Abstract
Arginase II catalyzes the conversion of arginine to urea and ornithine in many extrahepatic tissues. We investigated the protective role of arginase II on lipopolysaccharide-mediated apoptosis in the macrophage cells. Adenoviral gene transfer of full length of arginase II was performed in the murine macrophage cell line RAW264.7. The role of arginase II was investigated with cell viability, cytoplasmic histone-associated DNA fragmentation assay, arginase activity, nitric oxide production, and Western blot analysis. Arginase II is localized in mitochondria of macrophage cells, and the expression of arginase II was increased by lipopolysaccharide (LPS). LPS significantly increased cell death which was inhibited by AMT, a specific inducible nitric oxide synthase (iNOS) inhibitor. In contrast, LPS-induced cell death and nitric oxide production were increased by 2-boronoethyl-L-cysteine, a specific inhibitor of arginase. Adenoviral overexpression of arginase II significantly inhibited LPS-induced cell death and cytoplasmic histone-associated DNA fragmentation. LPS-induced iNOS expression and poly ADP-ribose polymerase cleavage were significantly suppressed by arginase II overexpression. Furthermore, arginase II overexpression resulted in a decrease in the Bax protein level and the reverse induction of Bcl-2 protein. Our data demonstrated that inhibition of NO production by arginase II may be due to arginine depletion as well as iNOS suppression though its reaction products. Moreover, arginase II plays a protective role of LPS-induced apoptosis in RAW264.7 cells.
Keywords: Arginase II, Bax, lipopolysaccharide, macrophage, nitric oxide
INTRODUCTION
Nitric oxide (NO) is an important biomolecule involved in many physiological and pathological processes. Overproduction of NO can contribute to tissue injury and toxicity. Especially, NO has been shown to mediate apoptosis in the macrophages (Dimmeler and Zeiher, 1997). Also in pathologic processes, excess NO production is the cause of several diseases, such as septic shock. Vasoactive mediators, such as NO, cause vasodilatation and increase the microvascular permeability (Stuehr and Marletta, 1985).
Arginase II is a mitochondrial arginase which is found in several extra-hepatic tissues (Wu and Morris, 1998). Arginase induction could diminish NO production by limiting the availability of L-arginine, the common substrate for both arginase and inducible nitric oxide synthase (iNOS). It is known that iNOS and arginase II are co-induced in lipopolysaccharide (LPS)-stimulated macrophages (Gotoh et al., 1996; Wang et al., 1995). When macrophage cells are treated with LPS, the cells undergo NO-dependent apoptosis (Gotoh and Mori, 1999). Since excess NO production is toxic to cells, the cellular mechanism to prevent NO overproduction may exist. Apoptosis of macrophages can be induced by numerous effector molecules, including NO (Albina et al., 1993). As iNOS expression and activity are induced by LPS in macrophages (Xie et al., 1992), the modulation of NO induced apoptosis in response to LPS appears to be a viable means of regulation.
Furthermore, as arginase II was expressed in mitochondria, mitochondrial anti-apoptotic genes can be influenced by arginase II expression during NO-induced apoptosis. However, it is still unknown whether anti-apoptotic molecules can be modulated by mitochondrial arginase II, thus affecting NO metabolism. Therefore, we investigated the ability of arginase II to reduce NO production and NO-mediated apoptosis in macrophage cells.
MATERIALS AND METHODS
Reagents
Antibodies for the detection of arginase I (sc-18351), arginase II (sc-20151), Bax (sc 493), Bcl-2 (sc 7382), iNOS (sc 8310) were obtained from Santa Cruz Biotechnology (USA). Antibodies against β-actin (A5316) and Flag were obtained from Sigma (USA). Poly ADP-ribose polymerase (PARP, 9541) was obtained from Cell signaling (USA). AMT (2-amino-5, 6-dihydro-6-methyl-4H-1, 3-thiazine hydrochloride), were purchased from Cayman (USA). 2-boronoethyl-L-cysteine, a specific inhibitor of arginase was purchased from Calbiochem (USA). All other relevant materials and reagents, including LPS (Escherichia coli O11:B4) were purchased from Sigma (USA).
