Abstract
Curcumin (diferuloylmethane), the yellow pigment of turmeric, is one of the most commonly used and extensively studied phytochemicals due to its pleiotropic effects in several human cancers. In the current study, the therapeutic efficacy of curcumin was investigated in human colorectal carcinoma HCT-15 cells. Curcumin inhibited HCT-15 cells proliferation and induced apoptosis in a dose- and time-dependent manner. Hoechst 33342 and DCFHDA staining revealed morphological and biochemical features of apoptosis as well as ROS generation in HCT-15 cells treated with 30 and 50 μM curcumin. Over-expression of pre-mRNA processing factor 4B (Prp4B) and p53 mutations have been reported as hallmarks of cancer cells. Western blot analysis revealed that curcumin treatment activated caspase-3 and decreased expression of p53 and Prp4B in a time-dependent manner. Transfection of HCT-15 cells with Prp4B clone perturbed the growth inhibition induced by 30 μM curcumin. Fractionation of cells revealed increased accumulation of Prp4B in the nucleus, following its translocation from the cytoplasm. To further evaluate the underlying mechanism and survival effect of Prp4B, we generated siRNA-Prp4B HCT15 clones. Knockdown of Prp4B with siRNA diminished the protective effects of Prp4B against curcumin-induced apoptosis. These results suggest a possible underlying molecular mechanism in which Prp4B over-expression and activity are closely associated with the survival and regulation of apoptotic events in human colon cancer HCT-15 cells.
Keywords: apoptosis, colorectal cancer, curcumin, Prp4B
INTRODUCTION
Curcumin is a turmeric polyphenol, isolated from the dried ground rhizome of the perennial herb curcuma species Curcuma longa, is known to exhibit potential anti-cancer activity against a variety of cancer models. Its preventive role in cancer management along with its ability to mediate apoptosis in cancer cells suggests its potential as a universal anti-cancer agent (Shehzad et al., 2013). Tumor suppressor protein p53 has been shown to be mutated in almost 50% of all human cancers, particularly in colorectal carcinomas. The underlying mechanism involves missense mutations or truncation, resulting in elevation of the p53 protein level as well as the arbitration of several cellular responses, including cell cycle arrest, DNA damage repair and induction of apoptosis (Liu and Bodmer, 2006). In addition to p53, intracellular caspases have been identified as major signaling molecules involved in apoptotic cell death. Caspases belong to a family of structurally related cysteine proteases, and their activity is directly or indirectly responsible for the proteolytic cleavage of proteins during apoptosis (Su et al., 2006).
Cancers of the colon and rectum, together with colorectal cancer, comprise the third leading cause of cancer-related deaths in men and women. An estimated 142,820 (73,680 male and 69,140 female) new cases as well as 50,830 (26,300 male and 24,530 female) deaths related to colorectal cancer will occur in the United States in 2013 (Siegel et al., 2013). In colon cancer, alternative splicing is misregulated, which exhibit behavior that usually elicit apoptosis (Letai, 2008). Transcription of apoptotic genes is regulated via alternative splicing, often producing isoforms variations with distinct functions that decide cell fate (Schwerk and Schulze-Osthoff, 2005). Therefore, modulation of apoptotic proteins via manipulation of alternative splicing will be an attractive strategy for cancer therapy.
Pre-mRNA processing is catalyzed by several splicing factors that form a complex with small nuclear ribonucleoprotein (snRNP) particles. Pre-mRNA processing factor 4B (Prp4B), an isoform of Prp4, is one of the earliest identified splicing gene products. As a component of U4/U6 snRNP, mutations in the central segment of Prp4 cause severe growth impairment (Hu et al., 1994). A mutational study on the amino acid sequence of Prp4 confirmed the presence of a serine/threonine protein kinase catalytic domain, which is essential for cell cycle transition. It is known that aberrant kinase activity or loss of function leads to the accumulation of pre-mRNAs, which perturb mitosis and subsequently elongate the cell cycle (Gross et al., 1997; Schwelnus et al., 2001). Specific sequential disorders and deregulated signaling is not only critical for regular growth and maturation, but also closely related with tumor initiation and progression (Silvera et al., 2010).
Despite broad research on the anti-tumor effects of curcumin, its ability to modulate colon cancer growth has not yet been well established. The present study primarily focused on the effects of different concentrations of curcumin on cell morphology and apoptosis in human colorectal carcinoma (HCT-15) cell lines as well as the expression of apoptosis-related genes such as p53 and caspase-3. According to our results, curcumin caused growth arrest and apoptosis in HCT-15 cell lines, and these inhibitory effects of curcumin mediated by the regulation of Prp4B, because specific transfection with Prp4 clone reverses curcumin-induced apoptosis.
Materials and Methods
Materials
Curcumin and propidium iodide (PI) were purchased from Sigma-Aldrich (USA). Hoechst 33342 dye was obtained from Calbiochem (USA). Dichlorofluorescein diacetate (DCFHDA) was obtained from Molecular Probes (USA). Electrophoresis reagents and Bio-Rad protein assay kit were purchased from Bio-Rad Laboratories (USA). Anti-p53 and pro-caspase-3 antibodies were obtained from Santa Cruz Biotechnology (USA). Prp4B antibody was obtained from Bethyl Laboratories (USA). These chemicals were used according to the manufacturer’s instructions.
