X, Gulari E, Gao X, Church G. Accurate multiplex gene synthesis from programmable DNA microchips. Nature. 2004;432:1050–1054. doi: 10.1038/nature03151. [DOI] [PubMed] [Google Scholar]
- Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature. 2009;459:442–445. doi: 10.1038/nature07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, Batzoglou S, Myers RM, Sidow A. Genomewide analysis of transcription factor binding sites based on ChIP-Seq data. Nat Methods. 2008;5:829–834. doi: 10.1038/nmeth.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg JR. Analysis of CRISPR in Streptococcus mutans suggests frequent occurrence of acquired immunity against infection by M102-like bacteriophages. Microbiology. 2009;155:1966–1976. doi: 10.1099/mic.0.027508-0. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wah DA, Hirsch JA, Dorner LF, Schildkraut I, Aggarwal AK. Structure of the multimodular endonuclease FokI bound to DNA. Nature. 1997;388:97–100. doi: 10.1038/40446. [DOI] [PubMed] [Google Scholar]
- Wang J, Friedman G, Doyon Y, Wang NS, Li CJ, Miller JC, Hua KL, Yan JJ, Babiarz JE, Gregory PD, et al. Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res. 2012a;22:1316–1326. doi: 10.1101/gr.122879.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tsutsumi S, Kawaguchi T, Nagasaki K, Tatsuno K, Yamamoto S, Sang F, Sonoda K, Sugawara M, Saiura A, et al. Whole-exome sequencing of human pancreatic cancers and characterization of genomic instability caused by MLH1 haploinsufficiency and complete deficiency. Genome Res. 2012b;22:208–219. doi: 10.1101/gr.123109.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Wei KY, Smolke CD. Synthetic biology: advancing the design of diverse genetic systems. Ann Rev Chem Biomol Eng. 2013a doi: 10.1146/annurev-chembioeng-061312-103351. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Shi ZY, Guo YY, Chen JC, Chen GQ. DNA fragments assembly based on nicking enzyme system. PLoS One. 2013b;8:e57943. doi: 10.1371/journal.pone.0057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westers H, Dorenbos R, van Dijl JM, Kabel J, Flanagan T, Devine KM, Jude F, Seror SJ, Beekman AC, Darmon E, et al. Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol Biol Evolution. 2003;20:2076–2090. doi: 10.1093/molbev/msg219. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen YJ, Makhijani V, Roth GT, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- Wilhelm BT, Marguerat S, Watt S, Schubert F, Wood V, Goodhead I, Penkett CJ, Rogers J, Bahler J. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453:1239–1243. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- Wold B, Myers RM. Sequence census methods for functional genomics. Nat Methods. 2008;5:19–21. doi: 10.1038/nmeth1157. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Lo TW, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, et al. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GW, Coufal NG, Liang TY, Peng GE, Fu XD, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nat Struct Mol Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BJ, Sung BH, Koob MD, Lee CH, Lee JH, Lee WS, Kim MS, Kim SC. Minimization of the Escherichia coli genome using a Tn5-targeted Cre/loxP excision system. Nat Biotechnol. 2002;20:1018–1023. doi: 10.1038/nbt740. [DOI] [PubMed] [Google Scholar]
- Zhang F, Maeder ML, Unger-Wallace E, Hoshaw JP, Reyon D, Christian M, Li X, Pierick CJ, Dobbs D, Peterson T, et al. High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc Natl Acad Sci USA. 2010;107:12028–12033. doi: 10.1073/pnas.0914991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chiodini R, Badr A, Zhang G. The impact of next-generation sequencing on genomics. J Genet Genomics. 2011;38:95–109. doi: 10.1016/j.jgg.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Werling U, Edelmann W. SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 2012;40:e55. doi: 10.1093/nar/gkr1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK, Chen G, Ye Z, Park IH, Daley GQ, et al. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]