Cell culture and viability assay
The murine macrophage cell line RAW264.7 was maintained in Dulbecco’s modified Eagle’s medium supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% fetal bovine serum (Gibco, USA). Cell viability was measured using the automated mammalian cell counter (ADAMMC, Digital Bio, Korea) which analyzed the cells stained with propidium iodide (Cho et al., 2011). The effect of Arginase II expression on apoptotic cell death was determined by quantification of cytoplasmic histone-associated DNA fragmentation, using a kit from Roche Diagnostics according to the manufacturer’s recommendations. Briefly, 5 × 104 cells per well were plated and infected with of Adβgal or AdArg II, and treated with LPS for 18 h at 37°C. Both floating and attached cells were collected and processed for analysis of cytoplasmic histone-associated DNA fragmentation as described by the manufacturer (Roche, Germany).
Immunofluorescent staining for Arginase II
For immunofluourecent staining, RAW 264.7 cells were grown on glass coverslips. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100, and blocked with 2% bovine serum albumin (BSA) for 1 h. Coverslips were then incubated overnight at 4°C in anti-arginase II (dilution 1:100) primary antibody in 1% BSA. Cells were then washed and incubated in FITC labeled secondary antibodies for 1 h. Mitotracker Red (Molecular probes) for 1 h was used for staining mitochondria. Coverslips were mounted on microscope slides, and fluorescence signals were visualized with an Olympus confocal microscope.
Adenovirus construction for human arginase II
To get full length human arginase II cDNA, PCR was performed with pfu polymerase from pDNR-LIB/hArgII (Open Biosystems), using following primers set linked with BamHI and XhoI : Arginase II sense primer 5′-CGC GGA TCC ATG TCC CTA AGG GGC-3′ Arginase II anti-sense primer 5′-CCG CTC GAG CTA AAT TCT CAC ACG TGC-3′. The PCR products containing of hArgII were subcloned into pENTR/CMV-FLAG. Site-specific recombination between entry vector (pENTR/CMV-Flag/hArgII) and adenoviral destination vector (pAd/PL-DEST) which contains the genome sequence of human adenovirus type 5 with E1 and E3 deleted as a viral vector backbone were performed by means of the Gateway system using LR clonase (Invitrogen/ Life technologies, USA)(Joo et al., 2012).
Western blot analysis
RAW264.7 cells were treated with LPS (300 ng/ml) for 18 h, and were lysed according to our previous publication (Kim et al., 2006). The cell lysate was cleared by centrifugation at 12,000 rpm for 20 min and the supernatant fraction was used for immunoblotting. Proteins were resolved by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. After blocking with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20, the membrane was incubated with the desired primary antibody (diluted 1:1000) overnight at 4°C. The membrane was then washed and treated with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (diluted 1: 3000) for 2 h at 22°C. The immunoreactive bands were visualized by an enhanced chemiluminescence method. Each membrane was stripped and re-probed with anti-actin antibody to ensure equal protein loading.
Arginase activity
Urea production was assayed as a functional assessment of arginase activity. Urea production from the cultured cell lysate was assayed in duplicate using a QuantiChrom Urea Assay kit (BioAssay Systems, USA) according to manufacturer’s protocol. Briefly, 40 μl of cell culture media was incubated in the presence or absence of arginase substrate buffer at 37°C for 2 h. The reaction mixture was incubated with 200 μl of urea reagent for 15 min at room temperature, and was determined at an optical density of 520 nm on a 96-well plate analyzer (Thermo LabSystems). The manufacturer’s urea standard (50 mg/dl) was used to calibrate the analyzer.
