Abstract
Human adipose-derived mesenchymal stem cells (hADMSCs) are a potential cell source for autologous cell therapy due to their regenerative ability. However, detailed cytological or phenotypic characteristics of these cells are still unclear. Therefore, we determined and compared cell size, morphology, ultrastructure, and immunohistochemical (IHC) expression profiles of isolated hADMSCs and cells located in human adipose tissues. We also characterized the localization of these cells in vivo. Light microscopy examination at low power revealed that hADMSCs acquired a spindle-shaped morphology after four passages. Additionally, high power views showed that these cells had various sizes, nuclear contours, and cytoplasmic textures. To further evaluate cell morphology, transmission electron microscopy was performed. hADMSCs typically had ultrastructural characteristics similar to those of primitive mesenchymal cells including a relatively high nuclear/cytosol ratio, prominent nucleoli, immature cytoplasmic organelles, and numerous filipodia. Some cells contained various numbers of lamellar bodies and lipid droplets. IHC staining demonstrated that PDGFR and CD10 were constitutively expressed in most hADMSCs regardless of passage number but expression levels of α-SMA, CD68, Oct4 and c-kit varied. IHC staining of adipose tissue showed that cells with immunophenotypic characteristics identical to those of hADMSCs were located mainly in the perivascular adventitia not in smooth muscle area. In summary, hADMSCs were found to represent a heterogeneous cell population with primitive mesenchymal cells that were mainly found in the perivascular adventitia. Furthermore, the cell surface markers would be CD10/PDGFR. To obtain defined cell populations for therapeutic purposes, further studies will be required to establish more specific isolation methods.
Keywords: adipose tissue, in situ localization, immunohistochemistry, mesenchymal stem cells, ultrastructure
INTRODUCTION
The morphology of adipose tissue has not been widely studied given the simple structure of this tissue and the fact that it is not generally associated with serious diseases except for liposarcoma. However, interest in adipose tissue has increased after mesenchymal stem cells, also known as “human adipose tissue derived stem cells (hADMSCs)”, were isolated from this tissue (Deslex et al., 1987; Hauner et al., 1987; Pettersson et al., 1984). hADMSCs are multipotent stem cells that are recovered from the stromal vascular fraction (SVF) of adipose tissue (Gimble et al., 2007; Nakagami et al., 2006; Ning et al., 2006; Psaltis et al., 2008; Schäffler et al., 2007; Wilson et al., 2011).
Despite the therapeutic potential of hADMSCs and many studies that characterized these cells, the definition, surface marker expression, morphologic characteristics, and in vivo location of hADMSCs are still being debated. Some investigations have shown that hADMSCs recovered from the SVF are likely to be considered “pericytes” or “perivascular progenitor cells” (Caplan et al., 2008; Crisan et al., 2008; Lin et al., 2008). However, it is still unclear whether or not isolated hADMSCs are truly “pericytes” and from where hADMSCs originate (Locke et al., 2011; Tholpady et al., 2006). Although “pericytes” are fully matured perivascular cells which include contractile filament and basement membrane attached to endothelial, this term has been broadly used to describe perivascular resident cells (Allt et al., 2001; Armulik et al., 2005; Dore-Duffy, 2011; Sims et al., 1986). The use of misnomers or unclear terminology when referring to hADMSCs could interfere with scientific communication as well as clinic or pathological evaluation performed during cell transplantation.
There are some barriers that make it difficult to clearly define hADMSCs. Many types of primitive cells in the vascular wall may share morphological properties or immunophenotypic markers (Klein et al., 2010; Majesky et al., 2011a; 2011b; Pacilli et al., 2009; Traktuev et al., 2008; Zimmerlin et al., 2010). These different types of resident progenitor cells include hemangiblasts, bone marrow-derived endothelial progenitors, vascular wall resident endothelial progenitors, vascular wall resident smooth muscle progenitors, and vascular wall resident mesenchymal stromal cells. Therefore, the results of flow cytometric evaluation or cell sorting could vary greatly among different specimens, persons, or passages. For instance, the expression of well-known surface markers including CD105, CD73, and CD90 can change according to the cell passage number (Gronthos et al., 2001; Lin et al., 2010; McIntosh et al., 2006; Mitchell et al., 2006) In order to resolve some of this confusion, some groups have attempted in vivo identification using immunofluorescence markers including ones specific for PDGFR, α-SMA, CD146, and NG2. Despite these efforts, precise identification of hADMSCs is still not possible (Crisan et al., 2008; Lin et al., 2008). Characterization of hADMSCs for use in therapeutic techniques is critical because it is essential to evaluate cells before and after transplantation into human body.
