Abstract
The transcription factor Pax6, which belongs to the paired box-containing gene family, regulates developmental processes, especially in the eyes, central nervous tissues and craniofacial structures. However, the role of Pax6 in bone has never been studied exclusively. Here we report that Pax6 is expressed at both the mRNA and protein level in the calvaria and long bones of adult mice as well as osteocyte-like MLOY4 cells and suppresses the canonical Wnt signaling pathway. Moreover, the expression levels of Pax6 were much higher in the calvaria than the long bones, and Pax6 was also expressed at E16 to E18 in both the calvaria and long bones. Knockdown of Pax6 in MLOY4 cells did not affect cell proliferation or survival; however, the expression of Sost, an osteocyte marker gene, was significantly decreased. In addition, the overexpression of Pax6 suppressed the canonical Wnt signaling pathway by enhancing the expression of Sost. Furthermore, we also demonstrated that Pax6 binds to the Sost promoter and that stimulation of Sost transcription by Pax6 was dependent on a specific Pax6-binding sequence within the promoter. In conclusion, the results of the present study suggest that Pax6 is expressed in bone and may play an important role in osteocyte differentiation by controlling canonical Wnt signaling.
Keywords: canonical Wntβ-catenin signaling, osteocytes, Pax6, Sost, Wnt3a
INTRODUCTION
Osteocytes are derived from matrix-producing osteoblasts. Osteoblasts move along the bone surface before localizing to a targeted, pre-determined location where they become embedded in the newly forming osteoid bone matrix. Once embedded, their dendritic processes can extend and retract, and the cell bodies are able to undulate within their lacunae (Dallas and Bonewald, 2010). Osteocytes play multifunctional roles as mechanosensors, regulators of mineral homeostasis and coordinators of bone remodeling processes carried out by osteoblasts and osteoclasts (Lanyon, 1993). Osteoblasts differentiate into osteocytes in a series of several stages (Franz-Odendaal et al., 2006). However, the molecular and cellular mechanisms governing osteoblast and osteocyte differentiation are largely unknown.
Sclerostin, the product of the Sost gene, was previously identified as an important negative regulator of bone formation in two rare bone sclerosing dysplasias, Sclerosteosis and Van Buchem disease (van Bezooijen et al., 2004). Sclerostin is a secretory glycoprotein that plays a key role in the regulation of bone formation through Wnt signaling (Semenov, 2005). Moreover, expression and secretion of sclerostin have been reported in terminally differentiated osteocytes (van Bezooijen et al., 2009). In our study, transcription factors that were upregulated with Sost were analyzed using gene expression omnibus (GEO) analysis (in silico analysis) (Barrett et al., 2007). Pax6 was identified as one of the transcription factors that were upregulated with the Sost gene in GEO analysis.
Pax6 belongs to the paired box-containing gene family, and acts as a transcription factor regulating developmental processes (Walther and Gruss, 1991). Pax6 is also known to regulate the development of eyes, central nervous tissues and craniofacial structures (Hogan et al., 1988; Kaufman et al., 1995; Stoykova et al., 1996; Theiler et al., 1978). Pax6, encoded by the Pax6 gene, contains two DNA-binding domains, the paired domain (PD) and the homeo-domain (HD), as well as a transactivation domain. In vertebrates, several isoforms of Pax6 are encoded by the Pax6 gene (Carriere et al., 1995; Jaworski et al., 1997; Kim and Lauderdale, 2006). Two major isoforms are produced by the Pax6 gene by alternative splicing, Pax6 (+5a) and Pax6 (−5a). Pax6 (+5a) differs from Pax6 (−5a) by the presence of an exon 5a-encoded 14 amino acid insertion in its PD. These two isoforms demonstrate distinct DNA-binding properties to the promoters of their downstream target proteins (Carriere et al., 1995; Jaworski et al., 1997; Tang et al., 1998; Walther and Gruss, 1991; Zhang et al., 2001). Pax6 (−5a) has been shown to influence both cell proliferation and neuronal differentiation, while Pax6 (+5a) has only been shown to affect proliferation (Berger et al., 2007; Cillo et al., 1991; Haubst et al., 2004; Lauderdale et al., 2000). The canonical Wnt signaling pathway is involved in many developmental processes (Wodarz and Nusse, 1998). Moreover, Pax6 is known to play an important role in lens morphogenesis through the inhibition of canonical Wnt/β-catenin signaling in the lens surface ectoderm. Previously, Pax6 was shown to regulate the expression of negative regulators of the Wnt signaling pathway, such as secreted frizzeled-related protein1 (sfrp1) and secreted frizzeled-related protein 2 (sfrp2) (Machon and Nusse, 2010). Although the canonical Wnt signaling is known to play a role in deciding the fate of osteoblast lineage cells, as well as in the regulation of bone mass and homeostasis (Johnson et al., 2004), the role of Pax6 in bone has never been reported.
In the present study, we experimentally showed the expression of Pax6 in bone tissues and MLOY4 established osteocyte-like cell lines, the regulation of the Sost gene by Pax6, and its role in inhibiting Wnt signaling.
MATERIALS AND METHODS
Animal study
Embryos used in this study were obtained from time-mated adult imprinting control region (ICR) pregnant mice. Embryonic day 0 (E0) was designated as the day on which vaginal plugs were confirmed. Embryos at E16, E18 were used in this study.
