Abstract
Carbon dioxide is a small, relatively inert, but highly volatile gas that not only gives beer its bubbles, but that also acts as one of the primary driving forces of anthropogenic climate change. While beer brewers experiment with the effects of CO2 on flavor and climate scientists are concerned with global changes to ambient CO2 levels that take place over the course of decades, many animal species are keenly aware of changes in CO2 concentration that occur much more rapidly and on a much more local scale. Although imperceptible to us, these small changes in CO2 concentration can indicate imminent danger, signal overcrowding, and point the way to food. Here I review several of these CO2-evoked behaviors and compare the systems insects, nematodes, and vertebrates use to detect environmental CO2.
Keywords: behavior, carbon dioxide, CO2, olfaction
CO2-EVOKED BEHAVIORS
Carbon dioxide is a byproduct of cellular respiration, which means animals constantly release it into the environment as waste. Plants, on the other hand, take up CO2 from the environment and fix its carbon atoms as carbohydrates via photosynthesis, acting as CO2 sinks. Thus, a given CO2 concentration encountered in the environment can have vastly different behavioral relevance to animal species depending on their ecological niche and location.
Social insects like bees, ants, and termites encounter ambient CO2 concentrations far above the atmospheric concentration of 0.038% (380 ppm) because their societies consist of many individuals living together in an enclosed space where CO2 can accumulate. CO2 concentrations inside bee hives have been measured at over 4% (40,000 ppm) (Seeley, 1974) and levels in termite nests range between 0.3–15% (3,000–150,000 ppm) (Ziesmann, 1996). Ants take advantage of this effect when they are outside their nests and use CO2 seeping from the nest entrance to find their way home (Buehlmann et al., 2012). Inside the nest, however, the same stimulus means something very different. Elevated CO2 levels can suffocate the nest’s inhabitants and, in the case of the fungus-farming leaf cutter ants, threaten the food supply. Thus, to prevent CO2 levels from getting dangerously high, social insects have evolved sensory equipment to track ambient CO2 levels and behaviors that prevent CO2 accumulation in their nests. For both honeybees and bumblebees, rising CO2 levels recruit worker bees to the entrances and exits of the hive to initiate a wing-fanning response that pushes fresh air through the hive until CO2 levels return to normal (Seeley, 1974; Weidenmüller et al., 2002). This behavior is not evoked via simple oxygen depletion in the hive and the number of fanning bees correlates directly with the CO2 concentration (Seeley, 1974). Ants and termites also detect ambient CO2 levels in their nests, but because most are wingless, they cannot rely on a fanning response like bees. Instead, ants and termites must modify their nests by opening or closing entrances and exits to optimize ventilation (Kleineidam and Roces, 2000; Ziesmann, 1996).
Unlike the social insects, which are particularly concerned with the ambient CO2 level, many solitary insects living in open environments pay special attention to gradients of CO2 in the form of filamentous plumes arising from individual point sources. Although CO2 is highly diffusible, the CO2 plumes arising from a sleeping human remain intact long enough to activate upwind search behaviors in mosquitoes from several meters away (Gillies, 1980). Nearly every medically relevant blood-feeding insect that transmits a disease-causing pathogen to humans detects and follows CO2 gradients as part of its host-seeking behavior. In addition to the malaria mosquito Anopheles gambiae and the dengue/yellow fever mosquito Aedes aegypti, other CO2-loving hematophagous Diptera include tsetse flies (Voskamp et al., 1999) (sleeping sickness), black flies (Fallis and Raybould, 1975) (river blindness and filariasis), and sandflies (Pinto et al., 2001) (leishmaniasis). The reduviid bug Triatoma infestans, which transmits the trypanosome that causes Chagas’ disease, also orients upwind to pulses of CO2 (Barrozo and Lazzari, 2006). Even ticks (Steullet and Guerin, 1992) (Lyme disease) and fleas (Benton and Lee, 1965) (bubonic plague), which seem to have independently evolved blood-feeding behavior, are attracted to the CO2 in our breath. Since CO2 is such a ubiquitous respiratory waste product, however, a whiff of CO2 alone is often not enough. Living, breathing humans also provide visual targets, produce thermal gradients, and release hundreds of compounds from the skin and in the breath that all contribute to a multimodal sensory stimulus that is species-specific and irresistible to hungry female mosquitoes and other biting insects (Gibson and Torr, 1999).