In the present study, we evaluated the ultrastructural and immunophenotypic characteristics of isolated hADMSCs as well as adipose tissues. We also identified characteristics of hADMSC that were shared with adipose tissues. Finally, we determined the in situ localization of hADMSCs in adipose tissues by performing immunohistochemical (IHC) staining and transmission electron microscopy (TEM). We suggest that the hADMSCs are exist on the adventitia of perivascular area in adipose tissues and CD10/PDGFR can be a sorting criteria of hADMSCs.
MATERIALS AND METHODS
Human adipose tissue isolation
Samples of abdominal subcutaneous adipose tissue were obtained from three healthy women at Seoul National University Dental Hospital (Korea). This procedure was performed after informed consent and approval from the Institutional Review Board of the Seoul National University School of Dentistry (IRB no. S-D20110003) were received. The tissues were fixed in cold 2% formaldehyde for 4 h at 56°C and embedded in paraffin. Paraffin embedded (PE) blocks were cut into sections 4-μm thick and subjected to routine hematoxylin and eosin (HE) staining. For IHC staining, the sections were incubated overnight at 4°C with primary antibodies (Table 1) and processed using an autostainer (XT System Benchmark, Ventana Medical System, USA) according to the manufacturer’s instruction.
Table 1.
Antibodies used for immunohistochemistry in the present study
| Target protein | Supplier | Dilution |
|---|---|---|
| CD31 | DAKO, Denmark | 1:50 |
| CD34 | DAKO, Denmark | 1:500 |
| CD146 | DAKO, Denmark | 1:200 |
| α-SMA | DAKO, Denmark | 1:400 |
| PDGFR | Epitomics, USA | 1:50 |
| CD68 | DAKO, Denmark | 1:2,000 |
| Oct-4 | Norvocast, UK | 1:100 |
| c-kit | DAKO, Denmark | 1:400 |
| CD10 | DAKO, Denmark | 1:25 |
| Vimentin | Zymed, USA | 1:250 |
Preparation of hADMSCs
Human adipose tissue was minced and digested with 0.1% type I collagenase (Gibco, USA) with 0.001% Dnase I (Sigma-Aldrich, USA) in Dulbecco’s modified Eagle’s medium-high glucose medium (DMEM-HG; Sigma) for 30 min at 37°C with shaking. The isolated cells were washed with Dulbecco’s modified Eagle’s Medium (DMEM; Welgene, Korea) and centrifuged at 400 G for 5 min at 4°C for three times. The resulting pellet containing the hADMSCs was resuspended in HBSS and filtered through a pore size-dependent manner. The cells were seeded and cultured in DMEM containing 20% fetal bovine serum (FBS; HyClone, USA) and 1% antibiotic-antimycotic solution (Gibco) at 37°C in 5% CO2. When 70% confluence was reached, the hADMSCs were passaged.
Fluorescence-activated cell sorting (FACS) analysis
We quantified the cell surface markers expression for the characterization of the hADMSCs by, fluorescence-activated cell sorting (FACS). We performed FACS analysis based on previously reported (Cho et al., 2012). The antibodies are listed as follows; fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD14, CD31, CD44 and CD45; phycoerythrin (PE)-conjugated mouse anti-human CD29, CD73, and CD117; PE/Cy5-conjugated mouse anti-human CD90; allophycocyanin (APC)-conjugated mouse anti-human CD34; HLA-DR; PE-conjugated streptavidin; biotin-conjugated HLA class II (all from BD PharMingen, USA); APC-conjugated mouse anti-human CD105 (eBioscience, USA). hADMSCs (100,000 cells/reaction) were incubated with primary antibodies for 30 min at 4°C. After incubated with the secondary antibody for 30 min, the cells were fixed with 4% paraformaldehyde. We detected the fluorescence intensity by a FACS CaliburTM and quantified with BD CellQuest Pro software (all from Becton Dickinson, USA).
Light microscopy analysis, cell size determination, and IHC profile evaluation
To evaluate hADMSC morphology, isolated cells were cultured on collagen-coated cover glasses until 70% confluency. After 48 h, the attached cells were fixed in 95% chilled-ethanol for 5 to 15 min. The fixed cells were stained with HE and Papanicolaou stain (Papanicolaou, 1942) using routine protocols. Briefly, the cells were incubated with hematoxylin (5 to 10 min), rinsed with distilled water, stained with Orange G 6 solution, rinsed five to 10 times with 95% alcohol, and stained with Eosin Azure (EA 36) solution (eosin yellowis and acid phosphotungstic) according to the manufacturer’s instruction (VWR International, Belgium). Alternatively, 1 × 107 hADMSCs were prepared for fixation in a PE block. The cells were centrifuged at 400 G for 3 min. The pellet was fixed in 95% ethanol for 30 min at RT and embedded in paraffin. The slides were subjected to both HE and Papanicolaue’s staining. For IHC staining, the slides were incubated overnight at 4°C with primary antibodies (Table 1) and processed using an autostainer (XT System Benchmark; Ventana Medical System, USA) according to the manufacturer’s instruction. The sizes of both attached and trypsinized cells were determined on digital images using an image analyzer (3DHISTECH, Hungary) as previously described (Krenacs et al., 2009).
