Abstract
Acteoside, an active phenylethanoid glycoside, has been used traditionally as an anti-inflammatory agent. The molecular mechanism by which acteoside reduces inflammation was investigated in lipopolysaccharide (LPS)-induced Raw264.7 cells and in a mouse model of cecal ligation and puncture (CLP)-induced sepsis. In vitro, acteoside inhibits high mobility group box 1 (HMGB1) release and iNOS/NO production and induces heme oxygenase-1 (HO-1) expression in a concentration-dependent manner, while HO-1 siRNA antagonizes the inhibition of HMGB1 and NO. The effect of acteoside is inhibited by the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 and Nfr2 siRNA, indicating that acteoside induces HO-1 via p38 MAPK and NF-E2-related factor 2 (Nrf2). In vivo, acteoside increases survival and decreases serum and lung HMGB1 levels in CLP-induced sepsis. Overall, these results that acteoside reduces HMGB1 release and may be beneficial for the treatment of sepsis.
Keywords: acteoside, heme oxygenase 1, high-mobility group box 1, nrf2, p38, Raw264.7 cell, sepsis
INTRODUCTION
The pathogenesis of severe sepsis and shock caused by invasive infection is characterized by the excessive release of early and late pro-inflammatory cytokines (Aziz et al., 2012). Among pro-inflammatory cytokines, high mobility group box 1 (HMGB1) accumulates in circulation during sepsis, leading to multiple organ collapse and a lethal outcome (Yang et al., 2004). HMGB1 is actively released by immune cells (macrophages, monocytes, natural killer cells, and platelets) into the extracellular environment (Andersson et al., 2000). Anti-HMGB1 antibodies and HMGB1 inhibitors significantly improve survival in septic animals, suggesting that HMGB1 is a possible therapeutic target in sepsis (Barnay-Verdier et al., 2011; Sama et al., 2004). Heme oxygenase-1 (HO-1), a stress-responsive protein induced by stimulants (inflammatory cytokines, heat shock, heavy metals, and oxidants) (Jang et al., 2012; Tsoyi et al., 2009) decreases circulating HMGB1 levels in animal models of sepsis and improves patient survival, demonstrating its therapeutic potential in inflammatory disorders (Wu et al., 2011). In addition, it is well known that the inducers of HO-1 expression inhibits the expression of the inflammatory genes (cyclooxygenase-2 and inducible nitric oxide synthase), and subsequently decreases PGE2 and NO production (Oh et al., 2006; Suh et al., 2006). It also was recently reported that the HO-1 system provides a therapeutic effect in many experimental pathological conditions (Bonelli et al., 2012; Farombi and Surh, 2006; Luz et al., 2012). In oxidative injury and inflammation conditions, an increase in the synthesis of the HO-1 gene is linked to the transcription factors NFkB, Nrf2, and AP-1 (Paine et al., 2010; Srisook et al., 2005; Surh et al., 2009). Among the transcriptional factors, Nrf2 nuclear translocation requires the activation of several signal transduction pathways, such as mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K) and0 Akt (Martin et al., 2004).
Despite little knowledge of their mechanisms of action, herbal medicines derived from plant extracts are increasingly utilized to treat a wide variety of diseases. Among them, acteoside (Fig. 1A), a well-studied phenylethanoid glycoside, has been used in Ayurvedic and Chinese medicine for hundreds of years and has a wide range of pharmacological and biochemical effects (He et al., 2011). A growing number of studies have revealed that acteoside has many biological effects, including anti-tumor (Inoue et al., 1998), anti-oxidant (Wong et al., 2001), anti-nephritic (Hayashi et al., 1994), anti-hepatotoxic (Lee et al., 2004) and antiseptic activity (Houghton, 1984; Lee et al., 2005). More recently, acteoside protected beta-amyloid-induced neurotoxicity via regulation of HO-1 expression by modulating the Nrf2 signaling pathways (Wang et al., 2012). Until now, the role of HMGB1 release and the related molecular mechanisms have not been completely clarified by acteoside. The present study demonstrates that acteoside induces HO-1 through activation of p38 and Nrf2 expression in LPS-activated Raw264.7 cells, which in turn reduces HMGB1 release both in macrophages and CLP-induced septic mice.