One of the most sensitive CO2 detection systems yet studied belongs to a moth. Using a series of pressure modulations to mimic the effect of minute changes in CO2 concentration, Gert Stange estimated that the labial palp organ of the moth Heliothis armigera may be able to detect deviations from the ambient CO2 concentration as low as 0.5 parts per million (0.00005%) (Stange, 1992). The behavioral relevance of this information to moths depends largely on the ecology of the species. The hawkmoth Manduca sexta uses elevations in CO2 to guide its choice of meals because the freshest blossoms release more CO2 and provide better nectar rewards than older flowers (Thom et al., 2004). Another moth, Cactoblastis cactorum, looks for decreases in CO2 to guide its feeding on the Opuntia cactus, because Opuntia opens its stomata at night to take up CO2, acting as a CO2 sink (Stange et al., 1995).
Rather than detecting CO2 as an attractant directing host-seeking behaviors, adult Drosophila release CO2 as a component of a highly aversive stress pheromone (Suh et al., 2004). Drosophila produce this stress odorant (dSO) in response to electric shock or violent shaking and presumably also to other more natural stressful stimuli in the wild. This novel use of CO2 as a stress pheromone seems at odds with the favorite foods of Drosophila. Rotting fruits are covered with yeast and other microbes fermenting sugars in the fruit and producing CO2 as a byproduct. As a behavioral aversion to one’s own favored food source would be strongly selected against, the Drosophila aversion to CO2 is probably contextual like that of ants inside and outside their nests. In support of this, Turner and Ray have identified specific odorants present in ripening fruit that modify the spiking of CO2 neurons and block the behavioral avoidance of CO2 (Turner and Ray, 2009).
Insects are not the only tiny invertebrates paying attention to small environmental gradients of CO2. CO2-evoked behaviors have also been identified in nematodes. Like in flies and ants, these behaviors are also contextual, as well-fed adult nematode worms (C. elegans) avoid 1% (10,000 ppm) CO2, whereas starved worms do not (Bretscher et al., 2008; Hallem and Sternberg, 2008). This avoidance behavior may help the nematodes escape soil environments that are becoming inhospitable. Even with such a simple nervous system, nematodes need a way to judge whether staying in a less hospitable environment that definitely has food is better than moving to a new environment that may not. Interestingly, CO2-evoked behaviors in worms are species-specific. In some parasitic nematode species, infective juveniles are attracted to CO2 instead of being repelled by it, presumably because it is released by their insect hosts and can be used to direct host-seeking behavior. Despite species-specific differences in sensitivity and behavioral relevance of CO2, all of the nematode species studied thus far seem to use the same set of sensory neurons and the same receptors to detect CO2 (Dillman et al., 2012; Hallem et al., 2011a).
OLFACTORY CO2 DETECTION MECHANISMS
The first hints at a molecular mechanism for the detection of environmental CO2 came from a rather unlikely place. Although the human olfactory system is insensitive to CO2, we do enjoy the taste of carbonated beverages. Mountaineers taking the carbonic anhydrase inhibitor acetazolamide to minimize the symptoms of altitude sickness often complain that the drug ruins the taste of their celebratory drinks upon reaching the summit (Graber and Kelleher, 1988). In more controlled tests, when acetazolamide was applied to half of their tongue, human volunteers consistently reported that the untreated side had a stronger sensation of carbonation (Dessirier et al., 2000).
Carbonic anhydrases are enzymes that catalyze the hydration of water to form carbonic acid, which rapidly dissociates in solution to protons and bicarbonate. These enzymes are among the most catalytically efficient yet discovered, and are known to play important roles in pH maintenance in many plant and animal systems (Tashian, 1989). The fact that a carbonic anhydrase inhibitor alters the taste of carbonation indicates that we are not tasting CO2 at all, but one of its metabolites: protons or bicarbonate.
