Abstract
Fuel ethanol production is far more costly to produce than fossil fuels. There are a number of approaches to cost-effective fuel ethanol production from biomass. We characterized stress response of thermotolerant Saccharomyces cerevisiae KNU5377 during glucose-based batch fermentation at high temperature (40°C). S. cerevisiae KNU5377 (KNU5377) transcription factors (Hsf1, Msn2/4, and Yap1), metabolic enzymes (hexokinase, glyceraldehyde-3-phosphate dehydrogenase, glucose-6-phosphate dehydrogenase, isocitrate dehydrogenase, and alcohol dehydrogenase), antioxidant enzymes (thioredoxin 3, thioredoxin reductase, and porin), and molecular chaperones and its cofactors (Hsp104, Hsp82, Hsp60, Hsp42, Hsp30, Hsp26, Cpr1, Sti1, and Zpr1) are upregulated during fermentation, in comparison to S. cerevisiae S288C (S288C). Expression of glyceraldehyde-3-phosphate dehydrogenase increased significantly in KNU5377 cells. In addition, cellular hydroperoxide and protein oxidation, particularly lipid peroxidation of triosephosphate isomerase, was lower in KNU5377 than in S288C. Thus, KNU5377 activates various cell rescue proteins through transcription activators, improving tolerance and increasing alcohol yield by rapidly responding to fermentation stress through redox homeostasis and proteostasis.
Keywords: cell rescue protein, high-temperature fermentation, redox state, Saccharomyces cerevisiae KNU5377, stress response
INTRODUCTION
Saccharomyces cerevisiae is an important industrial microorganism. It has a long history of use in bakeries to raise dough and in the production of alcoholic beverages; it is used to produce pharmaceuticals such as insulin and for fermenting sugars from rice, wheat, barley, corn, and grape juice (Pizarro et al., 2008). In particular, it has been used as an agent of bioethanol production as well as for wine, bread, beer, and sake fermentation (Belloch et al., 2008). At the beginning of fermentation, yeast cells are subjected to the osmotic and oxidative stress of high sugar concentrations and resolved oxygen; as fermentation progresses, other stresses such as high concentrations of ethanol and nutrient depletion become relevant (Belloch et al., 2008).
One of the most common stresses encountered during fermentation is the increased ethanol concentration. The high toxicity of endogenously produced ethanol reduces cell viability, growth rate, and fermentation rate. Many mechanisms have been developed to help organisms withstand and/or prevent ethanol-induced damage during fermentation, including cross-stress protection; yeast hybrids based on enological characterization (Belloch et al., 2008); membrane remodeling via changes in membrane (palmitoleic acid, oleic acid, and ergosterol) and cell wall composition (fatty acid, lipid, and isoprenoid metabolism); accumulation of amino acids (proline and tryptophan) and storage solutes (trehalose and glycogen) (Zhao and Bai, 2009); expression of molecular chaperones; transcriptional activation of V-ATPase and peroxisomal functions; enhancement of NADPH regeneration and redox balance (Cebollero et al., 2007; Ding et al., 2009; Orozco et al., 2012); genetic improvement through sexual cycle, parasexual hybridization and genetic engineering; and transcriptome remodeling of transcription factors, stress-related genes, and genes involved in signal transduction (Gibson et al., 2007; Ma and Liu, 2010a; Stanley et al., 2010). However, this approach has the intrinsic limitation that yeast adapts to different metabolic environments such as a high concentration of ethanol during fermentation.
Fuel bioethanol production is being scaled up rapidly world-wide due to the substantial rise in oil prices and concerns regarding the depletion of available oil and climate change. Many researchers have claimed that efforts should now be directed toward identifying thermostable, ethanol-tolerant strains with a broad spectrum of substrates and the ability to produce substantial amounts of ethanol (Abdel-Banat et al., 2010). Ethanol production at high temperature has received much attention because fermentation processes conducted at elevated temperatures could significantly reduce cooling costs in summer. Other advantages include more-efficient saccharification and fermentation, a continuous shift from fermentation to distillation, reduced risk of contamination, and suitability for use in tropical countries (Nonklang et al., 2008). However, temperatures routinely used for yeast growth are 25°C to 30°C (Belloch et al., 2008). Temperature shifts affect a variety of cellular processes including protein translation rate, membrane fluidity, RNA stability, rescue enzyme activity, impacting cell growth (Lee et al., 2012) and eventually decreasing fermentation yield and by-product quality (Salvado et al., 2011). Although the use of thermophilic bacteria in ethanol fermentation has been reported (Wiegel et al., 1980), the yeast S. cerevisiae is the most widely used microorganism in fuel ethanol production. Several cost reductions could be achieved if fermentation could be performed with thermotolerant yeasts at higher temperatures (Abdel-Banat et al., 2010). In this regard, thermotolerant, ethanol-resistant S. cerevisiae KNU5377 isolated in spoilage is a good candidate for fuel ethanol production. S. cerevisiae KNU5377 grows at 50°C, and produces ethanol at 40°C (Kim et al., 2011). Despite these advantages, a systematic analysis of the stress response of S. cerevisiae KNU5377 during alcoholic fermentation has not been reported, making it difficult to determine which yeast strain is best for any given conditions.
