Abstract
Berberine (BBR) is one of the major alkaloids and has been reported to have a variety of pharmacologic effects, including inhibition of cell cycle progression. Here, we investigated the mechanisms of BBR protection of neuronal cells from cell death induced by the Parkinson’s disease-related neurotoxin 6-hydroxydopamine (6-OHDA). Pretreatment of SH-SY5Y cells with BBR significantly reduced 6-OHDAinduced generation of reactive oxygen species (ROS), caspase-3 activation, and subsequent cell death. BBR also upregulated heme oxygenase-1 (HO-1) expression, which conferred protection against 6-OHDA-induced dopaminergic neuron injury and besides, effect of BBR on HO-1 was reversed by siRNA-Nrf2. Furthermore, BBR induced PI3K/Akt and p38 activation, which are involved in the induction of Nrf2 expression and neuroprotection. These results suggest that BBR may be useful as a therapeutic agent for the treatment of dopaminergic neuronal diseases.
Keywords: berberine, heme oxygenase-1, neuroprotection, NF-E2 related factor, phosphatidylinositol 3-kinase
INTRODUCTION
Parkinson’s disease (PD), which is characterized by a progressive loss of dopamine-containing neurons, is a progressive neurodegenerative disorder of unknown etiology. Substantial evidence suggests that free radical formation and oxidative stress might play an important role in the pathogenesis of PD (Greenamyre and Hastings, 2004). Thus, inhibition of reactive oxygen species (ROS) and regulation of oxidative stress may reduce or prevent the loss of dopaminergic neurons. It is well known that 6-hydroxydopamine (6-OHDA), an hydroxylated analog of dopamine, can induce massive oxidative stress leading to the damage of dopaminergic neurons (Tobon-Velasco et al., 2010). Moreover, 6-OHDA-induced ROS impairs mitochondrial membrane function through the inhibition of the electron-transport chain by blocking complex I (Chowdhury et al., 2005; Schober, 2004). Consequently, the collapse of mitochondrial membrane potential results in a decrease of Bcl-2 protein, which inhibits cell death by preventing mitochondrial membrane depolarization and increasing Bax proteins that promote cell death by inducing mitochondrial membrane depolarization and cytochrome C release (Shrivastava et al., 2012). Neuronal cells have developed several protective mechanisms to prevent ROS formation or to detoxify ROS. These mechanisms involve both antioxidants and protective enzymes (Chen and Kunsch, 2004). Among the various cytoprotective enzymes, it is well known that heme oxygenase (HO)-1 plays an important role in neuroprotective functions (Jazwa and Cuadrado, 2010; Schober, 2004). In oxidative injury and inflammation conditions, the synthesis of the HO-1 gene is increased and is linked to the transcription factor NF-E2-related factor 2 (Nrf2) (Alam and Cook, 2003; Srisook et al., 2005).
Recent studies have reported that Nrf2 is a master redox regulator by up-regulating HO-1 to protect dopaminergic neurons against 6-OHDA-induced neurotoxicity (Hwang and Jeong, 2008). Studies have also suggested that Nrf2 nuclear translocation requires the activation of several signal transduction pathways, such as mitogen-activated protein kinases (MAPKs), Akt, and phosphatidylinositol 3-kinase (PI3K) (Feng et al., 2011; Hwang and Jeong, 2008; Martin et al., 2004). Recently, it reported that HO-1 may be a therapeutic target in neurodegenerative disease and brain infection (Chen and Kunsch, 2004; Hu et al., 2012). In addition, increasing evidence indicates that a pharmacological inducer of HO-1 expression may maximize the intrinsic antioxidant potential of cells and therefore represent a novel target for therapeutic intervention.
Berberine (BBR), an alkaloid derived from Coptis chinensis, is used for treating diarrhea in traditional Chinese medicine. A growing number of studies reveal that BBR has a wide variety of biological effects, including anti-tumor (Tong et al., 2012) and cardiovascular-protective actions (Lau et al., 2001). Moreover, recent studies suggest that BBR inhibited LPS-induced inflammation in microglia cells (Hsu et al., 2012) and H2O2-induced cytotoxicity in motor neuron-like cells (Hsu et al., 2012) via regulation of HO-1 expression. However, the mechanism underlying its potential protective effects on dopaminergic neurons has not yet been fully studied. We here demonstrate that the BBR-induced activation of PI3K/Akt and p38 upregulates the expression and activity of HO-1, which, in turn, protects against 6-OHDA-induced oxidative damage in SH-SY5Y cells.