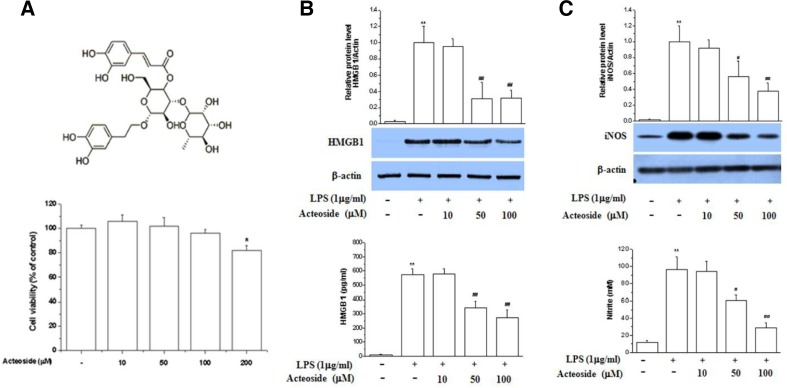
Fig. 1.
Effect of acteoside on HMGB1 release and NO production in LPS-stimulated Raw264.7 cells. Cells were treated with acteoside for 24 h (10, 50, and 100 μM). Cell viability was measured by MTT assay (A). Cells were pretreated with acteoside for 1 h (10, 50, and 100 μM) and then stimulated with LPS (1 μg/ml) for 24 h. Cells were lysed, harvested and subjected to Western blotting for detection of HMGB1 (B, upper) and iNOS (C, upper). The culture medium was collected and subjected to HMGB1 release (B, lower) and NO production analysis (C, lower). Significance compared to control, *p < 0.05; significance compared to LPS, #p < 0.05 and ##p < 0.01.
MATERIALS AND METHODS
Materials
Reagents used in this study were purchased from the following sources: Acteoside from Chromadex (USA); MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazol iumbromide] and LPS from Sigma-Aldrich (USA); SB203580, PD98059, and SP600125 were purchased from Calbiochem (USA); RPMI-1640, fetal bovine serum and Trizol were supplied by Gibco BRL (USA); antibodies for p38, phospho-p38 MAPK, β-actin and HRP-conjugated anti-rabbit IgG from Cell Signaling Technology (USA); antibodies for HMGB1, iNOS, Nrf2 and HO-1 from Calbiochem (USA). The HO-1-ARE-luciferase reporter gene was kindly provided by Dr. J. Alam (Tulane University School of Medicine, USA). All chemicals and reagents were of analytical grade.
Cell culture
Raw 264.7 cells were cultured in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovein serum (FBS), 100 μg/ml penicillin and 100 μg/ml streptomycin, in 5% CO2 at 37°C.
Cell treatment and viability assay
Acteoside was dissolved in dimethylsulfoxide (DMSO) and the stock solutions were added directly to the culture media. The control cells were treated with culture medium only. The cells (5 × 103/well) in 10% FBS-DMEM were seeded into the 48-well plates. After incubation for 24 h, various concentrations of acteoside and LPS were added to the well, and the plates were incubated at 37°C for an additional 24 h. The cells were used for the MTT-based assay by measuring the according to the manufacture’s instructions. Relative cytotoxicity was quantified by absorption measurements at 550 nm using a microtiter plate reader (Molecular Devices, USA). This wavelength was not found to interfere with acteoside.
Nitric oxide assay
The nitrite and nitrate concentration in the medium was measured, as an indicator of NO production. One hundred ml of each supernatant was mixed with the same volume of Griess reagent; the absorbance of the mixture at 545 nm was determined with an ELISA plate reader.
HMGB1 release assay
The levels of HMGB1 in the culture medium and blood were determined using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, USA) and were performed according to the manufacturer’s instructions.
RNA preparation and mRNA analysis by real-time quantitative PCR
The total RNA was isolated from cells using Trizol (GibcoBRL, USA). Accumulated PCR products were directly detected by monitoring the increase in reporter dye (SYBR®). The expression levels of HO-1 in the exposed cells were compared to those in the control cells at each time point using the comparative cycle threshold (Ct) method (Johnson et al., 2000). The following primer sequences were used: the HO-1 primers were sense 5′-CGCCTTCCTGCTCAACATT-3′ and antisense 5′-TGTGTTCCTCTGTCA GCATCAC-3′. Ribosomal protein S18 (S18) sense 5′-AAGTTTCAGCACATCCTGCGAGTA-3′; and S18 antisense 5′-TTGGTG AGGTCAATGTCTGCTTTC-3′. The quantity of each transcript was calculated as described in the instrument manual and was normalized to the amount of S18, a housekeeping gene.