Cell culture
Osteocyte-like MLOY4 cells were cultured as previously described (Kato et al., 1997; Ma et al., 2012). Mouse primary calvarial cells were prepared from the calvaria of neonatal mice as previously described (Park et al., 2007). Mouse primary calvarial cells and mouse pre-osteoblast MC3T3-E1 cells were cultured as described elsewhere (Park et al., 2007). To induce osteoblast differentiation, MC3T3-E1 cells were supplemented with α-MEM containing 50 μg/ml ascorbic acid (Sigma, USA) and 10 mM β-glycerophos-phate (Sigma, USA). The differentiation medium was replaced every two days.
Alkaline phosphatase (ALP) staining
To study the osteoblast differentiation, ALP staining was done using ALP staining kit (Sigma, USA) according to the manufacturer’s protocol.
RNA analysis
Total RNA isolation and cDNA synthesis were performed as previously described (Kato et al., 2007). In total, 1 μl of cDNA was used as the template for PCR amplification of β-Actin, Pax6, Pax6 isoform, Sost, DMP1 and E11 along with 10 pmole of primer and Go-Taq DNA polymerase (Promega, USA). Amplification was carried out at 95°C for initial denaturation, followed by appropriate cycles at 95°C for 30 s (denaturation), 55°C (for β-Actin, Pax6, Pax6 isoform and E11) or 57°C (for DMP1 and Sost) for 30 s (annealing) and 72°C for 30 s (polymerization). Pax6 isoform primers were designed in exon5a of Pax6. Amplified products were resolved on a 1.7% agarose gel. Quantitative real-time PCR was performed for the quantitation of Pax6, Sost, E11 and DMP1 with the Applied Biosystems Real-time PCR system with SYBR green mix (Promega, USA) as a double-stranded DNA-specific binding dye. The primers used in this study are listed in Table 1. We used the same primers to amplify Pax6, E11 and DMP1 for both real-time PCR and RT-PCR.
Table 1.
RT-PCR and real-time PCR primers
| Target name | Forward primer | Reverse primer |
|---|---|---|
| RT-PCR | ||
| Pax6 | 5′-AGTTCTTCGCAACCTGGCTA-3′ | 5′-GAGCCT CAATCTGCTCTTGG-3′ |
| Pax6 isoform | 5′-TGCGACATTTCCCGAATT-3′ | 5′-TCTGTCTCGGATTTCCCAAG-3′ |
| Sost | 5′-CCTATGACGCCAAAGATGTG-3′ | 5′-GTTGTGGAAGCGGGTGAG-3′ |
| E11 | 5′-AGTGTTGTTCTGGGTTTTGG-3′ | 5′-GATTCCGACCAGGGTCACTA-3′ |
| DMP1 | 5′-AGTGAGGAGGACAGCCTGAA-3′ | 5′-TCCCTGTGGAGTTGCTCTCT-3′ |
| β-actin | 5′-TTCAACACCCCAGCCATGT-3′ | 5′-TGTGGTACGACCAGAGGCATAC-3′ |
| Real-time PCR | ||
| Gapdh | 5′-AATGTGTCCGTCGTGGATCTG-3′ | 5′-CAACCTGGTCCTCAGTGTAGC-3′ |
| Sost | 5′-TCCTGAGAACAACCAGACCA-3′ | 5′-GCAGCTGTACTCGGACACATC-3′ |
Plasmid constructs and transfection
To clone Pax6 (+5a) and Pax6 (−5a), we amplified these two isoforms using MLOY4 cDNA with the following primer sequences: forward sequence, 5′-GCGGTGAGCAG ATGTGTG-3′, and reverse sequence, 5′-TCTCCTTCTCTCTTTACTGTA ATC-3′. The PCR products were cloned into the T&A cloning vector (RBC Life Sciences, Real Biotech Corporation, Taiwan) and subcloned into a pcDNA3.1 mammalian expression vector (Invitrogen, USA) after digestion with HindIII restriction enzyme to generate expression constructs [i.e., pcDNA3.1-Pax6 (+5a) and pcDNA3.1-Pax6 (−5a)]. Cells were then seeded in 6-well plates and transfected with 0.8 μg of pax6 plasmid at 70–80% confluency using Lipofectamine Plus™ reagent (Invitrogen, USA). At 48 h after transfection, the cells were processed to determine mRNA and protein expression levels of Pax6 and other osteocyte marker genes.
Sost reporter plasmid construction
The 1, 811 bp of the human Sost promoter (spanning nucleotides -1, 956 to -145 bp of the Sost gene locus relative to the position (+1) of the initiation methionine for the Sost open reading frame) was amplified from liver cells with the following primer sequences forward sequence (5′-TCTCCCCCGGGTG TGGATCATTTAGAGGTTCA AG-3′) and reverse sequence (5′-GCCCTAGATCTCCCAAAGACTTCTCCTCTAGCT C-3′). The promoter was cloned into a pGL3-basic luciferase reporter vector after digestion of the PCR product with SmaI and BglII to construct the pGL3-Sost promoter (wt) plasmid. The primers used to generate mutations in the transcription factor binding site were as follows (mutated nucleotides are indicated in bold letters): forward-5′-CTCTGGGTCACCTGGAAGTACAAACAGCAATTTGGAAGTTTGC-3′; and reverse-5′-GCAAACTTCCA AATTGCTGTTTGTACTTCCAGGTGACCCAGAG-3′. A pGL3-Sost promoter (mut) plasmid was generated by site direct mutagenesis using Expand long range dNTPack enzyme (Roche, Germany) utilizing the pGL3-Sost promoter (wt) plasmid as a template.