TEM analysis of hADMSCs and human adipose tissues
For ultrastructural morphology analysis, hADMSCs were detached from culture plates, pelleted by centrifugation at 4°C in 400 G for 10 min, and fixed in cold 4% glutaraldehyde for 1 h at RT. The pellets were then dehydrated in graded acetone, and embedded in Epon 812. Adipose tissues were prepared in the same manner as the cells. Ultrathin sections were stained with uranyl acetate and alkaline bismuth subnitrate before being examined with a JEM 1200 transmission electron microscope (TEM; Joel, Japan) at 80 Kv. TEM data were acquired and interpreted.
Localization of hADMSCs in adipose tissue according to histological and IHC profiles
Interpretation of histological and IHC data was performed. Regions in the adipose tissue positive for factors that correlated with the IHC profile of hADMSCs were designated as target areas. We classified hADMSC-positive vessels according to size as previously described (Lin et al., 2008): large (> 150 μm), medium (75–150 μm), small (10–75 μm), and capillaries (< 10 μm).
RESULTS
Phenotypic characteristics of hADMSCs and cells in human adipose tissues
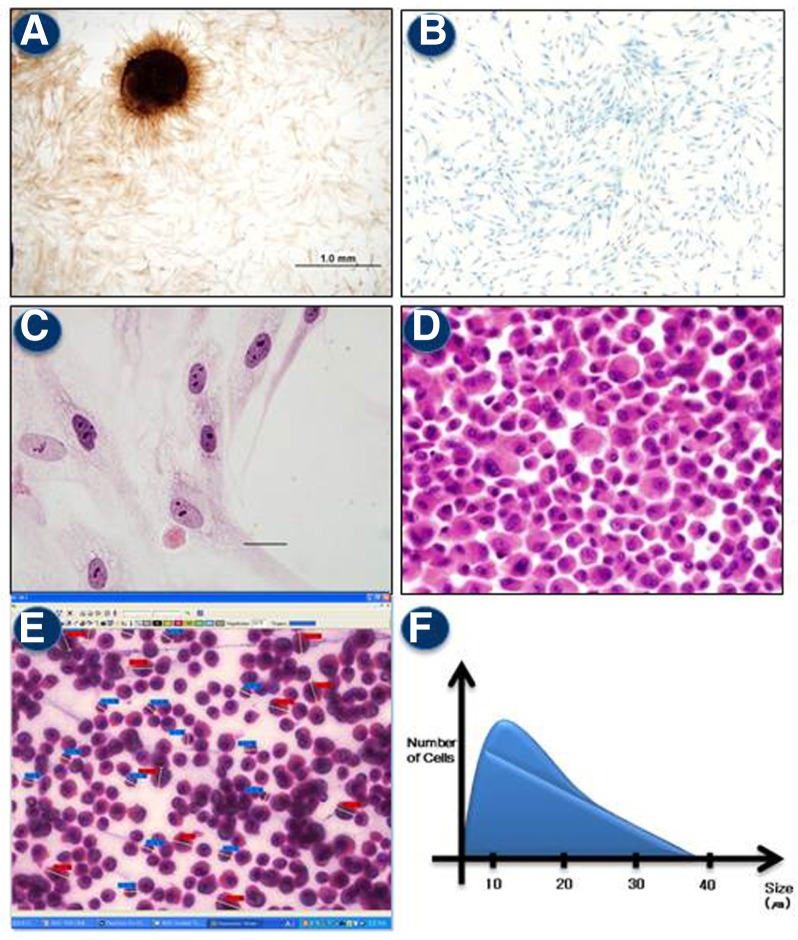
On low power light microscopy views, attached hADMSCs were found to form colonies (Fig. 1A) and were spindles-shaped or elongated (Fig. 1B). We also found several types of cells with various sizes, shapes, and color variation of the nuclei and cytosol (Fig. 1C). Evaluation of the sectioned PE block further revealed that the embedded cells had morphological features similar to those of the attached cells (Fig. 1D). Sizes of the cells ranged from 5 to 40 μm with an average diameter of about 22 μm. The majority of cell were between 15–25 μm in size (Figs. 1E and 1F). No nuclear/cytoplasmic inclusions or rare mitotic features were observed. Likewise, nuclear atypia as well as pleomorphism were not noted.
Fig. 1.