Western immunoblot analysis
Cells were harvested and washed 3 times with cold phosphate-buffered saline (PBS). The cytoplasmic and nuclear protein fractions were extracted using NE-PER extraction reagents according to the manufacturer’s protocol (Pierce Biotechnology, USA). Cytoplasmic/nuclear protein extracts or whole protein extracts were used for Western blot analysis. Western blotting was performed using anti-iNOS, anti-HO-1, anti-HMGB1, antip-38, anti-phospho-p38, anti-nrf2 and anti- β-actin antibodies. Protein samples were heated at 95°C for 5 min and were analyzed using SDS-PAGE. Immunoblot signals were developed by enhanced chemiluminescence (Pierce Biotechnology, USA).
siRNA knockdown
siHO-1 and scrambled siRNA were purchased from Invitrogen. siNrf2, and scrambled siRNA were acquired from Santa Cruz Biotechnology. siRNA was transfected into macrophages according to the manufacturer’s protocol and using the transfection reagent, Lipofectamine 2000© (Invitrogen, USA). The cells were incubated with 100 nM of target siRNA or scramble siRNA for 4 h in serum- and antibiotic-free media. The cells were then incubated for 18 h in media containing antibiotics and FBS, and cells were washed, pretreated with or without acteoside for 1 h, and treated with LPS.
Transient transfection and luciferase assay
Cells (3 × 105 cells/well) were seeded in 24-well plates, incubated overnight and transiently co-transfected with ARE-promoter- luciferase construct and pRL-SV40 plasmid (Renilla luciferase expression for normalization) (Promega, USA) using LipofectAMINE™ 2000 reagent (Invitrogen, USA). Relative luciferase activities were calculated by normalizing ARE-promoter-driven firefly luciferase activity to Renilla luciferase activity.
Animal model of sepsis
Specific pathogen-free BALB/C mice (male aged 8 weeks, 25 ± 3 g) were obtained from Central Laboratory Animal Inc. (Korea). Animals were housed under normal laboratory conditions, i.e. at 21–24°C and 40-0% relative humidity under a 12 h light/dark cycle with free access to standard rodent food and water. To induce sepsis, BALB/C mice were anesthetized with zoletil (80 mg/kg) and Xylazine (10 mg/kg) and then a 2 cm midline incision was performed to allow exposure of the cecum with adjoining intestine. The cecum was tightly ligated with a 3.0-silk suture at 5.0 mm from the cecal tip and punctured once with a 22- gauge needle (top and bottom). The cecum was then gently squeezed to extrude a small amount of feces from the perforation sites and returned to the peritoneal cavity. The laparotomy site was then stitched with 4.0-silk. In the sham control animals, the cecum was exposed but not ligated or punctured and then returned to the abdominal cavity. All animals were raised under specific pathogen-free conditions, and the protocol was reviewed and approved by the Animal Subjects Committee of Asan Medical Center (Korea).
Statistical analysis
All the experiments were repeated at least three times. The results are expressed as a mean ± SD, and the data were analyzed using one-way ANOVA followed by a Student’s t-test for significant difference. The Kaplan-Meier method was used to compare the differences in mortality rates between groups. A p value < 0.05 were considered significant.
RESULTS
Acteoside inhibits HMGB1 release and iNOS expression/NO production
The optimal concentration range of acteoside was assessed in Raw264.7 cells (> 95% cell viability, Fig. 1A). To identify the potential anti-inflammatory mechanism of action of acteoside, cells were incubated with acteoside in the presence of LPS. As shown in Fig. 1, acteoside inhibited HMGB1 protein expression and release in a concentration-dependent manner (Fig. 1B) and reduced nitric oxide synthase (iNOS) protein expression and production of nitric oxide (NO) (Fig. 1C) in LPS-activated Raw264.7 cells. These results confirm a previous report according to which acteoside inhibits the expression of iNOS (Lee et al., 2005).
