Abstract
S100A8 and S100A9 (S100A8/A9) are low-molecular weight members of the S100 family of calcium-binding proteins. Recent studies have reported S100A8/A9 promote tumorigenesis. We have previously reported that S100A8/A9 is mostly expressed in stromal cells and inflammatory cells between gastric tumor cells. However, the role of environmental S100A8/A9 in gastric cancer has not been defined. We observed in the present study the effect of S100A8/A9 on migration and invasion of gastric cancer cells. S100A8/A9 treatment increased migration and invasionat lower concentrations that did not affect cell proliferation and cell viability. S100A8/A9 caused activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB). The phosphorylation of p38 MAPK was not affected by the NF-κB inhibitor Bay whereas activation of NF-κB was blocked by p38 MAPK inhibitor SB203580, indicating that S100A8/A9-induced NF-κB activation is mediated by phosphorylation of p38 MAPK. S100A8/A9-induced cell migration and invasion was inhibited by SB203580 and Bay, suggesting that activation of p38 MAPK and NF-κB is involved in the S100A8/A9 induced cell migration and invasion. S100A8/A9 caused an increase in matrix metalloproteinase 2 (MMP2) and MMP12 expression, which were inhibited by SB203580 and Bay. S100A8/A9-induced cell migration and invasion was inhibited by MMP2 siRNA and MMP12 siRNA, indicating that MMP2 and MMP12 is related to the S100A8/A9 induced cell migration and invasion. Taken together, these results suggest that S100A8/A9 promotes cell migration and invasion through p38 MAPK-dependent NF-κB activation leading to an increase of MMP2 and MMP12 in gastric cancer.
Keywords: gastric cancer cells, MMP, NF-κB, p38MAPK, S100A8/A9
INTRODUCTION
Gastric cancer is one of the most frequent malignancies in the world. Gastric cancer cells express a variety of factors, such as growth factors and cytokines, through interaction with microenvironment (Smith et al., 2006). Interactions between tumor and stromal cells have been reported to create a unique microenvironment that is essential for tumor growth and metastasis (Whiteside, 2008). These reports reinforce the importance of understanding the tumor microenvironment for tumor targeted therapy.
S100A8 and S100A9 (S100A8/A9), a family of low-molecular-weight intracellular EF-hand motif calcium-binding proteins, are abundantly expressed in cells of the myeloid lineage, including monocytes and neutrophils and early-differentiation states of macrophages. These proteins function predominantly as S100A8/A9 hetero-dimers and are highly chemotactic for leukocytes recruitment, adhesion and migration, thereby amplifying the local proinflammatory microenvironment (Nacken et al., 2003; Rychman et al., 2003). These proteins have been implicated in the inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, cystic fibrosis and psoriasis (Foell et al., 2007; Roth et al., 2001; 2003).
Upregulation of these proteins has also been observed in many tumor, including gastric, colon, pancreatic, bladder, ovarian, thyroid, breast, and skin cancers (Gebhardt et al., 2006; Salama et al., 2008) and is seen in tumor-infilterating cells in many tumors (Gebhardt et al., 2006; 2008). Consistent with this, our earlier studies reported that S100A8 and S100A9 were mostly expressed in infiltrating cells in the stroma between gastric tumor cells (Choi et al., 2012). These proteins have been reported to promote cancer proliferation (Turovskaya et al., 2008) and cause cancer metastasis by stimulating the migration of monocytes and tumor cells to metastatic sites (Hiratsuka et al., 2006; 2008). Although the role of S100A8/A9 has been reported in smaller studies of gastric cancer cells, the role of stromal S100A8/A9 on migration and invasion of gastric cancer cell has not been defined. In addition, the underlying mechanism of S100A8/A9-induced cell migration and invasion remains to be elucidated in gastric cancer cells. Therefore, the present study was undertaken to examine the effect of exogenous S100A8/A9 on migration and invasion of gastric cancer cells and to define its underlying mechanism.
MATERIALS AND METHODS
Reagents
Recombinant human S100A8 and S100A9 were purchased from ATGen Co. (Korea) and each recombinant proteins were mixed and incubated for 1 h at 4°C0 to form S100A8/A9 complex (Nacken and Kerkhoff, 2007). Fibronectin, Hoechst 33258 and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenylte-trazolium bromide (MTT) were purchased from Sigma-Aldrich Chemical (USA). SB203580 and Bay were obtained from Calbiochem (USA). Antibodies were obtained from Cell Signaling Technology Inc. (USA). All other chemicals were of the highest commercial grade available.
