Abstract
Glutathione (GSH) plays a critical role in cellular defense against unregulated oxidative stress in mammalian cells including neurons. We previously demonstrated that GSH decrease using [D, L]-buthionine sulphoximine (BSO) induces retinal cell death, but the underlying mechanisms of this are still unclear. Here, we demonstrated that retinal GSH level is closely related to retinal cell death as well as expression of an anti-apoptotic molecule, Bcl-2, in the retina. We induced differential expression of retinal GSH by single and multiple administrations of BSO, and examined retinal GSH levels and retinal cell death in vivo. Single BSO administration showed a transient decrease in the retinal GSH level, whereas multiple BSO administration showed a persistent decrease in the retinal GSH level. Retinal cell death also showed similar patterns: transient increase of retinal cell death was observed after single BSO administration, whereas persistent increase of retinal cell death was observed after multiple BSO administration. Changes in the retinal GSH level affected Bcl-2 expression in the retina. Immunoblot and immunohistochemical analyses showed that single and multiple administration of BSO induced differential expressions of Bcl-2 in the retina. Taken together, the results of our study suggest that the retinal GSH is important for the survival of retinal cells, and retinal GSH appears to be deeply related to Bcl-2 expression in the retina. Thus, alteration of Bcl-2 expression may provide a therapeutic tool for retinal degenerative diseases caused by retinal oxidative stress such as glaucoma or retinopathy.
Keywords: Bcl-2, BSO, glutathione, oxidative stress, retina
INTRODUCTION
Glutathione (GSH), a tripeptide (L-γ-glutamyl-L-cysteinyl glycine) thiol, exists in most mammal cells and plays an important role in various biological processes (Dalle-Donne et al., 2007). GSH is a critical component of the antioxidant defense system. It also plays a role in cysteine transport and storage, maintains cellular redox status, and fulfills other important cellular functions (Anderson, 1998). Recent studies suggest that a decrease in retinal GSH below a threshold level may constitute a signal that initiates death receptor activation (Franco and Cidlowski, 2009) or mitochondria-dependent apoptotic signaling (Armstrong and Jones, 2002). In addition, γ-glutamylcysteine synthetase knockout mice in which GSH synthesis was inhibited and GSH was depleted displayed significant apoptotic cell death in multiple tissues (Dalton et al., 2004). Therefore, maintenance of retinal GSH level appears to be an important mediator of signal transduction in cell survival.
A member of the Bcl-2 (B-cell CLL/lymphoma 2) family, Bcl-2 is known to play a critical role in cellular defense against unregulated oxidative stress and apoptosis in neurons (Holm and Isacson, 1999; Reed et al., 2004). Also, expression of Bcl-2 prevents neuronal death in vitro (Allsopp et al., 1993; Zhong et al., 1993). The effect of Bcl-2 in neuronal survival in vivo has been reported using transgenic mice over-expressing the bcl-2 gene in neurons (Martinou et al., 1994). Two months after the transection of the optic nerve, the bcl-2 transgenic mice displayed 13-fold higher retinal ganglion cell survival. An increasing body of evidence about the connection between GSH and Bcl-2 has been established. Expression of GSH is much higher in cells expressing Bcl-2, suggesting that Bcl-2 may promote GSH retention, stability, or synthesis (Voehringer, 1999). In addition, over-expression of Bcl-2 leads to an increase in the cellular content of GSH (Ellerby et al., 1996; Voehringer and Meyn, 2000). In contrast, bcl-2 knockout mice show reduced GSH levels and GSH peroxidase activity in brain tissues and demonstrate enhanced susceptibility to mitochondrial oxidative stress-induced neuronal cell death (Hochman et al., 1998).
Retina is a part of the central nervous system (CNS) and it is the primary interest of vision. It is one of the most vascularized tissues in the body and has one of the highest oxidative metabolic rates per tissue weight (Martinou et al., 1994). Therefore, the retina is highly susceptible to oxidation, and may contain cellular defensive mechanisms against ROS elevation (Chen and Kadlubar, 2003; Ferreira et al., 2004; Gherghel et al., 2005; Moreno et al., 2004; Sternberg et al., 1993; Unal et al., 2007; Yang et al., 2001; Yildirim et al., 2004). The retina may contain a cellular defense system, such as GSH, against uncontrolled elevation of oxidative stress (Ganea and Harding, 2006). Several ROS scavengers, including Bcl-2 proteins, also have been implicated in such functions (Ellerby et al., 1996; Voehringer and Meyn, 2000).
We previously demonstrated that GSH decreases by application of BSO, an inhibitor of the first step in GSH synthesis, causing retinal cell death (Roh et al., 2007) and accompanying reduced gene expression of bcl-2 family members in the retina (Song et al., 2008). However, the mechanisms of cell death induced by decreased GSH and connections between retinal GSH and Bcl-2 in the retina have not been thoroughly studied. Thus, we here examined the effects of retinal GSH level alteration after single or multiple administration of BSO and changes of cell survival and Bcl-2 expression in the retina.
MATERIALS AND METHODS
Animals
Adult male mice (C57BL/6J, 6–8 week old) were purchased from KOATECH (Korea). The animals were housed in a temperature-controlled environment with free access to food and water under a 12-h light-dark cycle. All animal experimental protocols were approved by Kyungpook National University Institutional Animal Care and Use Committees. All applicable guidelines from the National Institute of Health Guide for the care and use of laboratory animals were followed.