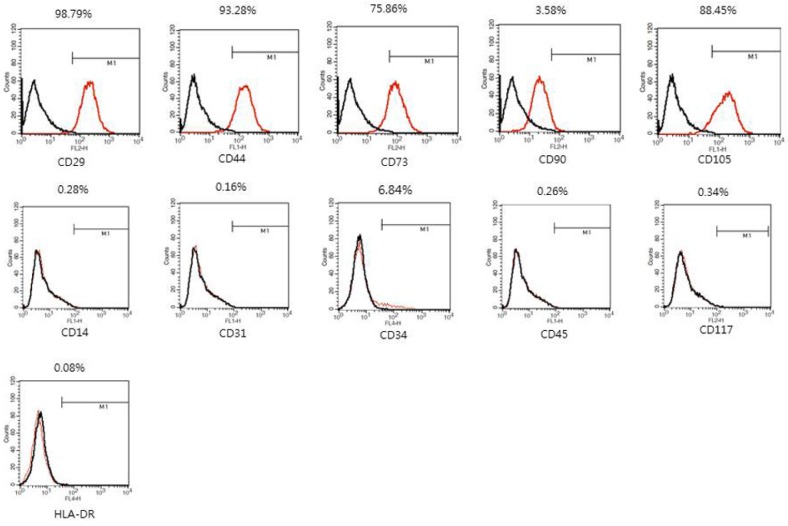
FACS analysis of hADMSCs. Data are shown as an overlay plot with immunoglobulin isotype control (in black) and different specific cell-surface markers (in red). Cell surface markers of hADMSCs at passage 4. The Cells were positive for mesenchymal stem cell markers (CD29, CD44, CD73, CD105) and negative for hematopoietic markers or endothelial marker (CD14, CD31, CD34, CD45, CD117, HLA-DR).
On high power light microscopy views, variations in the morphological characteristics of the attached and PE blocked hADMSCs were observed (Figs. 3A and 3B). First, some cells had relatively a small size (< 15 μm) with hyperchromatic nuclei and dense cytoplasm. Multiple and distinct nucleoli were also found (Figs. 3B and 3G). Second, cells that were medium-sized (15–25 μm) with hyperchromatic nuclei were also observed. The nuclear texture of these cells was similar to that of the small cells, but the cytoplasm was relatively less dense (Figs. 3C and 3H). Third, large cells (25–35 μm) with isochromatic nuclei were identified. The nuclei of these cells were more hypo-chromatic than the small and medium-sized cells, but the cytoplasmic texture was similar to that of the medium-sized cells. Multiple and distinct nucleoli were also observed (Figs. 3D and 3I). Finally, extremely large cells (> 35 μm) with isochromatic nuclei were present. The cytoplasmic and nuclear texture was similar to that of the medium-sized cells, but amount of cytoplasm was greater. Multiple and distinct nucleoli were identified (Figs. 3E and 3J).
Fig. 3.

Variations of hADMSC morphology. Attached (A–E) and PE blocked hADMSCs (F–J) were divided into four different types including small cells with hyperchromatic nuclei (B, G), medium-sized cells (C, H), large cells with isochromatic nuclei (D, I), and extremely large cells (E, J).
Cell surface marker expression of hADMSCs by FACS analysis
hADMSCs expressed the mesenchymal stem cell markers such as CD29 (98.79%), CD44 (93.28%), CD73 (75.86%), CD90 (3.58%), and CD105 (88.45%). however, few cells expressed hematopoietic marker such as CD14 (0.28%), CD34 (6.84%), CD45 (0.26%), CD117 (0.34%), and HLA-DR (0.08%), or the endothelial marker CD31 (0.16%) (Fig. 1).
IHC profiles of the hADMSCs and adipose tissues
Results of the IHC analysis are summarized in Table 2. hADMSCs strongly expressed PDGFR (Fig. 4C) and CD10 (Fig. 4D) during passaging. In contrast, expression of α-SMA was focal and weak while CD146 expression was absent at passage 4 (Figs. 4A and 4B). CD31, CD34, and Oct-4 were expressed in a few cells only during early passage, and the levels of expression rapidly diminished thereafter. The majority of the cells weakly expressed CD68 (data not shown).
Table 2.
Expression of various factors in adipose tissue and correlation with isolated hADMSCs determined by immunohistochemical (IHC) staining
| Location | Large vessels | Medium vessels | Small vessels | Capillaries | Isolated hADMSCs | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| M. wall | Adv. | M. wall | Adv. | M. wall | Adv. | Pericap. | Endoth. | ||
| α-SMA | + | − | + | − | + | − | +, Focal | +, Focal | +, Focal |
| CD146 | +, Focal | − | +, Focal | − | +, Focal | − | +, Weak | +, Weak | − |
| PDGFR | +, Outer | + | +, Outer | + | +, Outer | + | + | − | + |
| CD10 | − | − | − | + | − | + | + | − | + |
M. wall, muscular layer of the vascular wall; Adv., adventitia of the vascular wall; Pericap., pericapillary cells; Endoth., endothelial cells
Fig. 4.
