Abstract
The THO complex (THO) is an evolutionary conserved protein required for the formation of export-competent mRNP. The growing evidence indicates that the metazoan THO plays important roles in cell differentiation and cellular stress response. But the underlying mechanisms are poorly understood. Herein we examined the relevance of THO to cellular signaling pathways involved in cell differentiation and cellular stress response. When we examined the endogenous p53 level in the testis, it was sustained much longer during spermatogenesis in the THO mutant compared to that of wild-type. In flies with impaired THO, overexpression of p53 by eye-specific GAL4 not only enhanced p53-mediated retinal degeneration, but p53 level was also elevated compared to the control flies. Since the body size of the THO mutant flies was significantly larger than control flies, we also examined whether the PI3K/AKT signaling is enhanced in the mutant flies. The results showed that the endogenous level of phosphorylated AKT, which is the active form, was highly elevated in the THO mutants. Taken together our results suggested that both p53 and PI3K/AKT signalings are up-regulated in the flies with impaired THO.
Keywords: AKT, Drosophila, p53, PI3K, THO
INTRODUCTION
The eukaryotic gene expression requires complex regulation at various stages. The THO complex (THO) participates in the eukaryotic gene expression at the interface between transcription and RNA export. THO was first discovered as a protein complex that connects transcription elongation with mitotic recombination in budding yeast (Chávez et al., 2000). Subsequent studies have revealed that THO is involved in forming an optimal mRNP for nuclear export, preventing the nascent RNA from interacting with the DNA template (Jimeno et al., 2002). Metazoans also have a functional homolog of THO, although their subunit composition is slightly different from that of budding yeast (Masuda et al., 2005; Rehwinkel et al., 2004). Metazoan THO consists of 5 subunits, THOC1/HPR1, THOC2/THO2, THOC5, THOC6 and THOC7. Now, it is clear that THO is required for the biogenesis of export-competent mRNPs at the single cell level (review by Jimeno and Aguilera, 2010). However, our understanding of THO function at the multicellular organism level is very limited. Several lines of recent evidence have suggested that metazoan THO has cell type-specific roles during development (Griaud et al., 2012; Kopytova et al., 2010; Mancini et al., 2010; Moon et al., 2011; Wang et al., 2009). In mice homozygous for a hyphomorphic Thoc1 allele, spermatogenesis was severely compromised due to defects in the expression of genes required for normal cell differentiation in the testis, while most other tissues appeared to be unaffected (Wang et al., 2009). Using conditional knock-out mice, Mancini et al. (2010) showed that THOC5 was an essential element in the maintenance of hematopoiesis, whereas it was less important in differentiated cell types such as hepatocytes and heart muscles. In addition, it has been shown that THOC1 was differentially expressed in human cancers; it was up-regulated in ovarian and lung tumors, while down-regulated in testis and skin tumors (Dominguez-Sanchez et al., 2011). Previously, we reported that thoc5 flies were viable but showed severe defects in the spermatocytes including meiotic arrest and nucleolar disruption (Moon et al., 2011). In addition to this, THO dysfunction in Drosophila could cause a significant shortening of life span as well as increased sensitivity to the environmental stresses (Kim et al., 2011). To understand the underlying mechanisms for these phenotypes, here we examined 2 cellular signaling pathways, p53 and PI3K/AKT pathways, which have been well known to play important roles in cell differentiation and in cellular stress responses.
In mammalian cells, it has been well established that p53 is maintained usually at a very low level under normal condition. However, if the level of p53 increases in response to DNA damage or other stresses, its accumulation leads to cell cycle arrest or apoptosis (Ryan et al., 2001). Because of its transcriptional activity, p53 activates or represses many genes for recovering the cell from cellular damage or leading cells to death (Oren, 2003). Therefore, keeping p53 at low level for normal cells is a critical issue for their proliferation and survival. Many studies showed that nucleoli play an important role in the regulation of p53 stability. p14ARF is required for relocalization of MDM2, which is an E3 ubiquitin ligase for p53, into the nucleoli. If p14ARF recruits MDM2 to nucleoli, p53 is dissociated from MDM2 and stabilized in the nucleoplasm (Kashuba et al., 2003). The disruption of nucleoli by treatment of cytotoxic drugs or knocking out TIF1A also induced p53 stabilization (Rubbi and Milner, 2003; Yuan et al., 2005). Nucleolar disruption caused ribosomal proteins to be released from the nucleoli and bound to MDM2, leading p53 to be disassociated from MDM2 and stabilized (Yuan et al., 2005).
Drosophila p53 was first identified by Brodsky et al. (2000). Comparison of Drosophila p53 with its mammalian homolog showed little conservation in both N-and C-termini, but the central DNA binding domain was highly conserved (25% identity, 43% similarity) (Brodsky et al., 2000). Genetic analyses showed that Drosophila p53 homolog was unable to induce the G1 cell cycle arrest upon X-ray irradiation but fully capable of inducing apoptosis after ectopic expression or X-ray irradiation (Brodsky et al., 2004; Lee et al., 2003). Interestingly Drosophila p53 might not require an MDM2-like activity for its activation in response to DNA damage, because MNK-mediated phosphorylation without protein level changes was sufficient to activate p53 (Brodsky et al., 2004). In Drosophila, however, the regulation mode of p53 is still unclear. In this report, we show a strong genetic interaction of THO with p53 signaling and a significant increase in the level of p53 in the THO mutant, providing a novel player involved in the regulation of p53 level in Drosophila.