Administration of BSO and preparation of total retinal samples
Mice were intraperitoneally injected with BSO (Sigma-Aldrich Corp., USA) at 1.5 mg/kg once single administration or multiple administrations (once every consecutive day). Control mice were injected with phosphate buffered saline (PBS, pH 7.4). At least three animals were used for each condition. After 0, 1, 4, and 7 days of BSO injection, the animals were sacrificed under anesthesia with 5 mg/kg ketamine hydrochloride with xylazen (Yuhan Co., Korea) for tissue preparation.
Immunoblot analysis
Immunoblot analysis was performed using retinal extract preparations to assess expression levels of Bcl-2 as previously reported with some modifications (Moon et al., 1998). Retinal extracts were prepared by homogenizing a single retina in 100 ul of PBS. Sample buffer was added to each 60 ug protein sample, and the protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% Tris-HCl acrylamide gels. Proteins were transferred to a PVDF membrane. After blocking with 1% skimmed milk (SM)-TBS for 1 h, the membrane was incubated with anti-Bcl-2 antibody (1:500, StressGen, Canada) in 3% bovine serum albumin (BSA) overnight at 4°C. The membrane was then rinsed three times with 1× TBST (50 mM Tris buffer, 150 mM NaCl, 0.1% Tween-20) over approximately a total period of 30 min. The membrane was incubated with horseradish peroxide conjugated secondary antibody diluted at 1:10, 000 in 1% SM-TBS for 1 h at room temperature. After incubation, the membrane was washed with TBST and developed using an enhanced chemiluminescence western blot analysis system (Pierce Chemical Co., USA). Antibody against GAPDH (glyce-raldehyde 3-phosphate dehydrogenase, 1:1, 000, StressGen, Canada) was used for normalizing total proteins.
Immunohistochemical analysis
Mouse retina tissues were separated, post-fixed in 4% paraformaldehyde (PFA) for 2 h on ice, soaked in 30% sucrose for 1 day, and agar-embedded as previously described with some modifications (Lim et al., 2007). The retina tissues were cut to 50 um thickness using a vibratome (Leica, Germany). After rinsing with 0.01 M PBS, the retina sections were incubated with 0.3% Triton X-100 in PBS. After rinsing with 0.01 M PBS the retina sections were incubated with a blocking solution containing 10% normal donkey serum (Jackson Laboratory, USA) for 1 h at room temperature. The tissues were incubated with the anti-Bcl-2 antibody (1:500, StressGen, Canada) overnight at 4°C. The next day, the tissues were washed three times with 0.01 M PBS and incubated f or 1h in PBS containing biotinylated secondary antibody. Bcl-2 immunoreactivity was visualized using a 3.3′ diaminibezidine (DAB) detection system (Vector Laboratories, USA). Immunoreactivities were analyzed microscopically and quantitatively using NIH Image J (version 1.46).
Glutathione assay
Retinal GSH in retinal tissues was determined as instructed in the protocol from a commercial GSH assay kit (Calbiochem, USA). Briefly, the mice were perfused with PBS (pH 7.4), and retinal tissues were removed. The retinal tissues were homogenized in a MES buffer [0.4 M 2-(N-morpholino)ethanesulphonic acid, 0.1 M phosphate, 2 mM EDTA, pH 6.0], and centrifuged at 10, 000 × g for 15 min at 4°C. The supernatants were deproteinated and subjected to a GSH assay.
Terminal uridine deoxynucleotidyl nick end labeling (TUNEL)
For monitoring apoptotic cells in the retina, TUNEL was performed. Retinal tissue sections were air dried, rehydrated with PBS for 10 min, and permeabilized in 0.1% Triton-100 in PBS (pH 8.0) for 10 min. The tissue sections were then incubated in 5 μg/ml protease K, and rinsed twice in TDT buffer (30 mM Tris-HCl, 140 mM sodium cacodylate. 1 mM cobalt chloride, pH 7.2) for 5 min for each rinse at room temperature. End-labeling of DNA fragments with biotylated dUTP was performed after incubating in 100 μl of the reaction mixture (40 μM biotinylated dUTP and 0.3 unit/ml terminal transferase in TDT buffer) for 1 h at 37°C. The reaction was terminated by rinsing with Tris buffer for 15 min. Biotinylated DNA was visualized with an ABC kit (Vector Labs).
Data analysis
For a statistical analysis, a one-way ANOVA followed by a Turkey’s post hoc test for unbalanced sample numbers was performed. *p < 0.05, **p < 0.01, or ***p < 0.001 was regarded as statistical significance.
