Abstract
T cell receptor (TCR) stimulation plays a crucial role in development, homeostasis, proliferation, cell death, cytokine production, and differentiation of T cells. Thus, in depth understanding of TCR signalling is crucial for development of therapy targeting inflammatory diseases, improvement of vaccination efficiency, and cancer therapy utilizing T cell-based strategies. TCR activation turns on various signalling pathways, one of the important one being the Ca2+-calcineurin-nuclear factor of activated T cells (NFAT) signalling pathway. Stimulation of TCRs triggers depletion of intracellular Ca2+ store and in turn, initiates store-operated Ca2+ entry (SOCE), one of the major mechanisms to raise the intracellular Ca2+ concentrations in T cells. Ca2+-release-activated-Ca2+ (CRAC) channels are a prototype of store-operated Ca2+ (SOC) channels in immune cells that are very well characterized. Recent identification of STIM1 as the endoplasmic reticulum (ER) Ca2+ sensor and Orai1 as the pore subunit has dramatically advanced the understanding of CRAC channels and provides a molecular tool to investigate the physiological outcomes of Ca2+ signalling during immune responses. In this review, we focus on our current understanding of CRAC channel activation, regulation, and downstream calcineurin-NFAT signaling pathway.
Keywords: CRAC channels, NFAT, Orai1, STIM1, T cell receptor
INTRODUCTION
Ca2+ is utilized as a second messenger by essentially all cells in unicellular and multicellular organisms, where it regulates diverse aspects of cellular function. Under resting conditions in T cells, cytoplasmic [Ca2+] is in the range of ∼100 nM while that in the endoplasmic reticulum, which serves as an intracellular Ca2+ store, is much higher (∼400 μM). Extracellular [Ca2+] reaches almost 2 mM, establishing a huge [Ca2+] gradient between the extracellular milieu, Ca2+ store, and the cytoplasm. Increases in intracellular Ca2+ concentration ([Ca2+]i) can affect many signaling pathways via activation of ubiquitous Ca2+ sensors including calmodulin (CaM); which in turn activate a large number of protein kinases/phosphatases and gene transcription, that together shape both the early and late phases of the subsequent cellular response. Ca2+ entry via store-operated Ca2+ (SOC) channels is a predominant mechanism to increase [Ca2+]i in non-excitable cells, while in excitable cells (e.g. muscle and neuronal cells), voltage-gated ion channels are important for regulation of [Ca2+]i (Cahalan and Chandy, 2009; Hogan et al., 2010; Lewis, 2011; Putney, 2009). SOC channels were so named because they are activated by depletion of intracellular Ca2+ stores (Putney, 1986; 2009). The Ca2+-release-activated-Ca2+ (CRAC) channel is a prototype and specialized class of SOC channel in immune cells. Because the volume of ER in T lymphocytes is much smaller than that of other cell types such as cardiac or skeletal muscle cells, SOCE via CRAC channels is particularly important to sustain increased [Ca2+]i necessary for activation of NFAT family of transcription factors. In this review, we will focus on our current understanding of the regulation and known functions of Ca2+ signalling in T cells and phenotypes of animal models lacking the components of CRAC channels.
INTEGRATIVE T CELL RECEPTOR SIGNALLING PATHWAYS
Upon pathogen infection, specialized innate immune cells (e.g. dendritic cells) and adaptive immune cells (e.g. B cells) present foreign antigens on their surface together with major histocompatibility complex (MHC) class II molecules. Interactions between TCRs and antigens presented by MHC class II molecules play an important role in T helper cell functions such as differentiation into effector and memory cells, proliferation, and massive cytokine production after full differentiation. In addition, interactions between self-peptides and TCRs are important for T cell development in the thymus, homeostasis, and pathological onset of autoimmune diseases (Sprent and Surh, 2011). Thus, understanding of TCR signalling is crucial for development of therapy to rescue patients with immune deficiencies and to develop pharmacological methods to ameliorate the pathological symptoms of numerous autoimmune diseases exemplified in type I diabetes, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, graft-versus-host disease, and transplant rejection, that are primarily mediated by inflammatory and autoreactive T cells.
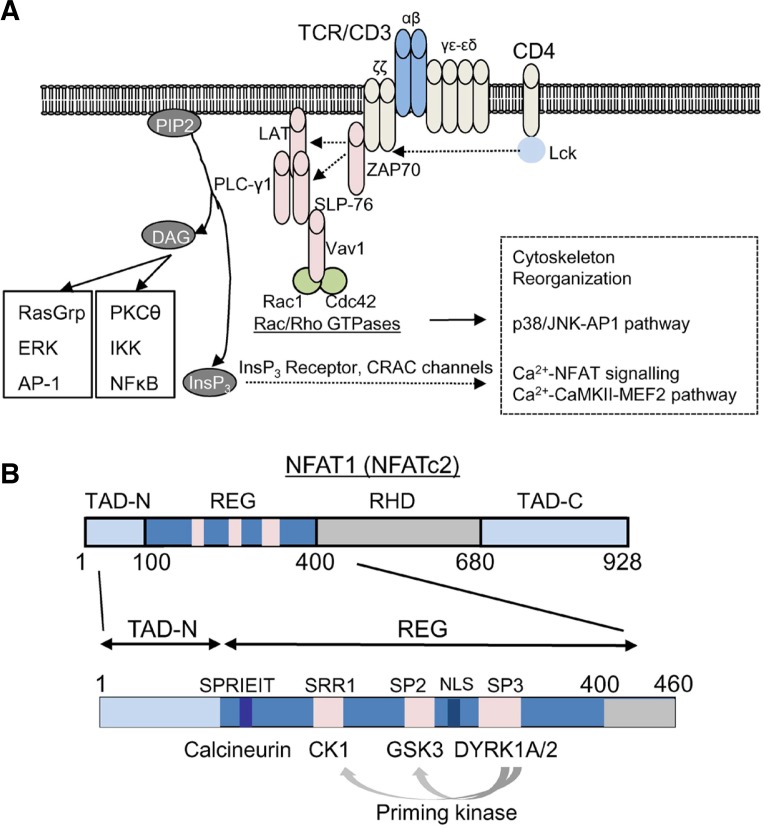
Antigen engagement of T cell receptor triggers a cascade of tyrosine phosphorylation events mediated by lymphocyte-specific protein tyrosine kinase (Lck) and zeta chain-associated protein kinase 70 (ZAP70) (Balagopalan et al., 2010; Samelson, 2011; Wang et al., 2010a). These events recruit phospholipase C (PLC) γ1 to the plasma membrane, which hydrolyzes phosphatidylinositol 4, 5-bisphosphate (PIP2) into inositol trisphosphate (InsP3) and diacyl glycerol (DAG). DAG predominantly activates NF-κB signalling pathway via activation of protein kinase C (PKC) θ, Bcl-10/Carma 1, NF-κB-inducible kinase (NIK), and inhibitor of NF-κB (IκB) kinase (IKK) complex that eventually phosphorylates IκB (Fig. 1A) (Muller and Rao, 2010; Smith-Garvin et al., 2009). Phosphorylation of IκB leads to its degradation and nuclear translocation of NF-κB transcription factors. DAG also activates Ras-mediated signalling pathway via activation of Ras guanine nucleotide releasing protein 1 (RasGRP1) which induces phosphorylation-induced activation of AP-1 (Fos-Jun) transcription factors mediated by kinases, dual specificity mitogen-activated protein kinase kinase (MEK) 1/2 and extracellular signal-regulated kinases (ERKs). The other product of PLCγ1 enzymatic activity, InsP3, binds to the InsP3 receptor (InsP3 R) on the ER membrane and releases Ca2+ from the ER into the cytoplasm and this store depletion leads to activation of CRAC channels on the plasma membrane. One of the most studied Ca2+-dependent signaling pathways in the immune system is the Ca2+-calmodulin/calcineurin-NFAT pathway. Upon increase of [Ca2+]ivia the CRAC channels, calmodulin binds Ca2+ and forms a complex with the protein phosphatase calcineurin, which in turn dephosphorylates the heavily-phosphorylated, cytoplasmic NFAT. Dephosphorylation of NFAT exposes its nuclear localization sequence (NLS) and induces its translocation from the cytoplasm to the nucleus (Gwack et al., 2007a; Hogan et al., 2003). Nuclear NFAT forms a multimeric protein complex with itself or with other transcription factors (e.g. AP-1) to induce gene transcription involved either in cytokine production, cell proliferation, growth arrest, or cell death, depending on the amplitude and duration of [Ca2+]i elevation as well as association with other transcription factors (Kim et al., 2011; Macian, 2005; Macian et al., 2002).
Fig. 1.