Cell culture and treatment
HCT-15 cells were obtained from ATCC, CCL-225. Cells were cultured at a density of 5 × 105 in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), L-glutamine and supplemented with 1% (v/v) antibiotic-penicillin streptomycin (Gibco, Invitrogen Corporation) at 37°C in a 5% CO2-humidified atmosphere. Exponentially growing cells were seeded at 1 × 106 cells per dish, followed by exposure to various concentrations of curcumin.
Cell morphology
Morphological analysis of curcumin-induced apoptosis was performed after staining using Hoechst 33342 dye. After treatment with 30 and 50 μM curcumin for 24 h, HCT-15 cells were fixed in 4% paraformaldehyde and permeabilized with PBS/0.5% Triton X-100, after which the nuclei were stained for 20 min using Hoechst dye. The cover slips were then washed, mounted onto slides and viewed with a fluorescence microscope.
Apoptotic ratio assay
Cells were trypsinized and collected by centrifugation at 1,000 × g for 10 min. For fixation, 70% ethanol was added and the cell suspension was kept overnight at 4°C. Cells were then stained with PI solution (50 μg/ml PI, 0.1% Triton X-100, 0.1 mM ethylenediamine tetra-acetic acid (EDTA) and 50 μg/ml of RNase) for 20 min at 4°C. Stained DNA was analyzed by flow cytometer (Becton Dickinson).
Measurement of reactive oxygen species (ROS)
Intracellular ROS concentration was measured using the oxidant-sensitive fluorescent probe, DCFHDA, and an inverted microscope. Cells were grown at a density of 2 × 105 cells per 35 mm culture dish and maintained in growth medium for 24 h. Cells were exposed to 10 mM N-acetylcysteine (NAC) for 1 h followed by 5 μM DCFHDA treatment for 20 min and then washed with 1× PBS. DCF fluorescence (excitation, 480 nm; emission, 520 nm) was imaged using an inverted microscope (Zeiss Axiovert 200).
Preparation of cytosolic and nuclear protein fractions
Cytosolic and nuclear fractions of cells were prepared according to a previously described method, with partial modification (Rosner and Hengstschläger, 2008). Briefly, cells were digested in buffer A [100 mM HEPES, 2 M potassium chloride (KCl), 0.1 M ethyleneglycol tetra-acetic acid (EGTA), 0.2 M EDTA, 1 M dithiothreitol (DTT), 1 mM sodium orthovanadate (Na3VO4), 100 mM phenylmethylsulfonyl fluoride (PMSF), and 6% IGEPAL (NP-40) of total volume] and centrifuged for 1 min at 13,000 × g to obtain supernatants, which were saved as the cytosolic fraction. Proteins in pellets were extracted with buffer B [100 mM HEPES, 5 M sodium chloride (NaCl), 0.1 M EGTA, 0.2 M EDTA, 1 M DTT, 1 mM Na3VO4, 100 mM PMSF, and various protease inhibitors]. Following centrifugation at 13,000 × g for 1 min, supernatants were obtained and used as nuclear fractions.
Western blotting
In brief, aliquots of protein extracts (30 μg) from cells of different treatment groups were suspended in 0.1 M Tris-HCl buffer (pH 7.4) containing 1% SDS, 0.05% β-mercaptoethanol, 2.5% glycerol and 0.001% bromophenol blue, followed by fractionation by 10% SDS-polyacrylamide gel electrophoresis. Proteins were transferred electrophoretically onto nitrocellulose membranes (0.2 μM, Schleicher and Schuell). Membranes were blocked using 5% non-fat dry milk and 0.1% Tween 20 in Tris-buffered saline (TBS) and subsequently probed with primary antibody in TBS containing 3% non-fat dry milk and 0.1% Tween 20. Antibody-antigen complexes were detected using goat anti-mouse IgG or goat anti-rabbit IgG peroxidase conjugates followed detection using an enhanced chemiluminescence (ECL) kit (Amersham Corp).
Reverse-transcription polymerase chain reaction (RT-PCR)
To determine transcriptional levels of Prp4B in HCT-15 cells, RT-PCR was performed. Total RNA was isolated from curcumin-treated HCT-15 cells using TRI reagent (Molecular Research Center, Inc.). RT-PCR was performed using an access RT-PCR kit (Promega). The following PCR conditions were employed: 1 cycle at 95°C for 5 min, 30 cycles at 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, and an additional extension step of 5 min at 72°C. The amplified PCR products were analyzed by 1% agarose gel electrophoresis and ethidium bromide staining. The following gene sequence were utilized: p53 5′-CCTCACCATCATCACACTGG-3′ (forward) and 5′-CCTCATTCAGCTCTCGGAAC-3′ (reverse), Prp4B 5′-AGATCCGAACGGACTAGACA-3′ (forward) and 5′-GCCTCCTAAGG GTAGGGGATTT-3′ (reverse) and 5′-AATCTGGCACCACACCTTCTAC-3′ (forward) and 5′-GTGATCTCCTTCTGCATCCTGT-3′ (reverse) for actin as an internal loading control (Oligo, Macrogen).