RESULTS
BSO decreased retinal glutathione levels and induced differential patterns of retinal cell death
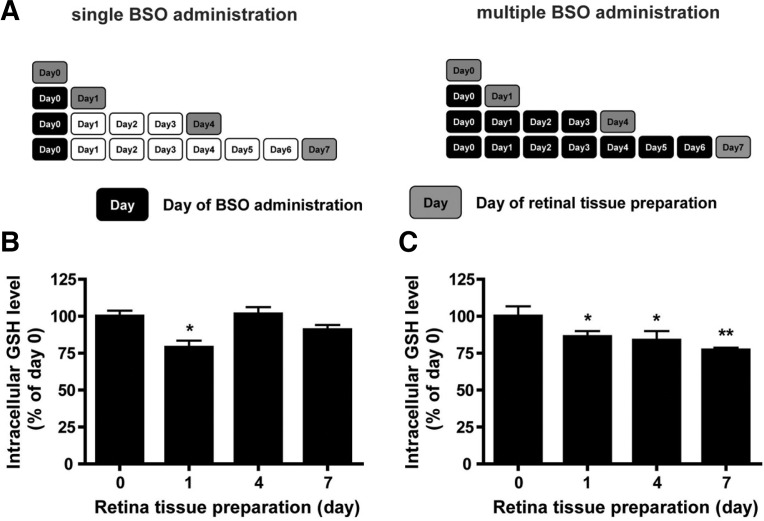
We sought to examine the effects of either acute or chronic oxidative stress in the retina. To this end, BSO, which inhibits γ-glutamylcysteine synthetase and results in lowered retinal GSH level (de Bernardo et al., 2004), was used. We decreased the retinal GSH level by BSO administration in two distinct manners: transient retinal GSH decrease by single administration of BSO and persistent retinal GSH decrease by multiple administration of BSO (Fig. 1A).
Fig. 1.
Changes in glutathione level after BSO administration in mouse retina. (A) Experimental scheme of BSO administration. Black boxes represent the days of BSO administration, whereas gray boxes indicate the days when retina tissue were collected. White boxes represent days of normal nurture. (B) Retinal GSH changes under single BSO administration condition. (C) Retinal GSH changes under multiple BSO administration condition. Retinal GSH levels were determined from retina tissues of six eyeballs (3 mice). Statistical significances are indicated as *p < 0.05 and **p < 0.01 (one-way ANOVA).
After single administration with BSO on day 0, retinal GSH levels were determined on days 0, 1, 4, and 7. We found that single BSO administration significantly lowered the retinal GSH level post one day of administration (Fig. 1B). The retinal GSH level then returned to the basal level after 4 days. In contrast, multiple BSO administration sustained the lowered retinal GSH level until day 7 (Fig. 1C). Taken together, the results show that distinct BSO administration schemes may expose retinal cells to oxidative stress in distinct manners, i.e. mimicking acute and chronic oxidative stress insults.
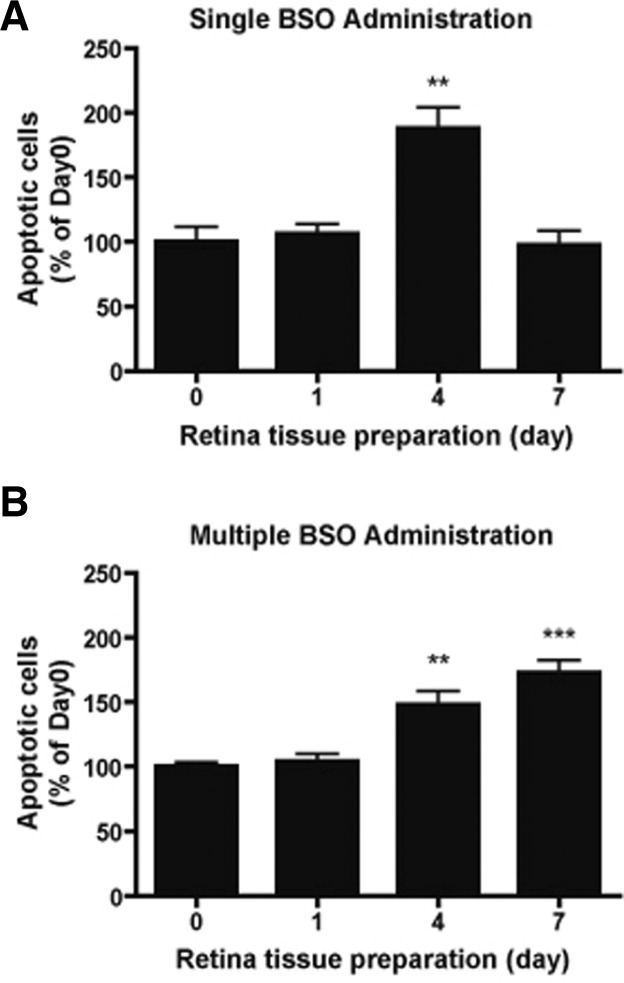
Distinct BSO administrations induced differential patterns of cell death in the retina
Next, we examined how differential GSH decreases would affect the retinal cell viability. Apoptotic cells were visualized using a TUNEL assay. In the case of single BSO administration, the number of TUNEL-positive cells in the retina was not significantly changed (Fig. 2A) one day after BSO administration. On day 4 after BSO administration, the number of TUNEL-positive cells significantly increased in the retina (**p < 0.01, one-way ANOVA), and then returned to the basal level by day 7 (Fig. 2A). In the case of multiple BSO administration, the number of TUNEL-positive cells in the retina was not significantly changed (Fig. 2B) one day after BSO administration, as also found for the case of single BSO administration. On day 4 after BSO administration, the number of TUNEL-positive cells significantly increased in the retina (**p < 0.01, one-way ANOVA), and further increased on day 7 (***p < 0.01, one-way ANOVA) (Fig. 2B).