Many signaling pathways have been proposed to govern the growth control in response to growth factors. One of such is the PI3K (phosphatidylinositol 3-kinase) signaling pathway (Britton et al., 2002; Brunet et al., 1999; Rulifson et al., 2002). After insulin or other growth factors stimulate their receptors, then PI3Ks convert the membrane lipid PIP2 (phosphatidylinositol 4, 5-biphosphate) into PIP3 (phosphatidylinositol 3, 4, 5-triphosphate). This conversion can be reversed by the tumor suppressor PTEN (Goberdhan and Wilson, 2003). The second messenger, PIP3, then activates AKT and p70 S6 kinase (S6K), ensuring downstream events such as increment of protein synthesis, and prevention of FOXO from being retained in the nucleus to suppress transcription of genes responsible for negative regulation of cellular growth or FOXO-induced apoptosis (Alvarez et al., 2001; Britton et al., 2002; Brunet et al., 1999; Rulifson et al., 2002). Since THO-defective flies showed a significant increase in body size, here we investigated the relevance of PI3K signaling pathway to this phenotype, and show that the level of endogenous phosphor-AKT (p-AKT), a downstream effector of PI3K/AKT signaling pathway, is significantly elevated in the THO mutant testis.
MATERIALS AND METHODS
Drosophila stocks and husbandry
The thoc5 allele thoc51 has been described previously (Moon et al., 2011). All other flies used in this study were obtained from the Bloomington Stock Center (Indiana University, USA), except the thoc7 allele thoc7d05792, which was from the Exelixis collection at the Harvard Medical School. Flies were reared on standard medium at 23–25°C.
Histology and antibody staining
For retinal section, adult heads were prepared in PBS [10 mM NaPO4 (pH 7.2), 150 mM NaCl], then fixed in 4% paraformaldehyde in PBS for 1 h at 4°C followed by washing three times with PBS. Then the heads were infiltrated with 12% sucrose in PBS at 4°C for overnight. On the following day, the preparations were embedded in O.C.T. (Sukura Finetek, # 4583) and frozen at −80°C. Serial sections with 10 μm in thickness were collected on the gelatin subbed slides. For antibody staining, the slides were post-fixed with 4% paraformaldehyde in PBS, and washed 3 times with PBST (0.3% Triton X-100 in PBS). After blocking with a blocking solution (2% bovine serum albumin and 2% normal donkey serum in PBS) for 1 h at room temperature, the slides were then incubated with primary antibodies at 4°C overnight. After washing 3 times with PBST, the slides were incubated with secondary antibodies for 2 h at room temperature. After washing 3 times with PBST, the slides were mounted with 80% glycerol and imaged with LSM510 confocal microscope (Carl Zeiss). For whole mount antibody staining, tissues were dissected in PBS, fixed with 4% paraformaldehyde in PBS, and then washed 3 times with PBST (Maeng et al., 2012). Thereafter the procedure was the same as that of retinal section.
For detection of cell death, pupal retinas were incubated with acridine orange (1.6 μM in PBS) for 5 min at room temperature, then examined directly under microscope after a brief washing with PBS.
Primary antibodies used in this study: anti-p53 (25F4, DSHB) at 1:50∼100, anti-p-AKT (#4054, Cell signaling) at 1:100. The secondary antibodies Alexa 546-conjugated goat anti-mouse (1:500) and Cy3-conjugated goat anti-Rabbit (1:500) were purchased from Molecular Probes (USA). Hoechst 33258 (Sigma-Aldrich, USA) was used to visualize DNA.
Western blotting
Testes from 1-day-old to 3-day-old male flies were dissected in PBS, homogenized, and boiled with SDS-PAGE sample buffer. After fractionation by SDS-PAGE, the proteins were transferred to PVDF membrane (BioRad, USA). Then, the blots were blocked with 5% non-fat milk in TBST [50 mM Tris (pH 7.5), 150 mM NaCl, 0.1% Tween-20] and incubated overnight with primary antibody at 4°C. The following primary antibodies were used: anti-p-AKT (#4054, Cell signaling) at 1:500, Actin (JLA20, DSHB at 1:200). Horseradish peroxidase (HRP)-coupled secondary antibody (1:10, 000; Jackson ImmunoResearch Laboratories) was used for ECL detection.
RESULTS
p53 level was sustained much longer during spermatogenesis in thoc5
Since thoc5 mutant flies showed very complicated defects in testis, including meiotic arrest and nucleolar disruption (Moon et al., 2011), we first investigated p53 levels in the testis to examine whether cellular signaling pathways involved in cell differentiation and cellular stress responses are normal in the THO mutant. In Drosophila, a coiled pair of testes is present in each male. Each testis is a blind-ended tube filled with cells at all stages of spermatogenesis. At its apical tip, germline stem cells (GSCs) are located in the niche. Each GSC divides asymmetrically to produce one cell that remains in the niche as a stem cell, and another cell that moves away from the apical tip (the niche) and differentiates to a gonialblast. A gonialblast undergoes 4 rounds of mitotic divisions with incomplete cytokinesis to produce 16 clustered spermatogonia, which become primary spermatocytes. These 16 primary spermatocytes undergo meiosis, and eventually produce 64 mature sperms. The gonialblast cells are found just distal to the stem cell niche, with later stages of spermatogenesis progressively apparent further down the testis.