Generation of the siRNA-Prp4 HCT15 clones
Clone cDNA of Prp4B (sc107962) was obtained from OriGene (USA), whereas siRNA-Prp4B pool was obtained from Santa Cruz Biotechnology (SC-76257) and utilized according to the manufacturer’s instructions. HCT-15 cells were transfected with both Lipofectamine LTX and Plus reagent for cDNA, whereas RNAiMAX was used for siRNA-Prp4 (Invitrogen, USA) according to the manufacturer’s recommendations. Cells were cultured for 24 h at a density of 2 × 105 cells per dish to 50–60% confluence. Cells were then transfected with 1 nmol of siRNA. At the indicated time after transfection, cells were treated with 30 μM curcumin for 24 h. The effects of scrambled siRNA (sc-37007) were also evaluated according to the modified protocol as previously described (Lee et al., 2012).
Statistical analysis
All data presented as the mean values ± SD (standard deviation) and originated from three separate experiments. Data were evaluated using SPSS for student’s t-test and subjected to one-way analysis of variance (ANOVA). Significance of the difference between the means was determined by Tukey’s range test and considered significant at P ≤ 0.05.
RESULTS
Curcumin induces apoptosis in HCT-15 cells
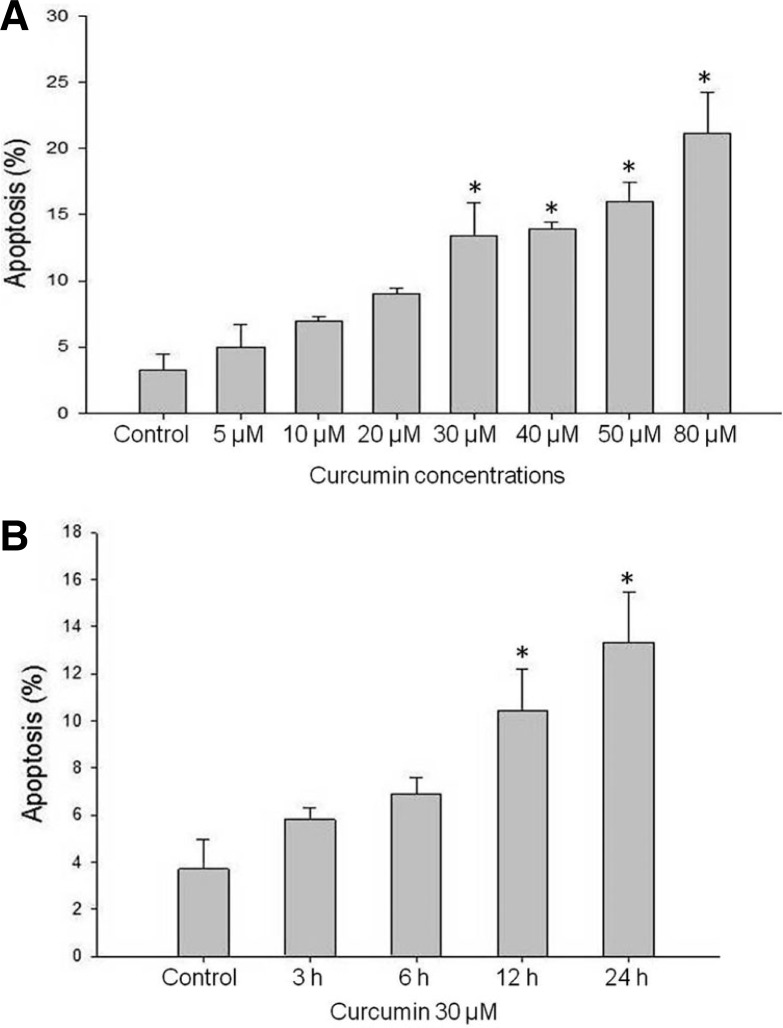
It is of great interest to target deregulated cell cycle progression by curcumin, which has potential to prevent the aberrant transition of cancer cells. To evaluate the dose-dependent effect of curcumin, HCT-15 cells were treated with various concentrations (0–80 μM) of curcumin for 24 h. The amount of sub-G1 DNA from fixed nuclei of HCT-15 cells was analyzed by PI staining and flow cytometry analysis. As shown in Fig. 1A, curcumin dose-dependently affected the growth of HCT-15 cells. Curcumin treatment significantly increased the proportion of cells in sub-G1 phase from 2.14% to 19.51%. Furthermore, we investigated the time-dependent effects of 30 μM curcumin for 24 h. Curcumin significantly increased cell death and its cell growth-inhibition rate was about 12.79% at 30 μM after 24 h (Fig. 1B). These results are consistent with a previously published work (Mukhopadhyay et al., 2002).
Fig. 1.
Quantification of sub-G1 phase in HCT-15 cells. (A) At the indicated time, the HCT-15 cells were harvested and stained with propidium iodide and their DNA content was analyzed by flow cytometry. The sub-G1 fractions of the cell (%) were plotted against different concentrations of curcumin for 24 h. (B) HCT-15 cell were treated with 30 μM curcumin for 24 h, and stained with propidium iodide and their DNA content was analyzed by flow cytometry. Curcumin treatment increased cell fraction at sub-G1 in time dependent manner. Cells treated with DMSO were used as a control. Data represent average ± SD of three independent experiments for each bar. *P < 0.05.