Fig. 2.
Effects of differential glutathione level changes on cell death of mouse retina. (A) Changes in apoptotic cell numbers in mouse retina under single BSO administration condition. (B) Changes in apoptotic cell numbers in mouse retina under multiple BSO administration condition. TUNEL(+) cell numbers were counted from retina tissues of six eyeballs (3 mice). Statistical significances are indicated as **p < 0.01 and ***p < 0.001 (one-way ANOVA)
We examined cell types in the retina that were mostly affected by single BSO treatment. To this end, we counted the TUNEL-positive cells in the outer nuclear, inner nuclear, and the ganglion cell layers. In the outer nuclear layer, the number of TUNEL-positive cells was not significantly changed on day 1 after BSO administration (Table 1). The number of TUNEL-positive cells significantly increased on day 4 after BSO administration (**p < 0.01, Table 1). On day 7, the number of TUNEL-positive cells was not significantly different from that on day 0 (Table 1). The effect of BSO on the inner nuclear layer showed a similar pattern: the number of TUNEL-positive cells significantly increased 4 days after BSO administration (**p < 0.01, Table 1). In contrast with the other layers, the number of TUNEL-positive cells in the ganglion cell layer was not statistically significant, although the number slightly increased 4 days after BSO administration (Table 1).
Table 1.
Summary of quantitative analysis of TUNEL(+) cells in the retina after single BSO administration
| Layer | Days
|
|||
|---|---|---|---|---|
| 0 | 1 | 4 | 7 | |
| GCL | 100.00 ± 29.21 | 94.14 ± 17.92 | 130.00 ± 31.91 | 91.36 ± 24.57 |
| INL | 100.00 ± 18.99 | 97.87 ± 9.71 | 177.30** ± 9.32 | 81.29 ± 17.20 |
| ONL | 100.00 ± 14.03 | 122.50 ± 6.67 | 236.70** ± 13.76 | 177.80 ± 11.90 |
TUNEL(+) cell were counted in mm2 of retina after BSO administration (mean ± standard error mean). Cell numbers are normalized to the value of day 0. Statistical significances are indicated (one-way ANOVA, **p < 0.01). GCL, INL, and ONL indicate ganglion cell layer, inner nuclear layer, and outer nuclear layer, respectively.
We next examined cell types in the retina that were mostly affected by multiple BSO treatment. In the outer nuclear layer, the number of TUNEL-positive cells was not significantly changed on day 1 after BSO administration (Table 2). The number of TUNEL-positive cells significantly increased on days 4 and 7 after multiple BSO administration (**p < 0.01, Table 2). In the inner nuclear layer, the number of TUNEL-positive cells significantly increased on 7 days after BSO administration (*p < 0.05, Table 2). In the ganglion cell layer, the number of TUNEL-positive cells significantly increased on days 4 (*p < 0.05) and 7 (**p < 0.01) after multiple BSO administration (Table 2).
Table 2.
Summary of quantitative analysis of TUNEL(+) cells in the retina after multiple BSO administration
| Layer | Days
|
|||
|---|---|---|---|---|
| 0 | 1 | 4 | 7 | |
| GCL | 100.00 ± 6.41 | 106.25 ± 7.96 | 174.34* ± 12.24 | 222.37** ± 14.01 |
| INL | 100.00 ± 6.68 | 98.20 ± 11.14 | 165.57 ± 12.84 | 183.23* ± 21.98 |
| ONL | 100.00 ± 2.55 | 108.27 ± 4.59 | 133.57** ± 10.38 | 155.56** ± 4.61 |
TUNEL(+) cell were counted in mm2 of retina after BSO administration (mean ± standard error mean). Cell numbers are normalized to the value of day 0. Statistical significances are indicated (one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001). GCL, INL, and ONL indicate ganglion cell layer, inner nuclear layer, and outer nuclear layer, respectively.
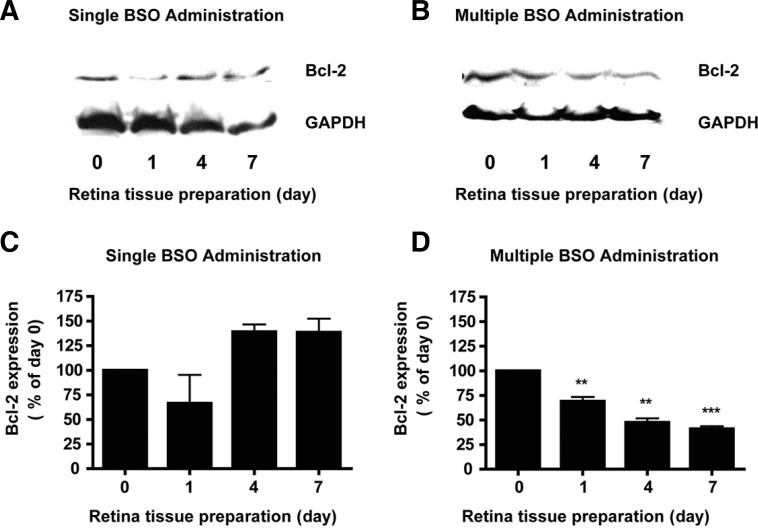
Differential expression of Bcl-2 by single and multiple administration of BSO
The level of anti-apoptotic protein Bcl-2 was determined using an immunoblot analysis of retinal tissues after BSO administration. The basal level of Bcl-2 expression was found in the control retinal tissues (Figs. 3A and 3B). However, Bcl-2 showed differential expression after single or multiple BSO administration (Figs. 3A and 3B). By one day after single administration of BSO, the amount of Bcl-2 had greatly decreased by 33% from the basal level (Fig. 3C) and returned to the basal level (to 139.2 % of the value on day 0, Fig. 3C) on day 4, and the effect was maintained until day 7 (to 138.5% of the value on day 0, Fig. 3C). Multiple BSO administration also caused a decrease in Bcl-2 expression (to 69.1% of the value on day 0, Fig. 3D) one day after administration of BSO. In contrast with the case of single administration, the decreased expression of Bcl-2 was maintained until day 7 (to 41.0% of the value on day 0, Fig. 3D). Equal loading was confirmed by GAPDH.