Signalling pathways of T cell receptor stimulation. (A) Antigen engagement of T cell receptor induces a series of phosphorylation events. Coreceptor (e.g. CD4) ligation in T cells activates protein tyrosine kinase Lck, which phosphorylates the ζ chain of TCR/CD3 complex to recruit ZAP-70 to the TCR/CD3 complex. ZAP70 phosphorylates two adaptor proteins LAT and SLP-76 that results in assembly of a signaling complex containing Vav1 and phospholipase C (PLC-γ1). This signaling complex recruits further downstream effector molecules including Rac1 and a Rho GTPase, cdc42 that have pleiotropic effects in cytoskeleton reorganization, p38/JNK, and Ca2+-NFAT signalling pathways. Cytoskeleton reorganization is important for formation of the immunological synapse between antigen presenting cells and T cells. Activated PLC-γ1 hydrolyzes PIP2 (phosphatidylinositol 4, 5-bisphosphate) into InsP3 (Inositol 1,4,5 trisphosphate) and DAG (diacyl glycerol). While DAG activates PKC (protein kinase C)-NF-κB and RasGRP1-AP-1 signalling pathways, InsP3 binds to the InsP3 receptor (InsP3 R) on the ER membrane to empty the ER Ca2+ store. ER Ca2+ depletion induces opening of CRAC channels, a prototype of store-operated Ca2+ channels. Eleva-ted [Ca2+]i triggers a broad range of downstream signalling pathways including the Ca2+-calmodulin/calcineurin-NFAT and the Ca2+-CaMKII-MEF2 signaling pathway. Ca2+-bound calmodulin forms a complex with a protein phosphatase calcineurin and dephosphorylates the heavily phosphorylated, cytoplasmic NFAT leading to its nuclear translocation. Nuclear NFAT forms a multi-meric protein complex with itself or with other transcription factors (e.g. AP-1) to induce gene transcription. (B) Schematic of the murine NFAT1 (NFATc2) protein. The transcription activation domains that interact with transcriptional cofactors are located at the N and C terminus (TAD-N and TAD-C). DNA binding domain shows the highest homology with the Rel homology domain of Rel-family transcription factors (RHD). The regulatory domain (REG) contains multiple phosphorylation sites to maintain cytoplasmic localization of NFAT under resting conditions and a docking site for Ca2+-calmodulin-calcineurin complex (SPRIET motif). After dephosphorylation by the protein phosphatase complex, the nuclear localization sequence (NLS) within the regulatory domain is exposed leading to nuclear import of NFAT. Serine-rich region (SRR) 1, Ser-Pro-X-X repeat motif (SP) 2, and SP3 within the regulatory domain are phosphorylated by casein kinase 1 (CK1), glycogen synthase kinase 3 (GSK3), and dual-specificity tyrosine-phosphorylation-regulated kinase (DYRK) family kinases, respectively. DYRKs play a role as a priming kinase for CK1 and GSK3-mediated phosphorylation.
In addition to Ca2+-calcineurin-NFAT pathway, increased [Ca2+]i activates Ca2+-calmodulin-dependent kinase II (CaMKII) that leads to activation of cyclic-AMP-responsive-element-binding protein (CREB) and myocyte enhancer factor 2 (MEF2) (Oh-hora, 2009). Elevated [Ca2+]i also regulates the Ras signalling pathway by binding to EF-hand motifs of RasGRP1 (Mor and Philips, 2006). The Ras guanine nucleotide exchange factor (RasGEF) activity of RasGRP1 on the Golgi depends on DAG and Ca2+ that eventually leads to activation of AP-1 transcription factor. Therefore, Ca2+ signalling is integrated with other signalling pathways at the DNA response elements of transcription factors, resulting in cell proliferation, cytokine gene expression, differentiation, or cell death depending on the intensity of diverse signalling pathways.
REGULATION OF NFAT IN T CELLS
Calcineurin is a Ca2+-calmodulin complex-dependent serine/threonine protein phosphatase, consisting of a catalytic subunit, calcineurin A (CnAα, CnAβ, and CnAγ) and a regulatory subunit calcineurin B (CnB1 and CnB2). NFAT consists of four homologous NFAT1 (NFATc2), NFAT2 (NFATc1), NFAT3 (NFATc4), and NFAT4 (NFATc3) (Hogan et al., 2003; Macian, 2005; Serfling et al., 2006; Wu et al., 2007). Most of the NFAT genes are expressed in lymphocytes, however NFAT1 is predominantly expressed in naïve, single-positive T cells. The level of active nuclear NFAT depends both on the intensity of Ca2+ influx and on the inducible kinases that are active under a particular stimulation condition. TCR stimulation also induces expression of a short isoform of NFAT2 (NFATc1), NFATc1/αA (Serfling et al., 2012). NFATc1/αA plays a more positive role in T cell activation than the other NFAT family members by supporting proliferation and protecting against cell death upon stimulation.
NFAT proteins contain an N-terminal transactivation domain (TAD), a regulatory domain, a highly conserved DNA-binding domain (Rel-homology domain, RHD) and a C-terminal TAD (Fig. 1B). The regulatory domain, which is moderately conserved among NFAT proteins, contains multiple serine-rich regions (SRRs) and Ser-Pro-X-X repeat motifs (SPs) that are phosphorylated by NFAT kinases including casein kinase I (CK1), glycogen synthase kinase 3 (GSK3), and dual-specificity tyrosine-phosphorylation-regulated kinase (DYRK) family (Fig. 1B) (Gwack et al., 2007a; Wu et al., 2007). DYRK-family kinases were identified from a genome-wide RNAi screen designed to identify regulators of NFAT localization (Gwack et al., 2006). In a parallel approach, DYRK1A was also identified as an NFAT kinase from the analyses of Down’s syndrome critical regions (DSCRs) (Arron et al., 2006). The authors showed that DYRK1A gene lies in the critical region of human chromosome 21 and its high expression caused by trisomy of chromosome 21 accounts for dysregulated NFAT signalling in Down’s syndrome. NFAT kinases can be subdivided into two categories based on their function-NFAT export kinases that re-phosphorylate nuclear NFAT for its export into the cytoplasm and NFAT maintenance kinases that phosphorylate cytoplasmic NFAT under resting conditions. CK1 phosphorylates the SRR-1 region of NFAT and functions as both, maintenance and export kinase. DYRKs phosphorylate the SP-3 motif of NFAT, thereby priming for GSK3- and CK1-mediated phosphorylation of the SP-2 and SRR-1 motifs, respectively. Cytoplasmic DYRK2 serves as the maintenance kinase while nuclear DYRK1A serves as the export kinase. GSK-3 functions as an export kinase and phosphorylates the SP-2 motif of NFAT1 and both the SP-2 and SP-3 motifs of NFAT2, and its activity is suppressed by Akt, a kinase activated in response to diverse signaling pathways in different cell types and by CD28 co-stimulatory signal in T cells (Gwack et al., 2007a). The substrate sites for GSK3 in NFAT are created after previous phosphorylation by a “priming” kinase that can be either protein kinase A (PKA) or DYRK1A (Fig. 1B) (Arron et al., 2006; Gwack et al., 2006). In addition to protein kinases, RNA also plays an important role in the regulation of NFAT localization. Under resting conditions, heavily phosphorylated NFAT proteins exist in a complex with the non-coding RNA Noncoding [RNA] Repressor of NFAT (NRON). NRON creates a platform for RNA-protein scaffold complexes containing NFAT, NFAT kinases [e.g. CK1, GSK3, DYRK, and leucine-rich repeat kinase 2 (LRRK2)], IQ motif-containing GTPase activating protein (IQGAP), and CaM to facilitate phosphorylation/dephosphorylation events (Liu et al., 2011; Sharma et al., 2011; Willingham et al., 2005). The regulatory domain of NFAT also contains a docking site for calcineurin, with a highly conserved consensus sequence Pro-X-Ile-X-Ile-Thr (in which X can be any amino acid) (Aramburu et al., 1999). Upon TCR stimulation-induced increase in [Ca2+]i, Ca2+-bound CaM activates calcineurin, which dephosphorylates multiple phosphoserines in the SRR and SP motifs of NFAT, exposing its NLS and facilitating nuclear translocation. In the nucleus NFAT can homodimerize or cooperate with multiple transcriptional partners, including AP-1 and forkhead box P-family proteins (FOXP2 and FOXP3) to activate or suppress specific transcriptional programs (Hogan et al., 2003; Wu et al., 2006b).
ESSENTIAL COMPONENTS OF CRAC CHANNELS IN T CELLS, ORAI1 AND STIM1
Although existence of CRAC channels was identified by electrophysiological methods based on their unique biophysical characteristics (Hoth and Penner, 1992), the molecular identity of the channel components was missing. After the completion of genomic sequencing, modern reverse genetics technologies such as genome-wide RNAi screening in Drosophila cells implemented by Dr. Norbert Perrimon and colleagues greatly supported identification of the molecular components of CRAC channels (Mohr et al., 2010). Genome-wide RNAi screens in Drosophila cells identified the Drosophila gene olf186-F (named Drosophila Orai) and the mammalian homologues Orai1, 2 and 3 as subunits of the CRAC channels (Feske et al., 2006; Gwack et al., 2007b; Vig et al., 2006; Zhang et al., 2006). Furthermore, a missense mutation of R91W was identified in the ORAI1 gene from severe combined immune deficiency (SCID) patients that lacked functional CRAC channels and expression of wild-type Orai1 recovered CRAC currents in the patient cells (Feske et al., 2006).