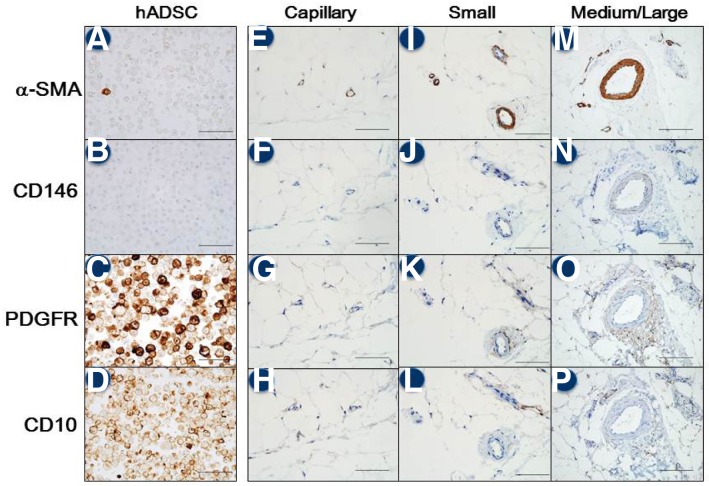
Comparison of immunohistochemical (IHC) staining patterns observed in hADMSCs and human adipose tissues. The columns represent hADMSCs (A–D) along with small (EH), medium (I–L), and large cells (M–P) in the human adipose tissues. hADMSCs strongly expressed PDGFR (4C) and CD10 (4D) during passaging. In contrast, expression of α-SMA was focal and weak while CD146 expression was absent at passage 4 (A, B). α-SMA, α-smooth muscle actin; PDGFR, PDGF receptor.
In the adipose tissue, differential expression of α-SMA, CD146, PDGFR, and CD10 was observed and depended upon the size of the vessels. α-SMA-positive cells were confined to the muscular wall of all vessels (regardless of size), and were located close to the endothelial layer and pericapillary cells (Figs. 4E, 4I, and 4M). Similarly, CD146-positive cells were observed only in the muscular wall of all vessels and were in close proximity to the pericapillary cells (Figs. 4F, 4J, and 4N). In contrast, PDGFR-positive cells were found in close proximity to the pericapillary cells in the outer muscular wall layer of the adventitia of all vessels (Figs. 4G, 4K, and 4O). CD10-positive cells were also distributed close to pericapillary cells in the adventitia which is located in outer layer of muscular wall of blood vessels (Figs. 4H, 4L, and 4P).
In immunohistochemical evaluation of the suggested stem cell surface markers (Table 1), we summarized and compared the expression pattern of the representative four markers, e.g., α-SMA, CD 146, PDGFR and CD10 (Table 2). Overlapping areas positive for both CD10 and PDGFR were located in the perivascular adventitia of all vessels as well as the pericapillary area, but not in the muscular wall. Some endothelial and pericapillary cells in the capillaries formed foci that were weakly positive for α-SMA. A few cells in the adventitia of small vessel expressed CD68. Endothelial cells in all vessels were positive for CD31 and CD34 (data not shown). hADMSCs showed PDGFR+/CD10+/CD146−/α-SMA+focal. The perivascular adventitia area and pericapilly area showed the similar expression pattern in the adipose tissues.
Ultrastructural features of the hADMSCs and adipose tissues
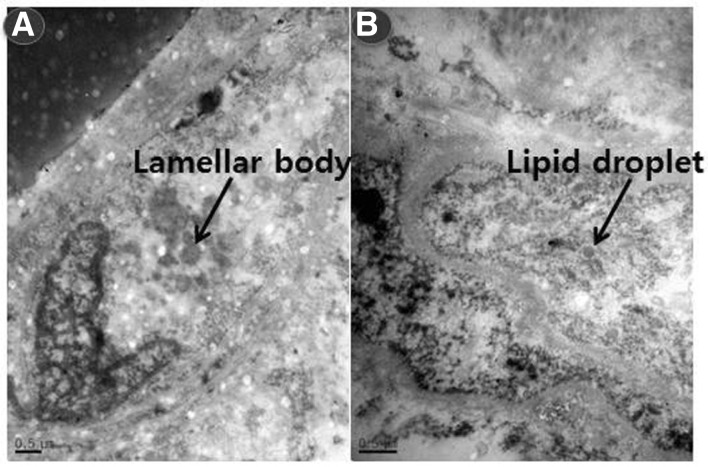
We analyzed and compared the intracellular morphology of isolated hADMSCs and adipose tissues using TEM. hADMSCs had typical ultrastructural features of undifferentiated mesenchymal cells. The cells contained large euchromatic nuclei with prominent nucleoli, sparse organelles, abundant free ribosomes, a distended rough endoplasmic reticulum (rER), and microvilli processes. Cytoplasmic vacuoles with lipid droplets and intracytoplasmic lamellar bodies with dark cytoplasm were also present (Fig. 5).