Transfection of cells with small interfering RNA
Control siRNA (sc-37007; Santa Cruz Biotechnology Inc., USA) and Pax6 siRNA (sc-36196; Santa Cruz Biotechnology Inc., USA) were used in this study. MLOY4 cells were seeded into 12-well plates and transfected with 50 pmoles of control siRNA or Pax6 siRNA using Lipofectamine RNAiMax reagent (Invitrogen, USA) according to the manufacturer’s protocol.
Cell proliferation and Cell number assay
MLOY4 cells were cultured in 12-well plates and transfected with Pax6 siRNA. After 60 h, cell proliferation levels were determined using a cell counting kit-8 (Dojindo Molecular Technologies Inc., Japan) according to the manufacturer’s instructions. The cell number (viability) was determined by measuring lactate dehydrogenase (LDH) activity with a Cytotox 96 nonradioactive cytotoxicity assay (Promega, USA). MLOY4 cells in 12-well plates were transfected with 0.6 μg of pcDNA3.1 empty vector, pcDNA3.1-Pax6 (−5a) or pcDNA3.1-Pax6 (+5a) plasmids using Lipofectamine Plus™ reagent. After 48 h, the effects of the Pax6 expression plasmids on cell proliferation and viability were studied.
Western blot analysis
Tissue samples and cell lysate samples were loaded and separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membrane was incubated with anti-Pax6 antibody (sc-53108; Santa Cruz Biotechnology Inc., USA) and anti-β-Actin antibody (sc-8432; Santa Cruz Biotechnology Inc., USA). After washing, the membrane was incubated with secondary antibody for 1 h. Immunoreactive proteins on the membrane were visualized with enhanced chemiluminescence (ECL) detection kits (Santa Cruz Biotechnology, USA).
Preparation of Wnt-3a conditioned medium
Wnt-3a conditioned medium was obtained from established L-Wnt-3a stable cell lines (CRL-2647), which were purchased from ATCC (ATCC, USA).
Chromatin immunoprecipitation (Chip) assay
The Sost promoter (∼2 kb) was analyzed for Pax6 binding elements using r-vista software analysis, which showed the putative Pax6 binding site (GGGAGTGCCAG) on the Sost promoter. This sequence was found to be a conserved region between mice and humans. For Chip assay, MLOY4 cells were cross-linked with 1% formaldehyde for 10 min at room temperature. Cross linking was stopped by using 0.125 M glycine, followed by washing with ice-cold PBS. After cell lysis, chromatin extracts were prepared and fragmented by sonication on ice. Protein A/G beads (Santa Cruz Biotechnology Inc., USA) were equilibrated with RIPA buffer and added to 50 μg of chromatin extract for preclearing. DNA-protein-antibody complexes were prepared using anti-Pax6 antibody following the Chip protocol (Abcam, USA) with minor modifications. After precipitation of the chromatin, bound DNA was resuspended in water and used as a template for PCR using Sost Chip primers, forward 5′-TCTGAAAACCACAGCCTGAC-3′; and reverse 5′-GTTTCCT CACCCTC CTCCTC-3′ to amplify the conserved Pax6 binding site of mouse Sost promoter (−317 to −87 bp upstream to translation initiation site). For the control, Chip primers were designed in the other region of mouse Sost promoter (−1, 910 to −1, 676 bp). The control Chip primers are as follows; forward 5′-TCTCCCAAGTCTGGAGCAAT-3′; and reverse 5′-GCAGACA GAAGTCCCCTCAG -3′.
Luciferase reporter assay for monitoring Sost promoter activity and Wnt signaling
To study the role of Pax6 on Sost promoter activity and Wnt signaling, MLOY4 cells were plated in 24-well and 12-well plates at a density of 55, 000 cells/well and 1.1 × 105 cells/well, respectively, and subsequently co-transfected using Lipofectamine Plus™ reagent with the indicated combinations of expression and reporter plasmids. In the Wnt signaling study, the Super TOPFlash plasmid (S-Top) (Clontech, USA) was co-transfected with Pax6 expression plasmids, and 24 h after transfection, the cells were treated with 100 μl of Wnt-3a conditioned medium. At 48 h after transfection, cell lysates were prepared, and luciferase assays were performed using the Dual-Luciferase Reporter Assay system (Promega, USA) according to the manufacturer’s protocol. Assays were normalized for transfection efficiency by co-transfecting with 2.5 ng of pRL-tk renilla luciferase reporter plasmid.
Induction of Pax6 expression by Wnt-3a treatment
MLOY4 cells were seeded in 12-well plates and treated with 100 μl of Wnt-3a conditioned media, and Pax6 and Sost mRNA expressions were measured at different time intervals.
Immunohistochemistry and HE staining
For immunohistochemical staining, mandibles from mice at each stage were fixed with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4) overnight at 4°C and demineralized with 10% ethylenediaminetetraacetic acid (pH 7.4) for 2 wks at 4°C. After being embedded in paraffin, these samples were sectioned to a thickness of 6 μm. Sections were then blocked in 3% hydrogen peroxide for 15 min. The tissue sections were boiled in 10 mM citrate buffer (pH 6.0) for 10 min and cooled at room temperature for 20 min. The immunostained sections were then counterstained with hematoxylin. These sections were used for immunohistochemistry. Specimens were incubated with a primary mouse monoclonal antibody against Pax6 (Santa Cruz) at 4°C overnight. After washing with PBS, the specimens were incubated with a secondary antibody at room temperature. The specimens were visualized using a 3, 3′-diaminobenzidine (DAB) reagent kit (Zymed laboratories, USA).