We characterized the molecular properties of S. cerevisiae KNU5377 during high-temperature fermentation because it has evolved to survive intensive, combinatorial stresses, and may be a source of thermotolerant yeast with a greater ability to adapt to the environmental hardship of industrial fermentation. We performed glucose-based batch fermentation to study ethanol production at high temperature (40°C). A wide range of genes including antioxidant systems, molecular chaperones, and energy-generating systems of S. cerevisiae KNU5377 were upregulated during fermentation at 40°C, conferring acquired tolerance to the environmental stresses of fermentation and improving fermentation capacity. Understanding the yeast stress response during fermentation will guide breeding of robust strains for efficient ethanol production, which is of particular importance to the production of fuel ethanol.
MATERIALS AND METHODS
Laboratory glucose-based batch fermentation
Yeast cells of S. cerevisiae S288C (parental wild-type strain in which many of the mutations have been derived; ATCC No. 204508) and S. cerevisiae KNU5377 (uncharacterized genotype; Patent No. KR 1020000024669) were grown for 20 h at 30°C in YPD (1% yeast extract, 2% peptone and 2% glucose) broth with shaking and then, fermented under aerobic fermentation conditions on a shaker (160 rpm) for 60 h at 40°C in YG medium containing 20% (w/v) glucose and 1% (w/v) yeast extract. The alcohol concentration was determined based on the percentage (v/v) of alcohol in the distillate after fermentation, measured using an alcohol hydrometer (REF 503; Korins, Korea). Residual total sugar concentrations were measured using a hand-held Refractometer N1 (Atago, Japan). Alcohol and residual glucose concentrations were obtained after removing cell debris by centrifugation (2, 000 rpm, 3 min). To measure cell survival during fermentation, cells were harvested at various time points (12, 24, 48, and 60 h) and serially diluted to 10−9 with YPD broth (1% yeast extract, 2% peptone, 2% glucose), after which 5 μl of the diluted solutions were loaded onto YPD agar plates, incubated for 3 days at 30°C, and then photographed. Optical density was measured at 600 nm at the indicated times during fermentation.
Two-dimensional gel electrophoresis and protein identification
Yeast cells were harvested at 24 h after the beginning of fermentation, washed 3 times with cold phosphate-buffered saline (PBS, pH 7.3 to pH 7.5; made from 10× tablets) (Invitrogen, USA) and resuspended in a lysis buffer containing 50 mM Tris-HCl, pH 7.5, 5% glycerol, 2% sodium dodecyl sulfate (SDS), 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and EDTA-free protease inhibitor cocktail (Roche, Germany) with an equal volume of glass beads (425–600 μm; Sigma, USA). After vigorously vortexing 5 times for 1 min each at 2-min intervals on ice, the protein extracts were cleared by centrifugation at 15, 000 rpm for 20 min at 4°C. To precipitate the protein, 2 volumes of cold acetone containing 0.1% β-mercaptoethanol (ME) were added; the mixtures were stored at −20°C overnight and then centrifuged at 15, 000 rpm for 30 min at 4°C. To wash the pellet, 2 volumes of ice-cold ethanol with 0.1% ME were added, mixed completely, and centrifuged at 15, 000 rpm for 10 min at 4°C. This step was repeated 3 times. The pellet was then vacuum-dried and resuspended in sample buffer containing 9.5 M urea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM Tris, 0.1 M DTT, and 0.2% Bio-Lyte (3–10; Bio-Rad, USA) at room temperature with shaking. The sample was centrifuged at 15, 000 rpm for 30 min, after which the cleared supernatant was carefully collected, and the protein concentration was measured using a modified Bradford assay (Kim et al., 2011). Next, 1 mg protein was loaded onto preparative gels and analyzed with pH 4–7 immobilized pH gradient (IPG) strips (17 cm, linear; Bio-Rad, USA) under the following focusing conditions: 250 V for 1 h, 250-10, 000 V for 6 h, and 90, 000 V-h at 10, 000 V. After isoelectric focusing (IEF), the gel strips were equilibrated and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 15% gels at 10 mA per gel for the first 1 h followed by 25 mA per gel, stained with Coomassie Brilliant Blue R-250 (CBB R-250; Sigma, USA), and then destained. Spots with significant changes were considered accumulated proteins. Overexpressed spots of interest were excised from the gel and subjected to in-gel digestion and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Kim et al., 2012). Finally, protein identification was conducted in MASCOT (http://www.matrixscience.com).