Humans cannot smell low concentrations of CO2, but high levels of CO2 (> 30%, 300,000 ppm) can activate trigeminal nociceptors to produce a burning sensation in our mucous membranes (Bensafi et al., 2008). Wang et al. (2010) recently reported that the CO2-evoked responses of trigeminal sensory neurons of mutant mice lacking the TRP channel TRPA1 are much lower than those of wild-type mice. TRPA1-expressing HEK-293 cells, unlike controls, generate calcium responses to CO2 and intracellular acidification. This suggests that CO2 diffuses into the mucosal nociceptors and is converted to carbonic acid by an intracellular carbonic anhydrase. Then, the protons produced by dissociation of the carbonic acid activate the proton-sensitive TRPA1 channel to generate excitatory changes in membrane voltage. The action of carbonic anhydrase seems to be one of the few common themes running throughout the different systems animals have evolved to detect environmental CO2.
In 1967, the Swedish scientist Holger Hansson described a histochemical staining technique using cobalt sulfate that deposits a black precipitate on sites of carbonic anhydrase (CA) enzymatic activity (Hansson, 1967). In 1984, Brown et al. used a modification of this technique to identify a population of ciliated CA-expressing neurons in the olfactory epithelia of rats. It took several more years, however, to identify these neurons as CO2 sensors and to discover which of the many vertebrate CA isoforms they express.
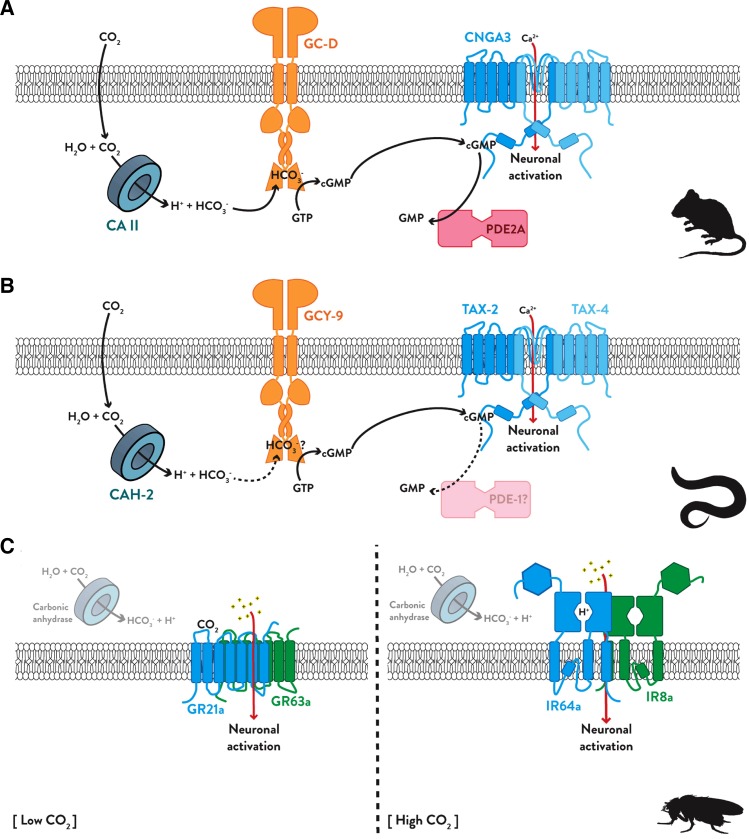
The elucidation of the signal transduction pathway underlying olfactory CO2 perception in vertebrates started in 1995 with the discovery of a population of cells in the rat olfactory epithelium that express a novel receptor guanylate cyclase gene called GC-D (Fülle et al., 1995). These neurons, which project to a group of specialized glomeruli on the caudal aspect of the olfactory bulb called the necklace glomeruli, express components of a cGMP-based signaling cascade instead of the typical olfactory cAMP-dependent cascade components: the rGC GC-D instead of the adenylyl cyclase ACIII (Fülle et al., 1995), the cGMP-sensitive phosphodiesterase PDE2A instead of the cAMP-sensitive PDE1C2 (Juilfs et al., 1997), and the cGMP-sensitive cyclic nucleotide gated ion channel CNGA3 (Han and Luo, 2010; Meyer et al., 2000). In 2007, Hu et al. (2007) added the final piece to the puzzle by confirming that these neurons innervating the necklace glomeruli respond to CO2 at near atmospheric levels and their response requires both GC-D and the intra-cellular carbonic anhydrase CAII. When GC-D is expressed in a cell culture system, its cyclase activity is stimulated by bicarbonate (Guo et al., 2009; Sun et al., 2009), pointing to GC-D as the actual receptor underlying dose-dependent CO2 olfactory responses. This may also explain why humans are unable to smell environmental CO2, as it was recently discovered that the crucial GC-D gene has become a pseudogene in primate lineages (Young et al., 2007). The mechanism underlying olfactory detection of CO2 by vertebrates is illustrated in Fig. 1A.