Nitric oxide production
Nitric oxide was measured as its stable end product, nitrite, using the Griess reagent as described previously (Jeon et al., 2004). In brief, 24 h after treatment, or 40 to 48 h after adenoviral infection, media from cells were processed for measurement of nitrite by specific light absorbance (540 nm wavelength) as per the manufacturer recommendations (Calbiochem/Merck, Germany). Culture media were deproteinized using a 10-kDa cutoff filter. Absorbance of cell media were adjusted to control for background levels determined in the culture media.
Statistical analysis
Values are expressed as the mean ± SEM. Statistical evaluation was performed using one-way analysis of variance followed by Dunnett’s test, with p < 0.05 considered significant.
RESULTS
Lipopolysaccharide induced arginase II expression
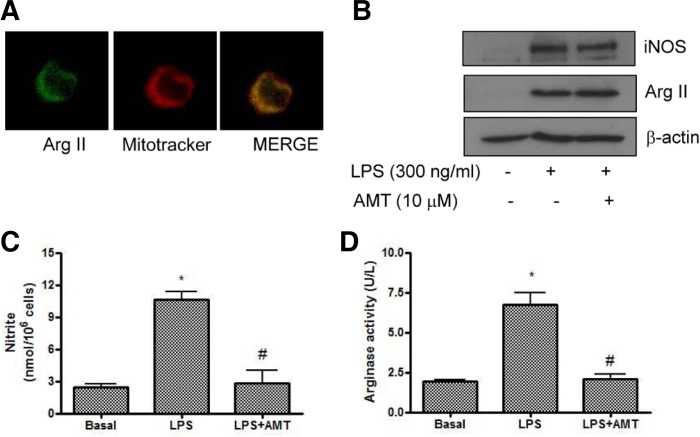
As shown in Fig. 1A, mitochondrial localization of endogenous arginase II was confirmed, which had merged fluorescent images for arginase and mitotracker in mitochondria in macrophage cells. Inflammatory cytokines such as LPS induced iNOS expression in macrophage cells. We examined whether LPS co-induced arginase II and iNOS expression. As shown in Fig. 1B, arginase II was minimally expressed in basal cells, however, LPS (300 ng/ml for 18 h) increased arginase II expression as well as iNOS expression. However, arginase I was not expressed in basal condition and was not induced by LPS in RAW264.7 cells (Data not shown). The pretreatment of AMT (10 μM), a selective iNOS inhibitor (Nakane et al., 1995) was not affect LPS-induced iNOS and arginase II expression, but it significantly suppressed LPS-induced NO production and arginase activity in macrophage cells (Figs. 1C and 1D).
Fig. 1.
Lipopolysaccharide (LPS) induced arginase II expression and activity in RAW264.7 cells. (A) Mitochondrial localization of arginase II. Arginase II (green, left), mitotracker (red, middle), and merged images (right). Note that fluorescent images for arginase II was similar with mitotracker suggesting the mitochondrial localization of arginase II. (B) Co-induction of arginase II and iNOS by LPS in RAW264.7 cells. Cells were exposed with LPS (300 ng/ml) or AMT (10 μM), an inhibitor of iNOS for 18 h. Western blot for arginase II and iNOS proteins was performed. (C) Effect of AMT on LPS-induced nitric oxide production in RAW264.7 cells. Cells were exposed to AMT for 18 h. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs basal, #P < 0.05 vs LPS. (D) Effect of AMT on LPS-arginase activity in RAW264.7 cells. Cells were exposed to AMT for 18 h. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs basal, #P < 0.05 vs LPS.