Statistics
Statistical significance was evaluated using Sigma Plot10 software. The results were calculated and expressed as the mean ± standard deviation (SD). Statistical significance was determined using Student’s t-test. P-values ≤ 0.05 were considered to be statistically significant.
RESULTS
Endogenous expression of Pax6 in various types of bone cells and tissues
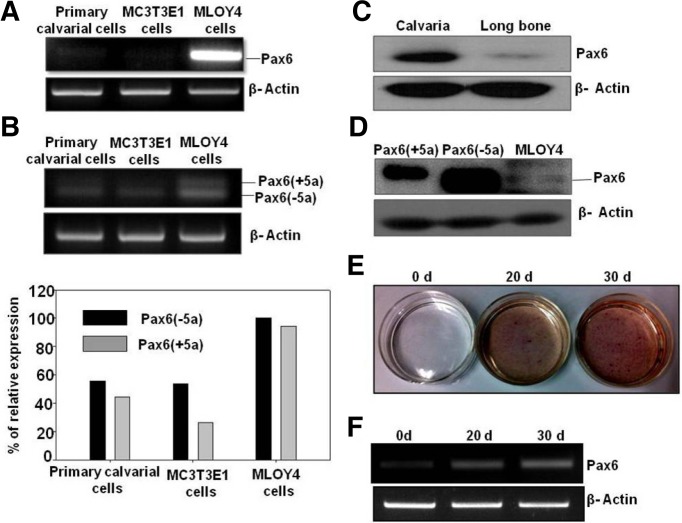
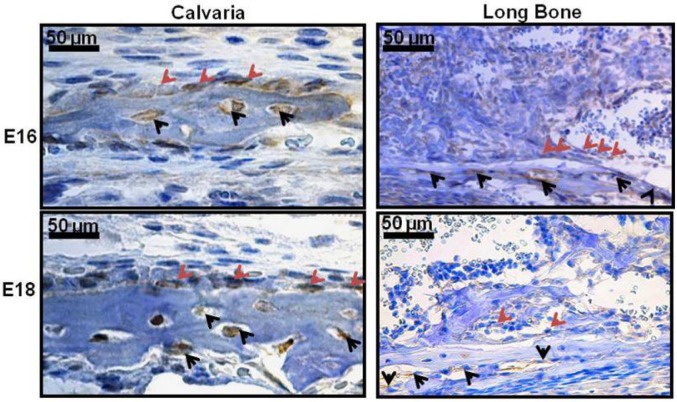
To study the endogenous expression of Pax6 in bone cells and tissues, we investigated the expression of Pax6 in primary calvarial cells, MC3T3E1 preosteoblast cells and osteocyte-like MLOY4 cells at the mRNA and protein level. Pax6 mRNA was expressed at high levels in MLOY4 cells compared to those in primary calvarial cells and MC3T3-E1 cells (Figs. 1A and 1B). Alternative splicing of Pax6 is known to result in two major Pax6 isoforms, both of which we detected in MLOY4 cells (Fig. 1B). In addition, our Western blot analysis demonstrated the in vivo expression pattern of Pax6 in calvaria and long bone tissues of adult mice (Fig. 1C). Pax6 expression at the protein level in MLOY4 cell lysates was confirmed by loading Pax6 (−5a)-and Pax6 (+5a)-overexpressing cell lysates. The Pax6 (−5a) isoform was found in MLOY4 cells (Fig. 1D). To examine the expression of Pax6 during osteoblast differentiation, MC3T3-E1 cells were differentiated using osteoblast differentiation media. Differentiation of osteoblast cells was shown by ALP staining (Fig. 1E). The expression of Pax6 was gradually increased as the differentiation progressed (Fig. 1F). Immunohistochemical studies showed the expression of Pax6 in osteoblasts and osteocytes in parietal bone region of calvaria sections at E16 and E18 embryonic stages. Whereas the long bone sections of E16 and E18 showed expression of Pax6 in both osteoblasts and osteocytes. Calvaria at E16 and E18 stages showed comparatively higher expression than long bone (Fig. 2).
Fig. 1.
Endogenous expression of Pax6 in bone cells and tissues. (A) RT-PCR analysis of Pax6 expression in mouse primary calvarial cells, MC3T3-E1 pre-osteoblast cells and osteocyte-like MLOY4 cells. (B) RT-PCR analysis of the endogenous expression of the two major isoforms of Pax6 [Pax6 (+5a) and Pax6 (−5a)]. Their relative expressions are shown in the bar graph [Pax6-5a (black bar), Pax6+5a (gray bar)]. (C) Endogenous Pax6 expression in calvaria and long bone tissues was analyzed with Western blotting and normalized to beta-actin expression. (D) Endogenous expression levels of Pax6 in MLOY4 lysates were compared with the overexpression of Pax6 (−5a) and Pax6 (+5a). (E) MC3T3-E1 cells differentiation was confirmed by ALP staining. (F) Pax6 expression was increased after induction of MC3T3-E1 cells with differentiation media.
Fig. 2.
Immunohistochemical analysis of endogenous Pax6 expression in the calvaria and long bone tissues at embryonic stage 16 and 18. Brown color stained positive cells for Pax6 are indicated by arrows in each embryonic stages. Osteoblasts are indicated by red arrows, osteocytes are indicated by black arrows. Scale bar = 50 μm.