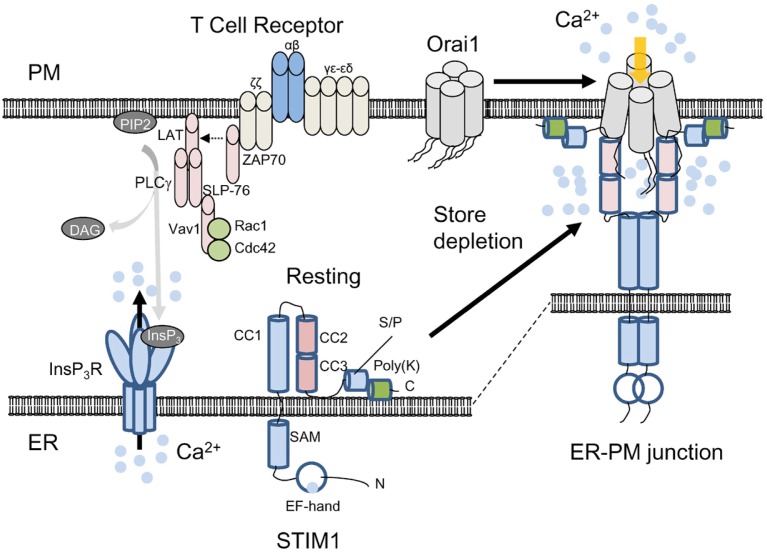
Prior to identification of Orai1, limited RNAi screen in Drosophila and HeLa cells identified STIM1, a Ca2+-binding protein localized predominantly in the endoplasmic reticulum (ER) as an important regulator of CRAC channel-mediated Ca2+ entry (Liou et al., 2005; Roos et al., 2005; Zhang et al., 2005). STIM1 contains an N-terminal EF-hand that detects luminal ER [Ca2+], a single transmembrane domain, and a long C-terminal cytoplasmic region (Fig. 2) [reviewed in (Soboloff et al., 2012)]. STIM1 plays a pivotal role in sensing ER [Ca2+] and CRAC channel opening. Upon ER Ca2+ depletion, STIM1 loses bound Ca2+, multimerizes, translocates to PM-proximal ER, mediates clustering of Orai proteins on the PM, and stimulates Ca2+ entry (Liou et al., 2005; Roos et al., 2005; Zhang et al., 2005). STIM1 interacts with Orai1 via CRAC-activating domain (CAD)/STIM1 Orai1 activating region (SOAR) (Muik et al., 2009; Park et al., 2009; Yuan et al., 2009). The CAD/SOAR fragment of STIM1 (coiled coil domain 2 and 3 in Fig. 2) was shown to play a pivotal role in activation of Orai1 by direct binding to the cytosolic N and C terminus of Orai1. Furthermore, the stoichiometry of STIM1 binding to Orai1 can affect the fast inactivation properties of CRAC channels, implicating STIM1 as a subunit of CRAC channels (Scrimgeour et al., 2009). These and other studies showed that CRAC channel activation involves multiple steps including STIM1 oligomerization, co-clustering of Orai1 and STIM1 at the ER-PM junctions, and gating of Orai1 (Liou et al., 2007; Muik et al., 2008; 2009; Navarro-Borelly et al., 2008; Park et al., 2009; Yuan et al., 2009).
Fig. 2.
Activation mechanism of Orai1 and STIM1. Schematic showing current understanding of CRAC channel activation. Under resting conditions, Orai1 and STIM1 are distributed at the PM and the ER membrane. The subunit stoichiometry of Orai1 under resting and stimulated conditions is currently unclear. For convenience, a tetrameric assembly of Orai1 is depicted here. Upon store depletion triggered by T cell receptor stimulation and InsP3 production via PLCγ1, STIM1 oligomerizes by sensing ER Ca2+ depletion with its ER-luminal EF-hand domain, clusters and translocates to the ER-PM junctions. By physical interaction with the cytoplasmic, N and C terminus of Orai1 through the CAD/SOAR domain (coiled coil domains 2 and 3), clustered STIM1 recruits and activates Orai1 in the ER-PM junctions. STIM1 contains an ER-luminal region comprising the EF-hand and SAM domains, a single transmembrane segment, and a cytoplasmic region. The cytoplasmic region has three coiled-coil domains (CC1, 2, and 3), serine/proline-rich domain (S/P) containing the residues involved in posttranslational modifications (see below), and a polybasic region (poly-K) at the C terminus that interacts with phosphoinositides after store depletion. The exact role of the poly-K tail of STIM1 in resting conditions is not known, but it may interact with phosphoinositides on the ER membrane to maintain its inactive, folded structure.
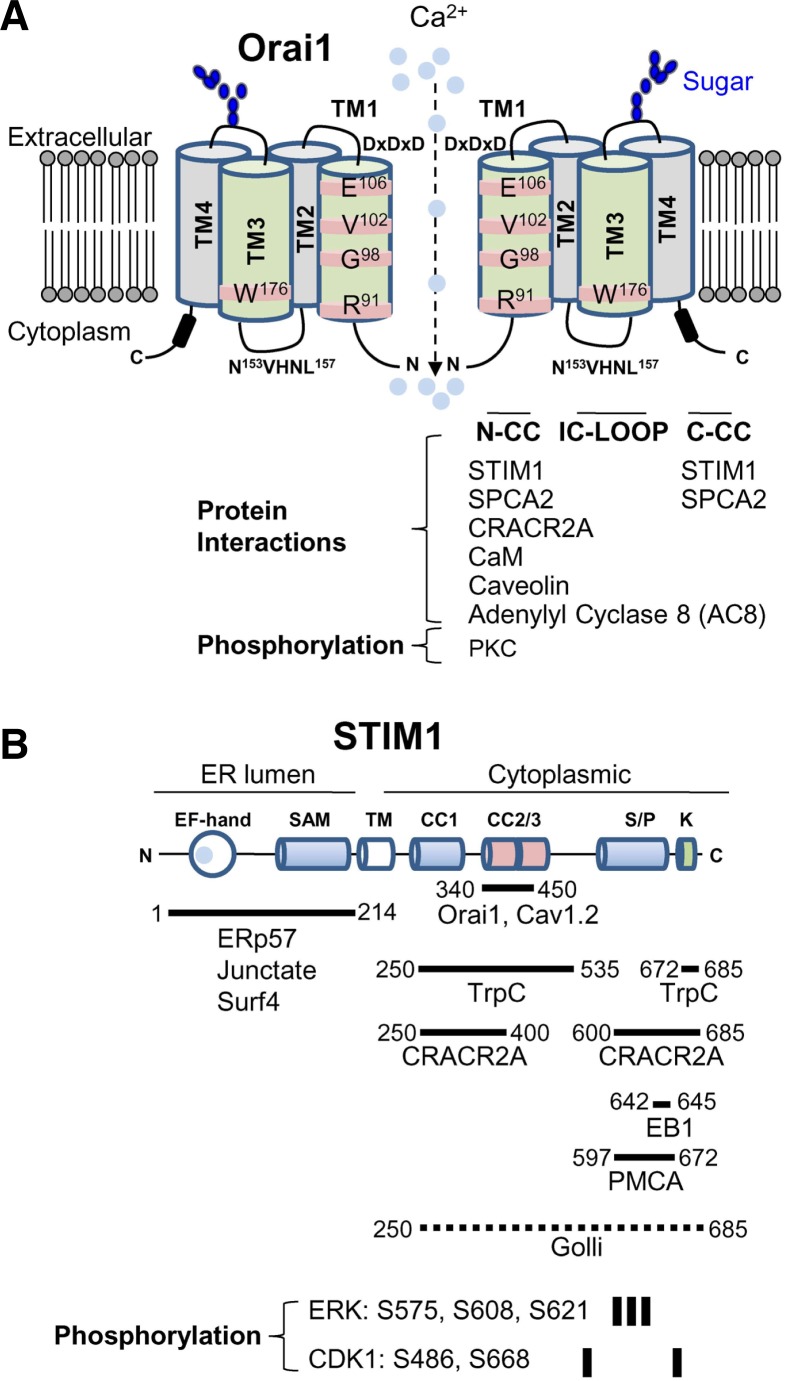
Orai1 has four transmembrane segments (TM1-TM4) with its N and C terminus facing the cytoplasm (Fig. 3A). The TM1 of Orai1 has been shown to line the pore, and residues R91, G98, V102, and E106 in TM1 are important for Ca2+ selectivity and gating (Cahalan and Chandy, 2009; Hogan et al., 2010; Lewis, 2011; McNally et al., 2012; Putney, 2009; Zhang et al., 2011). Although TM3 does not line the pore, it was shown that the residues within the TM3 segment including E190 and W176 influence channel gating and ion selectivity indicating a functional relation between TM1 and TM3 (Prakriya et al., 2006; Srikanth et al., 2011). The cytoplasmic N and C terminus of Orai1 mediate channel opening by interaction with STIM1 after store depletion. CRAC channels are also negatively regulated by excess Ca2+, resulting in Ca2+-dependent inactivation (CDI) of the channels (Hoth and Penner, 1992; 1993; Zweifach and Lewis, 1995). In addition to channel gating, mutational studies showed that all the cytoplasmic regions of Orai1 including the N terminus, the intracellular loop, and the C terminus are involved in CDI (Lee et al., 2009; Mullins et al., 2009; Srikanth et al., 2010b). Thus, intracellular domains of Orai1 are not only important for channel gating, but also crucial for channel inactivation, to avoid deleterious consequences of excessive Ca2+ such as cell death.