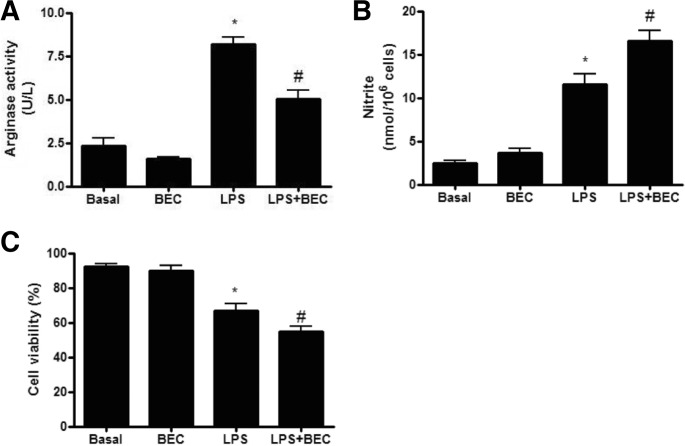
Arginase inhibition increased LPS-induced cell death
To explore the functional role of endogenous arginase II on the LPS-induced cell death, we investigated the effect of BEC, an arginase inhibitor, on the LPS-induced NO production and cell viability in RAW264.7 cells. As shown in Fig. 2A, BEC (100 μM) did not affect basal arginase activity, however, it significantly inhibit LPS-induced up-regulation of arginase activity. Furthermore, BEC significantly inhibited LPS-induced NO production and cell death in RAW264.7 cells (Figs. 2B and 2C). This data suggested that up-regulated arginase II in response to LPS plays a protective role against LPS-induced cell death in macrophages.
Fig. 2.
Arginase inhibition increased LPS-induced cell death in RAW264.7 cells. (A) Effect of arginase inhibition on LPS-induced arginase activity. Cells were treated with LPS (300 ng/ml) in the presence or absence of BEC, arginase inhibitor for 18 h. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs basal, #P < 0.05 vs. LPS. (B) Effect of arginase inhibition on LPS-induced nitrite production. Cells were treated with LPS (300 ng/ml) in the presence or absence of BEC, arginase inhibitor for 18 h. Data are presented as mean ± SEM (n = 5). *P < 0.05 vs basal, #P < 0.05 vs LPS. (C) Effect of arginase inhibition on LPS-induced cell death. Cells were treated with LPS (300 ng/ml) in the presence or absence of BEC, arginase inhibitor for 18 h. Data are presented as mean ± SEM (n = 5). *P < 0.05 vs basal, #P < 0.05 vs LPS.
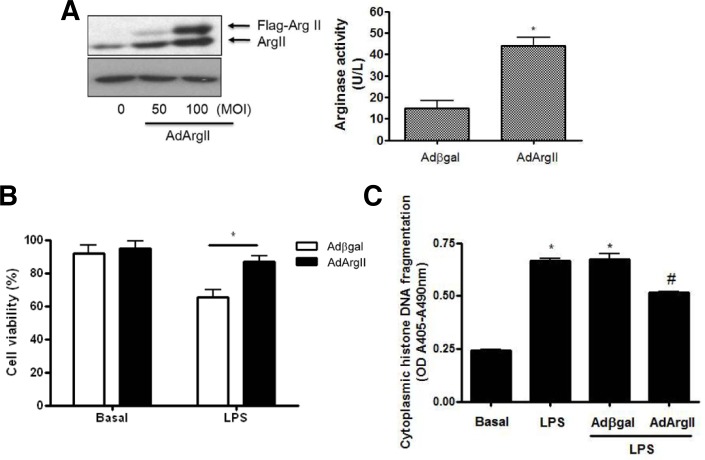
Gene transfer of arginase II inhibits LPS-induced cell viability and apoptosis in marcophages
To investigate whether arginase II inhibits LPS-induced cell death in macrophages, adenoviral gene transfer for arginase II (ArgII) was performed in Raw264.7 cells. The adenoviral overexpression of Flag-tagged ArgII was confirmed by Western blot and increased arginase activity, compared with Adβgal-transfected cells (Fig. 3A). As shown in Fig. 3B, adenoviral overexpression of arginase II significantly inhibited LPS-induced cell death, which was assessed with propridium iodide staining, compared with Adβgal-transfected cells. Also, the overexpression of arginase II in LPS-treated cells resulted in a significant decrease in cytoplasmic histone-associated DNA fragmentation (Fig. 3C). The DNA fragmentation in AdArgII-transfected cells treated with LPS was decreased by 33%, compared with Adβgal-transfected cells (Fig. 3C). These results indicated that the increased viability of arginase II expressing cells in the presence of LPS was indeed due to the attenuation of apoptotic cell death.