Curcumin induces oxidative-stress apoptosis
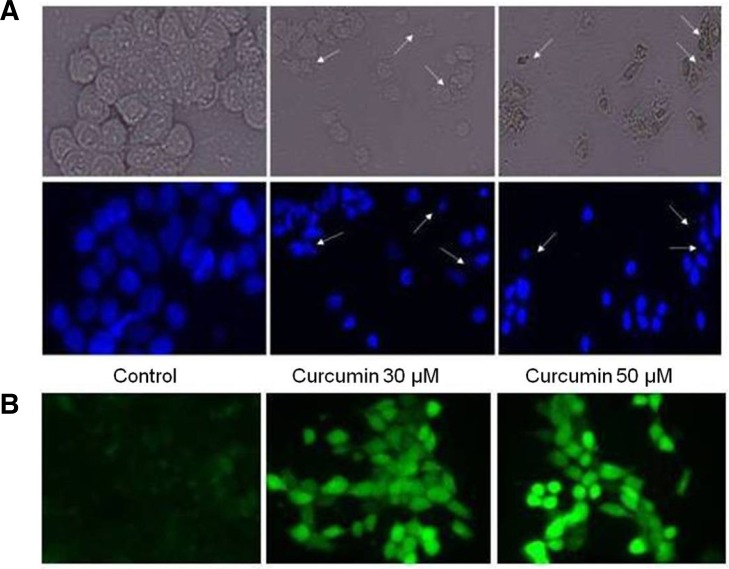
In order to confirm apoptosis in HCT-15 cells, exponentially growing cells were treated with 30 and 50 μM of curcumin for 24 h, to examine the effects of curcumin on cell morphology. Morphological analysis of apoptosis was performed after staining with Hoechst 33342 dye. Curcumin treatment caused HCT-15 cell size reduction, rounding and fractional detachment, thus indicating that curcumin has cytotoxic effects on HCT-15 cells as shown in Fig. 2A.
Fig. 2.
Effects of curcumin treatment on the cell morphology and ROS generation. (A) HCT-15 cells were treated with 30 and 50 μM curcumin and control with DMSO and then stained with 10 μM Hoechst 33342 for 10 min and the images were taken by fluorescence microscopy. (B) Intracellular ROS generation was determined by treating HCT-15 cells with 30 and 50 μM curcumin for indicated time, and further incubated with DCFHDA for 20 min. Accumulation of the probe in the cells was measured by an increased emission at 520 nm when the sample was excited at 480 nm. Images of DCF loaded cells were obtained under a fluorescence microscope.
ROS generation plays an important role in cell proliferation as well as in apoptosis, and it triggers signal transduction, culminating in cell cycle arrest and ultimately cell death (Antosiewicz et al., 2008). ROS generation has been reported to be associated with curcumin-induced apoptosis in many cancer cells. In order to measure the role of oxidative stress in curcumin-induced apoptosis, we used a specific cell-permeable fluorescent dye DCFHDA, which is known to induce fluorescence followed by intracellular oxidation of reactive metabolites. After treatment of cells for 24 h, we examined ROS generation in response to curcumin stimulation. A considerable increase in oxidant-induced 2′,7′-dichlorofluorescein fluorescence was observed in HCT-15 cells within 10 min after curcumin treatment as shown in Fig. 2B.
Curcumin inhibits expressions of Prp4B at protein and mRNA level
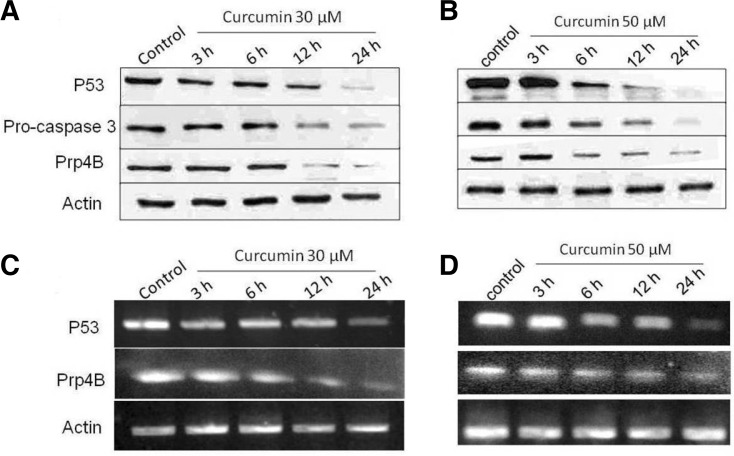
To investigate whether or not curcumin treatment alters procaspase-3 activity and expression of p53 and Prp4B, HCT-15 cells were treated for indicated time. Doses of curcumin were selected that would be physiologically relevant as well as induce maximal inhibition, as revealed by growth assays. As shown in Fig. 3A, curcumin (30 μM) treatment for 24 h downregulated p53 expression in a time-dependent manner. It is known that tumor suppressor protein p53 not only controls the transition of cells from G1 to S phase, but is also capable of inducing programmed cell death (Choudhuria et al., 2002). When treated with a high dose (50 μM) of curcumin, remarkable p53 inhibition was induced even after 12 h (Fig. 3B). Furthermore, RT-PCR was performed to further determine the preventive role of curcumin on p53 expression at mRNA level. As shown in Fig. 3C, curcumin down-regulated p53 mRNA expression, which was more vigilant in the dose of 50 μM (Fig. 3D).
Fig. 3.