Fig. 1.
(A) In the mouse system, CO2 diffuses into the CO2 receptor neurons. Its hydration is catalyzed by the carbonic anhydrase CAII to form carbonic acid, which immediately dissociates to form protons and bicarbonate. The bicarbonate activates the receptor guanylate cyclase GC-D, which converts bound GTP to cGMP. The cGMP then binds to the cyclic nucleotide gated channel CNGA3 and causes it to open and permit the entry of calcium ions that initiate action potentials that travel to the necklace glomeruli in the olfactory bulb. The response to CO2 is then terminated when PDE2A converts the cGMP into GMP. (B) Nematodes employ a remarkably similar system for CO2 detection. The carbonic anhydrase CAH-2 produces the bicarbonate that is detected by GCY-9. GCY-9 produces the cGMP that activates the TAX-2/TAX-4 CNG channel. The role of a PDE in terminating the nematode response to CO2 has not yet been confirmed. (C) In fruit flies, a role for carbonic anhydrase in CO2 detection is likely but not yet confirmed. In low CO2, the GR21a and GR63a gustatory receptors are required, but in high CO2, the ionotropic receptors IR64a and IR8a work together to detect a drop in sensory lymph pH.
Although they are only distantly related to rodents, the model organism Caenorhabditis elegans was recently found to employ a very similar system to detect gradients of CO2 in the soil. In fact, several neurons in nematodes including the AFD and ASE neurons can respond to CO2, but the ciliated BAG neurons seem to be the most sensitive and important for behavioral responses to CO2 (Bretscher et al., 2011). In the BAG neurons, responses to CO2 require expression of the CNG channel subunits TAX-2 and TAX-4 (Bretscher et al., 2008; Hallem and Sternberg, 2008) and the receptor guanylate cyclase GCY-9, which is surprisingly dissimilar to the vertebrate GC-D (Hallem et al., 2011b). Since the BAG neurons have also been found to express the carbonic anhydrase CAH-2 (Bretscher et al., 2011) and the cGMP-sensitive phosphodiesterase PDE-1 (Hallem et al., 2011b), nematodes seem to possess all the major players of the rodent CO2 detection pathway (Fig. 1B). Still, it is not yet clear whether GCY-9 is sensitive to molecular CO2 or one of its metabolites (likely bicarbonate). It also still remains to be determined whether CAH-2 or PDE-1 are actually required for CO2 detection in the BAG neurons and whether the other CO2-responsive neurons use the same mechanism.
In addition to the mechanistic similarities in CO2 detection in nematodes and rodents, the CO2 neurons of both species are multimodal. Not only do the nematode BAG neurons respond to CO2, they are also sensitive to oxygen and contribute to oxygen-evoked behaviors. Decreases in environmental oxygen activate the soluble guanylate cyclases GCY-31 and GCY-33, which then produce cGMP to open the same TAX-2/TAX-4 CNG channel (Zimmer et al., 2009). This means that increases in CO2 and decreases in O2 cause a similar increase in BAG neuron firing, but since increases in CO2 and decreases in O2 both indicate that a worm’s current habitat is becoming less desirable, detecting both stimuli with the same multimodal neurons makes a certain amount of sense. In mice, the CO2-sensitive GC-D neurons also respond to urinary peptides that are thought to play a role in fluid and salt balance (Leinders-Zufall et al., 2007). But the GC-D neurons respond even more sensitively to CS2, which is a component of mouse breath that has been implicated in the social transfer of acquired food preferences from experienced mice to naïve mice (Munger et al., 2010). Hopefully, further studies will clarify how the rodent brain can make sense of the output of the GC-D neurons as they respond to such seemingly disparate stimuli.