Fig. 3.
Interacting partners and posttranslational modification of Orai1 and STIM1. (A) Schematic of Orai1. Orai1 has four transmembrane segments (TM1-TM4). It has two extracellular domains and the second extracellular domain between TM3 and TM4 contains the asparagine (N223) residue involved in glycosylation (indicated in blue). The TM1 lines the pore and the residues in TM1 involved in Ca2+ selectivity and gating are depicted. The TM3 does not line the pore, but affects ion selectivity by possible interaction with TM1. Subunits of Orai1 form CRAC channels, but the molecular stoichiometry of CRAC channels is still in question. Schematic depicts a dimeric form for convenience of drawing. Orai1 contains three intracellular domains including the N terminus (N-CC), intracellular loop (IC-LOOP), and C-terminal coiled-coil domain (C-CC) that are important for protein interactions and channel activation/inactivation. Known molecular interactors of Orai1 in these intracellular domains are summarized. The N terminus of Orai1 is phosphorylated by protein kinase C (PKC). (B) Schematic of STIM1. STIM1 contains Ca2+-binding EF hands and a sterile α motif (SAM) domain in the ER-luminal region, a single transmembrane segment, and a long cytoplasmic region. The cytoplasmic region has three coiled-coil domains, serine/proline-rich domain, and a polybasic segment at the C terminus. Proteins associating with each of these domains are indicated. The fragment of STIM1 (340-450) involved in Orai1 interaction/gating is indicated. Golli proteins interact with the cytoplasmic region of STIM1, but the detailed interaction domain is not determined (dotted line). Residues phosphorylated by extracellular signal-regulated kinase (ERK) and cyclin-dependent kinase (CDK1) are indicated.
PATTERN AND LOCATION OF Ca2+ SIGNALS IN IMMUNE CELLS
A general misconception of Ca2+ signalling arises from its broad role in downstream events as a universal second messenger. However, many evidences suggest that Ca2+ can play a more specialized role in activation of specific signalling pathways depending on the amplitude, oscillation frequency, and the route of entry. In physiological conditions unlike treatment with ionophore or a blocker of SERCA (sarcoplasmic and endoplasmic reticulum Ca2+ ATPase) thapsigargin, T cells show Ca2+ oscillation after TCR engagement that is regulated by a balance between cytoplasmic and ER Ca2+ concentrations (Dolmetsch and Lewis, 1994; Dolmetsch et al., 1998). NFAT, AP-1 and NF-κB transcription factors were shown to be optimally activated in response to different oscillation patterns of Ca2+ in T cells. Transient high Ca2+ spikes evoked sustained activation of JNK and NF-κB, but not NFAT, whereas prolonged low increases in [Ca2+]i, which were insufficient to activate JNK or NF-κB, sufficed to activate NFAT (Dolmetsch et al., 1997). Recently, Kar et al. (2012a) showed that different agonists can induce store depletion via activating different STIM proteins in rat basophil leukemia (RBL) cells and this process may generate unique Ca2+ oscillation patterns. These studies suggest that based on differences in amplitude, duration and stimuli, the same second messenger, Ca2+ can activate distinct downstream signalling pathway(s).
Another interesting observation was obtained from comparative studies between Orai1-and transient receptor potential cation (TrpC) 1 channels-mediated Ca2+ entry induced by agonist such as carbachol (Ong et al., 2012). Both Orai1 and TrpC1 mediate agonist-induced Ca2+ entry; however, only Orai1-mediated Ca2+ oscillations efficiently induced nuclear translocation of NFAT, while TrpC1 channel activation led to sustained Ca2+ entry at higher agonist concentrations and activated NF-κB-mediated transcription. It was recently shown that [Ca2+] in the microdomains near the CRAC channels are more important for nuclear translocation of NFAT than global increase of Ca2+ emphasizing the importance of local Ca2+ concentrations in T cells (Kar et al., 2011). These observations are consistent with the results from comparative studies between CaV1 and CaV2 in activating CaMKII and phosphorylating the cAMP response element-binding protein (CREB) transcription factor, where CaV1 has a specialized role in activation of CaMKII by formation of nanodomain(s) of high [Ca2+] (Wheeler et al., 2012). Together, these results indicate that not only the amplitude of Ca2+ signalling, but also the pattern (e.g. oscillation frequency, sustained levels, and microdomains) can influence stimulation of diverse downstream signalling pathways.
Upon antigen engagement of CD4+ T cells, Orai1 and STIM1 translocate into the immunological synapse, a site of contact between TCRs and MHC class II molecules with antigen (Barr et al., 2008; Lioudyno et al., 2008). These results indicate that Ca2+ entry via CRAC channels occurs at specific locations in T cells. The location of Ca2+ entry is proven to be the site of Orai1 and STIM1 clustering (Luik et al., 2006). Because mitochondria translocate into the immunological synapse to buffer local Ca2+ and inhibit the negative feedback mechanism via plasma membrane Ca2+ ATPase (PMCA) (Quintana et al., 2011), the specific location of Orai1 and STIM1 clustering can be important for stimulation of specific signaling pathway(s). Ca2+ does not play a direct role in the formation of the immunological synapse in a short term because overexpression of the dominant negative mutant of Orai1 did not influence formation of immunological synapse (Lioudyno et al., 2008). However, it may influence the stability of the immunological synapse by suppressing T cell motility indirectly. The physiological role of polarized Ca2+ signalling and the molecular mechanism of Orai1 and STIM1 aggregation and retention at the immunological synapse need further investigation. Since the immunological synapse is the site for aggregation of signalling molecules including many tyrosine and serine/threonine kinases, it is possible that CRAC channel clustering and functions are regulated by TCR signalling molecules depending on the signal intensity and costimulation (e.g. CD28 or CTLA-4).
INTERACTING PARTNERS OF ORAI1
After identification of Orai1, numerous regulators and interacting partners of Orai1 have been identified [summarized in Fig. 3A, (Srikanth and Gwack, 2012)]. It will be of interest to determine their role in T cell functions. Proteins interacting with the N terminus of Orai1 include a novel cytoplasmic EF-hand-containing protein, CRAC channel regulator 2A (CRACR2A) (Srikanth et al., 2010a). It was shown that CRACR2A forms a ternary complex by direct interaction with Orai1 and STIM1. Interestingly, CRACR2A also regulates Ca2+ oscillation in T cells by serving as a cytoplasmic Ca2+ sensor. Overexpression of CRACR2A mutant defective in Ca2+ binding disrupted Ca2+ oscillation pattern (Srikanth et al., 2010a). Recent studies have shown that inhibition of Orai1 and STIM1 activity greatly diminishes cervical and mammary tumor cell migration and metastasis in vitro and in vivo (Chen et al., 2011; Feng et al., 2010; Yang et al., 2009). Interestingly, an isoform of the secretary pathway Ca2+-ATPase, SPCA2 was shown to enhance mammary tumor cell growth by raising [Ca2+]i via a direct interaction with Orai1 in a STIM-independent manner (Feng et al., 2010).
Calmodulin was identified as a negative regulator of CRAC channels. CaM binds the N terminus of Orai1 at elevated [Ca2+]i and induces fast Ca2+-dependent inactivation of the channel (Mullins et al., 2009). Another negative regulator of Orai1 function is caveolin. Machaca and colleagues used xenopus oocyte as a model and identified a role for caveolin in internalization of Orai1 (Yu et al., 2010). It was shown that during meiosis, SOCE is inhibited due to internalization of Orai1 and impairment of STIM1 clustering (Yu et al., 2009). The authors showed that Orai1 internalization occurred via a caveolin and dynamin-dependent endocytic pathway and mapped a caveolin-binding site in the N terminus of Orai1 and demonstrated protein interaction between Orai1 and caveolin (Yu et al., 2010).
Recently, a crosstalk between the CRAC channel pathway and cyclic adenosine monophosphate (cAMP) signaling pathway has been identified. An important intracellular signaling molecule cAMP is generated by adenylyl cyclase (AC)-mediated cleavage of adenosine triphosphate, which in turn activates downstream PKA pathway. Among the nine membrane-bound ACs, four of them (AC1, AC8, AC5 and AC6) are known to be regulated by Ca2+. Of those four, AC1 and AC8 are stimulated through an interaction with Ca2+-CaM complex. A role of STIM1 in activation of AC activity has been demonstrated (Lefkimmiatis et al., 2009). It was shown that intracellular store depletion, independent of cytosolic Ca2+ concentration, led to recruitment of ACs in a STIM1-dependent mechanism referred by the authors as “store-operated cAMP signalling”. Another study demonstrated a direct protein interaction between Ca2+-CaM activated AC8 and Orai1 N terminus (Willoughby et al., 2012). Using Forster resonance energy transfer (FRET) technique, GST pulldown, and immunoprecipitation analyses, the authors detected a constitutive association between Orai1 and AC8, which was not affected by store depletion and Orai1 activation (Willoughby et al., 2012). Both these studies together show that STIM1 itself or Ca2+ entry via Orai1 plays an integral role in regulating crosstalk between SOCE and cAMP signaling pathways.