Effect of Pax6 on Wnt inhibitor Sost and other osteocyte markers
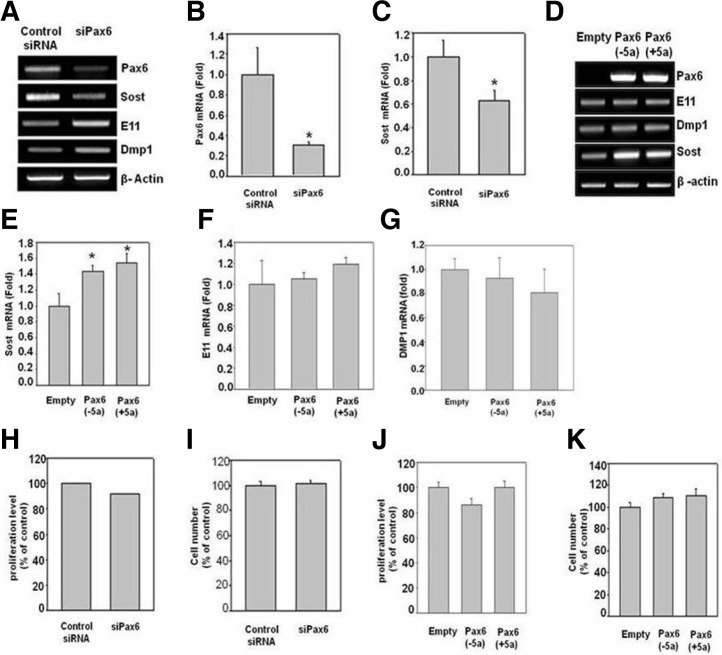
We then studied the effect of Pax6 on osteocyte marker genes via the knockdown or overexpression of Pax6 in MLOY4 cells. The gel images and semi-quantitative RT-PCR and real-time PCR data showed a significant knockdown of Pax6 (Figs. 3A and 3B). Knockdown of Pax6 increased the expression of E11 and DMP1 and decreased the expression of Sost (Figs. 3A and 3C). In contrast, the overexpression of Pax6 (−5a) and Pax6 (+5a) in MLOY4 cells resulted in an increase in the expression of Sost, which is a mature osteocyte marker gene; meanwhile, there were no changes in the other marker genes, as observed with RT-PCR and real-time PCR (Figs. 3D–3G). Together, these results suggest that osteocyte-specific expression of Pax6 might be involved in the transition of pre-osteocytes to mature osteocytes and may also play an important role in inhibiting Wnt signaling through enhanced Sost expression. In addition to these results, we studied the effect of Pax6 on the proliferation and cell number (viability) of MLOY4 cells. We found that neither knockdown nor overexpression of Pax6 in MLOY4 cells had a significant effect on proliferation or cell number (Figs. 3H–3K).
Fig. 3.
The effects of Pax6 on osteocyte marker genes and proliferation. The expression of osteocyte marker genes was analyzed with RT-PCR or real-time PCR. (A-C) Effect of siPax6 on osteocyte marker genes. RT-PCR and real-time PCR analysis showed significantly decreased expression of Sost and increased expression of DMP-1 and E11 in siPax6 transfected cells compared to control siRNA transfected cells. (D-G) Effect of Pax6 overexpression on osteocyte marker genes. RT-PCR and real time PCR analysis showed significantly increased expression of Sost by Pax6 overexpression compared to empty vector transfection. (H-I) Effect of siPax6 on cell proliferation and cell number. There is no effect on cell proliferation and cell number in cells treated with siPax6 compared to control siRNA. (J) Represents the effect of overexpression of Pax6 (+5a) or Pax6 (−5a) expression plasmids on cell proliferation compared to empty vector transfection. (K) Represents the effect of transient transfection of Pax6 expression plasmids on cell number compared to control vector. *Indicates a p-value ≤ 0.05 relative to the control.