MATERIALS AND METHODS
Chemicals and materials
Reagents used in this study were purchased from the following sources; berberine, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide], 6-OHDA and zinc protoporphyrin (ZnPP) from Sigma-Aldrich (USA); SB203580, LY294002, PD98059, and SP600125 were purchased from Calbiochem (USA); DMEM, fetal bovine serum, sodium pyruvate, and Trizol were supplied by Gibco BRL (USA); [γ-32P]ATP was the product of NEN Life Science (USA); DeadEnd™ Fluorometric TUNEL system and caspase-3, -9 activity assay kit from Promega (USA); 2,7-dichlorofluorescein diacetate (H2DCFDA) from Molecular Probes (USA); antibodies for Akt, phospho-Akt, p38, phospho-p38 MAPK, and HRP-conjugated anti-rabbit IgG from Cell Signaling Technology (USA); antibodies for β-actin, Nrf2 and HO-1 from Calbiochem (USA). The HO-1-ARE-luciferase reporter gene was kindly provided by Dr. J. Alam (Tulane University School of Medicine, USA). All other chemicals and reagents were of analytical grade.
Cell culture
Human neuroblastoma SH-SY5Y cells were obtained from the American Type Culture Collection (ATCC, USA) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/mL streptomycin at 37°C in an atmosphere of 5% CO2.
Cell treatment and cell viability assay
BBR was dissolved in dimethylsulfoxide (DMSO) and the stock solutions were added directly to the culture media. The control cells were treated with DMSO only. The final concentration of solvent was always < 0.1%. The cells (5 × 103/well) in 10% FBS-DMEM were seeded into the 48-well plates. After incubation for 24 h, various concentrations of BBR and 6-OHDA were added to the well, and the plates were incubated at 37°C for an additional 24 h. The cells were used for the MTT-based assay by measuring according to the manufacturer’s instructions. Relative cytotoxicity was quantified by absorption measurements at 5 50 nm u sing a m icrotiter plate r eader (Molecular Devices, USA). This wavelength was found not to interfere with BBR.
Caspase-3 activities and TUNEL assay
Cells were plated in 12-well plates at a density of 4 × 104 cells/well. After a 24 h chemical treatment, cells treated with the indicated combinations of BBR and 6-OHDA were lysed in hypotonic buffer [20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 100 μM PMSF, 2 μg/ml each of aprotinin, pepstatin, and leupeptin] (Hwang and Jeong, 2008). The supernatants were collected and incubated with 100 μM DEVD-pNA as a substrate at 37°C. The change in absorbance was measured at 405 nm using a plate reader. Apoptosis was detected by the TUNEL assay using the DeadEnd™ Fluorometric TUNEL System (Promega, USA) according to the manufacturer’s instructions. Cells were then washed twice with PBS, fixed with 4% methanol-free paraformaldehyde for 10 min, washed twice with PBS, and made permeable with 0.2% Triton X-100 for 5 min. Following two more washes, each glass slide was covered with equilibration buffer for 10 min. Next, the buffer was aspirated, and the glass slides were incubated with TdT buffer at 37°C for 1 h in the dark. Chromosomal DNA was stained with DAPI; stained cells were mounted on glass slides, and examined using a Carl Zeiss Axiovert 200 M microscope. The TUNEL apoptotic index (total number of apoptotic nuclei per total number of nuclei multiplied by 100) was calculated in various zones three times.
Determination of ROS production
ROS production in SH-SY5Y cells was measured using the redox-sensitive fluorescent dye H2-DCFDA. After treatment with 50 μM 6-OHDA or vehicle for 6 h, cells were incubated with 25 μM H2-DCFDA for 20 min. The cells were rinsed twice with phenol-red-free DMEM containing 1% FBS, and fluorescence was detected on a fluorescence reader (Varioskan, Thermo Electon Co., USA) by measuring the emission at 530 nm after excitation at 485 nm.
Electrophoretic mobility shift assay
A synthetic double-stranded oligonucleotide containing the Nrf2-binding domain (ARE) was labeled with [γ-32P]-ATP using T4 polynucleotide kinase and separated from unincorporated [γ-32P]-ATP by gel filtration on a nick spin column. Prior to addition of the [γ-32P]-labeled oligonucleotide, 10 μg of nuclear extract was incubated on ice for 15 min in gel shift-binding buffer [20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, and 50 mM Tris-HCl, pH 7.5, with 0.25 μg/ml poly(dIdC)]. DNA-protein complexes were resolved by 6% polyacrylamide gel electrophoresis at 150 V for 2 h and visualized by autoradiography.
siRNA knockdown
siNrf2 and scrambled siRNA were acquired from Santa Cruz Biotechnology (sc-37030). siRNA was transfected into SH-SY5Y according to the manufacturer’s protocol and using the transfection reagent, Lipofectamine 2000© (Invitrogen, USA). The cells were incubated with 100 nM of target siRNA or scramble siRNA for 4 h in serum- and antibiotic-free media. The cells were then incubated for 18 h in media containing antibiotics and FBS, and cells were washed, treated with or without BBR for 24 h.