In wild-type, antibody staining showed that endogenous p53 was detectable only in the narrow region at the apical tip of testis, where GSCs and the differentiating germ cells at the early stages (gonialblasts and spermatogonia) are resided (Fig. 1A). In each cell, p53 was highly enriched in a small restricted nuclear region. The immuoreactivity was not detectable in the testis of the p53 null mutant, confirming the specificity of the antibody (Fig. 1C). In thoc5, however, the area of p53-positive cells was expanded further down the testis (Fig. 1B). This suggests that p53 is sustained at the later stages of spermatogenesis. Alternatively, it is also possible that the number of cells, which normally maintain high level of p53, is increased in the mutant testis. To address this issue, we generated the thoc5 mutant cell clones in the testis using heat-induced FRT/FLP system, and compared directly the same stages of wild-type and mutant cells. The result showed that p53 immunoreactivities were seen in the distally located thoc5 mutant cells, while it was almost undetectable in their neighboring wild-type cells at the similar stages of spermatogenesis. This suggests that in a cell-autonomous manner p53 level is sustained longer during spermatogenesis in thoc5 than wild-type.
Fig. 1.
Endogenous level of p53 was sustained during spermatogenesis in thoc5. (AC) Antibody staining of testes using anti-p53 antibody. In the wild-type testis, p53 signal was restricted to the apical tip of testis (two-sided arrow in A). In the testis of trans-heterozyous for thoc51/thoc5e00906, the p53-positive area was significantly expanded toward basal side (two-sided arrow in B). No signals were detected in the testis of a p53 null mutant, p5311-1B-1. The outlines of testes were indicated by the dotted lines. (D) Clonal analysis by FRT-FLP-mediated mitotic recombination in the testis. The picture shows combined images of anit-p53 (red), GFP (green) and DNA (blue) in the testis tip region. The GFP-negative cells indicate the homozygous cell clone for thoc51 mutation (outlined by dotted lines). The immunoreactivities against p53 were detectable in the distally located mutant clone, whereas adjacent wild-type clones showed no obvious signals. Genotype: y w P{hs-FLP}; P{FRT42D} thoc51/P{FRT42D} P{Ubi-GFP}. Scale bars: 50 μm (A-C) or 10 μm (D).
The ectopic p53 expression caused eye pigmentation loss in the THO mutants
To verify whether the up-regulation of p53 in thoc5 is a testis-specific phenotype or a more general phenotype, we examined other tissues such as larval wing imaginal discs and eyeantennal imaginal discs. However, we failed to detect endogenous p53 immunoreactivity in these tissues from both wild-type and thoc5 (data not shown). For this reason, next we overexpressed p53 by using eye-specific GAL4 driver, and examined whether p53 was also up-regulated under overexpression conditions in the genetic background of thoc5. If the level of p53 is generally increased in the THO mutant, we would see the enhanced p53 activity in the thoc5 mutant background after ectopic expression of p53. Consistent with this hypothesis, we observed a severe loss of the eye pigmentation in the genetic background of thoc5, whereas eye pigmentation itself was normal in the wild-type background (Fig. 2). This pigmentation loss phenotype was also observed in the genetic backgrounds of thoc6 and thoc7, although the phenotypes were milder compared to that of thoc5 (Fig. 2), suggesting that the effect of p53 over-expression is more sensitive in the THO-defective eyes than the wild-type ones.
Fig. 2.

Eye pigmentation loss by ectopic expression of p53 in the homozygous backgrounds of the THO subunit genes. Ectopic expression of p53 by longGMR-GAL4 caused a complete pigmentation loss in the homozygous thoc51 background, and partial pigmentation loss in the homozygous thoc6e00298 and thoc7d05792 backgrounds. No pigmentation loss was seen in either the wild-type background (WT) or the control fly (GUS-p53 only). Genotypes: P{GUS-p53}/CyO; P{longGMR-GAL4}/+ (WT), thoc51 P{GUS-p53}/thoc51; P{longGMR-GLA4}/+ (thoc51), P{GUS-p53}/+; P{long GMR-GAL4} thoc6e00298/thoc6e00298 (thoc6e00298), P{GUS-p53}/+; P{long GMR-GAL4} thoc7d05792/thoc7d05792 (thoc7do5792), and thoc51 P{GUSp53}/thoc51 (GUS-p53 only).
The collapse of internal eye structure caused by p53 overexpression was enhanced in thoc5
For a detailed analysis of p53-mediated eye defects in the THO mutants, we examined the internal eye structure using phalloidin staining that marks the actin-based structure, the rhabdomere. Retinal sections showed that the pigmentation loss in the thoc5 mutant background was caused by severe loss of cells in the internal eye compartment (asterisk in Fig. 3b). In addition, the level of p53 was more elevated in the thoc5 mutant background, suggesting p53 might be hyper-stabilized in the mutant eyes (arrows in Fig. 3b).