Effect of Pax6 on Sost promoter activity in MLOY4 cells
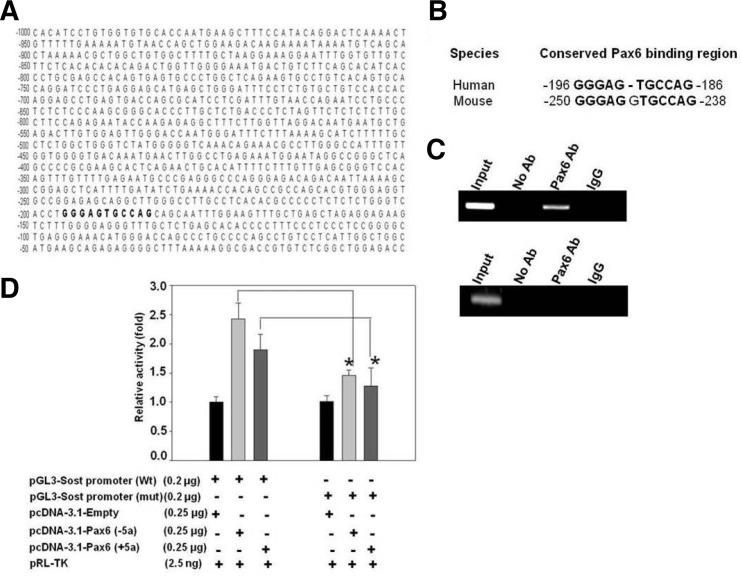
From the Insilico analysis we found one Pax6 binding site on the Sost promoter (∼2 kb) (Figs. 4A and 4B). This was further confirmed by Chip-PCR analysis. Pax6-bound chromatin was isolated from MLOY4 cells and immunoprecipitated with anti-Pax6 antibody. The Pax6-bound DNA fragment shows amplifycation with specific primer set (Fig. 4C), whereas the control primers designed in the other region shows no amplification (Fig. 4C). This result confirmed the specificity of Pax6 binding site on the Sost promoter. Since we found that Pax6 regulates the expression of the Sost gene, we further investigated the possible mechanism of Pax6 on Sost expression. To confirm the regulatory mechanism, we cloned 1, 811 bp of the human Sost promoter containing the Pax6-binding site (−1, 956 to −145 bp) into a pGL3-basic luciferase reporter vector, and evaluated the ability of this promoter fragment to drive luciferase expression in MLOY4 cells. Co-transfection of Pax6 (+5a) or Pax6 (−5a) plasmids with the wild type and mutant pGL3-Sost-promoter vectors led to a significant increase in promoter activity after overexpression of Pax6 in the wild type promoter and a decrease in activity in the mutant promoter in the presence of both isoforms of Pax6 (Fig. 4D). Since the Pax6 could not bind to the mutated region of the promoter, hence there was a significant decrease of reporter activity after overexpression of both forms of Pax6 compared to the wild type promoter (Fig. 4D). This result suggested that Pax6 regulates Sost expression by binding to its promoter region specifically.
Fig. 4.
The binding of Pax6 to the 5′ upstream promoter region of Sost on Chip assay and the effect of Pax6 on Sost promoter activity. (A) Sequence analysis of the 2 kb upstream regulatory region of human Sost promoter (1 kb region shown here). Putative Pax6 conserved binding site was represented in bold. (B) The conserved Pax6 binding site. (C) Chip PCR analysis in MLOY4 cells. PCR amplification was performed by using Pax6 binding element region specific primers (upper panel) and negative control primers (lower panel) as described in the “Materials and Methods”. This result confirms the specificity of Pax6 binding element on Sost promoter. (D) MLOY4 cells were transfected with the indicated combinations of the wild type and mutant Sost promoter and Pax6 expression plasmids. After 48 h, the relative luciferase activity was measured. *Indicates a p-value ≤ 0.05 relative to the control.
Effect of Wnt-3a on Pax6 mRNA expression in MLOY4 cells
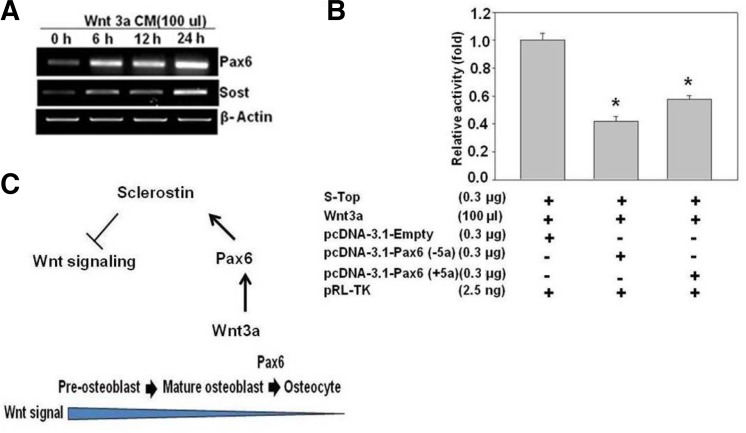
Sost is a negative regulator of Wnt signaling. Because Pax6 regulates Sost expression, we investigated the effect of Wnt-3a on Pax6 mRNA expression in MLOY4 cells. Interestingly, we found that Wnt-3a stimulated the expression of Pax6 mRNA at different time points, which further stimulated the expression of Sost mRNA (Fig. 5A). In addition, we studied the effects of Pax6 on the S-Top reporter vector, which is driven by T-cell factor (TCF)/lymphoid enhancer factor (LEF)-binding elements and is responsive to Wnt signaling. However, the co-expression of the S-Top plasmid with Pax6 isoforms showed an inhibition of Wnt/β-catenin signaling in MLOY4 cells (Fig. 5B). These results suggested that Pax6 plays an important role in the inhibition of Wnt signaling mediated by the Sost gene in MLOY4 cells. Additionally, we found that Wnt-3a enhanced Pax6 expression. In turn, overexpression of Pax6 inhibited Wnt signaling by increasing the expression of Sost, which is a potent inhibitor of Wnt signaling (Fig. 5C).
Fig. 5.
Effect of Pax6 on Wnt signaling. (A) MLOY4 cells were treated with Wnt-3a conditioned media (100 μl/well), and the effect on Pax6 and Sost mRNA expression was measured at different time points with RT-PCR. (B) MLOY4 cells were transfected with the indicated combinations of the Super TOPFlash reporter DNA and Pax6 expression plasmids. After 48 h, the relative luciferase activity was measured. (C) Schematic representation of the role of Pax6 in inhibiting Wnt signaling through Sost. *Indicates a p-value ≤ 0.05 relative to the control.