Transient transfection and luciferase assay
Cells (3 × 105 cells/well) were replated in 24-well plates over-night and transiently co-transfected with HO-1-ARE-promoter-luciferase construct and pRL-SV plasmid (Renilla luciferase expression for normalization) (Promega, USA) using LipofectAMINE ™ 2000 reagent (Invitrogen, USA). Relative luciferase activities were calculated by normalizing HO-1-ARE-promoter-driven firefly luciferase activity to Renilla luciferase activity.
RNA preparation and mRNA analysis by real-time quantitative PCR
Total RNA was isolated from the cells using Trizol (GibcoBRL, USA). Accumulated PCR products were detected directly by monitoring the increase in reporter dye (SYBR®). The expression levels of HO-1 in the exposed cells were compared to those in control cells at each time point using the comparative cycle threshold (Ct) method (Johnson et al., 2000). The following primer sequences were used: HO-1 forward, 5′-CG CCTTCCTGCTCAACATT-3′; reverse, 5′-TGTGTTCCTCTGTC AGCATCAC-3′; and Ribosomal protein S18 (S18) forward, 5′-AAGTTTCAGCACATCCTGCGAGTA-3′, reverse, 5′-TTGGTG AGGTCAATG TCTGCTTTC-3′. The quantity of each transcript was calculated as described in the instrument manual and normalized to the amount of S18, which is a housekeeping gene.
Western blotting analysis
Western blotting was performed using anti-HO-1, anti-Akt, anti-phospho-Akt, anti-p38, anti-phospho-p38, and anti-β-actin antibodies. Protein samples were heated at 95°C for 5 min and analyzed by SDS-PAGE. Immunoblot signals were developed by enhanced chemiluminescence (Pierce Biotechnology, USA). Nucleus and cytosol were separated by Nuclear Extract Kit from Active Motif (USA).
Statistical analysis
All the experiments were repeated at least three times. The results are expressed as a mean ± SD, and the data were analyzed using one-way ANOVA followed by a Student’s t-test for significant difference. A p value < 0.05 was considered significant.
RESULTS
BBR protects against 6-OHDA-induced neuronal cell death
The protective effect of BBR against 6-OHDA-induced oxidative stress in SH-SY5Y cells was assessed. BBR treatment dose-dependently increased cell survival following 6-OHDA treatment (Fig. 1A). The protective effects of BBR against 6-OHDA-induced apoptosis were determined using caspase-3 activity and TUNEL assays. TUNEL apoptotic index was increased by treatment of SH-SY5Y cells with 6-OHDA for 24 h, whereas BBR significantly reduced 6-OHDA-induced TUNEL apoptotic index (Fig. 1B). To examine whether BBR prevented caspase-3 activity, DEVDase activity was determined to measure caspase-3 activation. BBR pre-treatment significantly reduced the level of 6-OHDA-induced DEVDase activity (Fig. 1C). These results suggest that BBR inhibits 6-OHDA-induced apoptosis in SH-SY5Y cells.
Fig. 1.

BBR prevents 6-OHDA-induced cell death. SH-SY5Y cells were treated with BBR (1, 5, and 10 μM) for 12 h then incubated with 6-OHDA (50 or 100 μM) for an additional 24 h. (A) Cell viability was measured by MTT assays. (B) TUNEL apoptotic index were performed according to the manufacturer’s instruction. (C) The catalytic activities of caspase-3 in cell lysates were assayed using the specific substrates DEVD-pNA. *Significantly different from BBR plus 6-OHDA-treated cells.
BBR reduces 6-OHDA-induced ROS generation
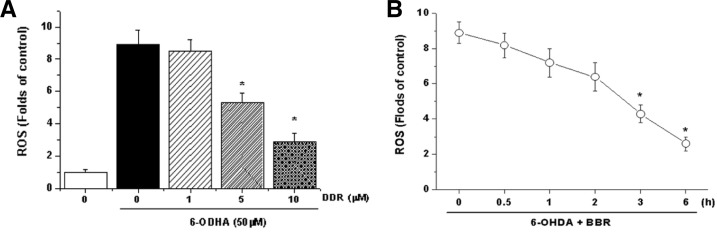
We determined the effect of BBR on 6-OHDA-induced ROS generation in SH-SY5Y cells. Pretreatment with BBR signifycantly reduced the 6-OHDA-induced ROS generation, indicating that BBR attenuates ROS production (Fig. 2A). Moreover, this effect was dependent on the duration of BBR pretreatment, that is, attenuation of ROS release by BBR required at least 3 hours of pretreatment with BBR prior to the addition of 6-OHDA (Fig. 2B). These results suggest that BBR induces the expression of a gene(s) essential to ROS antagonism.