Fig. 3.
Effects of differential glutathione level changes on Bcl-2 expression in mouse retina. (A) Immunoblot analysis of Bcl-2 in mouse retina under single BSO administration condition. (B) Immunoblot analysis of Bcl-2 in mouse retina under multiple BSO administration condition. (C) Quantitative analysis of Bcl-2 expression changes in mouse retina under single BSO administration condition. Equal loadings were confirmed by immunoblot analysis with GAPDH. (D) Quantitative analysis of Bcl-2 expression changes in mouse retina under multiple BSO administration condition. Apparent intensity of Bcl-2 immunoreactivities was determined by comparison with intensity of GAPDH immunoreactivities. Apparent changes of immunoreactivities were determined from retina tissues of six eyeballs (3 animals). Statistical significances are indicated as *p < 0.05 and ***p < 0.001 (one-way ANOVA).
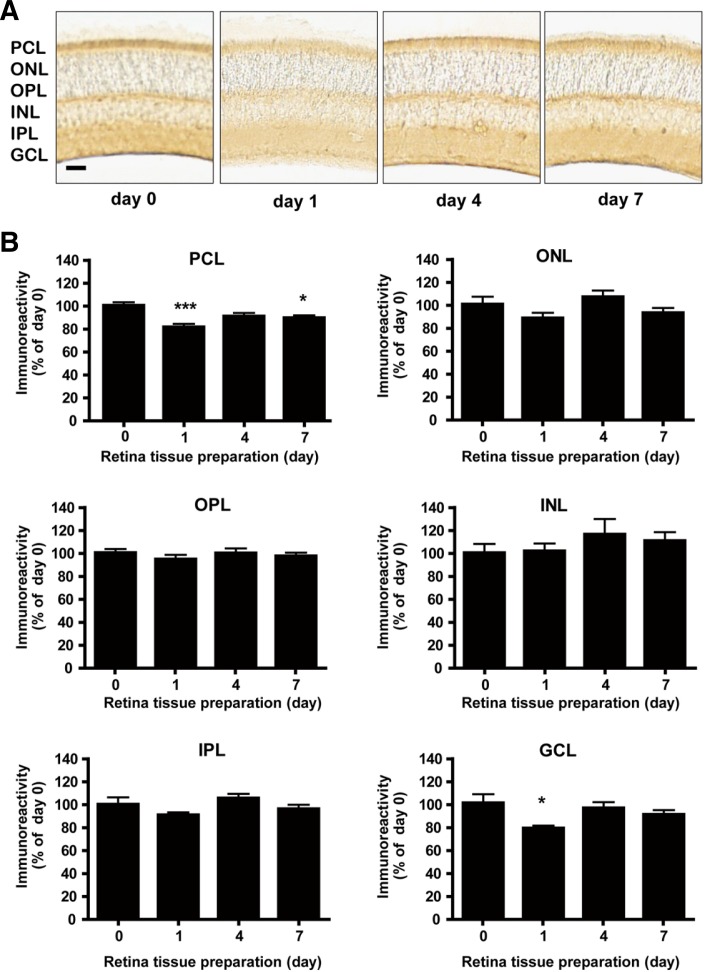
Differential expression of Bcl-2 in the region of the mouse retina by single and multiple administration of BSO
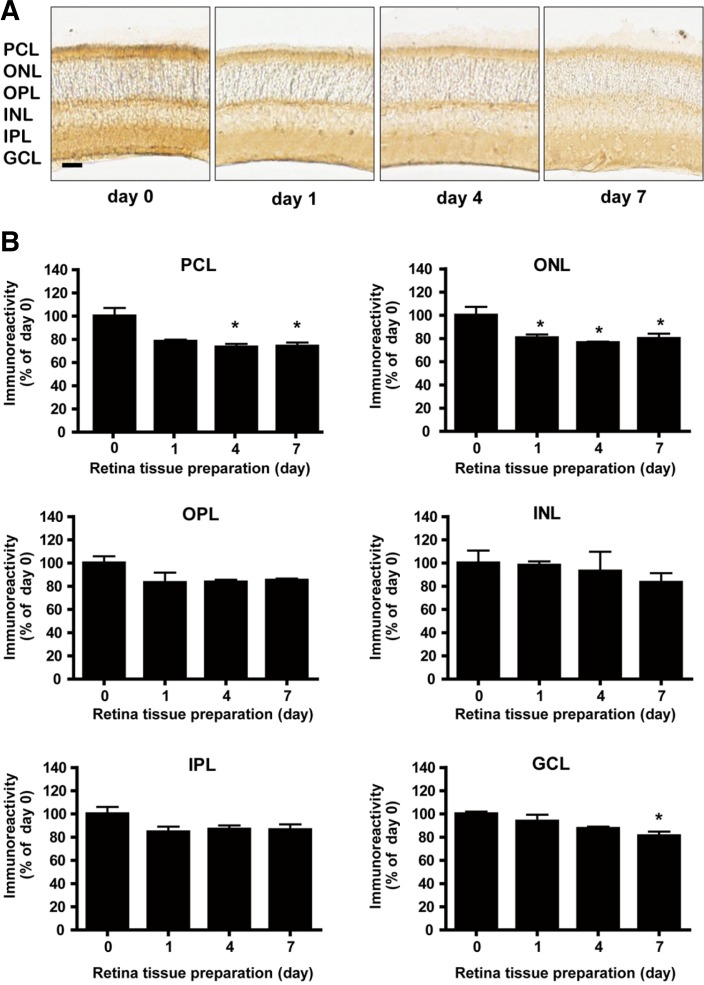
We next determined which cell types were most affected in terms of Bcl-2 expression after administration of BSO. We quantified Bcl-2 expression using NIH Image J (version 1.46). Bcl-2 immunoreactivity in the control retina was observed in the majority of the photoreceptor cell layer and in a few cells of the inner nuclear layer (Figs. 4A and 5A). Gross changes in thickness and morphology of the retina after administration of BSO were not observed within the 7 day window of our observation (Figs. 4A and 5A). One day after single BSO administration, Bcl-2 immunoreactivity significantly decreased in the photoreceptor cell layer (to 81.2% of the value on day 0, Fig. 4B, PCL, p < 0.001) and ganglion cell layer (to 82.0% of the value on day 0, Fig. 4B, GCL, p < 0.05), but was not changed in the inner nuclear layer, outer nuclear layer, outer plexiform layer, and inner plexiform layer (Fig. 4B). The decreases were recovered to the basal level on day 4, and the effect was maintained until day 7 (Figs. 4A and 4B). On the other hand, multiple BSO administration also caused a significant decrease in Bcl-2 expression