Western blot analysis
Crude protein extracts were prepared using glass beads. Cells were harvested at the indicated times during fermentation, then washed 3 times with cold PBS (pH 7.3 to pH 7.5; made from 10 tablets) (Invitrogen, USA) and resuspended in a lysis buffer containing 50 mM Tris-HCl, pH 7.5, 5% glycerol, 1% Triton X-100, 0. 1% d eoxycholic a cid, 1 mM DTT, 1 mM PMSF, and EDTA-free protease inhibitor cocktail (Roche, Germany) with an equal volume of glass beads (425–600 μm; Sigma, USA). After vigorously vortexing 5 times for 1 min each at 2-min intervals on ice, the protein extracts were cleared by centrifugation at 13, 000 rpm for 20 min at 4°C. Finally, protein concentrations were determined using a Pierce® BCA Protein Assay Kit (Thermo Scientific, USA). Protein extracts (25 μg) were loaded onto a 12% or 15% SDS-PAGE gel and separated at 50 V. The proteins were then transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA), which were incubated in blocking buffer containing 5% non-fat skim milk and 0.02% sodium azide in TBST (0.05% Tween-20, 10 mM Tris-HCl, pH 7.6; and 150 mM NaCl) for 1.5 h at room temperature, and then incubated overnight at 4°C with the primary antibodies diluted in blocking buffer. The primary antibodies were as follows: anti-Yap1, -Msn2, -Msn4, -tubulin (Tub), -isocitrate dehydrogenase (IDH), -malondialdehyde (MDA) (Santa Cruz Biotechnology, USA), anti-porin (Por) (Invitrogen, USA), anti-hexokinase (Hxk), -alcohol dehydrogenase (Adh), -aldehyde dehydrogenase (Ald) (Rockland Chemicals Inc., USA), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Ab Frontier, Korea), anti-glucose-6-phosphate dehydrogenase (G6PDH), -Zpr1, -dinitrophenyl (DNP) (Sigma, USA), anti-Hsp104, -Hsp60 (Stressgen, Belgium), anti-thioredoxin 2 (Trx2), -Trx3, -thioredoxin reductase (Trr), -thioredoxin peroxidase (Tsa1), -heat shock protein 90 (Hsp90 or Hsp82), -Ssa, -Ssb, -Hsp42, -Hsp30, -Hsp26, -cyclophilin isoform 1 (Cpr1), -Sti, and -Grp (Kim et al., 2011). The blots were washed 4 times for 40 min with TBST, then incubated with anti-rabbit, -rat, -goat, and -mouse secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology, USA) diluted with blocking buffer lacking 0.02% sodium azide for 1.5 h at room temperature. After washing with TBST, antibody binding was visualized with Enhanced Chemiluminescence Western blotting detection reagent (GE Healthcare, USA). Tubulin protein, a major cytoskeleton component, was used as a loading control.
Redox state analysis
To measure cytosolic reactive oxygen species (ROS) during fermentation, yeast cells were collected, washed twice with cold PBS, resuspended in a lysis buffer containing 20 mM HEPES, 1 mM PMSF, and EDTA-free protease inhibitor cocktail, and lysed using glass beads as described above. Cleared crude extracts (50 μg) were incubated for 20 min at 30°C with 50 μM 2′, 7′-dichlorofluorescein diacetate (DCFHDA; Invitrogen, USA). Fluorescence intensity was measured by spectrophotometry (excitation, 488 nm; emission, 525 nm). Protein oxidation was performed using the Protein Carbonyls Western Blot Detection kit (Cosmo Bio Co. Ltd., Japan) according to the manufacturer protocols.
Statistical analysis
All experiments were independently performed at least 3 times and results are expressed as the mean ± standard deviation (SD). The results of the spotting assay, growth kinetics, and fermentation ability are representative of at least 2 independent experiments carried out under identical conditions.
RESULTS
Estimation of alcohol yield and cell survival under batch fermentation conditions
Laboratory-scale fermentation using S. cerevisiae KNU5377 (KNU5377) and S. cerevisiae S288C (S288C) yeast cells was performed at 40°C under aerobic conditions. KNU5377 and S288C distinguishably differed in alcohol yield and residual glucose content at 40°C. During fermentation for 60 h at 40°C, the alcohol yield of KNU5377 was approximately 43% higher than that of S288C. The final alcohol concentration was approximately 9.2% and 5.2% after fermentation with KNU5377 and S288C, respectively. The residual glucose concentration was inversely proportional to the alcohol concentration during fermentation (Fig. 1A), and cell survival distinctly differed between KNU5377 and S288C during the same fermentation period. Specifically, the survival of KNU5377 was significantly during fermentation for 60 h in YG medium, although cell viability decreased with time (Fig. 1B). The optical density of KNU5377 and S288C cells followed a similar pattern. KNU5377 cells reached stationary phase with approximately A600 = 10.0 after the beginning of fermentation, while S288C cells had reached moderate log-phase (A600 = 2.0) (Fig. 1C). In addition, mid-log phase cells of KNU5377 were more tolerant than S288C to 12% ethanol exposure for 1 h with shaking (Fig. 1D). Therefore, KNU5377 cells enhance fermentation capacity, cell survival, and growth kinetics at high temperature; this is important for responding to the increased alcohol concentration produced by KNU5377 cells during batch fermentation.
Fig. 1.