Fig. 3.
p53-mediated apoptosis was enhanced in the homozygous thoc51 background. (A-C) External morphology of eyes over expressing p53 in the genetic backgrounds of wild-type (A) or thoc51 (B), or control eye (GUS-p53 only) (C). (a-c) Retinal sections stained with anti-p53 (green) and phalloidin (magenta). In the thoc51 mutant background (b), the internal structure of eye overexpressing p53 was severely disrupted (asterisk in b). In addition, immunostaining showed that p53 signals are much stronger in the genetic background of thoc51 (arrows in b) than in that of wild-type (a). In the control eye which harbors GUS-p53 only without GAL4, the signals were almost undetectable (c). (D-F) Acridine orange (AO) staining of pupal retinas overexpressing p53 in the genetic backgrounds of wild-type (E) or thoc51 (F), or control retina with longGMR-GAL4 driver only (D). Not only the number of AO-positive cells, but the intensity of signals was also significantly increased in the thoc51 background (arrows in D). Genotypes: P{GUS-p53}/CyO; P{longGMR-GAL4 }/+ (A, a and E), thoc51 P{GUS-p53}/thoc51; P{longGMR-GLA4}/+ (B, b and F), P{GUS-p53} (C and c), and P{longGMR-GAL4 }/+ (D). Scale bars: 100 μm (a-c) or 50 μm (D).
Since ectopic expression of p53 usually causes apoptosis, we examined whether the severe disruption of internal eye structure in the thoc5 mutant background was due to increased apoptosis. When pupal retina overexpressing p53 in the wildtype and thoc5 mutant backgrounds was stained with acridine orange (AO), which detects dying cells, we found indeed that the ectopic expression of p53 increased the number of AO-positive cells in both the wild-type and the thoc5 mutant backgrounds (Figs. Fig. 3D-Fig. 3F). However, the increment was much greater in thoc5 than in wild-type (arrows in Fig. 3F). Taken together, the ectopic expression of p53 induced much more apoptotic cells in the thoc5 mutant than in wild type backgrounds, and the pigmentation loss was caused by increased apoptosis. This finding also suggests that the up-regulation of p53 in the THO mutant background might be a more general phenotype rather than a testis-specific phenotype.
Age-dependent eye pigmentation loss by overexpression of p53 in the heterozygous genetic background for the THO mutations
The genetic interaction between p53 and THO was also seen in the flies heterozygous for the THO mutations. In the heterozygous genetic background either for thoc51 or thoc7d05972, adult fly eyes overexpressing p53 showed mild but apparent loss of eye pigmentation in an age-dependent manner (Fig. 4). However, the pigmentation loss in the heterozygous background for thoc6e00298 was not apparent (data not shown) probably because thoc6e00298 is a hypomorphic mutant allele. Consistent with this idea, the pigmentation loss phenotype was the mildest in the homozygous background for thoc6e00298 among mutations for the THO subunit genes (see Fig. 2).
Fig. 4.
Sensitization to p53-induced loss of eye pigmentation in the heterozygous backgrounds for thoc51 and thoc7d05792. (A-D) Age-dependent pigmentation loss was examined in the eyes overexpressing p53 in the genetic backgrounds of wildtype (A), thoc51/+ (B), thoc7d05792/+ (C), or the eye with GUS-p53 only (D). In both thoc51/+ and thoc7d05792/+ backgrounds, p53-induced pigmentation loss was getting worse with age. At all ages examined, however, no significant loss of eye pigmentation was observed in the wild-type background (A) or control eye (GUS-p53 only) (D). Genotypes: P{GUS-p53}/CyO; P{longGMR-GAL4}/+ (A), thoc51 P{GUS-p53}/+; P{longGMR-GLA4}/+ (B), P{GUS-p53}/+; P{longGMR-GAL4} thoc7d05792/+ (C), and P{GUS-p53} (D).