one day after administration of BSO in the photoreceptor cell layer (to 78.3% of the value on day 0, Fig. 5B, PCL, p < 0.05). Different from the case of single administration, the decrease in Bcl-2 expression was maintained until day 7 in the photoreceptor cell layer (to 74.2% of the value on day 0, Fig. 5B, PCL, p < 0.05), outer nuclear layer (to 79.9% of the value on day 0, Fig. 5B, ONL, p < 0.05), and ganglion cell layer (to 81.2% of the value on day 0, Fig. 5B, GCL, p < 0.05) (Figs. 5A and 5B). Bcl-2 immunoreactivity was observed mostly in the photoreceptor cell layer and plexiform layer of the control retina (Figs. 4A and 5A).
Fig. 4.
Differential Bcl-2 expression in mouse retina after single BSO administration. (A) Immunohistochemical analysis of Bcl-2 expression in mouse retina under single BSO administration condition. Immunoreactivity intensities were determined by NIH ImageJ. Scale bar represents 1 mm. (B) Immunohistochemical analysis of Bcl-2 expression in mouse retina layers under single BSO administration condition. Intensity of immunoreactivities was determined from at least 12 retina tissue sections of 8 eyeballs (4 animals). PCL, ONL, OPL, INL, IPL, and GCL represent photoreceptor cell layer, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, and ganglion cell layer, respectively. Statistical significances are indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 (one-way ANOVA).
Fig. 5.
Differential Bcl-2 expression in mouse retina after multiple BSO administration. (A) Immunohistochemical analysis of Bcl-2 expression in mouse retina under multiple BSO administration condition. Immunoreactivity intensities were determined by NIH ImageJ. Scale bar represents 1 mm. (B) Immunohistochemical analysis of Bcl-2 expression in mouse retina layers under multiple BSO administration condition. Immunoreactivities were determined from at least 10 retina tissue sections of 6 eyeballs (3 animals). PCL, ONL, OPL, INL, IPL, and GCL represent photoreceptor cell layer, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, and ganglion cell layer, respectively. Statistical significances are indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 (one-way ANOVA).