Curcumin modulates apoptotic proteins at protein and mRNA level. (A) Western blot analysis of caspase-3 activation and p53 and Prp4B expression in HCT-15 cells. HCT-15 cells were treated with 30 μM of curcumin and whole-cell lysates were extracted from cells and separated by SDS-PAGE and the resulting proteins were detected by immunoblotting. (B) HCT-15 cells were treated with 50 μM of curcumin and whole-cell lysates were extracted from cells and separated by SDS-PAGE and the resulting proteins were detected by immunoblotting (C) RTPCR analysis of Prp4B and p53. HCT-15 cells were treated with 30 μM of curcumin and total RNA was isolated from cells, and RT-PCR was performed. PCR products were separated on 1% agarose gel and photographed. (D) HCT-15 cells were treated with 50 μM of curcumin and PCR products were separated on 1% agarose gel and photographed. Actin was also examined as a reference.
Prp4 is a serine-threonine protein kinase that is widely expressed in both normal and cancer tissues, and specific mutations in Prp4 are known to lead to the accumulation of pre-mRNAs (Gross et al., 1997; Kojima et al., 2001). To explore a possible role for Prp4B in colon cancer, we treated HCT-15 cells with 30 and 50 μM curcumin for 24 h. The results were analyzed by Western blot and RT-PCR in order to observe the effects of curcumin at the protein and mRNA levels in HCT-15 cell lines. Curcumin time-dependently decreased the expression of Prp4B at 30 and 50 μM in HCT-15 cells (Figs. 3A and 3B). However, curcumin had weaker effect at a dose of 30 μM. Thus, 50 μM curcumin induced apoptosis and decreased the expression of Prp4B not only at the protein level but also at the mRNA level in HCT-15 cell lines (Figs. 3C and 3D). Furthermore, actin expression remained constant upon curcumin treatment and served as a loading control in the Western blot and RT-PCR experiments (Figs. 3A–3D).
Summing up, these results clearly demonstrate that curcumin activated caspase-3 as well as decreased the expression of truncated p53 and accumulation of mRNA through the down-regulation of Prp4B, which all are required for the induction of apoptosis in HCT-15 cells.
Curcumin induces Prp4-dependent apoptosis in HCT-15 cells
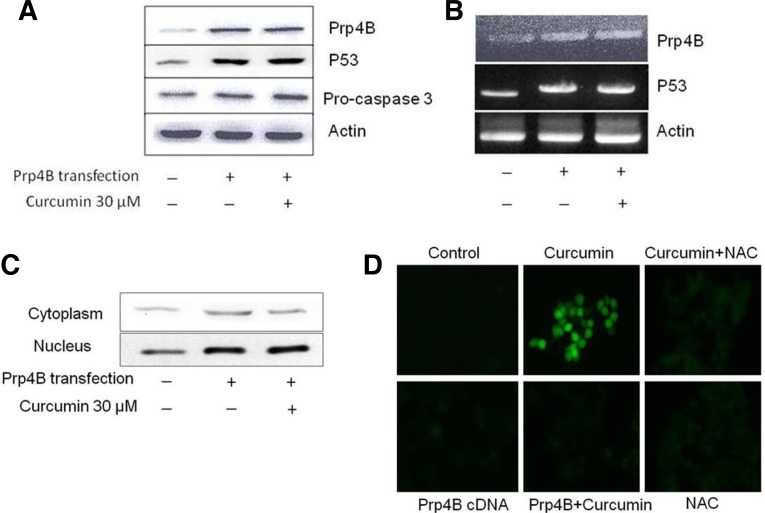
To further investigate the role of Prp4B in apoptosis of colon cancer, we evaluated the effect of curcumin against Prp4B over-expression in HCT-15 cells. Transfection of Prp4B into HCT-15 cells followed by cloning selection of transfectants demonstrated over-expression at the protein and mRNA levels (Figs. 4A and 4B). Western blot and RT-PCR analyses after treatment with 30 μM of curcumin revealed that these same transfectants showed no change in Prp4B protein expression or mRNA level as compared with the aforementioned parental HCT-15 cell line. Additionally, upon transfection with Prp4B clone, curcumin failed to down-regulate p53 expression and activation of caspase-3 (Figs. 4A and 4B). This means that specific over-expression of Prp4B after transfection reversed curcumin-induced apoptosis in HCT-15 cells. Furthermore, transfectant HCT-15 cells were fractionated into cytosolic and nuclear portions, in order to investigate the role of Prp4B with regards to curcumin-induced apoptosis. As shown in Fig. 4C, a higher level of Prp4B was observed in the nuclear fraction than in the cytosol, suggesting translocation of Prp4B from the cytosol to the nucleus. Curcumin treatment also failed to alter Prp4B expression in the nuclear fraction of HCT-15 cells.
Fig. 4.