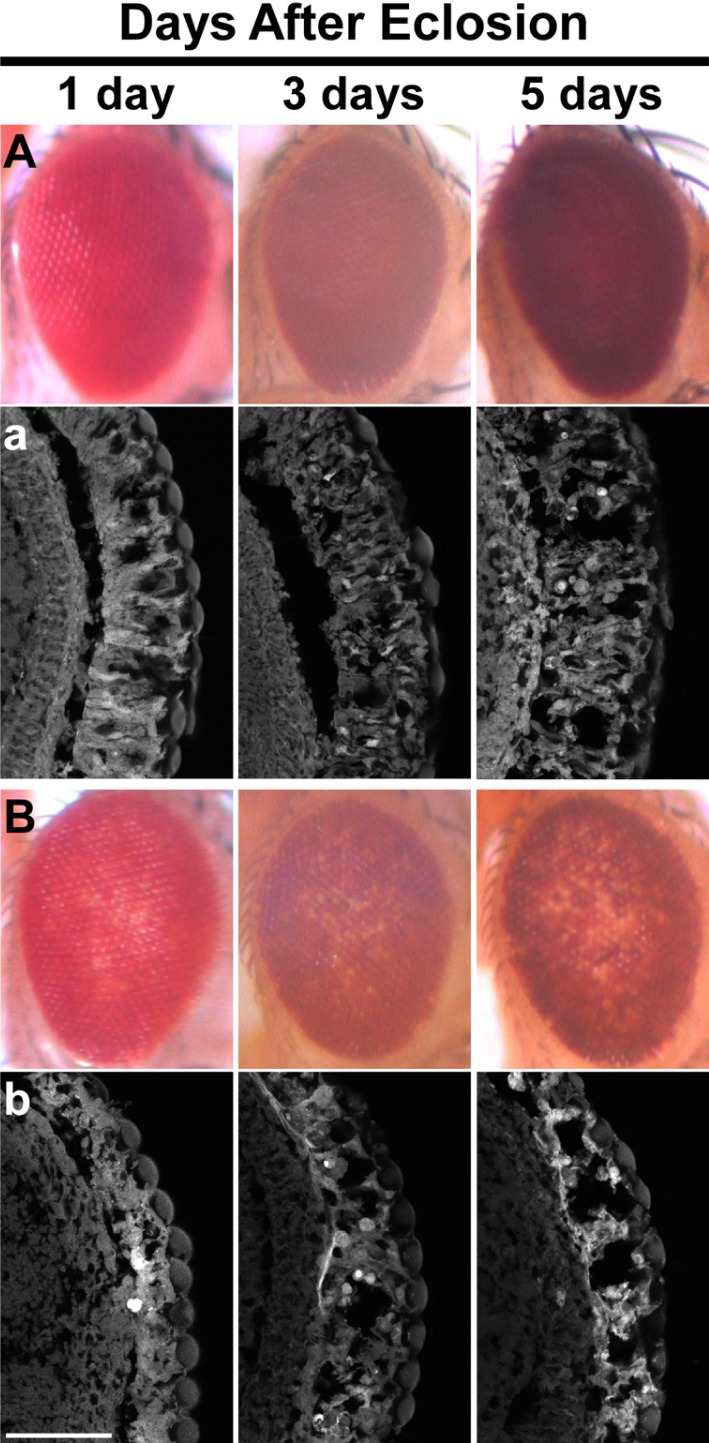
To validate that this partial pigmentation loss was caused by the collapse of internal eye architecture, the retinal sections of progressively aged adult files were analyzed by a confocal microscopy after phalloidin staining. The results showed that the disruption of internal eye architecture was directly correlated with the pigmentation loss; they both were getting worse with age in the heterozygous backgrounds for thoc51 and thoc7d05792 (Fig. 5). Interestingly, the internal eye architecture was degenerated with age by overexpression of p53 even in the wild-type background, although the loss of eye pigmentation was not obvious in the same genetic background. Taken together, these results consistently suggest that the decrease in the THO activity causes sensitization to p53-mediated cell death due to upregulation of p53.
Fig. 5.
Sensitization to p53-mediated retinal degeneration in the heterozygous background for thoc51. (A, B) External morphology of eyes overexpressing p53 in the genetic background of wild-type (A), or thoc51/+ (B). Loss of eye pigmentation is evident only in the thoc51 heterozygous background. (a, b) Internal structure of eyes revealed by phalloidine staining. Although disruption of internal eye structure became evident with age in the wild-type background (a), it is much worse in the thoc51 heterozygous background (b). Scale bar: 100 μm.
Body size and PI3K/AKT signaling is increased in thoc5
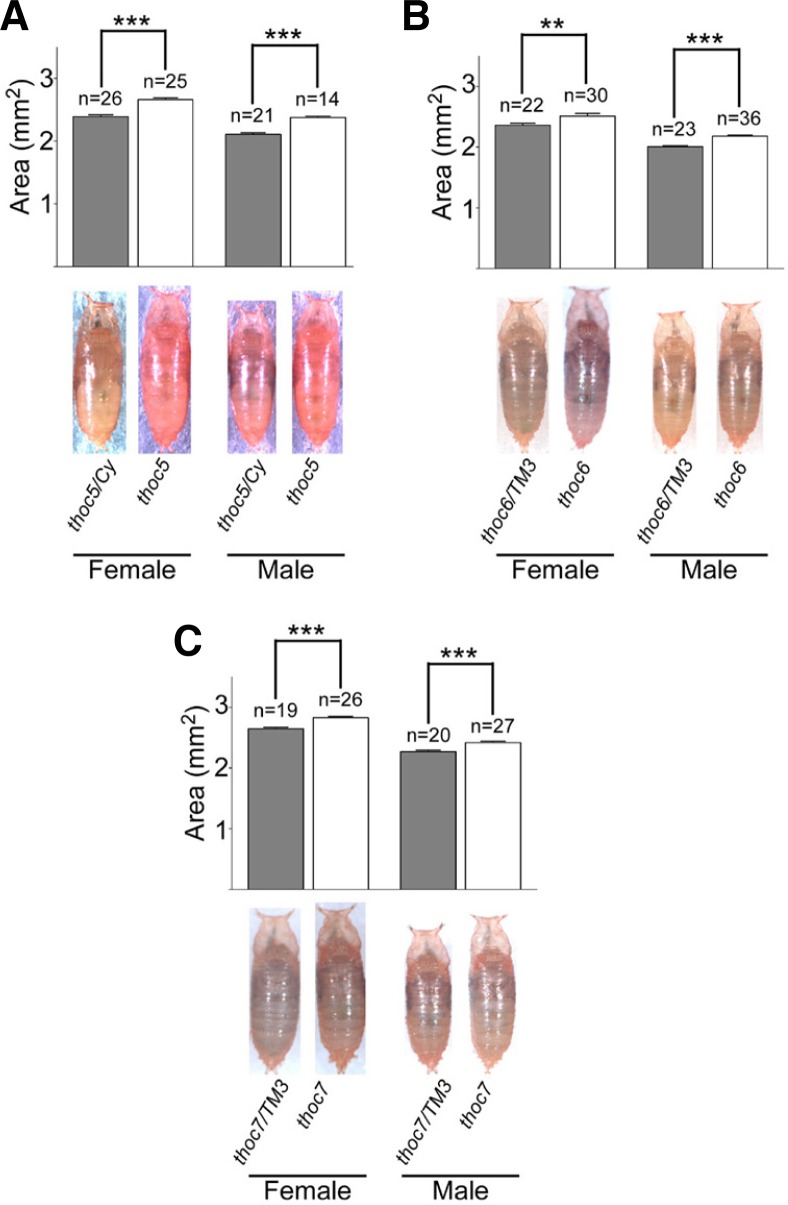
Another obvious phenotype found in the THO mutants was a significant increase in body size. In the mutant flies for all three THO subunits examined, thoc51, thoc6e00298 and thoc7d05792, we found that body size was significantly increased compared to control flies (Fig. 6). Since it has been well known that PI3K/AKT signaling is important for the control of growth (Oldham and Hafen, 2003), we examined the level of p-AKT. Unfortunately, however, Western blot analysis of whole-fly extracts showed no significant difference in the level of p-AKT between THO mutants and control (Supplementary Fig. S1). Because it is likely that different types of cells may be affected differently by THO mutations (Moon et al., 2011), next we examined the level of p-AKT in different types of cells. In the testis, which is one of the most severely affected organs by THO dysfunction, p-AKT was first detected in GSCs and its level was gradually reduced as spermatogenesis proceeds in the genetic background of wild-type (Fig. 7A). But the level of p-AKT was not only elevated but also sustained longer in thoc5 (Fig. 7B). A similar, but much milder, phenotype was found in the testis of thoc7 (Fig. 7B). But the elevated level of p-AKT was not so evident in the testis