Fig. 3.
Arginase II overexpression inhibited LPS-induced cell death in RAW264.7 cells. (A) Typical Western blot for arginase II (left) and arginase activity (right) in AdArgII-trasfected cells. Total adenoviral multiplicity of infection (100 MOI) was balanced with Adβgal. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs. Adβgal. (B) Arginase II overexpression significantly inhibited LPS-induced cell death, which was assessed with propridium iodide staining, compared with Adβgal-transfected cells. Data are presented as mean ± SEM (n = 5). *P < 0.05 vs LPS-treated Adβgal-transfected cells. (C) Effect of arginase II expression on apoptosis induction in RAW264.7 cells assessed by quantitation of cytoplasmic histone-associated DNA fragmentation. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs basal, #P < 0.05 vs LPS in Adβgal-transfected cells.
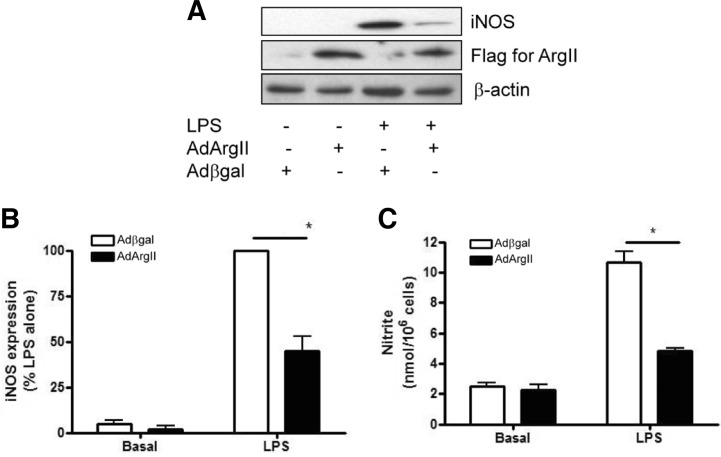
Overexpression of arginase II counter-regulated LPS-induced iNOS expression
We next investigated whether arginase II regulated iNOS protein expression. As shown in Fig. 4A, LPS (300 ng/ml) for 18 h induced iNOS expression. However, LPS-induced iNOS expression was significantly suppressed by ArgII overexpression in RAW264.7 cells. Also, adenoviral overexpression of arginase II significantly inhibited LPS-induced NO production compared with Adβgal-transfected cells (Fig. 4C). These data suggest that arginase-mediated suppression of LPS-induced NO production was mediated by iNOS suppression as well as limiting the availability of L-arginine.
Fig. 4.
Arginase II overexpression inhibited LPS-induced iNOS expression in RAW264.7 cells. (A) Typical immunoblot for iNOS and arginase II protein using lysates from RAW264.7 cells treated with 300 ng/ml LPS for 18 h. (B) Effect of arginase II overexpression on LPS-induced iNOS expression Data are presented as mean ± SEM (n = 3). *P < 0.05 vs LPS in Adβgal-transfected cells. (C) Effect of arginase II overexpression on the nitric oxide production. Nitrite production was measured in supernatant of cultured medium using Griess reagent. Data are presented as mean ± SEM (n = 3). *P < 0.05 vs LPS in Adβgal-transfected cells.