Anti-inflammatory effect of acteoside is mediated through HO-1
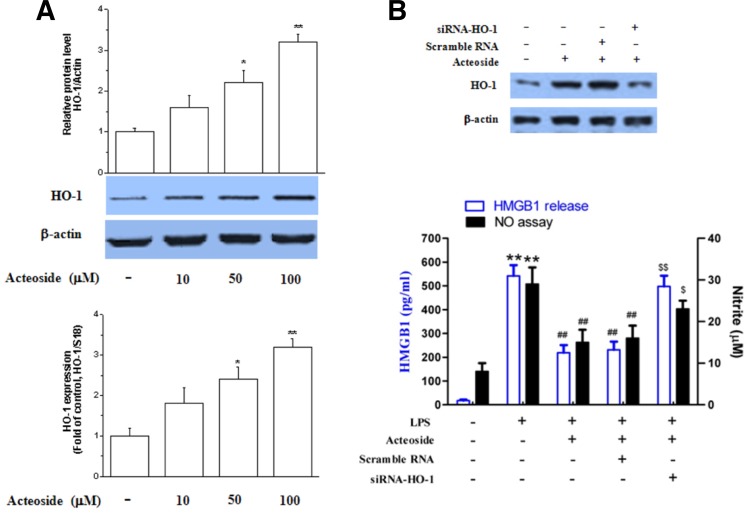
Since HO-1 decreases HMGB1 levels in septic conditions, acteoside was tested for potential induction of HO-1 in Raw264.7 cells (Wu et al., 2011). After cells were treated with acteoside for 24 h, the treatment dose-dependently increased both HO-1 mRNA and protein expression (Fig. 2A). RNA silencing was used to determine whether HO-1 induction reduced NO production and HMGB1 release. Results showed that HO-1 siRNA significantly reversed the anti-inflammatory effect of acteoside (Fig. 2B).
Fig. 2.
Effect of acteoside on the expression of HO-1 and role in anti-inflammatory activity. Raw264.7 cells were exposed to acteoside for 24 h, after which HO-1 protein expression and mRNA were analyzed by Western blotting and RT-PCR (A). Significance compared to control, *p < 0.05 and **p < 0.01. Cells were transfected with scrambled siRNA or HO-1 siRNA and subjected to Western blotting to confirm the effect of HO-1 siRNA upon exposure to acteoside (100 μM) for 24 h (B, upper). Cells were stimulated with LPS (1 μg/ml) in the presence or absence of acteoside (100 μM) for 24 h. Supernatants were subjected to HMGB1 release and NO production analysis (B, lower). Significance compared to LPS, *p < 0.05; significance compared to LPS + acteoside, #p < 0.05.
Acteoside induces HO-1 expression through p38 MAPK, but not ERK or JNK
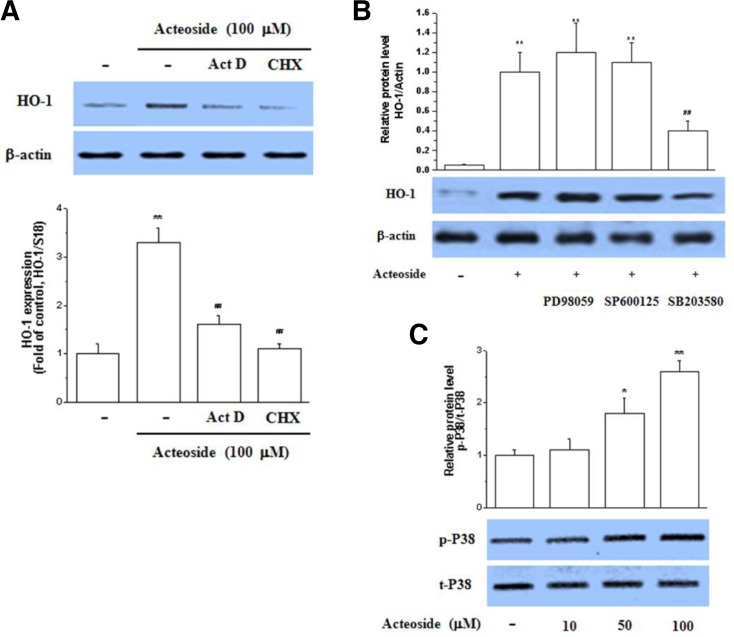
As shown in Fig. 3A, the HO-1 increase by acteoside was sensitive to pretreatment with actinomycin D or cycloheximide, suggesting that acteoside increases the expression of the inducible heme oxygenase isoform. The sensitivity of the mRNA increase to cycloheximide suggests that induction of HO-1 transcription involves de novo protein synthesis, which is consistent with the results of a previous study (Hill-Kapturczak et al., 2000). Next, the signaling cascade of HO induction by acteoside was assessed. To identify which MAPK mediates the acteoside induction of HO-1, pharmacological inhibitors were used. As shown in Fig. 3B, the induction of HO-1 in Raw264.7 cells by acteoside was inhibited by the p38 inhibitor SB203580 but not by the MAPK inhibitor SP600125 or the JNK inhibitor PD98059. Moreover, acteoside significantly induced p38 activation in Raw264.7 cells in a concentration-dependent manner (Fig. 3C). These results show that p38 plays a key role in acteoside-mediated HO-1 induction.