Despite lying somewhere between nematodes and rodents in terms of organismal complexity, insects appear to employ very different CO2 detection systems. CO2-sensitive neurons have been identified in many insects and studied both electrophysiologically and ultra-structurally (Stange and Stowe, 1999), but the underlying receptors responsible for detecting CO2 have only been identified recently in the model insect Drosophila melanogaster (Jones et al., 2007; Kwon et al., 2007). Unlike mosquitoes, which keep their CO2 neurons in structures above the mouthparts called maxillary palps (Omer and Gillies, 1971), Drosophila CO2 neurons are located in the antennae (de Bruyne et al., 2001). Suh et al. (2004) used calcium imaging in the fly brain to identify a single antennal lobe glomerulus that responds to small increases in CO2. This glomerulus, named the V glomerulus because of its ventral-most position, was earlier identified as being innervated by neurons expressing the gustatory receptor Gr21a (Scott et al., 2001). Suh et al. (2004) were able to confirm the identity of the CO2 neurons by showing that although wild-type flies innately avoid even small increases of CO2 in a t-maze, flies with genetically silenced Gr21a neurons fail to detect and avoid CO2.
Soon, a second gustatory receptor, Gr63a, was found to be co-expressed with Gr21a in the CO2 neurons. Gr63a null mutant flies fail to respond to elevated CO2 and ectopic expression of both Gr21a and Gr63a together, but not either one alone, confers CO2-sensitivity on non-CO2 neurons (Jones et al., 2007). The malaria mosquito Anopheles gambiae has clear homologues of Gr21a and Gr63a, which are co-expressed in the CO2 neurons of its maxillary palps (Jones et al., 2007) and which function as CO2 receptors when ectopically expressed in Drosophila (Lu et al., 2007). Phylogenetic analysis of the insect gustatory receptors reveals that Gr21a and Gr63a are more related to each other than any of the other Grs and that mosquitoes, moths, and beetles actually have three CO2 receptor genes (i.e., Gr1, Gr2, and Gr3) (Robertson and Kent, 2009). Thus, the primary olfactory CO2 receptor in insects is a heteromeric complex of unknown stoichiometry consisting of members of a highly conserved gustatory receptor subfamily (Fig. 1C).
While most insect gustatory receptors are expressed in taste organs (i.e., the mouth parts, forelegs, and wing margins) and olfactory receptors are expressed in olfactory organs (i.e., the antennae and maxillary palps), the fact that the insect CO2 receptors are gustatory receptors expressed in olfactory organs begs the question of their true ligand. Is it the volatile CO2 or a more soluble CO2 metabolite like bicarbonate, which is the ligand for the CO2-responsive guanylate cyclases in nematodes and rodents? If the GR21a/GR63a receptor responds to bicarbonate rather than CO2 itself, it would suggest the involvement of a carbonic anhydrase like the ones required in the nematode and vertebrate systems.
According to early electrophysiological experiments, treatment with acetazolamide dramatically reduces the response of honeybee CO2 neurons (Stange, 1974), strongly suggesting a role for carbonic anhydrase in the olfactory CO2 detection mechanism of at least some insects. The mechanism of CO2 detection in bees, however, remains a mystery because the honeybee genome does not contain Gr21a or Gr63a orthologues (Robertson and Kent, 2009). Although bees are sensitive to a wide range of CO2 concentrations (Stange and Diesendorf, 1973), it is possible that they instead employ variants of a second class of receptors recently implicated in the detection of high concentrations of CO2 by the Drosophila antenna.
Ai et al. found that flies with silenced Gr63a neurons fail to avoid low concentrations of CO2, which is consistent with previous reports, but that they are still capable of avoiding environments with CO2 concentrations above 5% (50,000 ppm). In addition to activating the V glomerulus, which is sensitive to much lower concentrations of CO2, odor streams containing high concentrations of CO2 also activate the more central DC4 glomerulus. The sensory neurons that innervate DC4 express a pair of variant ionotropic glutamate receptors, Ir64a and Ir8a, which mediate a response to acid. The fact that CO2 activates these neurons strongly suggests the involvement of a carbonic anhydrase catalyzing the conversion of CO2 to carbonic acid (Fig. 1C). Relatively few remaining experiments should suffice to identify the true ligand of the GR21a/GR63a heteromeric receptor complex and to confirm a role for a carbonic anhydrase in insect CO2 detection, but many questions remain to be answered regarding the relevant signal transduction mechanisms.