DISCUSSION
Canonical Wnt/β-catenin signaling plays important role in skeletal development, tissue regeneration and bone formation (Krishnan et al., 2006). Postnatally, it also regulates bone homeostasis and bone mass (Knothe et al., 2004). Sost encodes sclerostin, which is specifically expressed by osteocytes and acts through a network of osteocyte canaliculi to reach the surface of the bone (Poole et al., 2005; Weidauer et al., 2009). Sclerostin inhibits canonical Wnt signaling by binding to LRP5, 6 (Li et al., 2005).
In our GEO analysis, Pax6 was found to be a transcription factor that was upregulated with the Sost gene (data not shown). Pax6 acts as an important regulator in the development of the eye and central nervous tissues (Stoykova et al., 1996; Theiler et al., 1978). Pax6 is abundantly expressed in developing eyes, the forebrain, the ventral spinal card and the endocrine pancreas (Walther and Gruss, 1991). Homozygous mutant mice died at E6 to E8 before somite formation. Pax6 heterozygote mice have a small body size (Theiler et al., 1978). A Pax6-homozygous human fetus with aniridia showed an absence of nasal bones and defects in parietal bones at necropsy (Hodgson and Saunders, 1980). However, the role of Pax6 in bone has never been studied. Therefore, we hypothesized that Pax6 might play an important role in bone development.
In this paper, we showed that compared with the expression of Pax6 in cultured primary calvarial cells or established MC3T3-E1 preosteoblast cells, Pax6 was exclusively expressed in MLOY4 osteocyte-like cells (Fig. 1A). Two major isoforms of endogenous Pax6 [Pax6 (−5a) and Pax6 (+5a)] were detected at the mRNA level in bone cells (Fig. 1B). However, we found that Pax6 (−5a) was the major Pax6 protein expressed in bone (Fig. 1D). Pax6 was expressed exclusively in the calvaria and long bone tissues at E16 and E18 (Fig. 2). Pax6 has been reported to be essential for the proliferation and the pluripotency of retinal progenitors (Hsieh and Yang, 2009). The proliferation and apoptosis of human retinoblastoma cells are regulated by Pax6 (Bai et al., 2011). To evaluate the role of Pax6 in bone, we overexpressed and knocked down Pax6. In our study, neither knockdown nor overexpression of Pax6 exerted any effects on the proliferation or survival of MLOY4 cells (Figs. 3H–3K). Additionally, we observed that the knockdown of Pax6 decreased the expression of Sost mRNA, which is a late osteocyte marker gene, and increased the expression of E11 and DMP1 (Figs. 3A-3C). After transient transfection with Pax6 (+5a) or Pax6 (−5a) DNA, the expression of Sost was significantly increased (Figs. 3D and 3E). The induction of Sost expression by Pax6 was further supported by the observation of enhanced luciferase reporter activity flanked by the 1, 811 bp promoter region of the Sost gene after overexpression of each isoform of Pax6 (Fig. 4D). In other studies, Pax6 was found to play an important role in lens fiber cell and neuronal cell differentiation (Gotz et al., 1998; Shaham et al., 2009). In this study, we observed that the gradual increase of Pax6 mRNA during the differentiation of pre-osteoblast cells into mature osteoblasts (Figs. 1E and 1F). All these data suggest that Pax6 might play an important role in the differentiation of osteoblasts and osteocytes.
In chicken telencephalic development, Wnt-3a induced the expression of the dorsal markers Pax6 and Ngn2 (Gunhaga et al., 2003). Moreover, Pax6 was shown to play an important role in lens morphogenesis through the inhibition of canonical Wnt/β-catenin signaling in the lens surface ectoderm (Machon et al., 2010). In our study, we also observed the induction of Pax6 expression in MLOY4 osteocyte-like cells after treatment with Wnt-3a (Fig. 5A). In addition, Wnt-3a/β-catenin reporter activity was significantly reduced by the overexpression of both Pax6 (+5a) and Pax6 (−5a) along with the co-transfection of the S-Top reporter vector in osteocyte-like MLOY4 cells (Fig. 5B). After transient transfection with Pax6 (−5a) or Pax6 (+5a) DNA, the expression of Sost increased significantly (Figs. 3D and 3E). Additionally, our in silico analysis showed that mouse and human Sost promoters exhibited a conserved, putative Pax6-binding element (GGGAGTGCCAG) at the 2 kb upstream region of the Sost promoter. Overexpression of Pax6 (+5a) or Pax6 (−5a) along with pGL3-Sost promoter (wt) and pGL3-Sost promoter (mut) DNA revealed that mutant promoter activity was significantly lower than wild type promoter activity in the presence of both isoforms of Pax6 (Fig. 4D). All of these findings suggested that Pax6 inhibits canonical Wnt signaling through the stimulation of Sost by binding to its promoter. Canonical Wnt signaling has been shown to stimulate osteoblast differentiation and inhibit the terminal differentiation of mature osteoblasts and apoptosis (Manolagas, 2000). In the present study, knockdown of Pax6 increased the expression of E11 and DMP1, and decreased the expression of Sost. In addition, Pax6 expression was increased according to further differentiation of osteoblasts. Therefore, we speculated that Pax6 may be an important transcription factor in stimulating further differentiation of mature osteoblasts to osteocytes. The potential role of Pax6 is schematically represented in Fig. 5C.
In conclusion, we showed that Pax6 is expressed in the calvaria and long bones of adult mice and osteocyte-like MLOY4 cells. By direct binding to the putative Pax6 binding sequence of the Sost promoter region, Pax6 suppressed the canonical Wnt signaling pathway. Further exploration of phenotype in osteocyte-specific Pax6 knockout mice could provide more definitive evidence of the role of Pax6 in differentiation of mature osteoblasts to osteocytes.