DISCUSSION
In our study, we induced a roughly 25% decrease of retinal GSH level. Retinal GSH is maintained by three major pathways, de novo synthesis, regeneration from glutathione disulfide (GSSG), and extracellular GSH uptake (Circu and Aw, 2012). BSO used for our study may affect de novo synthesis of GSH in the retina. Muller cells, intra-retinal glial cells, have a large pool of GSH in normal conditions. Once stressful stimuli trigger, Muller cells redistribute GSH to retinal ganglion cells and protect them (Schutte and Werner, 1998). We found retinal cell apoptosis 4 days after single BSO injection and 4 and 7 days after multiple BSO injection. Apoptotic cells were found in the outer and inner nuclear layers after single BSO injection. But by multiple BSO injection (chronic oxidative damage), apoptotic cells also were found in the ganglion cell layers. This difference may be caused by the GSH derived from Muller cells. Transient decrease of de novo GSH synthesis may be compensated by extracellular GSH uptake. Because GSH in the Muller cells is redistributed selectively to ganglion cells, ganglion cells may be less susceptible to oxidative damage than photoreceptor cells are. However, when GSH de novo synthesis in Muller cells is sustained by multiple BSO treatment, Muller cells may not protect ganglion cells any longer.
We demonstrated that retinal GSH decrease altered Bcl-2 expression in the retina. And again the pattern of Bcl-2 expression change in each retinal layer almost precisely coincided with that of apoptosis. Our previous report demonstrated altered bcl-2 mRNA expression 1 day after single administration of BSO (Song et al., 2008) and we showed here that Bcl-2 expression decreased 1 day after single or multiple administration of BSO. Bcl-2 has been reported to play a critical role in tolerance to oxidative stress (Hockenbery et al., 1990). From a study using Bcl-2 deficient mice, the depletion of endogenous Bcl-2 expression can lead to apoptosis in retina (Kotulska et al., 2003). Transgenic mice over-expressing the bcl-2 gene displayed 13-fold higher retinal ganglion cell survival after 2 months of optic nerve transaction (Martinou et al., 1994). Thus cell death induced by GSH decrease could be closely related to decreased Bcl-2 expression.
Our experiment demonstrated that normal retina has high basal Bcl-2 expression under conditions with daily photo-oxidation or physiologic intracellular stress. Unlike other parts of the central nervous system, the retina is directly exposed to ionizing radiation accompanied with high levels of oxidative stress, which causes various degenerative diseases, because the retina’s role is to perceive environmental information in the form of light. Since the retina has one of the highest oxidative metabolic rates per tissue weight (Martinou et al., 1994), the roughly 30% decrease of Bcl-2 expression after GSH decrease under our experimental scheme could be sufficient to affect retinal cell viability. Moreover, an approximately 30% decrease of Bcl-2 expression combined with a 25% decrease of retinal GSH may be enough to alter the sensitivity to apoptosis in normal mouse retina.
The decrease in Bcl-2 expression in the retina after BSO single and multiple administrations is very intriguing, because Bcl-2 is also related with retinal GSH homeostasis. BSO-induced neuronal cell death was prevented in cells with Bcl-2 over-expression by decreasing the net cellular generation of reactive oxygen species (Kane et al., 1993). Over-expression of Bcl-2 increases the cellular content of GSH and inhibits neuronal cell death induced by GSH-depleting reagents (Ellerby et al., 1996; Voehringer and Meyn, 2000). Bcl-2 also regulates the mitochondrial GSH pool by interacting with 2-oxoglutarate carrier, which carries cytosolic GSH into the mitochondria (Wilkins et al., 2012). Thus, the ability to regulate cellular GSH status accounts for the major part of the antioxidant-like function of Bcl-2. The decrease in Bcl-2 expression in the retina after BSO-induced GSH decrease may further affect the ability of Bcl-2 to regulate retinal GSH redox status, and thus may initiate a vicious cycle. Since the ratio of Bcl-2/Bax is known to be critical in cell survival in neurons (Song et al., 2008), further study on the expression of Bax in the retina and regulation of cellular GSH by the ratio of Bcl-2/Bax would be worthwhile.
In our study, the highest expression of Bcl-2 was observed in the photoreceptor outer segment, followed by the outer plexiform layer and the inner plexiform layer (Figs. 4 and 5). We could not compare our findings to other studies, because most studies on Bcl-2 expression in the retina have been performed by means of immunoblot or reverse transcription polymerase chain reaction analyses. Since photoreceptor outer segments display the highest oxygen exhaustion rate of the retina and contain a high content of polyunsaturated fatty acids (Martinou et al., 1994), strong protective mechanisms against oxidative damage are necessary in this layer, and Bcl-2 is one of such mechanisms.
The correlation between cell death and Bcl-2 expression implies that Bcl-2 may play a critical role in maintaining retinal cell survival. In the case of single BSO administration, Bcl-2 expression in photocell and ganglion cell layers significantly decreased on day 1 after BSO administration (Fig. 4B). In the photocell layer, rod and cone cells exist, and ganglion cells are present in ganglion cell layers. Retinal cell death increased in the inner and outer nuclear layers on day 1 after BSO administration (Table 1). Decreases in Bcl-2 expression in the cone and rode cells may affect the viability of these cells, and in turn cell death in the outer nuclear layer may increase. Retinal cell death increase in the inner nuclear layer may be caused by GSH decrease. Similarly, Bcl-2 expression decreased in the photocell, outer nuclear, and ganglion cell layers by multiple BSO administration from day 1 after BSO administration (Fig. 5B), and consequently retinal cell death in most layers increased from day 4 (Table 2). These results suggest that intracellular GSH and GSH related Bcl-2 expression in retinal cells may be critical for maintaining retinal cell survival.