of thoc6e00298, showing again the hypomorphic nature of this mutation (Fig. 7B). Western blot analysis of testis extracts showed a consistent result; the level of p-AKT was increased in both thoc5 and thoc7 but not in thoc6, and the increment was much greater in thoc5 than thoc7 (Fig. 7C). To further confirm these findings, we directly compared p-AKT levels between wild-type and mutant cells by using FRT/FLP-mediated clonal analysis. The result showed that the level of p-AKT was highly increased in the thoc51 mutant mature primary spermatocytes compared to their neighboring wild-type cells with similar stage (Fig. 7D), suggesting the cell-autonomous function of THO in the regulation of p-AKT level. Taken together, our results suggest that the impairment in THO function may cell-autonomously up-regulate PI3K/AKT signaling in certain types of cells, and this may partly be responsible for the increased body size of the THO mutant flies.
Fig. 6.
Increased body sizes in the THO mutants. Body sizes of pharate adults as measured by body area in square millimeters were compared between controls and mutants for the THO genes, thoc51 (A), thoc6e00298 (B) and thoc7d05972 (C). Body sizes were directly compared between homozygous mutants with their heterozygous siblings. Statistical analysis confirmed significant differences in body size between homozygous mutants and their heterozygous siblings for both sexes. ***P < 0.001, **P < 0.01.
Fig. 7.
Increased level of p-AKT in the THO mutant testes. (A) Antibody staining of testes with anti-p-AKT antibody in wild-type (WT), thoc51/thoc5e00906 (thoc5), thoc6e00298 (thoc6) and thoc7d05792 (thoc7). In wild-type, p-AKT was highly accumulated in the tip region where cells at the early-stages of germline development reside, and gradually disappeared as spermatogenesis proceeded. In thoc5, however, the level of p-AKT was not only elevated, but also sustained over spermatogenesis. Similar, but much milder, defects were seen in the thoc7 mutant testis. No significant differences from wild-type and thoc6. were observed. (B) Western blot analysis of testis extracts from wildtype, thoc51/thoc5e00906, thoc6e00298 and thoc7d05792. The level of p-AKT was increased in thoc5 and thoc7, but not in thoc6. The result was well matched with that of antibody staining (C) FLP/FRT-mediated clonal analysis of thoc51 mutant cells in the testis. In the thoc51 mutant cells indicated by the absence of GFP signals (green), the immunoreactivities against p-AKT (Red) were highly elevated compared to their neighboring wild-type cells which showed only faint signals of p-AKT. Hoechst 33258 was used to visualize DNA (blue). Genotype: y w P{hs-FLP}; P{FRT42D} thoc51/P{FRT42D} P{Ubi-GFP}. Scale bars, 20 μm (A) and 10 μm (C).