Fig. 2.
Effects of BBR on 6-OHDA-induced cellular ROS production. (A) Cells were pretreated with BBR (1–10 μM) or vehicle for 12 h. After the medium was removed, SH-SY5Y cells were exposed to 6-OHDA (50 μM) for 6 h and then fluorescence was measured as described in the “Materials and Methods”. (B) Prevention of 6-OHDA-induced ROS by BBR depends on the duration of BBR pretreatment. *Significantly different from BBR plus 6-OHDA-treated cells.
BBR induces HO-1 expression
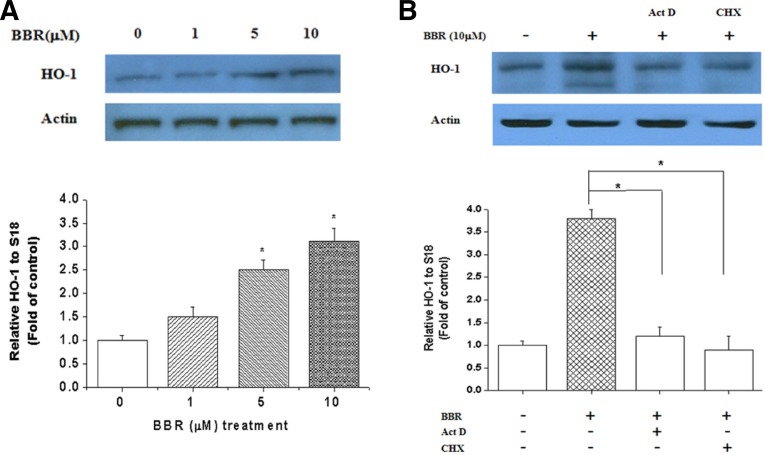
As HO-1 is an important component of the cellular defense against oxidative stress, we next assessed whether BBR could induce HO-1 expression. Cells were treated with BBR for 12 h, and BBR treatment dose-dependently increased HO-1 mRNA and protein expression (Fig. 3A). This increase was sensitive to pretreatment with actinomycin D or cycloheximide, suggesting that BBR enhances the expression of the inducible heme oxygenase isoform, HO-1. Moreover, the increase in mRNA was also sensitive to cycloheximide, suggesting that induction of HO-1 transcription involves de novo protein synthesis (Fig. 3B).
Fig. 3.
Effect of BBR on HO-1 mRNA and protein expression. (A) Cells were exposed to various concentrations of BBR for 12 h and total RNA was extracted. HO-1 mRNA and protein expression were analyzed by RT-PCR and Western blotting. (B) Cells were either untreated or pretreated with 50 μM cycloheximide or 10 μg/ml actinomycin D for 2 h prior to the addition of 10 μM BBR for an additional 12 h. (C) Cells were transfected with scramble (siRNA) or siNrf2 as described in the “Materials and Methods”. After 24 h incubation with BBR (10 μM), cells were harvested and subjected to Western blot for HO-1 expression. Transfection efficiency was confirmed by checking Nrf2 expression.