Bioinformatic sequence analysis has revealed enough similarity between the insect gustatory receptors and the insect olfactory receptors to place both subfamilies together in a larger superfamily of insect chemoreceptors (Robertson et al., 2003). Since most family members are predicted to have seven transmembrane domains, the insect chemoreceptors were long assumed to be canonical GPCRs like the vertebrate odorant receptors. Surprisingly, though, both the insect gustatory and olfactory receptors act as ligand-gated cation channels when expressed in heterologous cell culture systems (Sato et al., 2008; 2011; Wicher et al., 2008). Perhaps even more surprisingly, several studies have reported conflicting results using different expression systems and experimental approaches and concluded that at least some members of the superfamily couple to G proteins (Kain et al., 2008; 2009; Wicher et al., 2008).
In the CO2-responsive Gr21a/Gr63a neurons specifically, both the knockdown and constitutive activation of Gαq reduce CO2 sensitivity (Yao and Carlson, 2010). The loss of function of another group of receptors, the TRPC class of Transient Receptor Potential cation channels, along with their canonical signaling partner phospholipase C (PLC21C) have also recently been found to reduce the sensitivity of the Gr21a neurons in Drosophila (Badsha et al., 2012). The most attractive hypothesis that attempts to reconcile all of these results is that all of the insect ORs and GRs, including the CO2 receptors, are ligandgated cation channels that are modulated by other signaling pathways, perhaps via phosphorylation or some other post-translational modifications (Nakagawa and Vosshall, 2009). Lastly, the full details of signal transduction via the newly discovered but evolutionarily ancient family of variant ionotropic glutamate receptors or IRs that also plays an important, but independent role in insect olfaction and CO2 detection have yet to be determined (Benton et al., 2009; Croset et al., 2010). Many further experiments will be necessary to fully understand all the relevant players in insect olfactory and gustatory signal transduction such that the entire pathway of environmental CO2 detection comes to light.
CONCLUSION
Although CO2 is a ubiquitous environmental stimulus, a given CO2 concentration can mean different things to different species in different ecological niches. For this reason, it is unsurprising that different animals respond to environmental CO2 with unique sets of behaviors. What is surprising is that animals as distantly related as nematodes and rodents can share such a similar CO2 detection mechanism, while evolution has clearly put forth multiple unique solutions to the problem of CO2 detection in insects. Once the molecular details of all the signal transduction mechanisms responsible for CO2 detection in more diverse species have been identified, it will be very interesting to trace the path evolution has taken in gaining and losing CO2 receptors. It is also my hope that a better understanding of CO2 detection mechanisms will not only help mountaineers avoid the “Champagne Blues”, but also reduce the transmission of infectious parasites by CO2-tracking, blood-feeding insects.
Acknowledgments
Work in my lab is supported by grants from the Korea National Research Foundation (2009-0090781 and 2010-0006217).
REFERENCES
- Ai M., Min S., Grosjean Y., Leblanc C., Bell R., Benton R., Suh G.S.B. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badsha F., Kain P., Prabhakar S., Sundaram S., Padinjat R., Rodrigues V., Hasan G. Mutants in Drosophila TRPC channels reduce olfactory sensitivity to carbon dioxide. PLoS One. 2012;7:e49848. doi: 10.1371/journal.pone.0049848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrozo R.B., Lazzari C.R. Orientation response of haematophagous bugs to CO2: the effect of the temporal structure of the stimulus. J. Comp. Phys. A. 2006;192:827–831. doi: 10.1007/s00359-006-0120-y. [DOI] [PubMed] [Google Scholar]
- Bensafi M., Iannilli E., Gerber J., Hummel T. Neural coding of stimulus concentration in the human olfactory and intranasal trigeminal systems. Neuroscience. 2008;154:832–838. doi: 10.1016/j.neuroscience.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Benton A.H., Lee S.Y. Sensory reactions of Siphonaptera in relation to host-finding. Am. Midland Nat. 1965:119–125. [Google Scholar]