Exogenous expression of Prp4B restricts curcumin-induced apoptosis in HCT-15 cells. (A) Effect of Prp4B stably transfected cells by Western blotting. HCT-15 cells were transfected with Prp4B clone and total protein was isolated from cells and analyzed by Western blotting with anti-Prp4B and anti-actin antibodies. (B) Prp4B expression inhibits curcumin-induced apoptosis in HCT-15 cells. Cells were transfected with Prp4B clone and then incubated with 30 μM curcumin, total protein was isolated from cells and analyzed by Western blotting with anti-Prp4B, -p53, pro-caspase-3, and -actin antibodies. (C) Prp4B translocates from the cytosol to the nucleus. Transfectant HCT-15 cells were treated with curcumin for the indicated time. Cells were fractionated into cytosolic and nuclear fractions and equal amounts of lysates were used for immunoblotting with Prp4B-specific anti-bodies. (D) Prp4B expression decreases ROS generation. HC-T15 cells were transfected with Prp4B clone or NAC and then incubated with curcumin or carrier for the indicated time. Prp4B over-expression and NAC treatment diminished curcumin-induced ROS generation as shown by DCFHDA fluorescence.
To confirm whether elevated ROS generation is related to curcumin-induced apoptosis, the effect of Prp4B transfection was examined against curcumin treatment in HCT-15 cells. After treatment with curcumin, cells were incubated with 5 μM DCFHDA for 20 min and analyzed under a fluorescence microscope. As shown in Fig. 4D, curcumin treatment increased oxidant-induced 2′,7′-dichlorofluorescein fluorescence only in parental cells, whereas transfection with Prp4B prevented curcumin-induced oxidative stress-associated apoptosis in transfectant cells. Very similar results were obtained by blocking ROS generation with NAC before exposure to curcumin (Fig. 4D). Taken together, these results demonstrate that Prp4B promote cell survival and prevents curcumin-induced apoptosis in HCT-15 cells.
Knockdown of Prp4B by siRNA induces apoptosis in HCT-15 cells
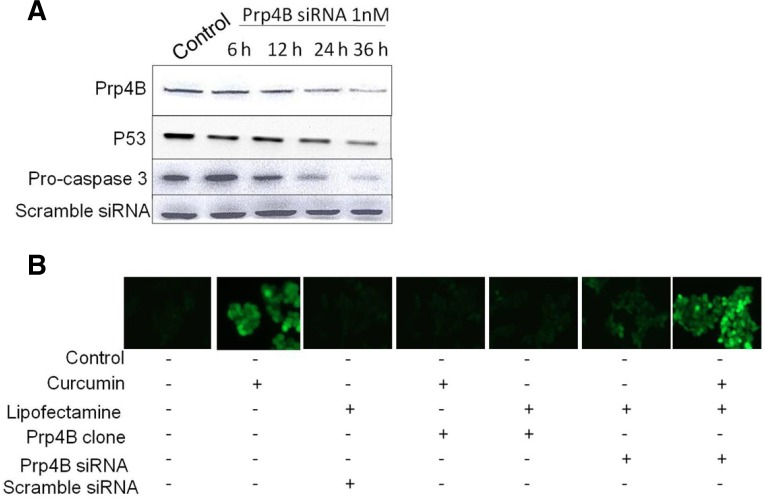
Utilization of small interfering RNA (siRNA) as a technique for functional gene silencing in cancer cells has enabled researchers to carry out genetic loss-of-function studies in cell culture systems (Bosher et al., 2000). Therefore, to determine the survival role of Prp4B in curcumin-induced apoptosis, targeted siRNA was used against Prp4B mRNA to knockdown Prp4B in HCT-15 cell lines. We selected siRNA concentration that would be physiologically relevant as well as comparable with previously reported studies (Lee et al., 2012). As shown in Fig. 5A, transfection with Prp4B-siRNA down-regulated the expression of Prp4B and p53 as well as activated caspase-3 in a time-dependent manner. Activation of caspase-3 and down-regulation of p53 are hallmarks of apoptosis in HCT-15 cells. Western blot analysis revealed that the frequency of apoptosis increased in cells transfected with Prp4B-siRNA as compared to scrambled siRNA.
Fig. 5.
RNA interference-mediated inhibition of Prp4B increases curcumin-induced apoptosis. (A) Silencing effect of siRNA-Prp4B cells. HCT-15 cells were transfected with 1 nM concentration of siRNA and then incubated for indicated time. Total cell lysates were prepared and used for Western blotting. (B) Prp4B inhibition induces ROS generation. HCT-15 cells were transfected with siRNA and then incubated with curcumin or carrier for indicated time. Prp4B siRNA diminished the protective effect of Prp4B and induced ROS generation as shown through DCFHDA fluorescence.
Much compelling evidence has shown that curcumin induces apoptosis by modulating different apoptotic signaling pathways. Among them, ROS generation plays a central role in mediating curcumin-induced apoptosis (Thayyullathil et al., 2008). In order to investigate the protective role of Prp4B in reversing curcumin-induced apoptosis associated with the generation of cellular oxidative stress; the levels of intracellular peroxides were evaluated by DCFHDA staining. We predicted that Prp4B inhibits curcumin-induced apoptosis in HCT-15 cells by reducing ROS production. However, Prp4B silencing before curcumin treatment diminished the protective effect of Prp4B and induced ROS generation (Fig. 5B). Additionally, HCT-15 cells transfected with scrambled siRNA showed the same effects as untransfected control cells (Fig. 5B). These results suggest that Prp4B provided protection to HCT-15 cells from curcumin-induced apoptosis by decreasing the steady-state levels of intracellular oxidants. Therefore, it may be possible that in addition to caspase-3 activation, curcumin-induced ROS generation, which leads to modulation of Prp4B and p53 expression and induced apoptosis in HCT-15 cells.