Fermentation capacity and cell survival during high-temperature fermentation. (A) Alcohol concentration (AC) and residual glucose (RG). Circle, RG in S288C; square, RG in KNU5377; upward triangle, AC in S288C; downward triangle, AC in KNU5377. (B) Cell survival during fermentation. Cell survival was estimated 12, 24, 48, and 60 h after the beginning of fermentation. S, S288C; K, KNU5377. (C) Growth kinetics. Growth kinetics was observed by measuring optical density (OD) at 600 nm. Square, OD in S288C; circle, OD in KNU5377. (D) Transient stress response to ethanol. Mid-log phase yeast cells were exposed to 12% ethanol for 1 h, diluted serially, and loaded onto YPD agar plates. S, S288C; K, KNU5377
Expression of transcription factors and cell rescue proteins during high temperature fermentation
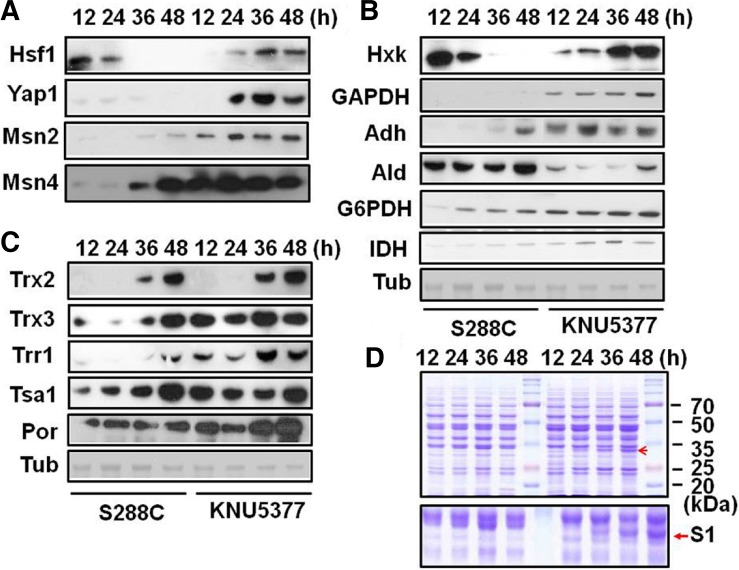
KNU5377 cells provided improved alcohol yield and cell survival during high-temperature fermentation in comparison to S288C cells under the same conditions. We introduced molecular techniques, including Western blotting and 2-D electrophoresis, to investigate the improved tolerance and alcohol yield of KNU5377 at high temperature. A range of proteins was upregulated in KNU5377 cells during high-temperature fermentation in comparison to S288C (Fig. 2). Identified proteins included (Table 1): adenylate kinase (spot 1), cytochrome c heme lyase (Cyc3p; spot 2), phosphoglycerate kinase (spots 3, 9, and 10), glyceraldehyde-3-phosphate dehydrogenase isoform 3 (Tdh3; spot 7), and translation elongation factor (spot 8). Three spots (4, 5, and 6) in KNU5377 were unidentified proteins. Changes in protein expression were also observed by Western blotting under the same fermentation conditions. Transcription factors including Hsf1, Msn2, Msn4, and Yap1 were time-dependently induced in KNU5377, but were inhibited or unchanged in S288C under the same conditions (except Msn4; Fig. 3A). Expression of metabolic enzymes including hexokinase (Hxk), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), alcohol dehydrogenase (Adh), glucose-6-phosphate dehydrogenase (G6PDH), and isocitrate dehydrogenase (IDH) was increased in KNU5377 cells (Fig. 3B). Aldehyde dehydrogenase (Ald) expression decreased with time in KNU5377 during fermentation (Fig. 3B). Also, time-dependent upregulation of antioxidant enzymes, including thioredoxin isoform 3 (Trx3), thioredoxin reductase (Trr1), and porin (Por) was observed in KNU5377 cells. However, expression of thioredoxin isoform 2 (Trx2) and thioredoxin peroxidase (Tsa1) was similar in KNU5377 and S288C (Fig. 3C). High expression of GAPDH (Tdh1, Tdh2, and Tdh3) in KNU5377 was identified by MALDI-TOF analysis following SDS-PAGE (S1) (Table 1); these proteins were not detected in S288C (Fig. 3D). Expression of a various molecular chaperones, heat shock proteins (HSPs), and cofactors was also increased in KNU5377 during fermentation. Overexpressed proteins included Hsp104, Hsp90 (or Hsp82), Hsp60, Hsp42, Hsp30, Hsp26, Cpr1, Sti1, and Zpr1. Although expression of the Hsp70 family (Ssa and Ssb) decreased with time in KNU5377 and S288C, the expression signal was lower in KNU5377 than in S288C. Expression of Grp (Grp94) increased time-dependently in KNU5377, but was lower than in S288C (Fig. 4). Thus, KNU5377 induces a range of cell rescue proteins through rapid, effective gene expression, enabling the strain to respond to environmental stresses generated during fermentation.
Fig. 2.
Two-dimensional gel electrophoresis. Crude protein extracts were prepared after 24 h fermentation. Solubilized protein (1 mg) was loaded on IPG strips (4–7), focused, equilibrated, run on 15% SDS-PAGE, and then stained with CBB R-250 dye. Overexpressed spots in KNU5377 were marked with arrows, and then numbered. pI, isoelectric point; MW, molecular weight.
Table 1.
Upregulated proteins identified in S. cerevisiae KNU5377 by MALDI-TOF MS analysis
| No. | Protein information | Score | Cov. (%) | pI | MW (kDa) |
|---|---|---|---|---|---|
| 1 | Structure of a mutant adenylate kinase | 2.41 | 55 | 6.2 | 24.02 |
| 2 | Cytochrome c heme lyase (Cyc3) | 2.39 | 27 | 5.3 | 30.29 |
| 3 | Phosphoglycerate kinse (Pgk1) | 2.37 | 76 | 5.4 | 13.86 |
| 7 | Glyceraldehyde-3-phosphate dehydrogenase (Tdh3) | 1.73 | 24 | 6.5 | 35.84 |
| 8 | Translation elongation factor | 1.75 | 30 | 5.2 | 22.38 |
| 9 | Phosphoglycerate kinse (Pgk1) | 2.13 | 64 | 5.4 | 13.86 |
| 10 | Phosphoglycerate kinse (Pgk1) | 1.99 | 49 | 5.4 | 13.86 |
| S1 | Glyceraldehyde-3-phosphate dehydrogenase (Tdh1) | 2.45 | 65 | 8.78 | 35.68 |
| S1 | Glyceraldehyde-3-phosphate dehydrogenase (Tdh2) | 2.44 | 65 | 8.32 | 35.84 |
| S1 | Glyceraldehyde-3-phosphate dehydrogenase (Tdh3) | 2.02 | 48 | 6.46 | 35.74 |
| S2 | Triosephosphate isomerase (Tpi1) | 1.90 | 31 | 5.7 | 26.65 |
Cov., coverage; pl, isoelectric point; MW, molecular weight
Fig. 3.