DISCUSSION
In the previous report, we showed the reduced life span and the increased susceptibility to the environmental stresses in mutant flies for Drosophila THO subunits (Kim et al., 2011). To understand the underlying mechanisms of these defects, here we investigated genetic interactions of THO with 2 cellular signaling pathways, p53 and PI3K/AKT pathways.
The following evidence suggests that defects in the function of THO cause up-regulation of p53 in a cell autonomous manner. First, endogenous level of p53 was sustained much longer during spermatogenesis in the male germline lacking THO compared to the control germline. FRT/FLP-based clonal analysis showed that p53 level was cell-autonomously sustained in the mutant clone. Second, mutations in the THO subunit genes elevated the level of overexpressed p53 in the eye, showing an increased sensitivity to p53-mediated apoptosis. Third, the sensitivity to p53-mediated apoptosis was directly correlated with the genetic background; the more severe defect in THO the genetic background had, the greater the sensitive to p53-mediated apoptosis was.
Why p53 is up-regulated in the flies with impaired THO? The fact that the nucleolar integrity was severely disrupted in flies lacking THO let us postulate that disruption of nucleolus might be a good candidate to answer for this question (Moon et al., 2011). It has been known that disruption of nucleolus mediated stabilization of p53 in response to DNA damage and other stresses in mammalian cells (Rubbi and Milner, 2003). In addition, it has been reported that genetic disruption of nucleolus by knocking out murine TIF-1A gene caused p53 to be stabilized by dissociating it from MDM2 (Yuan et al., 2005). However, it is doubtful that the same is true in our Drosophila model. First, mutations in THO subuints caused nucleolar disruption only in certain types of cells including male germline and salivary gland cells (Moon et al., 2011). Moreover, we failed to find any signs of nucleolar disruption in the THO-deficient eyes which were sensitized to p53-mediated apoptosis (data not shown). Second, it has recently been reported that p53 level was not significantly increased in the eyes of virito mutant in which nucleolar architecture was severely compromised (Marinho et al., 2011). Finally, the Drosophila homolog of MDM2, which plays a key role in nucleolar disruption-mediated p53 stabilization in mammalian cells, has not been found to date. Consistent with these facts, in Drosophila, it has been shown that posttranslational modification rather than abundance was sufficient to activate p53 signaling in response to DNA damage (Brodsky et al., 2004). For these reasons, we speculate that nucleolar disruption is not directly involved in the up-regulation of p53 in the THO mutant flies. An alternative possibility is that the phenotype of condensed chromatin structure in thoc5 might represent genomic instability, and the genomic instability could lead to activation of MNK, Drosophila homolog of CHK2, which activates p53 following DNA damage (Brodsky et al., 2004). But the fact that DNA damage activates p53 without significant changes in protein level (Brodsky et al., 2004) is inconsistent with our findings which showed obvious changes of p53 level in the testis. Another alternative explanation for these ambiguities is that upregulation of p53 in the THO mutant may be restricted to certain limited types of cells, and the mechanisms underlying this may also be different depending on cell types. To clarify these issues further studies are certainly required.
In addition to p53 signaling, we examined PI3K/AKT signaling in the THO mutant flies. It has been well established that PI3K/AKT signaling pathway is a key player in regulating life span as well as body size in Drosophila (Edgar, 2006). Combined with the previously reported lifespan reduction (Kim et al., 2011), increased body size in the THO mutant flies compared to control is well matched with the known phenotypes of mutant flies with defects in PI3K/AKT signaling pathway (Edgar, 2006). Although we failed to detect a global increase in PI3K/AKT signaling in the THO mutant flies (Supplementary Fig. S1), a cell-autonomous elevation of endogenous p-AKT level in the mutant male germline provided a piece of evidence for the relevance of Drosophila THO with PI3K/AKT signaling.
Another interesting finding in this report is that the levels of both p53 and p-AKT are very high in the wild-type male germ germline. If p53 is important for spermatogenesis, why p53-null flies are not sterile? With regard to the female germline, it has recently been reported that DNA double strand breaks formed during meiotic recombination provoked activation of p53, and unrepaired DNA breaks during meiotic recombination led to sustained p53 activity (Lu et al., 2010). But it has been known that meiotic recombination is very rare in the Drosophila male germline, and we showed that p53 was detected only in the pre-meiotic germline. Thus, it is unlikely that the role of p53 in male germline is similar to that in female germline. Certainly these issues will be a good topic for our future study.
Taken together, here we found the significant genetic interactions of THO with 2 cellular signaling pathways, p53 and PI3K/AKT signaling pathways. Both signalings were up-regulated by THO dysfunction in a cell autonomous manner. However, it seems unlikely that THO generally plays a major role in regulating these signaling pathways, because not only Western blot analysis of whole-fly extract, but also FRT/FLP-mediated clonal analysis in the imaginal discs (Supplementary Fig. S2) shown no significant changes in the endogenous levels of both p53 and p-AKT in the THO mutants. It seems rather likely that the effect of THO dysfunction on these 2 signaling pathways is different depending on cell types; it might be generally mild in most cells except certain types of cells such as germline.