DISCUSSION
Previous compelling evidence has provided in-depth analysis of multiple targets through which curcumin induces apoptosis and protects against variety of cancers including colorectal cancer (Shehzad et al., 2013). Curcumin disrupts the signaling pathways and molecular targets involved in the initiation and progression of various cancers, but the exact molecular mechanism underpinning the potency of curcumin against treatment of colon cancer remains under investigation. The data presented in this paper provide the novel insights into the regulation of Prp4B with regards to curcumin-induced apoptosis in human colorectal carcinoma cells. To gain deeper insights into the mechanism of curcumin-induced apoptosis through ROS generation, we investigated the actions of this dietary agent in colorectal carcinoma HCT-15 cells.
In this study, curcumin significantly induced rapid ROS generation in HCT-15 cells, resulting in multiple apoptotic signals, including caspase-3, p53 and Prp4B signaling pathways. Importantly, curcumin activated caspase-3 as well as down-regulated p53 and Prp4B expression. Additionally, transfection of HCT-15 cells with Prp4B clone perturbed curcumin-induced cell death by inhibiting ROS generation. Therefore, curcumin has potent anticancer effects, although the exact mechanism of action underlying curcumin-induced apoptosis needs to be investigated. Indeed, the roles of different signaling pathways have been well documented in curcumin-induced apoptosis (Shehzad and Lee, 2013). Curcumin induced cell death in a time and dose-dependent manner (Figs. 1A and 1B) and caused morphological changes in HCT-15 cells (Fig. 2A). In contrast, it has been reported that curcumin inhibits cell cycle transition, rather than induced apoptosis (Hanif et al., 1997). However, our results clearly demonstrated that curcumin-induced biochemical and morphological features of apoptosis. Additionally, the synergistic effect of curcumin and catechin has been shown in HCT-15, which displayed typical apoptotic features upon treatment (Manikandan et al., 2012). Curcumin also induced rapid ROS generation as shown by DCFHDA oxidation, implying a potent anticancer effect (Fig. 2B). These results are consistent with the previously reported studies (Thayyullathil et al., 2008).
This study also confirmed that the growth inhibition and apoptosis of HCT-15 cells induced by curcumin was due to caspase-3 activation. Pro-caspase-3 cleaves to form active caspase-3, which has been reported to play a central role in the initiation and modulation of apoptotic signaling pathways (Su et al., 2006). Curcumin treatment at both concentrations induced the activation of caspase-3 in a time-dependent manner at the protein and mRNA levels (Figs. 3A–3D). It is thought that ROS generation leads to activation of caspase-3, and this enrichment might further enhance curcumin-induced apoptosis (Moungjaroe et al., 2006). In contrast, p53 mutation in cancer promotes uncontrolled cell proliferation and progression of tumors, including colorectal cancer (Liu and Bodmer, 2006). Treatment of HCT-15 cells with curcumin resulted in marked down-regulation of p53 at the protein and mRNA levels (Figs. 3A–3D). This result supports the notion that curcumin prevents cancer progression through p53 inhibition (Han et al., 1999).
In addition to p53, curcumin also down-regulated the expression of Prp4B, which is a spliceosomal factor playing a dominant role in pre-mRNA splicing and RNA maturation. Mutation of Prp4 leads to pre-mRNA accumulation, which impairs the cell cycle transition. Although studies in HeLa cells have shown that Prp4 expression is important for chromosomal alignment (Montembault et al., 2007), the precise cellular function of Prp4 in cancer cells remains to be elucidated. Curcumin treatment to HCT-15 cells down-regulated the expression of Prp4 in a time and dose-dependent manner (Figs. 3A and 3C). Hence, curcumin at a dose of 30 μM had a weak effect on Prp4B expression at both the protein and mRNA levels, whereas a remarkable effect was observed upon treatment at 50 μM curcumin (Figs. 3B and 3D). Prp4 has been reported to play a role in cell differentiation and cellular signaling. To further validate the survival function of Prp4B in colon cancer, HCT-15 cells were transfected with Prp4B, showing elevated transcript levels of Prp4B expression (Fig. 4A). Curcumin treatment at concentration of 30 μM failed to induce apoptosis through down-regulation of p53, caspase-3, and Prp4B in transfected cells after 24 h (Fig. 4B). Additionally, transfection of HCT-15 cells with Prp4B clone resulted in translocation of Prp4B from the cytosol to the nucleus, where Prp4B localizes in the nucleus. As shown in Fig. 4C, increased Prp4B accumulation in the nucleus, prevented curcumin-induced apoptosis. We also confirmed the reversal of curcumin-induced apoptosis by measuring oxidant-induced 2′,7′-dichlorofluorescein fluorescence intensity. It was observed that over-expression of Ppr4B protein in transfected cell lines led to a modest decrease in curcumin-induced oxidative stress apoptosis (Fig. 4D). The same results were further confirmed by blocking curcumin-induced ROS generation with NAC (Thayyullathil et al., 2008).