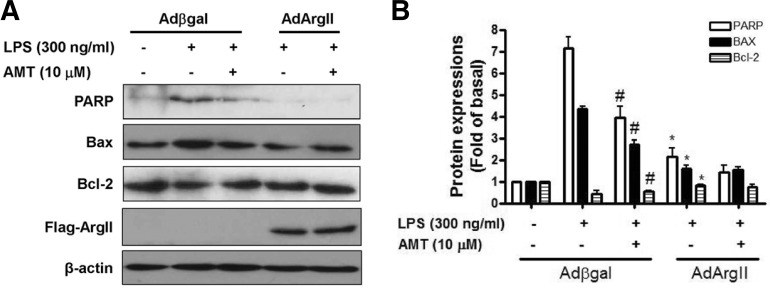
Arginase II inhibited LPS-induced mitochondrial apoptosis
As it was established that LPS treatment induced apoptotic cell death in macrophages, we raised the question as to whether LPS-induced apoptosis in AdArgII-transfected cells was associated with alteration of poly ADP-ribose polymerase (PARP) cleavage, and Bcl-2 family proteins expression. As shown in Fig. 5A, LPS (300 ng/ml) induced PARP cleavage which was significantly inhibited in AdArgII-transfected cells. We addressed this possibility by determining the effect of arginase II on levels of Bcl-2 and Bax proteins by immunoblotting (Fig. 5A). The arginase II overexpression resulted in a decrease in Bax protein levels while the reverse induction of Bcl-2 protein was evident in LPS-treated cells when compared with LPS-treated Adβgal-transfected cells (Fig. 5B). Also AMT, an inhibitor of iNOS, inhibited LPS-induced PARP cleavage and Bax in Adβgal-transfected cells, but did not significantly suppressed in AdArgII-transfected cells. This data suggested that an inhibition of apoptotic molecules by iNOS inhibitor or arginase II shares common pathways in macrophages. Collectively, these results indicated that LPS-induced apoptosis was rescued by regulation of Bcl-2/Bax in arginase II over-expressed macrophages, through reduction of PARP cleavage.
Fig. 5.
Arginase II overexpression inhibited mitochondrial apoptosis protein and apoptosis in RAW264.7 cells. (A) Immunoblotting for cleavaged PARP, Bax, and Bcl-2 proteins in RAW264.7 cells. LPS (300 ng/ml) or AMT (10 μM) was treated for 18 h in Adβgal or AdArgII-transfected cells. (B) Summarized data for Fig. 5A. Data are presented as mean ± SEM (n = 4). *P < 0.05 vs LPS in Adβgal-transfected cells. #P < 0.05 vs. LPS in Adβgal-transfected cells.
DISCUSSION
This study demonstrated that gene transfer of arginase II inhibited NO-induced apoptosis via suppression of iNOS/NO production and apoptosis molecules in RAW264.7 cells.
In septic shock, iNOS is strongly induced, and a large amount of NO can subsequently be produced. The excessive production of NO can lead to various symptoms, especially hypotension, multiple organ failure (Stuehr and Marletta, 1985). Arginase would be a good target molecule for septic shock and other conditions due to the excessive NO production. NOS inhibitors usually inhibit all types of NOS, and inhibition of constitutive types of NOS may cause side effects. On the other hand, the demand of iNOS for its substrate arginine is much higher than those of constitutive NOS because of its very high activity. The induction of arginase may lead to selective inhibition of the high output NO production by iNOS (Gotoh and Mori, 1999). Several of the harmful effects of bacteria are mediated by proinflammatory cytokines induced by bacterial cell wall components. The most toxic component of the gram-negative bacteria is the lipopolysaccharide (LPS). LPS causes damage to mitochondrial electron transport, leading to disordered energy metabolism and causes the induction of proinflammatory gene such as inducible nitric oxide synthase, resulting in a profound increase of NO (Gross and Wolin, 1995).
In RAW264.7 cells, arginase II appears to be constitutively active, but its activity can be up-regulated by exposure to LPS. In the present study, arginase activity was increased by LPS (Fig. 1), suggesting that increased arginase activity could diminish NO production by limiting the availability of L-arginine, the common substrate for both arginase II and iNOS (Wang et al., 1995). Also in the present study, AMT inhibited LPS-induced arginase activity suggesting LPS-induced iNOS induction is required to activate arginase II induction, although a more detailed early time course analysis is required to fully address this point.