BINDING PARTNERS OF STIM1
Only a few positive regulators of STIM1 function have been identified so far (Fig. 3B). Previous results showed that Orai1 and STIM1 translocate into pre-existing junctional areas, a space of 10–25 nm between the PM and ER membranes (Varnai et al., 2007; Wu et al., 2006a). STIM1 contains a polybasic stretch of amino acids in its C terminus that bind phosphoinositides, and is important for its clustering at ER-PM junctions (Korzeniowski et al., 2009; Liou et al., 2007; Walsh et al., 2010). Truncation of this polybasic domain abolished STIM1 accumulation at ER-PM junctions, and overexpression of Orai1 recovered accumulation of STIM1-ΔK mutant into the ER-PM junctions (Liou et al., 2007; Park et al., 2009). In excitable cells (e.g. neurons and muscle cells), proteins including junctophiins, junctin, and junctate localize to the junctions between the PM and ER/sarcoplasmic reticulum (SR) membranes and form a structural foundation for regulating the intracellular Ca2+ stores and Ca2+ entry (Berridge et al., 2003; Carrasco and Meyer, 2011; Takeshima et al., 2000; Weisleder et al., 2008). It was shown that that junctate is a structural component of the ER-PM junctions in T cells and recruits STIM1 into these junctions after store depletion suggesting a conserved function of the components of the ER-PM junctions in excitable and non-excitable cells (Srikanth et al., 2012).
Numerous negative regulators of STIM1 have been identified. An ER resident protein SARAF (SOCE-associated regulatory factor; TMEM66) was identified as an interacting partner of STIM1 that facilitates the Ca2+-dependent slow inactivation of CRAC channels (Palty et al., 2012). SARAF was shown to interact with STIM1 via its cytoplasmic C terminus containing a serine-proline rich domain and polybasic region under resting conditions. After store depletion, SARAF was shown to translocate to the ER-PM junctions with STIM1 and facilitate the slow inactivation of CRAC channels. Surfeit locus protein 4 (Surf4) was also identified as an interacting partner of STIM1 (Fujii et al., 2012). Surf4 interacted with the ER-luminal region of STIM1 and its deficiency increased STIM1 translocation, thus SOCE. A surface plasmon resonance screen monitoring the changes in resonance signal between immobilized ER-luminal N terminus of STIM1 on a chip and various ER resident proteins identified a 58-kDa thiol oxidoreductase ERp57 as an ER-intraluminal binding partner and a negative regulator of STIM1 (Prins et al., 2011). Polycystin-1 fragment P100 and Stanniocalcin 2 (STC2) have been shown to interact with STIM1 and negatively regulate its translocation. Mutations in polycystin-1 (PC-1) or polycystin-2 (PC-2) encoding genes result in autosomal dominant polycystic kidney disease (ADPKD). PC-1 cleavage fragment of 100-kDa, P100, inhibited SOC currents when expressed in Xenopus oocytes (Woodward et al., 2010). Another study identified stanniocalcin 2, a homologue of stanniocalcin 1, a secreted glycoprotein involved in Ca2+ uptake during hypercalcemia, as a negative regulator of SOCE (Zeiger et al., 2011). Stanniocalcin 2 was shown to interact with STIM1, however, the molecular mechanism of how it inhibited SOCE remains unknown. STIM1 was identified as binding partner of EB1, a microtubule tip-binding protein, and shown to play a role in ER tubulation (Grigoriev et al., 2008). Recently, a novel protein of unknown function, POST (partner of STIM1, TMEM20) was also identified as a STIM1 interactor by affinity purification of Orai1 from Jurkat T cells (Krapivinsky et al., 2011). POST is a 10-transmembrane containing protein localized to the PM and ER membrane. While POST expression did not affect STIM1-Orai1 induced CRAC currents, the authors suggested a role of POST in regulating plasma membrane Ca2+ ATPase (PMCA) activity.
STIM1 is also known to interact with and regulate ion channels in addition to Orai1. An interaction between STIM1 and TrpC channels has been studied [(Huang et al., 2006; Ong et al., 2007; Worley et al., 2007), but also see (DeHaven et al., 2009)]. The arachidonic acid-regulated Ca2+ (ARC) channels, a class of highly Ca2+-selective ion channels, activated by low concentrations of arachidonic acid are opened by the pool of STIM1 that constitutively resides in the PM (Mignen et al., 2007). In addition to the ARC channels, two groups showed an interaction between STIM proteins and CaV1.2 channels (Park et al., 2010; Wang et al., 2010b). The CAD/SOAR fragment of STIM1 required for Orai1 activation was shown to inactivate and inhibit surface expression of CaV1.2 (Fig. 3B) (Park et al., 2010; Wang et al., 2010b).
MULTIPLE Ca2+ SENSORS REGULATE CRAC CHANNEL ACTIVITY
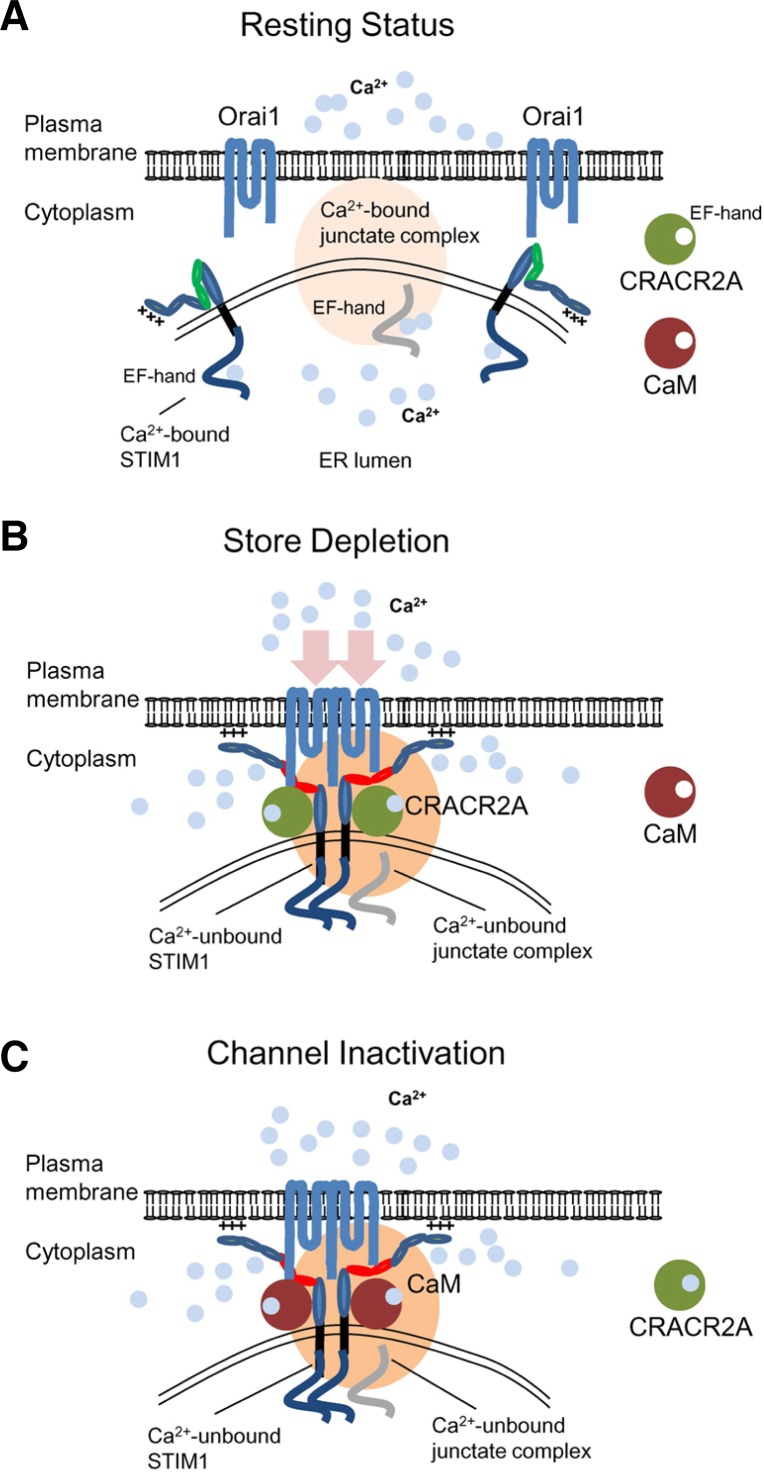
The protein interaction studies suggest an interesting scenario where multiple Ca2+-sensing molecules are involved in regulation of CRAC channel activity. Under resting conditions, Orai1 and STIM1 are distributed at the PM and the ER membrane, respectively. Ca2+-bound STIM1 exists as a folded structure mediated by intramolecular interaction between the CAD/SOAR domain and the autoinhibitory region within the coiled-coil domain (Korzeniowski et al., 2010). Junctate also exists as a Ca2+-bound form via its Ca2+ binding domains in the ER lumen (Fig. 4A). Cytoplasmic Ca2+ sensors such as CaM and CRACR2A do not bind Ca2+ under resting conditions. Upon store depletion, STIM1 loses bound Ca2+, unfolds itself, oligomerizes, and translocates to form clusters at the ER-PM junctions premarked by junctate protein (Fig. 4B). ER store depletion causes junctate to lose bound Ca2+ and trap STIM1 into the ER-PM junctions by direct interaction. At this stage, cytoplasmic CRACR2A stabilizes Orai1-STIM1 complex by protein interaction. As [Ca2+]i increases, CRACR2A binds Ca2+ via its EF-hands and dissociates from the Orai1-STIM1 complex and Ca2+-bound CaM is recruited into the Orai1-STIM1 complex to inactivate the CRAC channels (Fig. 4C). When ER Ca2+ is re-filled by SERCA following the increase of [Ca2+]i, the protein complex of Orai1 and STIM1 dissociates and returns to the resting state.