BBR induces Nrf2 nuclear translocation
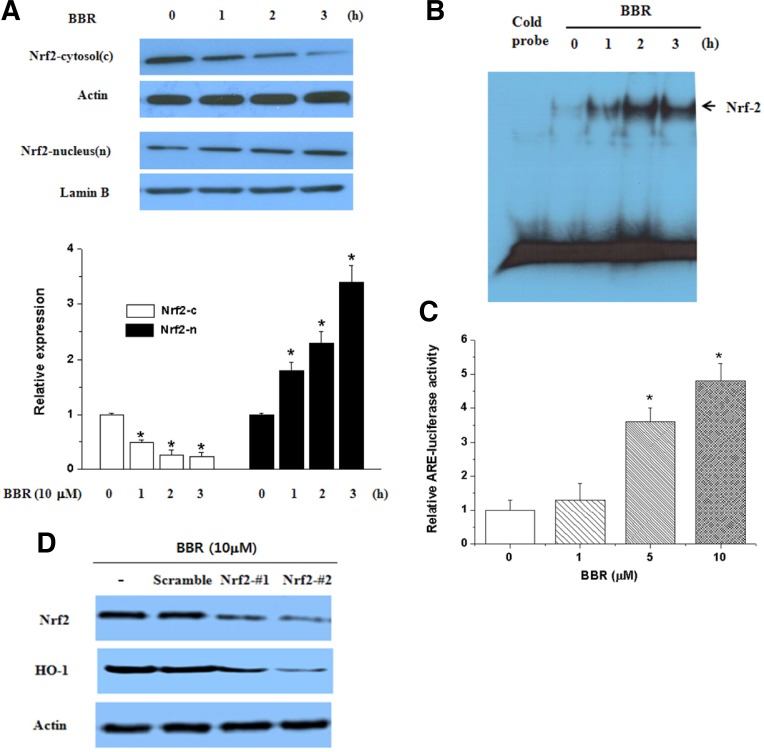
Among the transcription factors, Nrf2 is an important transcription factor that regulates ARE-driven HO-1 gene expression (Alam and Cook, 2003; Srisook et al., 2005). Therefore, we examined whether BBR activated Nrf2 in association with its up-regulation of HO-1 in SH-SY5Y cells. BBR treatment increased Nrf2 translocation in the nucleus (Fig. 4A). Nrf2 activation in BBR-treated cells was assessed by EMSA with an oligonucleotide harboring a consensus Nrf2 binding element. BBR-treated cells exhibited high levels of Nrf2 binding (Fig. 4B). Also, BBR increased the transcriptional activity of Nrf2 (Fig. 4C). To further determine whether BBR-mediated HO-1 expression mediated by Nrf2 activation, we used siNrf2. As shown in Fig. 4D, BBR significantly reduced HO-1 expression in siNrf2 transfected cells suggesting the critical role of Nrf2 signaling in HO-1 expression.
Fig. 4.
Effect of BBR on Nrf2 translocation into the nucleus and its transcriptional activity. (A) Effect of BBR on translocation of Nrf2. SH-SY5Y cells were treated with BBR (10 μM) for the indicated time and then nuclear and cytosolic extracts were prepared for western blotting. The Nrf2 protein level was normalized using lamin B (nuclear) and actin (cytosol). Each blot is representative of three independent experiments. (B) Effect of BBR on ARE-binding activity of Nrf2. Nuclear extracts that were prepared from cells were treated with 10 μM of BBR for the indicated times. (C) Effect of BBR on the transcriptional activity of HO-1. Cells were transfected with the ARE reporter plasmid and treated with BBR (1–10 μM). After 18 h of treatment, luciferase activities were determined. *Significantly different from BBR plus 6-OHDA-treated cells.
Involvement of PI3K/Akt and p38 pathway in HO-1 expression by BBR
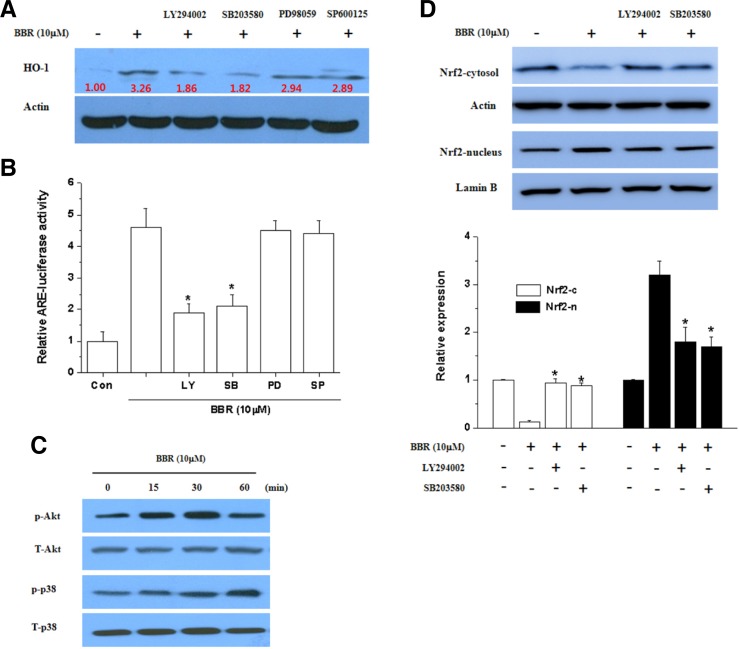
To further explore the upstream signaling pathway involved in BBR-mediated Nrf2 activation and induction of HO-1, the activation of PI3K/Akt and p38 was examined in SH-SY5Y cells. Interestingly, HO-1 expression (Fig. 5A) and the transcriptional activity of Nrf2 (Fig. 5B) were reduced by LY294002 (PI3K inhibitor) and SB203580 (p38 inhibitor) but not other kinases such as PD98059 (MEK inhibitor) and SP600125 (JNK inhibitor). Consistantly, Akt and p38 were phosphorylated by BBR (Fig. 5C). Likewise, Nrf2 nuclear translocation was effectively blocked by LY294002 and SB203580 (Fig. 5D). These results indicate a role for PI3K/Akt and p38 signaling in BBR-mediated HO-1 induction through nuclear translocation of Nrf2.