Acknowledgments
Mrs. Jami Ajita is a graduate student supported by a Brain Korea 21 scholarship. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government Ministry of Education and Science Technology (No. 20120000486).
REFERENCES
- Bai SW, Li B, Zhang H, Jonas JB, Zhao BW, Shen L, Wang YC. Pax6 regulates proliferation and apoptosis of human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2011;52:4560–4570. doi: 10.1167/iovs.10-5487. [DOI] [PubMed] [Google Scholar]
- Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar R. NCBI GEO: mining tens of millions of expression profiles database and tools update. Nucleic Acids Res. 2007;35:D760–D765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Berger S, Tuoc TC, D’Amelio M, Cecconi F, Gorski JA, Jones KR, Gruss P, Stoykova A. Conditional activation of Pax6 in the developing cortex of transgenic mice causes progenitor apoptosis. Development. 2007;134:1311–1322. doi: 10.1242/dev.02809. [DOI] [PubMed] [Google Scholar]
- Carriere C, Plaza S, Caboche J, Dozier C, Bailly M, Martin P, Saule S. Nuclear localization signals, DNA binding, and transactivation properties of quail Pax6 (Pax-QNR) isoforms. Cell Growth Differ. 1995;6:1531–1540. [PubMed] [Google Scholar]
- Cillo C, Faiella A, Cantile M, Boncinelli E. Homeobox genes and cancer. Exp Cell Res. 1999;248:1–9. doi: 10.1006/excr.1999.4451. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–443. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: How osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Gotz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Hodgson SV, Saunders KE. A probable case of homozygous condition of the aniridia gene. J Med Genet. 1980;17:478–480. doi: 10.1136/jmg.17.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Hirst EM, Horsburgh G, Hetherington CM. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development. 1988;103:115–119. doi: 10.1242/dev.103.Supplement.115. [DOI] [PubMed] [Google Scholar]
- Hsieh YW, Yang XJ. Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors. Neural Dev. 2009;4:32. doi: 10.1186/1749-8104-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski C, Sperbeck S, Graham C, Wistow G. Alternative splicing of Pax6 in bovine eye and evolutionary conservation of intron sequences. Biochem Biophys Res Commun. 1997;240:196–202. doi: 10.1006/bbrc.1997.7623. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling a union made for bone. J Bone Miner Res. 2004;19:1749–1757. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte -like cell line, MLOY4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Chang HH, Shaw JP. Craniofacial abnormalities in homozygous Small eye (Sey/Sey) embryos and newborn mice. J Anat. 1995;186:607–617. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol. 2006;292:486–505. doi: 10.1016/j.ydbio.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;36:1–8. doi: 10.1016/s1357-2725(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53:102–107. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T. 3’ deletions cause aniridia by preventing Pax6 gene expression. Proc. Natl. Acad. Sci USA. 2000;97:13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wu X, Li X, Fu J, Shen J, Li X, Wang H. Corticosterone regulates the expression of neuropeptide Y and reelin in MLOY4 cells. Mol Cells. 2012;33:611–616. doi: 10.1007/s10059-012-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, Kreslova J, Ruzickova J, Vacik T, Klimova L, Fujimura N, Lachova J, Kozmik Z. Lens morphogenesis is dependent on Pax6 mediated inhibition of the Canonical Wnt/beta-Catenin signaling in the lens surface ectoderm. Genesis. 2010;48:86–95. doi: 10.1002/dvg.20583. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells basic regulatory mechanisms for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim SJ, Rhee Y, Byun JH, Kim SH, Kim MH, Lee EJ, Lim SK. Fidgetin-like1 gene inhibited by basic fibroblast growth factor regulates the proliferation and differentiation of osteoblasts. J Bone Miner Res. 2007;22:889–896. doi: 10.1359/jbmr.070311. [DOI] [PubMed] [Google Scholar]
- Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a WNT signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Shaham O, Smith AN, Robinson ML, Taketo MM, Lang RA, Ashery-Padan R. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- Tang HK, Singh S, Saunders GF. Dissection of the transactivation function of the transcription factor encoded by the eye developmental gene Pax6. J Biol Chem. 1998;273:7210–7221. doi: 10.1074/jbc.273.13.7210. [DOI] [PubMed] [Google Scholar]
- Theiler K, Varnum DS, Stevens LC. Development of Dickie’s small eye, a mutation in the house mouse. Anat. Embryol. (Berl) 1978;155:81–86. doi: 10.1007/BF00315732. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte expressed negative regulator of bone formation but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ, Van Hul W, Hamersma H, Dikkers FG, et al. Sclerostin in mineralized matrices and van buchem disease. J Dent Res. 2009;88:569–574. doi: 10.1177/0022034509338340. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Weidauer SE, Schmieder P, Beerbaum M, Schmitz W, Oschkinat H, Mueller TD. NMR structure of the Wnt modulator protein sclerostin. Biochem Biophys Res Commun. 2009;380:160–165. doi: 10.1016/j.bbrc.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Zhang W, Cveklova K, Oppermann B, Kantorow M, Cvekl A. Quantitation of Pax6 and Pax6 (5a) transcript levels in adult human lens, cornea and monkey retina. Mol Vis. 2001;7:1–5. [PMC free article] [PubMed] [Google Scholar]