Expression of cell rescue proteins during fermentation. Proteins were prepared from yeast cells at 12, 24, 36, and 48 h. Western blotting was performed with primary antibodies. Expression of transcription factors (A), metabolic enzymes (B), and antioxidant enzymes (C). Tubulin (Tub) protein was used as a loading control. (D) SDS-PAGE (12%) analysis following fermentation. Red arrow indicates a single band overexpressed in KNU5377 cells. Upper panel, original image; lower panel, enlarged image.
Fig. 4.
Expression of heat shock proteins, molecular chaperones, and cofactors during fermentation. Protein expression was analyzed by Western blotting with primary antibodies. Tubulin (Tub) protein was used as a loading control.
Redox state analysis during high-temperature fermentation
Various proteins were upregulated during high-temperature fermentation of KNU5377. To examine whether these cell rescue proteins influence the redox state during fermentation, hydroperoxide levels, and protein and lipid oxidation were observed in KNU5377 and S288C. DCFHDA fluorescence measuring the oxidative conversion of DCFHDA to the highly fluorescent compound dichlorofluorescin (DCF) was lower in KNU5377 than in S288C, although signal intensity in both strains decreased over time (Fig. 5A). Protein oxidation was detected by protein carbonyls Western blot detection kit using anti-DNP antibody. As shown in Fig. 5B, protein carbonylation in S288C was higher than that in KNU5377 and increased over time. Loading calibration was performed by Ponceau staining (Fig. 5B). Lipid peroxidation was assessed by Western blotting with anti-MDA antibody following SDS-PAGE. A single band (S2) detected in both strains was identified as triosephosphate isomerase (Tpi1p) by MLADI-TOF spectroscopy (Table 1). Coverage, isoelectric point (pI), and molecular weight of Tpi1p were 31%, 5.7, and 26.65 kDa, respectively. Lipid oxidation of Tpi1p in S288C occurred from the beginning (12 h) of fermentation, but was observed in KNU5377 after 24 h (Fig. 5C). These results indicate KNU5377 improves redox homeostasis by minimizing oxidation of macromolecule such as lipids and proteins following the reduction of cellular hydroperoxide concentrations during fermentation (Fig. 6).
Fig. 5.
Redox state during fermentation. (A) Cellular hydroperoxide levels were measured by measuring DCFHDA fluorescence intensity by spectrophotometry. White bar, intensity in S288C; black bar, intensity in KNU5377. (B) Protein carbonylation was visualized by Western blotting with anti-DNP. Protein housekeeping was adjusted by Poceau staining (lower panel). (C) Lipid peroxidation was detected by Western blotting with anti-MDA (right panel) following SDS-PAGE (left panel). The band (grey arrow) corresponding to the signal was excised and identified by MALDI- TOF (right, lower panel).
Fig. 6.
Schematic diagram of a specific mechanism through proteins overexpressed in KNU5377 strain during high-temperature fermentation.
DISCUSSION
Ethanol production has mostly been adapted to S. cerevisiae strains. S. cerevisiae growth at 30–35°C is considered optimal for fuel ethanol fermentation. However, if fermentation could be performed at higher temperatures, for example within 40–50°C range, several cost reductions could be applied to large-scale fuel ethanol production (Abdel-Banat et al., 2010). S. cerevisiae KNU5377 is thermotolerant and tolerant of ROS-induced oxidative stress (Kim et al., 2011). We have demonstrated that KNU5377 has a very specific stress response mechanism during fermentation at high temperature (40°C). Under glucose fermentation, the stress response of KNU5377 was demonstrated by growth kinetics and viability. Alcohol yield and growth kinetics of KNU5377 were higher than in the reference strain, S288C (Figs. 1A and 1C), and was more tolerant of the increased alcohol concentrations produced by fermentation (Fig. 1B). In addition, KNU5377 activated a better stress response during transient exposure to a high concentration of ethanol (12%) for 1 h (Fig. 1D). These results show that KNU5377 is a thermo-, stress-tolerant yeast capable of high-temperature fermentation of glucose into ethanol.
To investigate whether the improved fermentation capacity at 40°C is due to a stress-responsive system, gene expression was analyzed by proteomic techniques. During glucose-based batch fermentation, KNU5377 upregulated transcription factors (Hsf1, Msn2/4, and Yap1), metabolic enzymes (Pgk1, Hxk, GAPDH, Adh, IDH, and G6PDH), antioxidant enzymes (Trx3, Trr1, and Cyc3), and elongation factors (Figs. 2 and 3, Table 1). However, Ald expression was downregulated under the same conditions. Expression of most proteins was time-dependent. Downregulation of antioxidant systems led to a number of oxygen-dependent phenotypes including oxygen sensitivity; slow growth; hypersensitivity to ROS-generating agents such as fermentation conditions, which are believed to accelerate aging; and auxotrophy for methionine and lysine (Tan et al., 2009). In addition, Hxk, Trx3, and Tsa1 mediate the ethanol stress response to ROS-induced abiotic stresses (Gasch et al., 2000; Tucker and Fields, 2004). As shown in S228C, repression of genes involved in the nonoxidative branch of the pentose phosphate pathway and glycolysis that are important for NADPH and ATP production, such as G6PDH, IDH, and Pgk1, is sensitive to exogenous stimuli and defective in adaptation to oxidative stress (Tan et al., 2009). However, KNU5377 increased porin (Por) expression during fermentation. Furthermore, mitochondrial function requires redox homeostasis, which is mediated primarily by the voltage-dependent anion channel (VDAC; porin pore). The VDAC releases superoxide anion from the mitochondria to the cytosol and increases cytosolic ROS (Han et al., 2003). The elevated expression of cell rescue systems makes it easy to modify neutralizing ROS or repair oxidative damage (Fig. 6), which is associated with intrinsic tolerance to environmental stresses produced during fermentation (heat shock, oxidative stress, and increased ethanol concentration).