Fig. 3.
Acteoside induces HO-1 through p38 MAPK. Raw264.7 cells were pretreated with cycloheximide (50 μM) or actionmycin D (10 μg/ml) for 2 h prior to the addition of acteoside (10 μM) for an additional period of 24 h. HO-1 protein expression and mRNA were analyzed by Western blotting and RT-PCR (A). Significance compared to acteoside, **p < 0.01. Cells were pretreated with SB203580 (10 μM), SP600125 (10 μM), and PD98059 (10 μM) for 1 h, and then treated with acteoside (100 μM) for 24 h. After incubation, cells were harvested and subjected to Western blotting (B). Significance compared to control, **p < 0.01; significance compared to LPS, ##p < 0.01. Cells were exposed to acteoside for 3 h and protein was extracted. Parallel immunoblots were analyzed for total kinase levels with anti-p38 antibodies (C). Significance compared to control, *p < 0.05, **p < 0.01.
Acteoside activates Nrf2 in LPS-activated Raw264.7 cells
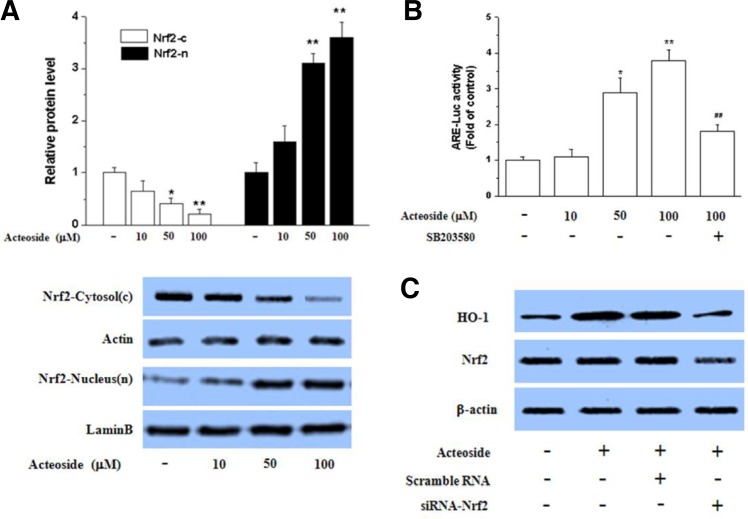
Among the HO-1 transcription factors, Nrf2 regulates ARE-driven HO-1 gene expression (Alam and Cook, 2003; Srisook et al., 2005). To understand whether p38 MAPK has a key role for inducting HO-1 through Nrf2 activation, we checked the Nrf2 nuclear translocation ARE-luciferase activity by acteoside. Figure 4A shows that acteoside stimulated the nuclear translocation of Nrf2 in Raw264.7 cells in a concentration-dependent manner. In addition, acteoside increased ARE-luciferase activity, which was significantly reduced by SB203580, indicating that acteoside- induced Nrf2 binding DAN is dependent on p38 activity (Fig. 4B). To assess further whether acteoside-mediated HO-1 expression occurs via Nrf2 activation, Nrf2 was silenced. As shown in Fig. 4C, acteoside significantly reduced HO-1 expression, demonstrating the critical role of Nrf2 signaling in HO-1 expression.
Fig. 4.
Effect of acteoside on Nrf2 translocation into the nucleus and transcriptional activity. Raw264.7 cells were treated with acteoside for 3 h, then nuclear and cytosol extracts were prepared for Western blotting (A). Significance compared to cytosolic (c) control, *p < 0.05, **p < 0.01. Significance compared to nuclear (n) control, ##p < 0.01. Cells were treated with acteoside (10 μM) for 24 h or pretreated with SB- 203580 (10 μM) for 1 h before adding acteoside (100 μM) for an incubation period of 24 h. Cells were then subjected to luciferase assay (B). Significance compared to control, *p < 0.05, **p < 0.01. Significance compared to acteoside (100 μM), #p < 0.05. Cells were transfected with scrambled siRNA or Nrf2 siRNA. After a 24 h incubation period with acteoside (100 μM), cells were harvested and subjected to Western blotting for HO-1. Transfection efficiency was confirmed by measuring Nrf2 expression.