Supplementary Material
Acknowledgments
This work was supported by the 2011 sabbatical year research grant of the University of Seoul to Y. D. Chung.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Alvarez B, Martinez AC, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744–747. doi: 10.1038/35099574. [DOI] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Chávez S, Beilharz T, Rondón AG, Erdjument-Bromage H, Tempst P, Svejstrup JQ, Lithgow T, Aguilera A. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 2000;19:5824–5834. doi: 10.1093/emboj/19.21.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sanchez MS, Saez C, Japon MA, Aguilera A, Luna R. Differential expression of THOC1 and ALY mRNP biogenesis/export factors in human cancers. BMC Cancer. 2011;11:77. doi: 10.1186/1471-2407-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. The functions of insulin signaling: size isn’t everything, even in Drosophila. Differentiation. 2003;71:375–397. doi: 10.1046/j.1432-0436.2003.7107001.x. [DOI] [PubMed] [Google Scholar]
- Griaud F, Pierce A, Sanchez MB, Scott M, Abraham SA, Holyoake TL, Tran DD, Tamura T, Whetton AD. A pathway from leukaemogenic oncogenes and stem cell chemokines to RNA processing via THOC5. Leukemia. 2012 doi: 10.1038/leu.2012.1283. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jimeno S, Aguilera A. The THO complex as a key mRNP biogenesis factor in development and cell differentiation. J Biol. 2010;9:6. doi: 10.1186/jbiol217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno S, Rondón AG, Luna R, Aguilera A. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 2002;21:3526–3535. doi: 10.1093/emboj/cdf335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashuba E, Mattsson K, Klein G, Szekely L. p14ARF induces the relocation of HDM2 and p53 to extranucleolar sites that are targeted by PML bodies and proteasomes. Mol. Cancer. 2003;2:18. doi: 10.1186/1476-4598-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cho B, Moon S, Chung YD. The THO complex is required for stress tolerance and longevity in Drosophila. Genes Genomics. 2011;33:291–297. [Google Scholar]
- Kopytova DV, Orlova AV, Krasnov AN, Gurskiy DY, Nikolenko JV, Nabirochkina EN, Shidlovskii YV, Georgieva SG. Multifunctional factor ENY2 is associated with the THO complex and promotes its recruitment onto nascent mRNA. Genes Dev. 2010;24:86–96. doi: 10.1101/gad.550010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee E, Park J, Kim E, Kim J, Chung J. In vivo p53 function is indispensable for DNA damage-induced apoptotic signaling in Drosophila. FEBS Lett. 2003;550:5–10. doi: 10.1016/s0014-5793(03)00771-3. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Chapo J, Roig I, Abrams JM. Meiotic recombination provokes functional activation of the p53 regulatory network. Science. 2010;328:1278–1281. doi: 10.1126/science.1185640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng O, Son W, Chung J, Lee KS, Lee YH, Yoo OJ, Cha GH, Paik SG. The BTB/POZ-ZF transcription factor dPLZF is involved in Ras/ERK signaling during Drosophila wing development. Mol. Cells. 2012;33:457–463. doi: 10.1007/s10059-012-2179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A, Niemann-Seyde SC, Pankow R, El Bounkari O, Klebba-Farber S, Koch A, Jaworska E, Spooncer E, Gruber AD, Whetton AD, et al. THOC5/FMIP, an mRNA export TREX complex protein, is essential for hematopoietic primitive cell survival in vivo. BMC Biol. 2010;8:1–17. doi: 10.1186/1741-7007-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho J, Casares F, Pereira PS. The Drosophila Nol12 homologue viriato is a dMyc target that regulates nucleolar architecture and is required for dMyc-stimulated cell growth. Development. 2011;138:349–357. doi: 10.1242/dev.054411. [DOI] [PubMed] [Google Scholar]
- Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 2005;19:1512–1517. doi: 10.1101/gad.1302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Cho B, Min SH, Lee D, Chung YD. The THO complex is required for nucleolar integrity in Drosophila spermatocytes. Development. 2011;138:3835–3845. doi: 10.1242/dev.056945. [DOI] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J, Herold A, Gari K, Köcher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E. Genome-wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster. Nat Struct Mol Biol. 2004;11:558–566. doi: 10.1038/nsmb759. [DOI] [PubMed] [Google Scholar]
- Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Chinnam M, Wang J, Wang Y, Zhang X, Marcon E, Moens P, Goodrich DW. Thoc1 deficiency compromises gene expression necessary for normal testis development in the mouse. Mol Cell Biol. 2009;29:2794–2803. doi: 10.1128/MCB.01633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Zhou Y, Casanova E, Chai M, Kiss E, Gröne H-J, Schütz G, Grummt I. Genetic inactivation of the transcription factor TIF-IA leads to nucleolar disruption, cell cycle arrest, and p53-mediated apoptosis. Mol. Cell. 2005;19:77–87. doi: 10.1016/j.molcel.2005.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.