In addition to metabolic and antioxidant enzymes, we observed time-dependent, increased expression of HSPs, molecular chaperones, and cofactors including Hsp104, Hsp90 (or Hsp82), Hsp60, Hsp42, Hsp30, Hsp26, Cpr1, Sti1, and Zpr1 in KNU5377 during fermentation. However, expression of the Hsp70 family (Ssa and SSb) and Grp decreased with time in KNU5377 (Fig. 4). Chaperones that participate broadly in protein refolding, such as Hsp90, Hsp104 and small HSPs (sHSPs) promote folding through cycles of substrate and cofactor binding (Hartl and Hayer-Hartl, 2002). Hsp104 acts with Hsp26 in protein refolding after stress-induced unfolding (Haslbeck et al., 2005). Hsp90 depends on its association with a variety of cochaperones [Hsp40 (or Hsp42) and cyclophilins], and cofactors (Mayr et al., 2000; Zhao et al., 2005). Hsp90 complex formation is mediated by an adaptor protein, Sti, and an essential ER chaperone, Grp (Grp94) (Chu et al., 2006), which functions as an interaction domain for Hsp90 (Wegele et al., 2003). Zpr1 is a zinc finger (ZnF) protein that translocates to the nucleus in response to growth stimuli and is a lethal partner with Cpr1p for PPIase activity. Zpr1 is essential for cell viability in diverse eukaryotic organisms including yeast and mice. Reduction of Zpr1 expression in mammalian cells by antisense or siRNA knockdown causes defects in transcription, prevents DNA synthesis, and results in an accumulation of cells in the G1 and G2 phases of the cell cycle (Ansari et al., 2002; Mishra et al., 2007). Although the chaperone network (63 chaperones) of physiological and genetic interactions has been well characterized under stress-free conditions through systematic biology (Gong et al., 2009), a comprehensive analysis of expression of chaperones and their cofactors (or substrates) has not yet been reported for high-temperature fermentation. Our findings indicate that a balance of chaperone machinery systems in KNU5377 facilitate protein (re)folding, thus improving proteostasis under fermentation.
Why does a higher stress tolerance via elevated induction of these cell rescue proteins occur in KNU5377 but not S288C? The difference seems to be caused by stress response factors. As shown in Fig. 3A, expression of the general and specific stress response factors Hsf1, Yap1, and Msn2/4 was higher in KNU5377 than in S288C. Under normal physiological conditions, glucose triggers the G protein-coupled receptor system to activate adenylate cyclase to increase cAMP production, which activates cAMP-protein kinase pathway (PKA) and inhibits the general stress response regulated by Msn2/4 and specific stress responses by Yap1. However, under stress conditions, cAMP levels are downregulated through the Ras-cAMP pathways and stress responses are sensed within cells. At this time, transcription factors such as Msn2/4 and Yap1 are translocated from the cytosol to the nucleus to induce the stress response (Ma and Liu, 2010a). Expression of many genes upregulated by ethanol shares the Msn2/4, Yap1, and Hsf1 transcription factors. Approximately 200 genes (e.g. PGK1, TDH, ADH, ZWF1, HSP104, HSP82, HSP42, HSP30, HSP26) are upregulated under ethanol stress (van Voorst et al., 2006), of which 58 are co-regulated by these transcription factors (Ma and Liu, 2010b). For example, inhibition of acid trehalase (ATH1) gene (Jung and Park, 2005) or overexprression of transcription factor MSN2 (Sasano et al., 2012) and Ras-cAMP pathway inhibitor 1 (RPI1) (Puria et al., 2009) promotes ethanol fermentation and tolerance in S. cerevisiae. Under ethanol stress, Msn2/4 triggers a so-called environmental-stress response, inducing gene expression through a stress response element (STRE) and activating transcription of downstream genes (Ma and Liu, 2010a). Oxidative stress is indirectly generated under ethanol stress. Yap1 is a basic leucine zipper required for oxidative stress tolerance. Many ethanol-induced genes contain a Yap1 binding motif (Eastmond and Nelson, 2006; Fernandes et al., 2007; Herrero et al., 2008). However, the functions of Yap1 in ethanol tolerance have been not characterized. Hsf1 is another candidate regulator of ethanol tolerance because it is also associated with many ethanol-induced genes regulated by Msn2/4. It regulates transcription of hundreds of targets, including genes involved in protein folding, detoxification, energy generation, carbohydrate metabolism, and cell wall organization (Hahn et al., 2004; Ma and Liu, 2010a). Our results suggest that improved activation of these transcription factors in KNU5377 enables rapid and efficient remodeling of yeast gene expression in response to environmental stresses induced by fermentation (Fig. 6), which plays very important roles in the cellular defense against ethanol and the stresses produced during fermentation, maintaining redox balance within the cells. As the key regulators, Msn2/4, Yap1, and Hsf1 are worthy of further investigation for ethanol and environmental tolerance in KNU5377.