LPS induced arginase II, but arginase I expression could not be detected in LPS-treated RAW264.7 cells, in agreement with the results of other groups (Gotoh et al., 1996; Morris et al., 1998). Arginase inhibitor, BEC, increased LPS-induced NO production and cell death even it has very weak effect in basal condition as shown in Fig 2. Because only arginase II was expressed and up-regulated in response to LPS, these results for BEC suggested that arginase II plays the protective role against LPS-induced cell death in RAW264.7 cells.
Arginase overexpression rescued LPS-induced apoptosis via inhibiting NO production. One possible outcome is a lowering of intracellular levels of L-arginine in macrophages. Because L-arginine is the substrate for iNOS-mediated excessive NO production, arginase II overexpression could inhibit the death of activated macrophage. Arginase catalyzes the conversion of arginine to urea and ornithine. The possibility of an inhibition of iNOS by arginase II would be due to arginase-mediated urea production. Urea and thiourea derivatives showed their inhibitory activities of NO production in LPS-activated macrophages. Urea inhibited NO production through the suppression of iNOS protein and mRNA expression (Kim et al., 2007; Moeslinger et al., 1999). Also, inhibition of iNOS-dependent NO production caused by urea enhances macrophage proliferation as a consequence of diminished NO-mediated apoptosis (Moeslinger and Spieckermann, 2001).
Excess NO induces apoptosis of some cell types, including macrophages. As NO is synthesized by NOS from arginine, a common substrate of arginase, these two enzymes compete for arginine. Arginase, which catalyzes the conversion of arginine to urea and ornithine, exists in two distinct isoforms. Arginase I is expressed almost exclusively in the liver where it serves as an essential enzyme of the urea cycle. In contrast, arginase II is expressed in many other extra-hepatic tissues. Arginase II is composed of a polypeptide chain of 354 amino acid residues, including the putative NH2-terminal pre-sequence for mitochondrial targeting and import (Shi et al., 1998).
Poly ADP-ribose polymerase (PARP) is a family of proteins involved in a number of cellular process involving apoptosis (Boulares et al., 1999). In the present study, PARP cleavage was increased by LPS, suggesting LPS-induced apoptosis. Arginase II inhibited LPS-induced PARP cleavage. Our data suggested that arginase II overexpression prevents against LPS-induced cell death via inhibiting PARP cleavage. Also, we provide experimental evidence that the rescue from LPS-mediated apoptosis in macrophages is attributed to arginase II over-expression, showing the regulation of levels of Bcl-2 family proteins. Bcl-2 family proteins have emerged as the critical regulators of mitochondria-mediated apoptosis (Adams and Cory, 1998; Chao and Korsmeyer, 1998). Previous studies indicated that LPS- induced apoptosis in macrophages was associated with an alteration in levels of Bcl-2 family proteins (Gotoh and Mori, 1999; Messmer et al., 1996a; 1996b; Seminara et al., 2007). For instance, cell death by LPS in macrophages caused an increase of NO production, modulating the stress-activated protein kinase (SAPK)/c-jun N-terminal kinase (JNK) signal transduction pathway. The activated JNK initiated mitochondrial-mediated apoptosis through Bax de-phosphorylation and down-regulation of Mcl-1, an anti-apoptotic Bcl-2 family member.