These data imply an important role for Prp4B in colon cancer metastasis. Therefore, targeted siRNA was used to knockdown Prp4B in HCT-15 cell lines. Prp4B-siRNA transfection down-regulated the expression of Prp4B and p53 as well as activated caspase-3 (Fig. 5A). Pprp4B-siRNA transfection also enhanced curcumin-induced apoptosis as compared to scrambled siRNA (Fig. 5B). Furthermore, silencing of cells with Prp4B-siRNA before curcumin treatment diminished the protective effect of Prp4B and induced ROS generation (Fig. 5A). The molecular mechanism of action of curcumin on Prp4B remains unclear, along with the substrates responsible for the regulation of Prp4B and how curcumin modulates this expression in HCT-15 cells. However, our results clearly indicate that curcumin downregulates the expression of Prp4B at protein as well as mRNA levels.
In conclusion, we verified that curcumin-induced apoptosis in HCT-15 cells involves a novel target Prp4B along with ROS generation as a key initiating signaling candidate. Considerably, over-expression of Prp4B in transfected HCT-15 cells was sufficient to inhibit curcumin-induced apoptosis, suggesting that Prp4B may directly participate in the execution of apoptosis. The distinct synergy observed between Prp4B silencing and curcumin sensitivity could be the basis for the molecular mechanism of curcumin. A combination of curcumin with specific inhibitors of Prp4 could be an effective anti-cancer strategy in-vivo. However, the determination of Prp4B function in cancer cells regarding modulation of multi-apoptotic signaling by curcumin awaits further precise investigation.
Acknowledgments
This work was supported by the Nuclear Research and Development Program of the National Research Foundation of Korea funded by the Korean Ministry of Education, Science and Technology (2011-0006331).
REFERENCES
- Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. (2008). Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 74, 1570–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher JM, Labouesse M. (2000). RNA interference: genetic wand and genetic watchdog. Nat Cell Biol. 2, E31–36 [DOI] [PubMed] [Google Scholar]
- Choudhuria T, Pala S, Munna L, Dasa T, Saa G. (2002). Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 512, 334–340 [DOI] [PubMed] [Google Scholar]
- Gross T, Lützelberger M, Weigmann H, Klingenhoff A, Shenoy S, Käufer NF. (1997). Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue. Nucleic Acids Res. 25, 1028–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S. (1999). Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 93, 152–161 [DOI] [PubMed] [Google Scholar]
- Hanif R, Qiao L, Shiff SJ, Rigas B. (1997). Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 130, 576–584 [DOI] [PubMed] [Google Scholar]
- Hu J, Xu Y, Schappert K, Harrington T, Wang A, Braga R, Mogridge J, Friesen JD. (1994). Mutational analysis of the PRP4 protein of Saccharomyces cerevisiae suggests domain structure and snRNP interactions. Nucleic Acids Res. 22, 1724–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Zama T, Wada K, Onogi H, Hagiwara M. (2001). Cloning of human PRP4 reveals interaction with Clk1. J Biol Chem. 276, 32247–32256 [DOI] [PubMed] [Google Scholar]
- Lee SK, Shehzad A, Jung JC, Sonn JK, Lee JT, Park JW, Lee YS. (2012). Protein kinase Cα protects against multidrug resistance in human colon cancer cells. Mol Cells 34, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai AG. (2008). Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer 8, 121–132 [DOI] [PubMed] [Google Scholar]
- Liu Y, Bodmer WF. (2006). Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc Natl Acad Sci USA. 103, 976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan R, Beulaja M, Arulvasu C, Sellamuthu S, Dinesh D, Prabhu D, Babu G, Vaseeharan B, Prabhu NM. (2012). Synergistic anticancer activity of curcumin and catechin: an in vitro study using human cancer cell lines. Microsc Res Tech. 75, 112–116 [DOI] [PubMed] [Google Scholar]
- Montembault E, Dutertre S, Prigent C, Giet R. (2007). PRP4 is a spindle assembly checkpoint protein required for MPS1, MAD1, and MAD2 localization to the kinetochores. J Cell Biol. 179, 601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moungjaroe J, Nimmannit U, Callery PS, Wang L, Azad N, Lipipun V, Chanvorachote P, Rojanasakul Y. (2006). Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. J Pharmacol Exp Ther. 319, 1062–1069 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. (2002). Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 21, 8852–8861 [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschläger M. (2008). Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 17, 2934–2948 [DOI] [PubMed] [Google Scholar]
- Schwelnus W, Richert K, Opitz F, Gross T, Habara Y, Tani T, Käufer NF. (2001). Fission yeast Prp4p kinase regulates pre-mRNA splicing by phosphorylating a non-SR-splicing factor. EMBO Rep. 2, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerk C, Schulze-Osthoff K. (2005). Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell 19, 1–13 [DOI] [PubMed] [Google Scholar]
- Shehzad A, Lee YS. (2013). Molecular mechanisms of curcumin action: signal transduction. Biofactors 39, 27–36 [DOI] [PubMed] [Google Scholar]
- Shehzad A, Lee J, Lee YS. (2013). Curcumin in various cancers. Biofactors 39, 56–68 [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. (2013). Cancer statistics, 2013. CA Cancer J Clin. 63, 11–30 [DOI] [PubMed] [Google Scholar]
- Silvera D, Formenti SC, Schneider RJ. (2010). Translational control in cancer. Nat Rev Cancer. 10, 254–266 [DOI] [PubMed] [Google Scholar]
- Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, Lin WC, Chen GW. (2006). Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 26, 4379–4389 [PubMed] [Google Scholar]
- Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. (2008). Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 45, 1403–1412 [DOI] [PubMed] [Google Scholar]