KNU5377 also exhibited reduced cellular hydroperoxide (Fig. 5A), protein carbonylation (Fig. 5B), and lipid peroxidation (Fig. 5C) during high-temperature fermentation. Triosephosphate isomerase (Tpi1p) was identified as the result of lipid peroxidation during glucose fermentation. Tpi1p oxidation was observed after 12 h after the start of fermentation in S288C and after 24 h in KNU5377 (Fig. 5C). Tpi1p activity is of special interest because this enzyme is an important branch point of the glycolytic pathway. Reduced Tpi1p activity causes an accumulation of dihydroxyacetone phosphate (DHAP). Increased DHAP alters cell metabolism by depleting ATP and trapping inorganic phosphate, forming a toxic methylglyoxal (MG) by non-enzymatic/enzymatic conversion of DHAP, leading to stress sensitivity (Compagno et al., 2001). The most remarkable effect in tpi1 mutant cells is that the final product of glucose catabolism is glycerol instead of ethanol. Gpd1 (Tdh1) and Gpd2 (Tdh2) convert DHAP to glycerol-3-phosphate (G3P). High Gpd activity maintains the cytosolic redox balance during batch growth on ethanol-containing medium (Compagno et al., 2001). Accordingly, high Gpd (GAPDH or Tdh) expression was observed in KNU5377 during batch fermentation (Figs. 3B and 3D). Therefore, increased Gpd expression and reduced loss of Tpi1p activity in KNU5377 reduce the concentrations of DHAP and MG during high-temperature fermentation, increasing acquired tolerance and alcohol concentration by maintaining redox homeostasis.
In conclusion, S. cerevisiae KNU5377 activated a broad range of cell rescue systems including metabolic enzymes, antioxidant enzymes, and molecular chaperones during high-temperature fermentation, increasing stress tolerance and alcohol yield by minimizing oxidative damage from ROS, improving redox homeostasis and proteostasis. These results suggest that fermentation at high temperature can greatly reduce fuel ethanol production costs and thermotolerant S. cerevisiae KNU5377 has considerable potential for the development of fermentation technologies.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008060022013), Rural Development Administration, and funded by the Global Frontier Program (2011-0031341) of the Ministry of Education, Science and Technology (MEST), Republic of Korea.
REFERENCES
- Abdel-Banat BM, Hoshida H, Ano A, Nonklang S, Akada R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl Microbiol Biotechnol. 2010;85:861–867. doi: 10.1007/s00253-009-2248-5. [DOI] [PubMed] [Google Scholar]
- Ansari H, Greco G, Luban J. Cyclophilin A peptidyl-prolyl isomerase activity promotes ZPR1 nuclear export. Mol Cell Biol. 2002;22:6993–7003. doi: 10.1128/MCB.22.20.6993-7003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloch C, Orlic S, Barrio E, Querol A. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol. 2008;122:188–195. doi: 10.1016/j.ijfoodmicro.2007.11.083. [DOI] [PubMed] [Google Scholar]
- Cebollero E, Gonzalez-Ramos D, Tabera L, Gonzalez R. Transgenic wine yeast technology comes of age: is it time for transgenic wine? Biotechnol Lett. 2007;29:191–200. doi: 10.1007/s10529-006-9236-y. [DOI] [PubMed] [Google Scholar]
- Chu F, Maynard JC, Chiosis G, Nicchitta CV, Burlingame AL. Identification of novel quaternary domain interactions in the Hsp90 chaperone, GRP94. Protein Sci. 2006;15:1260–1269. doi: 10.1110/ps.052065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno C, Brambilla L, Capitanio D, Boschi F, Ranzi BM, Porro D. Alterations of the glucose metabolism in a triose phosphate isomerase-negative Saccharomyces cerevisiae mutant. Yeast. 2001;18:663–670. doi: 10.1002/yea.715. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang X, Zhang L, Zhao N, Yang D, Zhang K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009;85:253–263. doi: 10.1007/s00253-009-2223-1. [DOI] [PubMed] [Google Scholar]
- Eastmond DL, Nelson HC. Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response. J Biol Chem. 2006;281:32909–32921. doi: 10.1074/jbc.M602454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes PN, Mannarino SC, Silva CG, Pereira MD, Panek AD, Eleutherio EC. Oxidative stress response in eukaryotes: effect of glutathione, superoxide dismutase and catalase on adaptation to peroxide and menadione stresses in Saccharomyces cerevisiae. Redox Rep. 2007;12:236–244. doi: 10.1179/135100007X200344. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BR, Lawrence SJ, Leclaire JP, Powell CD, Smart KA. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev. 2007;31:535–569. doi: 10.1111/j.1574-6976.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- Gong Y, Kakihara Y, Krogan N, Greenblatt J, Emili A, Zhang Z, Houry WA. An atlas of chaperone-protein interactions in Saccharomyces cerevisiae: implications to protein folding pathways in the cell. Mol Syst Biol. 2009;5:275. doi: 10.1038/msb.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Miess A, Stromer T, Walter S, Buchner J. Disassembling protein aggregates in the yeast cytosol. The cooperation of Hsp26 with Ssa1 and Hsp104. J Biol Chem. 2005;280:23861–23868. doi: 10.1074/jbc.M502697200. [DOI] [PubMed] [Google Scholar]