This study demonstrated that arginase II inhibited LPS-induced apoptosis via suppression of NO production in RAW264.7 cells. Inhibition of NO production by arginase II may be due to arginine depletion as well as iNOS suppression though its reaction products. Also, data presented herein indicate that arginase II overexpression is required for cell survival from LPS-mediated apoptotic cell death. This conclusion is based on the following observation, a decrease of pro-apoptotic protein Bax, an increase of anti-apoptotic protein Bcl-2 level, and PARP cleavage in arginase over-expressed cells.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education, Science and Technology) (2011-0006231, 2011-0016797 to B.H.J).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Cho EJ, Park MS, Kim SS, Kang G, Choi S, Lee YR, Chang SJ, Lee KH, Lee SD, Park JB, et al. Vasorelaxing activity of ulmus davidiana ethanol extracts in rats: activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011;15:339–344. doi: 10.4196/kjpp.2011.15.6.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J Cell Biol. 1999;144:427–434. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett. 1996;395:119–122. doi: 10.1016/0014-5793(96)01015-0. [DOI] [PubMed] [Google Scholar]
- Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- Joo HK, Lee YR, Lim SY, Lee EJ, Choi S, Cho EJ, Park MS, Ryoo S, Park JB, Jeon BH. Peripheral benzodiazepine receptor regulates vascular endothelial activations via suppression of the voltage-dependent anion channel-1. FEBS Lett. 2012;586:1349–1355. doi: 10.1016/j.febslet.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Kim SN, Son SC, Lee SM, Kim CS, Yoo DG, Lee SK, Hur GM, Park JB, Jeon BH. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006;105:105–110. doi: 10.1097/00000542-200607000-00019. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ryu JH, Cheon YJ, Lim HJ, Jeon R. Design and synthesis of urea and thiourea derivatives and their inhibitory activities on lipopolysaccharide-induced NO production. Bioorg Med Chem Lett. 2007;17:3317–3321. doi: 10.1016/j.bmcl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Messmer UK, Reed UK, Brune B. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem. 1996a;271:20192–20197. doi: 10.1074/jbc.271.33.20192. [DOI] [PubMed] [Google Scholar]
- Messmer UK, Reimer DM, Reed JC, Brune B. Nitric oxide induced poly(ADP-ribose) polymerase cleavage in RAW 264.7 macrophage apoptosis is blocked by Bcl-2. FEBS Lett. 1996b;384:162–166. doi: 10.1016/0014-5793(96)00311-0. [DOI] [PubMed] [Google Scholar]
- Moeslinger T, Spieckermann PG. Urea-induced inducible nitric oxide synthase inhibition and macrophage proliferation. Kidney Int Suppl. 2001;78:S2–8. doi: 10.1046/j.1523-1755.2001.59780002.x. [DOI] [PubMed] [Google Scholar]
- Moeslinger T, Friedl R, Volf I, Brunner M, Baran H, Koller E, Spieckermann PG. Urea induces macrophage proliferation by inhibition of inducible nitric oxide synthesis. Kidney Int. 1999;56:581–588. doi: 10.1046/j.1523-1755.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol. 1998;275:E740–747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- Nakane M, Klinghofer V, Kuk JE, Donnelly JL, Budzik GP, Pollock JS, Basha F, Carter GW. Novel potent and selective inhibitors of inducible nitric oxide synthase. Mol Pharmacol. 1995;47:831–834. [PubMed] [Google Scholar]
- Seminara AR, Ruvolo PP, Murad F. LPS/IFN-gamma-induced RAW 264.7 apoptosis is regulated by both nitric oxide-dependent and -independent pathways involving JNK and the Bcl-2 family. Cell Cycle. 2007;6:1772–1778. doi: 10.4161/cc.6.14.4438. [DOI] [PubMed] [Google Scholar]
- Shi O, Kepka-Lenhart D, Morris SM, Jr, O’Brien WE. Structure of the murine arginase II gene. Mamm Genome. 1998;9:822–824. doi: 10.1007/s003359900874. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WW, Jenkinson CP, Griscavage JM, Kern RM, Arabolos NS, Byrns RE, Cederbaum SD, Ignarro LJ. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochem Biophys Res Commun. 1995;210:1009–1016. doi: 10.1006/bbrc.1995.1757. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.