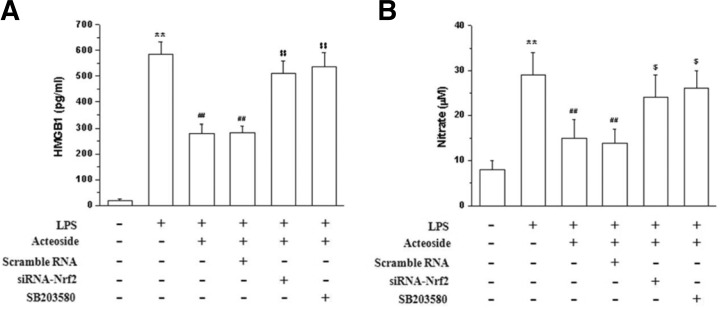
Anti-inflammatory effect of acteoside is mediated through p38 MARK/Nrf2 signal cascade pathways
The role of HO-1 induction in the anti-inflammatory activity of acteoside (Lee et al., 2004) was investigated Results showed that acteoside-induced HO-1 expression mediates the inhibition of HMGB1 release (Fig. 4A) and NO production (Fig. 4B), since these effects were antagonized by SB203580 and Nrf2 siRNA, thus demonstrating the importance of p38/Nrf2 signaling in acteoside-induced HO-1 expression and anti-inflammatory activity.
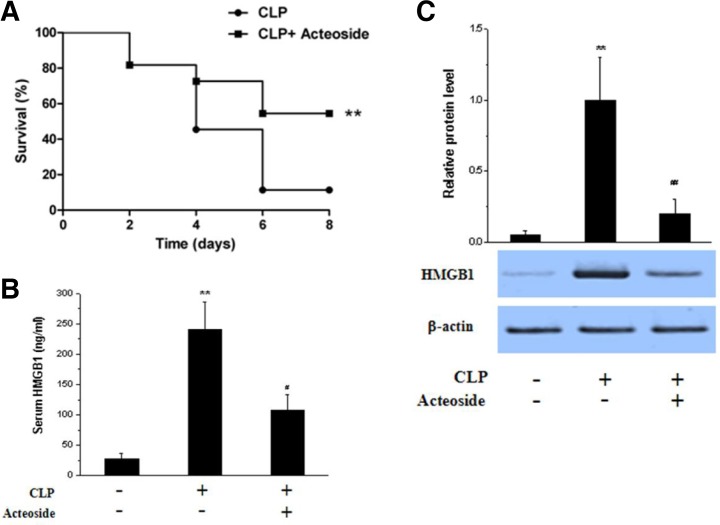
Acteoside inhibits HMGB1 accumulation in vivo
To assess the effect of acteoside on HMGB1 accumulation in vivo, the CLP preclinical mouse model of sepsis, which more closely resembles human sepsis than LPS-induced endotoxemia, as reported previously (Tsoyi et al., 2011), was used. As shown in Fig. 6, in septic animals, the 6 day survivial rate of vehicle-treated control mice after CLP was 10%, while acteoside significantly increased survival to 60% (Fig. 6A). Moreover, HMGB1 levels, which were higher in the lung tissues and sera of CLP-induced septic mice than in those of non-CLP-induced (sham) mice, were significantly reduced by acteoside (100 mg/kg, i.p. 200 μl, twice) (Figs. 6B and 6C).
Fig. 6.
Acteoside increases survival and inhibits HMGB1 levels in CLP-induced septic mice. BALB/c WT mice (n = 10) were subjected to CLP and received acteoside (n = 10, 100 mg/kg i.p.) or an equal volume of vehicle (n = 10, saline) at 0 and 24 h after the onset of sepsis. Survival was monitored daily for up to 8 days (A). To measure HMGB1 levels, WT mice were treated with acteoside (100 mg/kg i.p., n = 3) or an equal volume of vehicle (n = 3, saline) at 0 and 12 h after the onset of sepsis (CLP). After 24 h, lung tissues were harvested under anesthesia from the sham, CLP, and CLP + acteoside groups. Protein was extracted from lung tissues and expression of HMGB1 was analyzed by Western blotting (B). Blood was collected by cardiac puncture and HMGB1 release was analyzed by ELISA (C). Significance compared to sham, *p < 0.05. Significance compared to CLP, #p < 0.05 serum (C).