- Herrero E, Ros J, Belli G, Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta. 2008;1780:1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Park HD. Antisense-mediated inhibition of acid trehalase (ATH1) gene expression promotes ethanol fermentation and tolerance in Saccharomyces cerevisiae. Biotechnol Lett. 2005;27:1855–1859. doi: 10.1007/s10529-005-3910-3. [DOI] [PubMed] [Google Scholar]
- Kim IS, Jin I, Yoon HS. Decarbonylated cyclophilin A Cpr1 protein protects Saccharomyces cerevisiae KNU5377Y when exposed to stress induced by menadione. Cell Stress Chaperones. 2011;16:1–14. doi: 10.1007/s12192-010-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Kim YS, Yoon HS. Rice ASR1 protein with reactive oxygen species scavenging and chaperone-like activities enhances acquired tolerance to abiotic stresses in Saccharomyces cerevisiae. Mol. Cells. 2012;33:285–293. doi: 10.1007/s10059-012-2253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yun HS, Kwon C. Molecular communications between plant heat shock responses and disease resistance. Mol. Cells. 2012;34:109–116. doi: 10.1007/s10059-012-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Liu ZL. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2010a;87:829–845. doi: 10.1007/s00253-010-2594-3. [DOI] [PubMed] [Google Scholar]
- Ma M, Liu LZ. Quantitative transcription dynamic analysis reveals candidate genes and key regulators for ethanol tolerance in Saccharomyces cerevisiae. BMC Microbiol. 2010b;10:169. doi: 10.1186/1471-2180-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Richter K, Lilie H, Buchner J. Cpr6 and Cpr7, two closely related Hsp90-associated immunophilins from Saccharomyces cerevisiae, differ in their functional properties. J Biol Chem. 2000;275:34140–34146. doi: 10.1074/jbc.M005251200. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Gangwani L, Davis RJ, Lambright DG. Structural insights into the interaction of the evolutionarily conserved ZPR1 domain tandem with eukaryotic EF1A, receptors, and SMN complexes. Proc. Natl. Acad. Sci. USA. 2007;104:13930–13935. doi: 10.1073/pnas.0704915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonklang S, Abdel-Banat BM, Cha-aim K, Moonjai N, Hoshida H, Limtong S, Yamada M, Akada R. High-temperature ethanol fermentation and transformation with linear DNA in the thermotolerant yeast Kluyveromyces marxianus DMKU3-1042. Appl Environ Microbiol. 2008;74:7514–7521. doi: 10.1128/AEM.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco H, Matallana E, Aranda A. Oxidative stress tolerance, adenylate cyclase, and autophagy are key players in the chronological life span of Saccharomyces cerevisiae during winemaking. Appl Environ Microbiol. 2012;78:2748–2757. doi: 10.1128/AEM.07261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro FJ, Jewett MC, Nielsen J, Agosin E. Growth temperature exerts differential physiological and transcriptional responses in laboratory and wine strains of Saccharomyces cerevisiae. Appl Environ Microbiol. 2008;74:6358–6368. doi: 10.1128/AEM.00602-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puria R, Mannan MA, Chopra-Dewasthaly R, Ganesan K. Critical role of RPI1 in the stress tolerance of yeast during ethanolic fermentation. FEMS Yeast Res. 2009;9:1161–1171. doi: 10.1111/j.1567-1364.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- Salvado Z, Arroyo-Lopez FN, Guillamon JM, Salazar G, Querol A, Barrio E. Temperature adaptation markedly determines evolution within the genus Saccharomyces. Appl Environ Microbiol. 2011;77:2292–2302. doi: 10.1128/AEM.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano Y, Haitani Y, Hashida K, Ohtsu I, Shima J, Takagi H. Overexpression of the transcription activator Msn2 enhances the fermentation ability of industrial baker’s yeast in frozen dough. Biosci Biotechnol Biochem. 2012;76:624–627. doi: 10.1271/bbb.110959. [DOI] [PubMed] [Google Scholar]
- Stanley D, Bandara A, Fraser S, Chambers PJ, Stanley GA. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J Appl Microbiol. 2010;109:13–24. doi: 10.1111/j.1365-2672.2009.04657.x. [DOI] [PubMed] [Google Scholar]
- Tan SX, Teo M, Lam YT, Dawes IW, Perrone GG. Cu, Zn superoxide dismutase and NADP(H) homeostasis are required for tolerance of endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol. Biol. Cell. 2009;20:1493–1508. doi: 10.1091/mbc.E08-07-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CL, Fields S. Quantitative genome-wide analysis of yeast deletion strain sensitivities to oxidative and chemical stress. Comp. Funct. Genomics. 2004;5:216–224. doi: 10.1002/cfg.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Voorst F, Houghton-Larsen J, Jonson L, Kielland-Brandt MC, Brandt A. Genome-wide identification of genes required for growth of Saccharomyces cerevisiae under ethanol stress. Yeast. 2006;23:351–359. doi: 10.1002/yea.1359. [DOI] [PubMed] [Google Scholar]
- Wegele H, Haslbeck M, Reinstein J, Buchner J. Sti1 is a novel activator of the Ssa proteins. J Biol Chem. 2003;278:25970–25976. doi: 10.1074/jbc.M301548200. [DOI] [PubMed] [Google Scholar]
- Wiegel J. Formation of ethanol by bacteria. A pledge for the use of extreme thermophilic anaerobic bacteria in industrial ethanol fermentation process. Experientia. 1980;36:1434–1446. [Google Scholar]
- Zhao XQ, Bai FW. Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. J Biotechnol. 2009;144:23–30. doi: 10.1016/j.jbiotec.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]