Fig. 5.
Induction of HO-1 and activation of Nrf2 by BBR via the PI3K/Akt and p38 pathways. (A) Effect of BBR on the phosphorylation of Akt and p38. Parallel immunoblots were analyzed for total kinase levels with anti-Akt, ERK1/2, p38 and JNK1/2 antibodies. (B) Effect of PI3K and p38 inhibitors on BBR-induced HO-1 expression. Whole cell lysates were subjected to Western blotting analysis by using anti-HO-1 and anti-actin antibodies. (C) Effect of PI3K and p38 inhibitors on BBR-induced Nrf2 translocation. Nuclear and cytosolic extracts were subjected to Western blot analysis using anti-Nrf2, anti-actin and anti-lamin B antibodies. The Nrf2 protein level was normalized using lamin B (nuclear) and actin (cytosol). Each blot is representative of three independent experiments. (D) Effect of PI3K and p38 inhibitors on BBR-induced ARE-luciferase activity. The luciferase activity was measured by luminometer. *Significantly different from BBR plus 6-OHDA-treated cells.
Involvement of HO-1 upregulation in neuroprotection by BBR
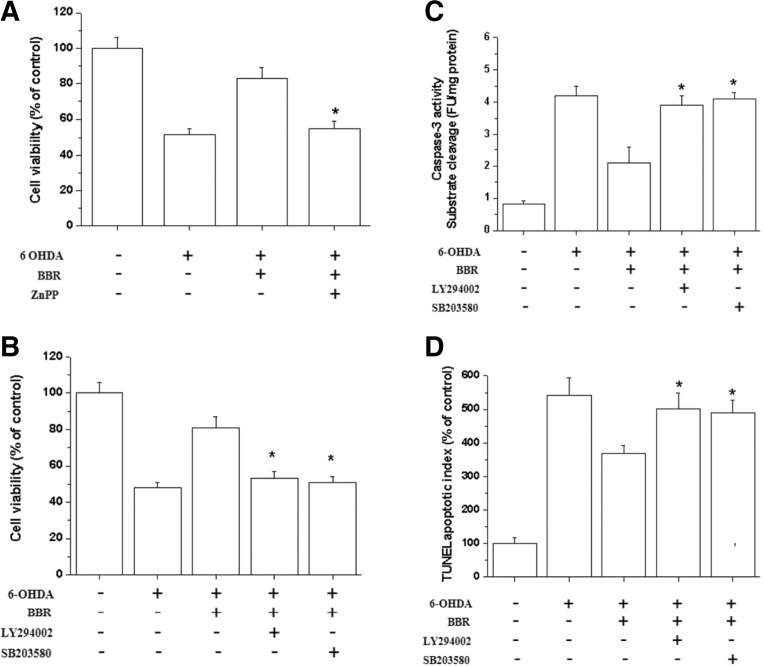
To determine whether the increased HO-1 activity induced by BBR could confer neuroprotection against oxidative stress, SH-SY5Y cells were pretreated with the ZnPP (HO inhibitor). ZnPP attenuated the protective effect of BBR on 6-OHDA-induced cytotoxicity (Fig. 6A), suggesting that the neuroprotective effect of BBR is partly mediated through HO-1 induction. Moreover, inhibitors of PI3K/Akt and p38 also attenuated the protective effect of BBR on 6-OHDA-induced caspase-3 activity (Fig. 6B), cytotoxicity (Fig. 6C) and apoptosis (Fig. 6D), suggesting the involvement of PI3K/Akt and p38 signaling in BBR mediated HO-1 gene induction and neuroprotection.
Fig. 6.
PI3K/Akt and p38 inhibitors atenuate the anti-apoptotic effect of BBR. (A) The HO-1 enzyme inhibitor ZnPP reversed the protective effect of BBR against 6-OHDA-induced cell death. SH-SY5Y cells were treated with BBR (10 μM) for 12 h and then incubated with different doses of ZnPP (5 μM) for 30 min; 6-OHDA (50 μM) was then added to the cells for an additional 24 h. Cell viability was detected by the MTT assay. (B) SH-SY5Y cells were pretreated for 30 min with LY294002 and SB203580 prior to the addition of BBR. Following a 12 h incubation with BBR, cells were treated with 6-OHDA for 24 h. Cell viability was assessed by the MTT assay after 6-OHDA treatment. (C) The catalytic activity of caspase-3 in cell lysates was assayed using the specific substrate DEVD-pNA. (D) TUNEL apoptotic index was performed as described in the materials and methods section. * Significantly different from BBR plus 6-OHDA-treated cells.