DISCUSSION
A previous study showed that acteoside up-regulates heme oxygenase-1 through phosphatidylinositol 3-kinase/Akt, ERK, and NF-E2-related factor-2 in neuronal cells (Wang et al., 2012). however, the precise anti-sepsis mechanism of action of acteoside in macrophages and septic animals remains unclear. The present study explored whether the anti-inflammatory action of acteoside was related to the induction of HO-1 in Raw264.7 cells and CLP-induced septic mice. We first showed direct evidence that acteoside contributes to the reduction of HMGB1 and NO production by inducing HO-1 in macrophages. Other studies suggested that HMGB1 mediates cognitive impairment in sepsis survivors (Chavan et al., 2012; Huang et al., 2010) and that HO-1 inhibits the release of HMGB1 in Raw264.7 cells activated by LPS and in LPS- or CLP-induced septic mice (Tsoyi et al., 2009). Next, we assessed the signaling pathway through which acteoside induces HO-1. Since activation of MAPKs plays a central role in the induction of HO-1 gene expression [32], we investigated which MAPKs are responsible for acteoside-mediated HO-1 induction. p38 MAPK, but not JNK or ERK, mediated acteoside-induced HO-1 expression. Furthermore, the inhibition of HO-1 induction and that of the anti-inflammatory effect of acteoside by SB203580 suggests that p38 plays a key role in acteoside-mediated HO-1 induction.
The HO-1 gene promoter contains multiple regulatory transcription factor binding sites, including ARE, NF-κB, AP-1, and AP-2 responsive elements (Boberek et al., 2010; Lavrovsky et al., 1994; Surh et al., 2009). Among the transcriptional factors, Nrf2 is the transcription factor that can activate the transcription of anti-oxidant proteins including HO-1. Under the Nrf2 activation, it dissociates from Kelch-like ECH-associated protein 1 (Keap1) and translocates into the nucleus and positively regulates the ARE-mediated expression of phase II detoxification enzyme genes, including HO-1(Hwang and Jeong, 2010; Zhang et al., 2004). Acteoside-induced HO-1 expression in Raw264.7 cells was significantly inhibited by Nrf2 siRNA, indicating the involvement of p38 MARK/Nrf2 signaling; however, others have produced compelling evidence for the lack of involvement of NFkB and p38 in HMGB1 release in LPS-treated macrophages, while emphasizing the role of Janus kinase 2/signal transducer and activator of transcription-1 and iNOS/NO signaling in HMGB1 release (Jiang and Pisetsky, 2006; Oh et al., 2009). Thus, further study is needed to identify the pathways involved in the inhibition of HMGB1 downstream of HO-1. HMGB1 is released from stimulated macrophages and works as a late mediator of sepsis (Chavan et al., 2012; Yang et al., 2004) In addition, HMGB1 neutralizing antibodies potently improve survival in septic animals, suggesting a potential therapeutic benefit. Finally, the present study showed that the release of HMGB1 was decreased by acteoside in LPS-stimulated Raw264.7 cells as well as in CLP-induced septic mice. These results suggest that the traditional use of acteoside as an anti-inflammatory agent can be explained partly by the induction of HO-1.
In summary, acteoside is able to induce HO-1 in macrophages through p38MAPK/Nrf2 signaling and to decrease HMGB1 release in LPS-stimulated Raw264.7 cells and in CLP-induced septic mice. To the best of our knowledge, this is the first report showing that acteoside inhibits HMGB1 release in LPS-activated macrophages and CLP-induced septic animals. Thus, HO-1 induction accounts for the anti-inflammatory action of acteoside, which may have therapeutic value in systemic inflammatory conditions such as sepsis.
Fig. 5.
Acteoside reduces inflammation by HO-1 down-regulation through p38 MARK and Nrf2 in Raw 264.7 cells. Cells were transfected with Nrf2 siRNA or scrambled siRNA and incubated with acteoside (100 μM) with or without SB203580 (10 μM) for 1 h and stimulated with LPS (1 μg/ml) for 24 h. Supernatants were subjected to HMGB1 release (A) and NO production assay (B). Significance compared to LPS, **p < 0.01. Significance compared to acteoside + LPS, ##p < 0.01.
Acknowledgments
This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science and Technology (2012R1A1A2008714). It was also supported by a g rant (2013–505) from the Asan Institute for Life Sciences, Seoul, Korea.