Fig. 4.
CRAC channel regulation by multiple Ca2+-sensing molecules in the ER and cytoplasm. (A) Schematic showing a possible mechanism of CRAC channel regulation. Under resting conditions, Orai1 and STIM1 are distributed at the PM and the ER membrane. Junctate is located at ER-PM junctions in a Ca2+-bound form via its ER-luminal EF hand domain (indicated in gray). Cytoplasmic Ca2+ sensors such as CRACR2A and calmodulin are not bound to Ca2+ in resting conditions. (B) Upon store depletion, STIM1 oligomerizes by sensing ER Ca2+ depletion with its ER-luminal EF-hand domain, and translocates to form clusters at the ER-PM junctions. By physical interactions with Orai1 through the CAD/SOAR domain (depicted in red), clustered STIM1 recruits and activates Orai1 in the ER-PM junctions. During the process, junctate loses bound Ca2+ and supports STIM1 recruitment into ER-PM junctions. CRACR2A is recruited into the Orai1-STIM1 complex to stabilize their interactions. (C) Following the increase of cytoplasmic [Ca2+], CRACR2A dissociates from the Orai1-STIM1 complex. Ca2+-bound calmodulin interacts with the N terminus of Orai1 and inactivates the channel via a mechanism called fast inactivation. The slow inactivation of CRAC channels depends on Ca2+ entry and interaction with SARAF. After channel inactivation, once the ER is refilled with Ca2+, Orai1 and STIM1 return to the resting status.
POSTTRANSLATIONAL MODIFICATION OF ORAI1 AND STIM1
Posttranslational modifications have been shown to regulate SOCE. Kawasaki et al showed that Orai1 was phosphorylated at S27 and S30 in the cytoplasmic N terminus by protein kinase C (PKC) β and this phosphorylation suppressed SOCE (Kawasaki et al., 2010) (Fig. 3A). Cells expressing Orai1 mutated at S27 and S30 showed a marginal enhancement of SOCE and CRAC currents (Kawasaki et al., 2010). Interestingly, Orai proteins contain several conserved serine residues, some in the N terminus close to the pore-forming TM1 (S75, S82, S89, and S90; amino acid numbering based on human Orai1), in the intracellular loop (S159 and S163), and C terminus (S260). Based on their location, it is possible that phosphorylation of these residues may influence the regulation of channel gating, inactivation, or protein interactions of Orai1. Future studies examining the mutations of these residues and their impact on SOCE and CRAC currents would be important to understand phosphorylation-mediated regulation of Orai proteins.
Putney and colleagues demonstrated phosphorylation mediated regulation of STIM1 and SOCE during mitosis (Smyth et al., 2009) (Fig. 3B). It was known that SOCE was suppressed during cell division, however, the molecular mechanism behind this event was not known. Smyth et al showed that S486 and S668 located within the serine/threonine-rich domain of STIM1 were phosphorylated during mitosis by cyclin dependent kinase 1 (CDK1) and these phosphorylations suppressed SOCE. Expression of STIM molecules that were truncated at position 482 or with mutations of S486 and S668 significantly rescued SOCE in mitotic cells (Smyth et al., 2009). In a different study, Machaca and colleagues showed that SOCE was suppressed even in meiotic cells. The authors observed trapping of Orai1 in intracellular vesicles and impaired STIM1 clustering in xenopus oocytes during meiosis (Yu et al., 2009). Overexpression of STIM1D76A, a constitutive active mutant also showed impaired cluster formation, suggesting that STIM1 multimerization was blocked during meiosis. A study by Pozo-Guisado et al. (2010) identified a positive role of phosphorylation of STIM1 on SOCE. The authors showed that ERK1 and ERK2 phosphorylate S575, S608, and S621 residues located within the C-terminal S/T-rich domains of STIM1 (Fig. 3B) (Pozo-Guisado et al., 2010). These phosphorylation events were induced by store depletion with thapsigargin as well as ERK agonist, 12-O-tetradecanoylphorbol-13-acetate (TPA). FRET experiments measuring interaction between Orai1 and STIM1 showed that phosphorylation enhanced STIM1 association with Oria1 when compared with a mutant STIM1 that cannot be phosphorylated by ERK1/2 (Pozo-Guisado et al., 2010). Another study reported a positive role of tyrosine phosphorylation of STIM1 in human platelets (Lopez et al., 2012). STIM1 was shown to be phosphorylated at tyrosine residues by immuno-blotting with a classical anti-phospho-tyrosine antibody, 4G10. This event is possibly mediated by Bruton’s tyrosine kinase (Btk) because a specific blocker of Btk, LFM-A13 suppressed tyrosine phosphorylation of STIM1 and protein interactions between Orai1 and STIM1 (Lopez et al., 2012).
DIVERSE ROLES OF CRAC CHANNEL-MEDIATED Ca2+ SIGNALLING IN IMMUNE CELLS
Several reports have described the phenotypes of Orai1-and STIM1-deficient immune cells (Bergmeier et al., 2009; Braun et al., 2009a; Gwack et al., 2008; Oh-Hora et al., 2008; Schuhmann et al., 2010; Vig et al., 2008). In humans, loss of Orai1 and/or STIM1 function causes immune deficiency and symptoms of these patients have been recapitulated in animal models lacking expression of Orai1 or STIM1 proteins. ORAI1-deficient mice showed a reduction in cytokine production by CD4+, CD8+ effector T cells and mast cells (Gwack et al., 2008; Vig et al., 2008). In addition, ORAI1 deficiency impaired Ca2+ influx and functions of neutrophils and platelets (Bergmeier et al., 2009; Braun et al., 2009b; Schaff et al., 2010). STIM1 deficiency showed a pronounced reduction in SOCE, and cytokine production in T cells resulting in resistance to experimental autoimmune encephalomyelitis (EAE) (Oh-Hora et al., 2008; Schuhmann et al., 2010). On the contrary, mice deficient in STIM2, another member of the STIM family, showed a mild defect in SOCE and correspondingly, succumbed to EAE, albeit with less severe symptoms (Schuhmann et al., 2010). These results clearly demonstrate a predominant role of Orai1 and STIM1 in effector T cell functions in vivo.
In addition to a positive role in immune activation, Ca2+ signalling also plays a role in negative regulation of the immune system [summarized in (Qu et al., 2011)]. Dysregulated Ca2+ signalling also induces autoimmune and lymphoproliferative disorders. SCID patients harboring mutations in STIM1 also showed autoimmune hemolytic anemia, thrombocytopenia and enlarged spleen and lymph nodes (Picard et al., 2009). Mice lacking both STIM1 and STIM2 showed lymphoproliferative disorder in addition to SCID phenotype (Oh-Hora et al., 2008). The lymphoproliferative phenotype of double knockout mice was attributed to a severe reduction in regulatory T cells (Tregs) which are crucial for immune tolerance (Oh-Hora et al., 2008). These observations identify an important role for Ca2+-NFAT signaling pathway in development of Tregs (Wu et al., 2006b). In addition, B-cell specific knockout of STIM1 and STIM2 showed decreased levels of the immune suppressive cytokine IL-10, that led to development of autoimmune diseases (Matsumoto et al., 2011). Together, these reports indicated that Ca2+ signalling plays an important role in both aspects of immune regulation - effector T cell activation and immune tolerance, and a block of CRAC channel activity can lead to completely opposite outcomes like immune deficiency and autoimmunity.