DISCUSSION
BBR, an isoquinoline alkaloid isolated from Chinese herbs such as the Coptidis Rhizome, has been shown to enhance cell survival through the reduction of reactive oxygen species (ROS), and the release of cytochrome c and apoptosis-inducing factors (AIFs) in PC12 cells damaged by oxygen-glucose deprivation (Zhou et al., 2008). Moreover, SH-SY5Y cell cultures induced by adding H2O2 are well established as models for damaged neuronal cells and ischemic studies (Hsu et al., 2012). However, the precise mechanisms of its dopaminergic neuroprotective effects remain unclear. In this report we have demonstrated the survival promoting effect of BBR in the 6HD-damaged neuronal cell model. We found that BBR increases cell viability of SH-SY5Y under oxidative stress by about two-fold. This result implies an antiapoptotic effect of BBR under oxidative stress conditions. Furthermore, this study documents a new physiological role for the PI3K/Akt and p38 survival pathway activated by BBR: control of intracellular levels of oxygen free radicals by regulating the expression of HO-1 through Nrf2 activation. Moreover, enhanced HO-1 expression is essential for BBR function in neuroprotection. Although PI3K/Akt is a well-documented pathway involved in protecting against apoptotic insults, including oxidative stress (Hsu et al., 2012), this is the first report linking this survival pathway with a specific enzyme involved in ROS detoxification in injured dopaminergic neuronal cells. The cytoprotective properties of antioxidants have been partially attributed to their ability to induce cytoprotective enzymes. Among the various cytoprotective enzymes, HO-1 expression has been considered an adaptive and beneficial response to oxidative stress in a wide variety of cells (Hsu et al., 2012; Kusunoki et al., 2012; Sun et al., 2012). Recent reports have shown that transgenic mice overexpressing moderate levels of HO-1 displayed augmented resistance to neural damage in animal models of cerebral ischemia (Chen et al., 2000; Panahian et al., 1999) or after H2O2- and glutamate-induced oxidant stress in vitro (Chen et al., 2000).
Our study demonstrated that BBR increased HO-1 mRNA and protein expression in SH-SY5Y cells and besides, effect of BBR on HO-1 was reversed by siRNA-Nrf2. In addition, the increase of HO-1 expression by BBR conferred cytoprotection against 6-OHDA-induced oxidative stress. These results suggest that BBR-induced HO-1 expression might be an important mechanism for the neuroprotective effects of BBR. The diversity of stimuli that can induce HO-1 suggests that the molecular mechanisms that regulate HO-1 are complex. Several studies have described the regulatory sites and transcription factors required for activation of the HO-1 promoter (Balogun et al., 2003; Gong et al., 2002). Previous studies have implicated Nrf2 in inducer-dependent activation of the HO-1 gene (Alam and Cook, 2003; Alam et al., 1999; Xu et al., 2005). The transcription factor Nrf2 positively regulates the ARE-mediated expression of genes encoding phase II detoxification enzymes, including HO-1 (Hwang and Jeong, 2010; Zhang et al., 2004). Consistently, our results showed that BBR increases the expression of HO-1 genes through Nrf2-mediated ARE activation.
PI3K/Akt and MAPK pathways have been reported to be involved in HO-1 expression (Gong et al., 2002; Maines, 2007; Ryter et al., 2002) and in Nrf2-dependent transcription (Surh et al., 2009). The current experiments were designed to determine a possible role for the PI3K/Akt and p38 pathways in BBR-induced HO-1 expression and, as a result, BBR was found to activate PI3K/Akt and p38 pathways. In addition, the use of specific inhibitors for PI3K/Akt and ERK pathways confirmed the involvement of PI3K/Akt and p38, but not of ERK1/2 and JNK1/2, in BBR-induced HO-1 expression, transcriptional activity, and neuroprotection. Consistently, inhibitors of PI3K/Akt or p38 blocked BBR-induced Nrf2 nuclear translocation. These results suggest that PI3K/Akt and p38 pathways are important for BBR-induced HO-1 expression and Nrf2 nuclear translocation.