Fig. 5.
Ultrastructural features of the hADMSCs viewed with transmission electron microscopy (TEM). (A) An indented nucleus with prominent nucleoli. (B) An intracytoplasmic fat vacuole. (C, D) Intracytoplasmic lamellar bodies.
To determine the in situ location of mesenchymal stem cells, we also performed TEM to analyze the ultrastructural morphology of adipose tissues. In the perivascular area, mature pericytes were identified as well as some primitive mesenchymal progenitor cells with large euchromatic nuclei and prominent nucleoli, sparse organelles, abundant free ribosomes, and a distended rough endoplasmic reticulum (rER) similar to hADMSCs, but did not include lamellar body neither lipid droplet in the cytosol.
Interestingly, the perivascular adventitia of the adipose tissue contained a number of primitive cells with lamellar bodies and lipid droplets similar to those of hADMSCs (Fig. 6).
Fig. 6.
Ultrastructural characteristics of human adipose tissues evaluated with TEM. (A) Primitive cells with lamellar bodies in the adventitia of a medium-sized vessel. (B) Primitive cells containing lipid droplets in the adventitia of a medium-sized vessel.
DISCUSSION
Adipose tissue is considered to be a source of mesenchymal stem cells. Up to now, fat tissues have not been given much attention by researchers. However, many studies have recently shown that hADMSCs can be used for stem cell research as well as cell-based therapy (Gimble et al., 2007; Nakagami et al., 2006; Ning et al., 2006; Psaltis et al., 2008; Schäffler et al., 2007; Wilson et al., 2011). In the present study, we compared the morphology and IHC characteristics of isolated hADMSCs and adipose tissues. We showed that hADMSCs are morphologically diverse and can be categorized as four different types. We propose that CD10 and PDGFR double-positive cells are hADMSCs. Furthermore, we found that these cells are localized not in the perivascular area but rather the adventitia.
We first evaluated the morphology of hADMSCs using light microscopy. The cells were shown to have different morphological features and could be divided into four different groups based on cell size and shape. The ability of hADMSCs to form colonies was noted during the early stage of primary culturing but diminished rapidly with subsequent passages. However, the spindle-shaped morphology was maintained. Furthermore, our novel classification system was maintained not only during the early passages but also later passages. These results indicate that some characteristics of hADMSCs change as the cells are passaged while others such as the spindle shape are maintained.
The isolated hADMSCs expressed the typical mesenchymal cell markers such as CD29, CD44, and CD73. The more than 90% expression of CD29, CD44 represent that the hADMSCs are mesenchymal stem cells. The cell population included the low population of hematopoietic markers and endothelial marker. The results means the major population of the isolated hADMSCs are mesenchymal stem cells similar to bone marrow derived-mesenchymal stem cells (Cho et al., 2012).
We also performed an IHC analysis to identify cell surface markers on the hADMSCs and determine the location of these cells in human adipose tissues. A few CD34-positive cells were identified in primary cultures, but the number of these cells diminished rapidly as the cells were passaged (data not shown). This phenomenon, especially among CD31- or CD34-positive cells, has been previously reported (McIntosh et al., 2006; Mitchell et al., 2006).
Since the technique for isolating primary cells was reported in the 1960s (Rodbell et al., 1968), this method has been modified but is essentially unchanged. Many types of primary cells are isolated from adipose tissue but the stromal vascular fractions predominantly contain circulating blood cells, endothelial cells, pericytes, adipocyte progenitor cells, and smooth muscle progenitor cells (Gimble et al., 2007). This heterogeneous cell population can be cultured under different conditions, but with standard culture conditions specifically tailored for hADMSCs the cells generally form “spindle” or “fibroblast-like” morphologies as they are passaged (Deslex et al., 1987; Hauner et al., 1987; Pettersson et al., 1984; Zuk et al., 2001). This finding indicates that some types of cells such as endothelial and circulating blood cells disappear under these culture conditions.
Evaluation of high power light microscopy views and TEM data demonstrated that isolated hADMSCs had basic primitive mesenchymal features. The cells had a relatively high nuclear/cytoplasm (N/C) ratio, prominent nucleoli, premature cytoplasmic organelles, and numerous filipodia. These characteristics are similar to those of bone marrow-derived mesenchymal stem cells (Pasquinelli et al., 2007). hADMSC morphology including cell size, shape, nuclear density, and cytoplasmic contents varied in this study. In particular, we observed that the hADMSCs had typical characters such as lipid droplet and lamellar body inclusions similar to those of macrophage progenitor cells (Vandenabeele et al., 2003).