Another role of Ca2+ signalling in immune suppression is observed when T cells undergo cell death. Cell death induced by TCR stimulation is critical for homeostasis of peripheral T cells after antigen clearance and for negative selection of auto reactive T cells in the thymus (Budd, 2001; Krammer et al., 2007; Strasser, 2005). Abrogation of T cell death leads to hypersensitive immune reaction and autoimmune disorders. Activation induced T cell death occurs through the death receptor- and mitochondria-mediated pathways. Death receptor-mediated apoptosis involves the Fas ligand/Fas signalling pathway, primarily regulated by NFAT (Hodge et al., 1996; Macian et al., 2002; Serfling et al., 2006) while mitochondria-mediated cell death occurs due to loss of mitochondrial membrane potential (Marsden and Strasser, 2003; Strasser, 2005). Mitochondria-mediated cell death pathway involving the Bcl-2 family members (e.g. Bcl-2 and Bcl-XL) and the BH3-only proteins (e.g. Bad, Bik, Bim, and Noxa) play an important role in T cell death and survival as seen in isolated T cells and in animal models (Budd, 2001; Hildeman et al., 2002; 2007; Marrack and Kappler, 2004; Strasser, 2005; Strasser and Pellegrini, 2004). It was shown that Orai1-deficient T cells are strongly resistant to cell death due to reduction in death receptor-and mitochondria-mediated cell death mechanisms by decreasing expression levels of proapoptotic genes and mitochondrial Ca2+ uptake (Kim et al., 2011). These results suggested that Ca2+ signalling contributes to both cell death mechanisms via accumulation of Ca2+ in the mitochondria and NFAT-mediated transcriptional regulation. A positive role of Orai1 in cell death is also observed in various cell types with different inducers of cell death such as soft substrate, oxidation, and ER stress (Chiu et al., 2008; Flourakis et al., 2010; Hawkins et al., 2010; Henke et al., 2013). However, its negative role in cell death with activation of CD95 (Fas) and type I interferon has also been demonstrated (Khadra et al., 2011; Yue et al., 2012). Therefore, it seems that the role of Ca2+ entry via Orai1 in cell death cannot be generalized; instead, different ligands and inducers may have unique Ca2+ patterns as discussed above that can influence the outcome of cell death or survival.
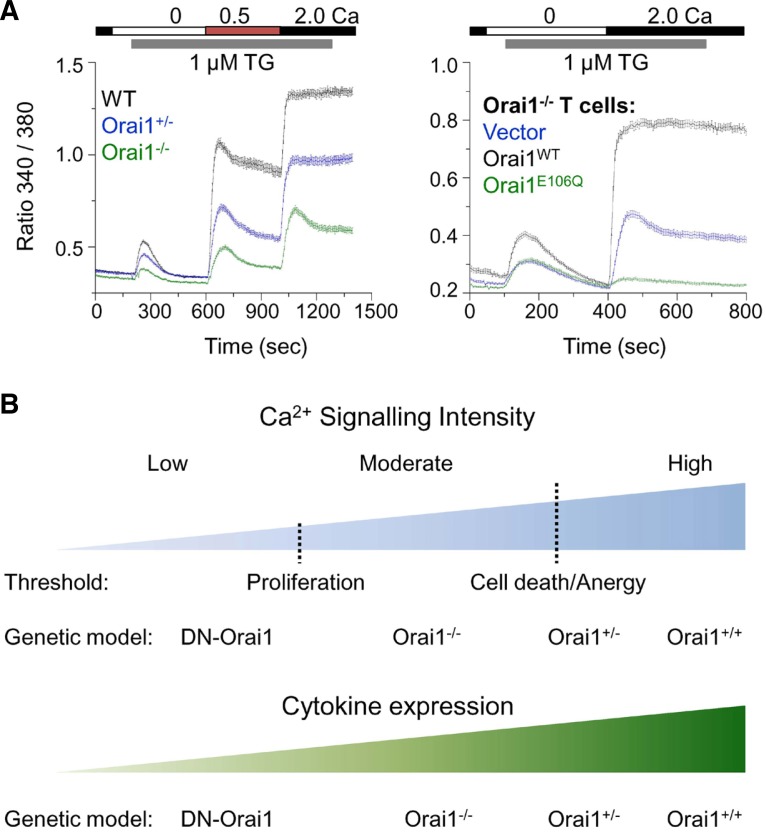
The role of CRAC channels in T cell proliferation seems to be more complex than in cytokine production or cell death because it differs significantly in various mouse models of CRAC channels. It was shown that Orai1−/− T cells proliferate normally although they show a severe reduction in cell death triggered by TCR stimulation (Kim et al., 2011). These results suggest that different thresholds of [Ca2+]i may be necessary for different physiological outcomes, with proliferation requiring much lesser [Ca2+]i than cell death. A further reduction in [Ca2+]i by expression of a dominant negative mutant of Orai1 in Orai1−/− T cells almost completely abolished TCR-induced cell proliferation (Fig. 5A). It was shown that other Orai family members, Orai2 and Orai3, possibly contribute the residual Ca2+ entry in Orai1−/− T cells. Hence the dominant negative mutant Orai1E106Q is likely to heteromultimerize with Orai2 and Orai3 in Orai1−/− T cells to further decrease SOCE (Kim et al., 2011). Orai1 deficiency shows a gene dosage effect in both human and mouse T cells with heterozygotes showing intermediate levels of Ca2+ entry (Feske et al., 2006; Gwack et al., 2008; Kim et al., 2011). Interestingly, Orai1+/− mice do not show any SCID phenotype or defect in cell death suggesting that intermediate levels of [Ca2+]i are sufficient to induce cell death. Ca2+ signaling also plays a pivotal role in the induction of anergy in T cells. Anergic T cells are incapable of proliferation and cytokine expression after antigen encounter (Baine et al., 2009). Repetitive TCR stimulation is unlikely to induce anergy in Orai1−/− T cells because proliferation and cell cycle progression were actively induced. Therefore, the threshold levels of [Ca2+]i for anergy induction is likely to be higher than that observed in Orai1−/− T cells. These studies delineate the thresholds of [Ca2+]i with extremely low [Ca2+]i (as seen in Orai1−/− T cells expressing dominant negative Orai1E106Q) blocking both proliferation and cell death, slightly higher levels (as observed in Orai1−/− T cells) sufficient for proliferation but not for cell death, and intermediate levels (as observed in Orai1+/− T cells) sufficient for both proliferation and cell death (Fig. 5B). These observations are similar with the concept proposed by Parekh and colleagues indicating that graded Ca2+ influx via CRAC channels (analog signal) induces all-or-none activation of gene transcription via NFAT (digital outcome) (Kar et al., 2012b). However, all the outcomes of graded Ca2+ influx are not digital, because T cells with different levels of [Ca2+]i showed a gradual effect in cytokine production with increasingly higher cytokine levels produced by Orai1−/− CD4+ T cells expressing Orai1E106Q, Orai1−/−, Orai1+/−, and Orai1+/+ T cells in an analogous manner. Further studies using transgenic animal models exhibiting graded Ca2+ influx will help in dissecting the digital and analog outcomes of Ca2+ signaling under physiological conditions.
Fig. 5.
Roles of Ca2+ signalling in diverse aspects of T cell activation. (A) Gradual levels of store-operated Ca2+ entry generated by genetic modifications. Left-CRAC channel activity was measured in effector CD4+ T cells from wild type (WT), Orai1 heterozygous (Orai1+/−) and Orai1-deficient (Orai1−/−) mice after store depletion with thapsigargin (TG) in the presence of extracellular solution containing 0.5 and 2 mM Ca2+. Right-CRAC channel activity was measured in Orai1−/− CD4+ T cells transduced with retroviruses expressing empty vector (vector, blue trace), wild type (Orai1WT, black trace) or dominant negative mutant of Orai1 (Orai1E106Q). Data modified from article originally published in (Kim et al., 2011). (B) Ca2+ requirement for T cell death, cytokine production and proliferation differs. T cell proliferation does not need high Ca2+. Instead, excessive Ca2+ concentrations induce cell death and anergy in T cells. Therefore, T cell proliferation requires moderate intracellular Ca2+ concentrations as observed in Orai1-deficient (Orai1−/−) T cells and a further reduction in [Ca2+]i by overexpression of dominant negative Orai1 (DN-Orai1) in Orai1−/− T cells inhibits proliferation. Thus the threshold of [Ca2+]i necessary for proliferation is much lower than that for cell death and anergy. However, cytokine production gradually increases with increase in [Ca2+]i, with DN-Orai1 cells showing minimal cytokine production and Orai1+/+ cells showing maximal cytokine levels. Thus, the pattern of Ca2+ signalling can modulate the outcomes of T cell fates such as cytokine production, proliferation, anergy, and cell death in a digital or analogue manner.