In summary, BBR induces HO-1 expression in dopaminergic SH-SY5Y neuronal cells, and this expression confers neuroprotection against oxidative injury. BBR also induces Nrf2 nuclear translocation, which is upstream of BBR-induced HO-1 expression, and activates the phosphorylation of Akt and p38. The PI3K/Akt and p38 pathways are involved in BBR-induced Nrf2 nuclear translocation, HO-1 expression, and neuroprotection.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (KRF-2008-313-C00748), and was supported by a grant (2011-505) from the Asan Institute for Life Sciences in Seoul, Korea.
REFERENCES
- Alam J., Cook J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.L., Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- Chen K., Gunter K., Maines M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J. Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury A.K., Watkins T., Parinandi N.L., Saatian B., Kleinberg M.E., Usatyuk P.V., Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang P., Chen X., He G. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J. Cell. Biochem. 2011;112:1524–1531. doi: 10.1002/jcb.23065. [DOI] [PubMed] [Google Scholar]
- Gong P., Stewart D., Hu B., Li N., Cook J., Nel A., Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antioxid. Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- Greenamyre J.T., Hastings T.G. Biomedicine. Parkinson’s-divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- Hsu Y.Y., Chen C.S., Wu S.N., Jong Y.J., Lo Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012;46:415–425. doi: 10.1016/j.ejps.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Hu X., Wang J., Jiang H. Heme oxygenase-1: An important therapeutic target for protecting against myocardial ischemia and reperfusion injury. Int. J. Cardiol. 2012. pii: S0167-5273(12) 01348-4. [DOI] [PubMed]
- Hwang Y.P., Jeong H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008;582:2655–2662. doi: 10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]
- Hwang Y.P., Jeong H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010;242:18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Jazwa A., Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- Johnson M.R., Wang K., Smith J.B., Heslin M.J., Diasio R.B. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal. Biochem. 2000;278:175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- Kusunoki C., Yang L., Yoshizaki T., Nakagawa F., Ishikado A., Kondo M., Morino K., Sekine O., Ugi S., Nishio Y., et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2012. pii: S0006-291X(12) 02107-9. [DOI] [PubMed]
- Lau C.W., Yao X.Q., Chen Z.Y., Ko W.H., Huang Y. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Maines M.D. Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3 K signaling pathways. Antioxid. Redox Signal. 2007;9:2187–2195. doi: 10.1089/ars.2007.1805. [DOI] [PubMed] [Google Scholar]
- Martin D., Rojo A.I., Salinas M., Diaz R., Gallardo G., Alam J., De Galarreta C.M., Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Panahian N., Yoshiura M., Maines M.D. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J. Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- Ryter S.W., Otterbein L.E., Morse D., Choi A.M. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell. Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Shrivastava P., Vaibhav K., Tabassum R., Khan A., Ishrat T., Khan M.M., Ahmad A., Islam F., Safhi M.M., Islam F. Anti-apoptotic and Anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s Rat model. J. Nutr. Biochem. 2012 doi: 10.1016/j.jnutbio.2012.03.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Srisook K., Kim C., Cha Y.N. Molecular mechanisms involved in enhancing HO-1 expression: derepression by heme and activation by Nrf2, the “one-two” punch. Antioxid. Redox Signal. 2005;7:1674–1687. doi: 10.1089/ars.2005.7.1674. [DOI] [PubMed] [Google Scholar]
- Sun G.B., Sun X., Wang M., Ye J.X., Si J.Y., Xu H.B., Meng X.B., Qin M., Sun J., Wang H.W., et al. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol. Appl. Pharmacol. 2012;265:229–240. doi: 10.1016/j.taap.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Surh Y.J., Kundu J.K., Li M.H., Na H.K., Cha Y.N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- Tobon-Velasco J.C., Carmona-Aparicio L., Ali S.F., Santamaria A. Biomarkers of cell damage induced by oxidative stress in Parkinson’s disease and related models. Cent. Nerv. Syst. Agents Med. Chem. 2010;10:278–286. doi: 10.2174/187152410793429719. [DOI] [PubMed] [Google Scholar]
- Tong N., Zhang J., Chen Y., Li Z., Luo Y., Zuo H., Zhao X. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012;3:1263–1267. doi: 10.3892/ol.2012.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Li C.Y., Kong A.N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Zhang X., Lu L., Dixon C., Wilmer W., Song H., Chen X., Rovin B.H. Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-delta12,14-prostaglandin J2 in human mesangial cells. Kidney Int. 2004;65:798–810. doi: 10.1111/j.1523-1755.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- Zhou X.Q., Zeng X.N., Kong H., Sun X.L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci. Lett. 2008;447:31–36. doi: 10.1016/j.neulet.2008.09.064. [DOI] [PubMed] [Google Scholar]