- Benton R., Vannice K.S., Gomez-Diaz C., Vosshall L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A.J., Busch K.E., de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A.J., Kodama-Namba E., Busch K.E., Murphy R.J., Soltesz Z., Laurent P., de Bono M. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69:1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehlmann C., Hansson B.S., Knaden M. Path integration controls nest-plume following in desert ants. Curr. Biol. 2012;22:645–649. doi: 10.1016/j.cub.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Croset V., Rytz R., Cummins S.F., Budd A., Brawand D., Kaessmann H., Gibson T.J., Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M., Foster K., Carlson J.R. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Dessirier J.M., Simons C.T., Carstens M.I., O’Mahony M., Carstens E. Psychophysical and neurobiological evidence that the oral sensation elicited by carbonated water is of chemogenic origin. Chem. Senses. 2000;25:277–284. doi: 10.1093/chemse/25.3.277. [DOI] [PubMed] [Google Scholar]
- Dillman A.R., Guillermin M.L., Lee J.H., Kim B., Sternberg P.W., Hallem E.A. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc. Natl. Acad. Sci. USA. 2012;109:E2324–E2333. doi: 10.1073/pnas.1211436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallis A.M., Raybould J.N. Response of two African simuliids to silhouettes and carbon dioxide. J. Med. Ent. 1975;12:349–351. doi: 10.1093/jmedent/12.3.349. [DOI] [PubMed] [Google Scholar]
- Fülle H.J., Vassar R., Foster D.C., Yang R.B., Axel R., Garbers D.L. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc. Natl. Acad. Sci. USA. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G., Torr S.J. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Ent. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Gillies M.T. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull. Entomol. Res. 1980;70:525–532. [Google Scholar]
- Graber M., Kelleher S. Side effects of acetazolamide: the champagne blues. Am. J. Med. 1988;84:979–980. doi: 10.1016/0002-9343(88)90091-5. [DOI] [PubMed] [Google Scholar]
- Guo D., Zhang J.J., Huang X.-Y. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Sternberg P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Dillman A.R., Hong A.V., Zhang Y., Yano J.M., DeMarco S.F., Sternberg P.W. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011a;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Spencer W.C., McWhirter R.D., Zeller G., Henz S.R., Rätsch G., Miller D.M., Horvitz H.R., Sternberg P.W., Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011b;108:254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Luo M. Loss of CO2 sensing by the olfactory system of CNGA3 knockout mice. Curr. Zool. 2010;56:793–799. [Google Scholar]
- Hansson H.P. Histochemical demonstration of carbonic anhydrase activity. Histochemistry. 1967;11:112–128. doi: 10.1007/BF00571716. [DOI] [PubMed] [Google Scholar]
- Hu J., Zhong C., Ding C., Chi Q., Walz A., Mombaerts P., Matsunami H., Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Jones W.D., Volkan P.C., Kadow I.G., Vosshall L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Juilfs D.M., Fülle H.J., Zhao A.Z., Houslay M.D., Garbers D.L., Beavo J.A. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc. Natl. Acad. Sci. USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain P., Chakraborty T.S., Sundaram S., Siddiqi O., Rodrigues V., Hasan G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain P., Chandrashekaran S., Rodrigues V., Hasan G. Drosophila mutants in phospholipid signaling have reduced olfactory responses as adults and larvae. J. Neurogenet. 2009;23:303–312. doi: 10.1080/01677060802372494. [DOI] [PubMed] [Google Scholar]
- Kleineidam C., Roces F. Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insectes Soc. 2000;47:241–248. [Google Scholar]
- Kwon J.Y., Dahanukar A., Weiss L.A., Carlson J.R. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T., Cockerham R.E., Michalakis S., Biel M., Garbers D.L., Reed R.R., Zufall F., Munger S.D. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc. Natl. Acad. Sci. USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Qiu Y.T., Wang G., Kwon J.Y., Rutzler M., Kwon H.-W., Pitts R.J., van Loon J., Takken W., Carlson J.R., et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.R., Angele A., Kremmer E., Kaupp U.B., Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc. Natl. Acad. Sci. USA. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger S.D., Leinders-Zufall T., McDougall L.M., Cockerham R.E., Schmid A., Wandernoth P., Wennemuth G., Biel M., Zufall F., Kelliher K.R. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr. Biol. 2010;20:1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Vosshall L.B. Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr. Opin. Neurobiol. 2009;19:284–292. doi: 10.1016/j.conb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer S.M., Gillies M.T. Loss of response to carbon dioxide in palpectomized female mosquitoes. Entomol. Exp. Appl. 1971;14:251–252. [Google Scholar]