CONCLUSION
Molecular understanding of CRAC channels is crucial for development of therapy that benefits patients with immune deficiencies, autoimmune diseases, and transplant rejection. Ca2+ signalling pathway comprising of CRAC channels-calcineurin-NFAT has been extensively studied due to its importance in immune cell functions. Blockers for calcineurin such as cyclosporin A and FK506 (Tacrolimus) are currently used as strong immunosuppressants to suppress transplant rejection and acute inflammation. However, ubiquitous expression of calcineurin makes long-term treatment with the calcineurin blockers technically challenging. Identification of CRAC channel subunits and understanding of their regulation now provide new targets for drug development to prevent transplant rejection and treat autoimmune diseases. Blockers of CRAC channels are less likely to have detrimental side effects because SCID patients with defective CRAC channel activity showed mild extra-immunological phenotype. Detailed structural studies targeting the pore region of Orai1 in the closed and open configuration (in the absence and presence of STIM1) will greatly help our understanding of channel regulation because this will provide useful information for designing of chemicals specifically targeting Orai1 pore. In addition, identification of interacting partners of CRAC channels, particularly in the immune system will provide new targets for therapeutic intervention of the Ca2+-calcineurin-NFAT signaling pathway. Ca2+ is a universal second messenger; however, accumulating evidences suggest that [Ca2+]i has specific effects depending on signaling patterns (e.g. amplitude and frequency of oscillation), site of accumulation (e.g. micro-or nanodomains), and cell types. In vivo studies using transgenic animal models exhibiting graded Ca2+ influx will provide tools to dissect the digital and analog outcomes of Ca2+ signaling under physiological conditions. In addition to the immune system, recent identification of the role of Orai1 in cervical and mammary tumor cell migration and metastases provides new opportunities for targeting Orai1 as cancer therapy and for investigating the function of intracellular [Ca2+]i in tumor formation.
Acknowledgments
This work was supported by National Institute of Health grants AI-083432 and AI-101569, a grant from the Lupus Research Institute (Y.G.), and a scientist development grant from the American Heart Association, 12SDG12040188 (S.S.).
REFERENCES
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- Baine I, Abe BT, Macian F. Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol Rev. 2009;231:225–240. doi: 10.1111/j.1600-065X.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ, Sommers CL, Oh-Hora M, Rao A, et al. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol. Biol. Cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–678. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Braun A, Gessner JE, Varga-Szabo D, Syed SN, Konrad S, Stegner D, Vogtle T, Schmidt RE, Nieswandt B. STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation. Blood. 2009a;113:1097–1104. doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009b;113:2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356–362. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:33.31–33.28. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WT, Tang MJ, Jao HC, Shen MR. Soft substrate up-regulates the interaction of STIM1 with store-operated Ca2+ channels that lead to normal epithelial cell apoptosis. Mol. Biol. Cell. 2008;19:2220–2230. doi: 10.1091/mbc.E07-11-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J Gen Physiol. 1994;103:365–388. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Flourakis M, Lehen’kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Shiota M, Ohkawa Y, Baba A, Wanibuchi H, Kinashi T, Kurosaki T, Baba Y. Surf4 modulates STIM1-dependent calcium entry. Biochem Biophys Res Commun. 2012;422:615–620. doi: 10.1016/j.bbrc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr, Hoogenraad CC, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007a;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007b;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, et al. Hair loss and defective T and B cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke N, Albrecht P, Bouchachia I, Ryazantseva M, Knoll K, Lewerenz J, Kaznacheyeva E, Maher P, Methner A. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 2013;4:e470. doi: 10.1038/cddis.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+(CRAC) channels. J Biol Chem. 2011;286:14795–14803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. USA. 2012a;109:6969–6974. doi: 10.1073/pnas.1201204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. CRAC channels drive digital activation and provide analog control and synergy to Ca(2+)-dependent gene regulation. Curr Biol. 2012b;22:242–247. doi: 10.1016/j.cub.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ueyama T, Lange I, Feske S, Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J Biol Chem. 2010;285:25720–25730. doi: 10.1074/jbc.M109.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra N, Bresson-Bepoldin L, Penna A, Chaigne-Delalande B, Segui B, Levade T, Vacher AM, Reiffers J, Ducret T, Moreau JF, et al. CD95 triggers Orai1-mediated localized Ca2+ entry, regulates recruitment of protein kinase C (PKC) beta2, and prevents death-inducing signaling complex formation. Proc. Natl. Acad. Sci. USA. 2011;108:19072–19077. doi: 10.1073/pnas.1116946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, Srikanth S, Yee MK, Mock DC, Lawson GW, Gwack Y. ORAI1 deficiency impairs activated T cell death and enhances T cell survival. J Immunol. 2011;187:3620–3630. doi: 10.4049/jimmunol.1100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc. Natl. Acad. Sci. USA. 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc. Natl. Acad. Sci. USA. 2009;106:14687–14692. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011;3:pii: a003970. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Natl. Acad. Sci. USA. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez E, Jardin I, Berna-Erro A, Bermejo N, Salido GM, Sage SO, Rosado JA, Redondo PC. STIM1 tyrosine-phosphorylation is required for STIM1-Orai1 association in human platelets. Cell Signal. 2012;24:1315–1322. doi: 10.1016/j.cellsig.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S, Bakal C, Perrimon N. Genomic screening with RNAi: results and challenges. Annu Rev Biochem. 2010;79:37–64. doi: 10.1146/annurev-biochem-060408-092949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc. Natl. Acad. Sci. USA. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231:210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Jang SI, Ambudkar IS. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca(2+)](i) sig-nals determine specificity of Ca(2+)-dependent gene expression. PLoS One. 2012;7:e47146. doi: 10.1371/journal.pone.0047146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, et al. STIM1 mutation associated with a syndrome of immuno-deficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 2010;123:3084–3093. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Prins D, Groenendyk J, Touret N, Michalak M. Modulation of STIM1 and capacitative Ca(2+) entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011;12:1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- Qu B, Al-Ansary D, Kummerow C, Hoth M, Schwarz EC. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium. 2011;50:261–269. doi: 10.1016/j.ceca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nunez L, Villalobos C, Meraner P, Becherer U, et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 2011;30:3895–3912. doi: 10.1038/emboj.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson LE. Immunoreceptor signaling. Cold Spring Harb Perspect Biol. 2011;3:pii: a011510. doi: 10.1101/cshperspect.a011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann MK, Stegner D, Berna-Erro A, Bittner S, Braun A, Kleinschnitz C, Stoll G, Wiendl H, Meuth SG, Nieswandt B. Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J Immunol. 2010;184:1536–1542. doi: 10.4049/jimmunol.0902161. [DOI] [PubMed] [Google Scholar]
- Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY. Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins. J Physiol. 2009;587:2903–2918. doi: 10.1113/jphysiol.2009.170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E, Klein-Hessling S, Palmetshofer A, Bopp T, Stassen M, Schmitt E. NFAT transcription factors in control of peripheral T cell tolerance. Eur J Immunol. 2006;36:2837–2843. doi: 10.1002/eji.200536618. [DOI] [PubMed] [Google Scholar]
- Serfling E, Avots A, Klein-Hessling S, Rudolf R, Vaeth M, Berberich-Siebelt F. NFATc1/alphaA: The other face of NFAT factors in lymphocytes. Cell Commun Signal. 2012;10:16. doi: 10.1186/1478-811X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z, Schmidt V, Hogan PG, Sacks DB, Rao A. Dephosphorylation of the nuclear factor of acti-vated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc. Natl. Acad. Sci. USA. 2011;108:11381–11386. doi: 10.1073/pnas.1019711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Gwack Y. Orai1, STIM1, and their associating partners. J Physiol. 2012;590:4169–4177. doi: 10.1113/jphysiol.2012.231522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Jung HJ, Kim KD, Souda P, Whitelegge J, Gwack Y. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol. 2010a;12:436–446. doi: 10.1038/ncb2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Jung HJ, Ribalet B, Gwack Y. The intracellular loop of Orai1 plays a central role in fast inactivation of Ca2+ release-activated Ca2+ channels. J Biol Chem. 2010b;285:5066–5075. doi: 10.1074/jbc.M109.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Yee MK, Gwack Y, Ribalet B. The third transmembrane segment of orai1 protein modulates Ca2+ release-activated Ca2+(CRAC) channel gating and permeation properties. J Biol Chem. 2011;286:35318–35328. doi: 10.1074/jbc.M111.265884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1) Proc. Natl. Acad. Sci. USA. 2012;109:8682–8687. doi: 10.1073/pnas.1200667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2010;425:159–168. doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010a;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010b;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder N, Takeshima H, Ma J. Immuno-proteomic approach to excitation--contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium. 2008;43:1–8. doi: 10.1016/j.ceca.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- Woodward OM, Li Y, Yu S, Greenwell P, Wodarczyk C, Boletta A, Guggino WB, Qian F. Identification of a polycystin-1 cleavage product, P100, that regulates store operated Ca entry through interactions with STIM1. PLoS One. 2010;5:e12305. doi: 10.1371/journal.pone.0012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006a;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006b;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17:251–260. doi: 10.1016/j.tcb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc. Natl. Acad. Sci. USA. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J Cell Biol. 2010;191:523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Soboloff J, Gamero AM. Control of type I interferon-induced cell death by Orai1-mediated calcium entry in T cells. J Biol Chem. 2012;287:3207–3216. doi: 10.1074/jbc.M111.269068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger W, Ito D, Swetlik C, Oh-hora M, Villereal ML, Thinakaran G. Stanniocalcin 2 is a negative modulator of store-operated calcium entry. Mol Cell Biol. 2011;31:3710–3722. doi: 10.1128/MCB.05140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Hu J, Amcheslavsky A, Zheng H, Cahalan MD. Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc. Natl. Acad. Sci. USA. 2011;108:17838–17843. doi: 10.1073/pnas.1114821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]