Aside from general cellular morphological features, we evaluated and compared the expression of surface markers on hADMSCs and in human adipose tissues. We occasionally found CD 68-positive cells among the hADMSCs and in the fat tissues, especially adjacent to vessels. Cells expressing CD68 might have arisen from cell differentiation during passaging (Zengin et al., 2006). α-SMA-positive cells were confined in the entire vascular wall of vessels including arteries, arterioles, veins, and venules. Interestingly, not only pericapillary cells but also some endothelial cells expressed α-SMA. However, the number of α-SMA-positive hADMSCs was relatively low. A previous study reported that α-SMA is a “pericyte marker” (Lin et al., 2008). However, according to our ultrastructural analysis results, these pericapillary cells could be not only pericytes but also primitive mesenchymal stem cells. Decreased α-SMA expression in hADMSCs might be attributed to passaging.
CD146 is also known as a “pericyte marker” (Crisan et al., 2008; Lin et al., 2008). This marker was also expressed around the vascular wall overlapping with the α-SMA-positive areas in the adipose tissue. This result suggests that CD146 can also serve as a perivascular mesenchymal stem cell marker. However, there were few CD146-positive cells among the cultured hADMSCs, suggesting that isolated hADMSCs may not arise from the vascular wall muscle layer or capillary SMA+/CD146+ endothelial cells.
PDGFR is a well-known perivascular stem cell marker (Ball et al., 2010; Crisan et al., 2008). In large vessels, PDGFR-positive cells were seen in the muscular wall and adventitia. In medium and small vessels, cells expressing PDGFR were noted in the outer layer of the muscular wall and adventitia. In capillaries, positive cells were found in the pericapillary and stromal layer adjacent to the vessel. We also found that most cultured hADMSCs expressed PDGFR. Our results suggested that the isolated hADMSCs can be arised form adventitia rather than perivascular muscular region.
CD10 is a known marker of mesenchymal stem cells (Mariotti et al., 2008; Musina et al., 2005). In the current study, CD10-positive cells were found in the adventitia rather than the perivascular regions similar to cells expressing PDGR. We also discovered that CD10-positive areas correlated with regions positive for PDGFR, but not with areas showing CD146 or α-SMA expression. Based on the results of our stem cell surface marker evaluation, we concluded that the isolated hADMSCs originated from the adventitia or capillary region rather than the perivascular muscular layer. The findings of our ultrastructural evaluation also supported the IHC data. hADMSCs were found to contain lipid droplets and lamellar bodies in the cytosol. We also observed these cells in the adventitia layer but not the perivascular layer in adipose tissues.
In summary, our study demonstrated that adipose-derived mesenchymal stem cells are found in capillaries and the adventitia rather than the perivascular region. This conclusion was based on IHC data showing that the cell surface markers CD10 and PDGFR (rather than CD146) were expressed in small to medium-sized blood vessels. We also found that even though the morphology of hADMSCs varies considerably, these cells can be classified based on cell size. Our results indicated that the isolated hADMSCs we obtained originated from a specific location in adipose tissues. Further studies are needed to refine cell isolation and sorting techniques based on the stem cell markers we evaluated in the present investigation. Additionally, the proliferation and differentiation of hADMSCs and other stem cells should be examined in greater detail.
Fig. 2.
Heterogeneity of isolated hADMSCs. (A) Colony formation of the cells observed during early passage. (B) Spindle shape of the cells in a low power view. (C) Various morphologies of the attached cells in a high power view. HE staining (400 × magnification) (D) Various morphologies of trypsinized cells in a high power view. (E) Image analyzer cell annotation. Each cell size was measured. Total counted cells was 1,000. (F) Size distribution of the hADMSCs. The sizes of both attached and trypsinized cells were determined on digital images using an image analyzer (3DHISTECH, Hungary).
Acknowledgments
This research was supported by the Bio and Medical Technology Development Program (no. 860-20110087) of the National Research Foundation (NRF) funded by the South Korean government [The Ministry of Education, Science and Technology (MEST)] and the Seoul National University Dental Hospital Research Fund (no. 07-2012-0001).