- Pinto M.C., Campbell-Lendrum D.H., Lozovei A.L., Teodoro U., Davies C.R. Phlebotomine sandfly responses to carbon dioxide and human odour in the field. Med. Vet. Ent. 2001;15:132–139. doi: 10.1046/j.1365-2915.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Robertson H.M., Kent L.B. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J. Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H.M., Warr C., Carlson J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Pellegrino M., Nakagawa T., Nakagawa T., Vosshall L.B., Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Sato K., Tanaka K., Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl. Acad. Sci. USA. 2011;108:11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K., Brady R., Cravchik A., Morozov P., Rzhetsky A., Zuker C.S., Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. J. Insect Physiol. 1974;20:2301–2305. doi: 10.1016/0022-1910(74)90052-3. [DOI] [PubMed] [Google Scholar]
- Stange G. The influence of a carbonic anhydrase inhibitor on the function of the honeybee antennal CO2-receptors. J. Comp. Phys. A. 1974;91:147–159. [Google Scholar]
- Stange G. High resolution measurement of atmospheric carbon dioxide concentration changes by the labial palp organ of the moth Heliothis armigera (Lepidoptera: Noctuidae) J. Comp. Phys. A. 1992;171:317–324. [Google Scholar]
- Stange G., Diesendorf M. The response of the honeybee antennal CO2-receptors to N2O and Xe. J. Comp. Phys. A. 1973;86:139–158. [Google Scholar]
- Stange G., Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc. Res. Tech. 1999;47:416–427. doi: 10.1002/(SICI)1097-0029(19991215)47:6<416::AID-JEMT5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Stange G., Monro J., Stowe S., Osmond C. The CO2 sense of the moth Cactoblastis cactorum and its probable role in the biological control of the CAM plant Opuntia stricta. Oecologia. 1995;102:341–352. doi: 10.1007/BF00329801. [DOI] [PubMed] [Google Scholar]
- Steullet P., Guerin P.M. Perception of breath components by the tropical bont tick, Amblyomma variegatum Fabricius (Ixodidae). I. CO2-excited and CO2-inhibited receptors. J. Comp. Phys. A. 1992;170:665–676. doi: 10.1007/BF00198976. [DOI] [PubMed] [Google Scholar]
- Suh G.S.B., Wong A.M., Hergarden A.C., Wang J.W., Simon A.F., Benzer S., Axel R., Anderson D.J. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sun L., Wang H., Hu J., Han J., Matsunami H., Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc. Natl. Acad. Sci. USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian R.E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989;10:186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Thom C., Guerenstein P.G., Mechaber W.L., Hildebrand J.G. Floral CO2 reveals flower profitability to moths. J. Chem. Ecol. 2004;30:1285–1288. doi: 10.1023/b:joec.0000030298.77377.7d. [DOI] [PubMed] [Google Scholar]
- Turner S.L., Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Voskamp K.E., Everaarts E., Den otter C.J. Olfactory responses to attractants and repellents in tsetse. Med. Vet. Ent. 1999;13:386–392. doi: 10.1046/j.1365-2915.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Chang R.B., Liman E.R. TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 2010;30:12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmüller A., Kleineidam C., Tautz J. Collective control of nest climate parameters in bumblebee colonies. Anim. Behav. 2002;63:1065–1071. [Google Scholar]
- Wicher D., Schäfer R., Bauernfeind R., Stensmyr M.C., Heller R., Heinemann S.H., Hansson B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Yao C.A., Carlson J.R. Role of G-proteins in odorsensing and CO2-sensing neurons in Drosophila. J. Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.M., Waters H., Dong C., Fülle H.J., Liman E.R. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS One. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziesmann J. The physiology of an olfactory sensillum of the termite Schedorhinotermes lamanianus: carbon dioxide as a modulator of olfactory sensitivity. J. Comp. Phys. A. 1996;179:123–133. [Google Scholar]
- Zimmer M., Gray J.M., Pokala N., Chang A.J., Karow D.S., Marletta M.A., Hudson M.L., Morton D.B., Chronis N., Bargmann C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]