REFERENCES
- Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2012;93:329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37:957–962. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- Boberek JM, Stach J, Good L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS One. 2010;5:e13745. doi: 10.1371/journal.pone.0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli M, Savitskaya A, Steiner CW, Rath E, Bilban M, Wagner O, Bach FH, Smolen JS, Scheinecker C. Heme oxygenase-1 end-products carbon monoxide and biliverdin ameliorate murine collagen induced arthritis. Clin Exp Rheumatol. 2012;30:73–78. [PubMed] [Google Scholar]
- Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey KJ, Diamond B. Response to “HMGB1 mediates cognitive impairment in sepsis survivors”. Mol Med. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nagamatsu T, Ito M, Hattori T, Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (2) effect of acteoside on leukocyte accumulation in the glomeruli of nephritic rats. Jpn J Pharmacol. 1994;66:47–52. doi: 10.1254/jjp.66.47. [DOI] [PubMed] [Google Scholar]
- He J, Hu XP, Zeng Y, Li Y, Wu HQ, Qiu RZ, Ma WJ, Li T, Li CY, He ZD. Advanced research on acteoside for chemistry and bioactivities. J Asian Nat Prod Res. 2011;13:449–464. doi: 10.1080/10286020.2011.568940. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem. 2000;275:40904–40909. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- Houghton PJ. Ethnopharmacology of some Buddleja species. J Ethnopharmacol. 1984;11:293–308. doi: 10.1016/0378-8741(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol. 2010;242:18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sakuma Z, Ogihara Y, Saracoglu I. Induction of apoptotic cell death in HL-60 cells by acteoside a phenylpropanoid glycoside. Biol Pharm Bull. 1998;21:81–83. doi: 10.1248/bpb.21.81. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kim YM, Tsoyi K, Park EJ, Lee YS, Kim HJ, Lee JH, Joe Y, Chung HT, Chang KC. Ethyl pyruvate induces heme oxygenase-1 through p38 mitogen-activated protein kinase activation by depletion of glutathione in RAW 264.7. cells and improves survival in septic animals. Antioxid Redox Signal. 2012;17:878–889. doi: 10.1089/ars.2011.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol. 2006;177:3337–3343. doi: 10.4049/jimmunol.177.5.3337. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278:175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc. Natl. Acad. Sci. USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Woo ER, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG. Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci. 2004;74:1051–1064. doi: 10.1016/j.lfs.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Lee JY, Woo ER, Kang KW. Inhibition of lipopolysaccharide- inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol. 2005;97:561–566. doi: 10.1016/j.jep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Luz NF, Andrade BB, Feijo DF, Araujo-Santos T, Carvalho GQ, Andrade D, Abanades DR, Melo EV, Silva AM, Brodskyn CI, et al. Heme oxygenase-1 promotes the persistence of Leishmania chagasi infection. J Immunol. 2012;188:4460–4467. doi: 10.4049/jimmunol.1103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3- kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182:5800–5809. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Sama AE, D’Amore J, Ward MF, Chen G, Wang H. Bench to bedside: HMGB1-a novel proinflammatory cytokine and potential therapeutic target for septic patients in the emergency department. Acad Emerg Med. 2004;11:867–873. doi: 10.1197/j.aem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Srisook K, Kim C, Cha YN. Molecular mechanisms involved in enhancing HO-1 expression: de-repression by heme and activation by Nrf2, the “one-two” punch. Antioxid Redox Signal. 2005;7:1674–1687. doi: 10.1089/ars.2005.7.1674. [DOI] [PubMed] [Google Scholar]
- Suh GY, Jin Y, Yi AK, Wang XM, Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006;35:220–226. doi: 10.1165/rcmb.2005-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Li MH, Na HK, Cha YN. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76:173–182. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, Kim HJ, Seo HG, Lee JH, Chung HT, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14:2057–2070. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21:368–378. doi: 10.1007/s12640-011-9292-5. [DOI] [PubMed] [Google Scholar]
- Wong IY, He ZD, Huang Y, Chen ZY. Antioxidative activities of phenylethanoid glycosides from Ligustrum purpurascens. J Agric Food Chem. 2001;49:3113–3119. doi: 10.1021/jf0100604. [DOI] [PubMed] [Google Scholar]
- Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Am J Cardiovasc Dis. 2011;1:150–158. [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lu L, Dixon C, Wilmer W, Song H, Chen X, Rovin BH. Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-delta12,14-prostaglandin J2 in human mesangial cells. Kidney Int. 2004;65:798–810. doi: 10.1111/j.1523-1755.2004.00454.x. [DOI] [PubMed] [Google Scholar]