In summary, GSH depletion alters cell survival and Bcl-2 expression in the retina. This finding indicates that the retina may alter Bcl-2 expression to defend against unregulated oxidative stress and that GSH may play a critical role in this process. It would be intriguing to determine if over-expression of Bcl-2 can indeed provide protection to the retinal cells against oxidative stress linked retinal degenerative diseases such as glaucoma and retinopathy.
Acknowledgments
This work was supported by the Ministry of Education, Science and Technology & Daegue Gyeongbuk Institute of Science and Technology (DGIST) (12-BD-04, DGIST Convergence Science Center) and the Ministry of Education, Science and Technology (2012K001350, Converging Research Center Program). The authors wish to thank Ms. Eunsun Kim for technical support.
REFERENCES
- Allsopp TE, Robinson M, Wyatt S, Davies AM. Ectopic trkA expression mediates a NGF survival response in NGF-independent sensory neurons but not in parasympathetic neurons. J Cell Biol. 1993;123:1555–1566. doi: 10.1083/jcb.123.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem. Biol. Int. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003;114:697–698. doi: 10.1016/s0002-9343(03)00199-2. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta. 2012;1823:1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Rad Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- de Bernardo S, Canals S, Casarejos MJ, Solano RM, Menendez J, Mena MA. Role of extracellular signal-regulated protein kinase in neuronal cell death induced by glutathione depletion in neuron/glia mesencephalic cultures. J Neurochem. 2004;91:667–682. doi: 10.1111/j.1471-4159.2004.02744.x. [DOI] [PubMed] [Google Scholar]
- Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Holm K, Isacson O. Factors intrinsic to the neuron can induce and maintain its ability to promote axonal outgrowth: a role for BCL2? Trends Neurosci. 1999;22:269–273. doi: 10.1016/s0166-2236(98)01352-6. [DOI] [PubMed] [Google Scholar]
- Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kotulska K, Lewin-Kowalik J, Jaroslaw-Jerzy B, Larysz-Brysz M, Marcol W, Fus Z. Bcl-2 deficiency deprives peripheral nerves of neurotrophic activity against injured optic nerve. J Neurosci Res. 2003;73:846–852. doi: 10.1002/jnr.10708. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Kim IB, Oh SJ, Chun MH. Identification and characterization of SMI32-immunoreactive amacrine cells in the mouse retina. Neurosci Lett. 2007;424:199–202. doi: 10.1016/j.neulet.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Moon C, Jaberi P, Otto-Bruc A, Baehr W, Palczewski K, Ronnett GV. Calcium-sensitive particulate guanylyl cyclase as a modulator of cAMP in olfactory receptor neurons. J Neurosci. 1998;18:3195–3205. doi: 10.1523/JNEUROSCI.18-09-03195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sáenz DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Reed JC, Doctor KS, Godzik A. The domains of apoptosis: a genomics perspective. Sci STKE. 2004;2004:re9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- Roh YJ, Moon C, Kim SY, Park MH, Bae YC, Chun MH, Moon JI. Glutathione depletion induces differential apoptosis in cells of mouse retina, in vivo. Neurosci Lett. 2007;417:266–270. doi: 10.1016/j.neulet.2007.02.088. [DOI] [PubMed] [Google Scholar]
- Schutte M, Werner P. Redistribution of glutathione in the ischemic rat retina. Neurosci Lett. 1998;246:53–56. doi: 10.1016/s0304-3940(98)00229-8. [DOI] [PubMed] [Google Scholar]
- Song HG, Moon C, Park MH, Moon JI, Moon C. Decrease in intracellular glutathione level alters expressions of B-cell CLL/Lymphoma 2 family members in the mouse retina. J Health Sci. 2008;54:464–470. [Google Scholar]
- Sternberg P, Jr, Davidson PC, Jones DP, Hagen TM, Reed RL, Drews-Botsch C. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest Ophthalmol Vis Sci. 1993;34:3661–3668. [PubMed] [Google Scholar]
- Unal M, Guven M, Devranoglu K, Ozaydin A, Batar B, Tamcelik N, Gorgun EE, Ucar D, Sarici A. Glutathione S transferase M1 and T1 genetic polymorphisms are related to the risk of primary open-angle glaucoma: a study in a Turkish population. Br J Ophthalmol. 2007;91:527–530. doi: 10.1136/bjo.2006.102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999;27:945–950. doi: 10.1016/s0891-5849(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Voehringer DW, Meyn RE. Redox aspects of Bcl-2 function. Antioxid Redox Signal. 2000;2:537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- Wilkins HM, Marquardt K, Lash LH, Linseman DA. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic Biol Med. 2012;52:410–419. doi: 10.1016/j.freeradbiomed.2011.10.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1273–1276. [PubMed] [Google Scholar]
- Yildirim O, Ates NA, Tamer L, Muslu N, Ercan B, Atik U, Kanik A. Changes in antioxidant enzyme activity and malondialdehyde level in patients with age-related macular degeneration. Ophthalmologica. 2004;218:202–206. doi: 10.1159/000076845. [DOI] [PubMed] [Google Scholar]
- Zhong LT, Sarafian T, Kane DJ, Charles AC, Mah SP, Edwards RH, Bredesen DE. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc. Natl. Acad. Sci. USA. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]