REFERENCES
- Allt G, Lawrenson JG. (2001). Pericytes: cell biology and pathology. Cells Tissues Organs 169, 1–11 [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. (2005). Endothelial/pericyte interactions. Circ Res. 97, 512–23 [DOI] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA, Kielty CM. (2010). Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: implications for neovascularization. Exp Opin Biol Ther. 10, 57–71 [DOI] [PubMed] [Google Scholar]
- Caplan AI. (2008). All MSCs are pericytes? Cell Stem Cell 11, 229–230 [DOI] [PubMed] [Google Scholar]
- Cho T-J, Kim J, Kwon S-K, Oh K, Lee J-A, Lee D-S, Cho J, Park S. (2012). A potent small-molecule inducer of chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. Chem Sci. 3, 3071–3071 [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 [DOI] [PubMed] [Google Scholar]
- Deslex S, Negrel R, Vannier C, Etienne J, Ailhaud G. (1987). Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Int J Obes. 11, 19–27 [PubMed] [Google Scholar]
- Dore-Duffy P, Cleary K. (2011). Morphology and properties of pericytes. Methods Mol Biol. 686, 49–68 [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. (2007). Adipose-derived stem cells for regenerative medicine. Circ Res. 100, 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. (2001). Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 189, 54–63 [DOI] [PubMed] [Google Scholar]
- Hauner H, Schmid P, Pfeiffer EF. (1987). Glucocorticoids and insulin promote the differentiation of human adipocyte pre-cursor cells into fat cells. J Clin Endocrinol Metab. 64, 832–835 [DOI] [PubMed] [Google Scholar]
- Klein D, Hohn HP, Kleff V, Tilki D, Ergün S. (2010). Vascular wall-resident stem cells. Histol Histopathol. 25, 681–689 [DOI] [PubMed] [Google Scholar]
- Krenacs T, Zsakovics I, Diczhazi C, Ficsor L, Varga VS, Molnar B. (2009). The potential of digital microscopy in breast pathology. Pathol Oncol Res. 15, 55–58 [DOI] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Sukhikh GT. (2005). Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 139, 504–509 [DOI] [PubMed] [Google Scholar]
- Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. (2008). Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 17, 1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. (2010). Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 25, 807–815 [DOI] [PubMed] [Google Scholar]
- Locke M, Feisst V, Dunbar PR. (2011). Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells 29, 404–411 [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. (2011a). The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 31, 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ. (2011b). Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 108, 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti E, Mirabelli P, Abate G, Schiattarella M, Martinelli P, Fortunato G, Di Noto R, Del Vecchio L. (2008). Comparative characteristics of mesenchymal stem cells from human bone marrow and placenta: CD10, CD49d, and CD56 make a difference. Stem Cells Dev. 17, 1039–1041 [DOI] [PubMed] [Google Scholar]
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, et al. (2006). The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 24, 1246–1253 [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al. (2006). Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24, 376–385 [DOI] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Sukhikh GT. (2005). Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 139, 504–509 [DOI] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. (2006). Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 13, 77–81 [DOI] [PubMed] [Google Scholar]
- Ning H, Lin G, Lue TF, Lin CS. (2006). Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 74, 510–518 [DOI] [PubMed] [Google Scholar]
- Pacilli A, Pasquinelli G. (2009). Vascular wall resident progenitor cells: a review. Exp Cell Res. 315, 901–914 [DOI] [PubMed] [Google Scholar]
- Papanicolaou GN. (1942). A new procedure for staining vaginal smears. Science 95, 438–439 [DOI] [PubMed] [Google Scholar]
- Pasquinelli G, Tazzari P, Ricci F, Vaselli C, Buzzi M, Conte R, Orrico C, Foroni L, Stella A, Alviano F, et al. (2007). Ultrastructural characteristics of human mesenchymal stromal (stem) cells derived from bone marrow and term placenta. Ultrastruct Pathol. 31, 23–31 [DOI] [PubMed] [Google Scholar]
- Pettersson P, Cigolini M, Sjöström L, Smith U, Björntorp P. (1984). Cells in human adipose tissue developing into adipocytes. Acta Med Scand. 215, 447–451 [DOI] [PubMed] [Google Scholar]
- Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. (2008). Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26, 2201–2210 [DOI] [PubMed] [Google Scholar]
- Rodbell M, Jones AB, Chiappe de Cingolani GE, Birnbaumer L. (1968). The actions of insulin and catabolic hormones on the plasma membrane of the fat cells. Recent Prog Horm Res. 24, 215–254 [DOI] [PubMed] [Google Scholar]
- Schäffler A, Büchler C. (2007). Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells 25, 818–827 [DOI] [PubMed] [Google Scholar]
- Sims DE. (1986). The pericyte-a review. Tissue Cell 18, 153–174 [DOI] [PubMed] [Google Scholar]
- Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, Katz AJ. (2006). Adipose tissue: stem cells and beyond. Clin Plast Surg. 33, 55–62 [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 102, 77–85 [DOI] [PubMed] [Google Scholar]
- Vandenabeele F, De Bari C, Moreels M, Lambrichts I, Dell’Accio F, Lippens PL, Luyten FP. (2003). Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 66, 145–153 [DOI] [PubMed] [Google Scholar]
- Wilson A, Butler PE, Seifalian AM. (2011). Adipose-derived stem cells for clinical applications: a review. Cell Prolif. 44, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S. (2006). Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133, 1543–1551 [DOI] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. (2010). Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 [DOI] [PubMed] [Google Scholar]