Cell culture
SNU216 and SNU484 cells were obtained from the Korean Cell Line Bank (Korea) and were maintained byserial-passages in 75-cm2 culture flasks (Costar, USA). The cells were grown in RPMI-1640 (HyClone, Thermo Scientific Inc., USA) containing 10% fetal bovine serum at 37°C in 95% air/5% CO2 incubator. When the cultures reached confluence, subculture was prepared using a 0.02% EDTA-0.05% trypsin solution. The cells were grown on tissue culture platesin RPMI-1640 medium containing 10% fetal bovine serum. Serum was removed from culture media at the time of agent addition.
RNAi transfection
Cells were transfected with siRNA using Lipofectamine RNAi-MAX reagent (Invitrogen, Life Technologies Corporation, USA) according to the manufacturer’s instructions. The sequences of siRNA are as follows: MMP2 siRNA (invitrogen), 5′-CCA GAU GUGGCCAACUACAACUUC U-3′, 5′-AGA AGU UGU AGUUGGCCACAUCUG G-3′; MMP12 siRNA (invitrogen), 5′-GCC AACCUUGCCAUCUGGCAUUGA A′, 5′-UUC AAU GCC AGAUGGCAAGGUUGG C-3′. RNAi negative control was obtained from invitrogen.
Measurement of cell proliferation
Cell proliferation was evaluated using a MTT assay. After washing the cells, culture medium containing 0.5 mg/ml of MTT was added to each well. The cells were incubated for 2 h at 37°C, the supernatant was removed and the formed form azan crystals in viable cells were solubilized with 0.11 ml of dimethyl-sulfoxide. A 0.1 ml aliquot of each sample was then transferred to 96-well plates and the absorbance of each well was measured at 570 nm with multiplatereader (Bio-Tek Synergy H1, Bio Teek, USA). Data were expressed as a percentage of control measured in the absence of S100A8/A9. Unless otherwise stated, the cells were treated with 1 μg/ml S100A8/A9 for 24 h. Test reagents were added to the medium 30 min before S100A8/A9 exposure.
Nuclei staining
Cells were grown on 22-mm glass coverslips in 6-well plates. After treatment with reagents as indicated, the cells were washed twice with phosphate-buffered solution (PBS) and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 30 min at 4°C. The fixed cells were stained with 10 μM Hoechst 33258 for 20 min at room temperature. Cells were then washed twice with PBS and examined by a fluorescence microscope (Axioskop2 plus, ZEISS, Germany). Apoptotic cells were identified by condensation and fragmentation of nuclei.
Cell cycle analysis
Cells were grown in 6-well plates and were treated as indicated. Then, attached and floating cells were pooled, pelleted by centrifugation, washed in phosphate-buffered saline, and fixed with cold 70% ethanol containing 0.5% Tween 20 at 4°C overnight. Cells were washed and resuspended in 1.0 ml of propidium iodide solution containing 100 μg of RNase A/ml and 50 μg propidium iodide/ml and incubated for 30 min at 37°C. Cells were assayed using FACS Aria (BDBiosciences, USA) at 488 nm and data were analyzed. Cells with sub-G1 propidium iodide incorporation were considered as apoptotic. The percentage of apoptotic cells was calculated as the ratio of events on sub-G1 to events from the whole population.
Cell migration assay
Migration of gastric cancer cells was assayed using a multiwell chambers (Neuroprobe, USA). The polycarbonate membrane of a 96-well chemotaxis chamber was precoated with 5 mg/ml fibronectin. Cells were suspended in serum-free RPMI at 2 × 106 cells/ml, and 50 μl cells were placed into the upper compartment of a chamber separated by an 8-μm precoated polyhydrocarbon filter. The lower wells of the chamber were filled with medium containing S100A8/A9 (1 μg/ml). In experiments for inhibitors, cells were preincubated with agents for 30 min and added to the upper chamber. After incubation for 18 h at 37°C, nonmigrated cells were removed by scraping, and cells that had migrated across the filter were washed with PBS, fixed with 4% paraformaldehyde for 20 min at room temperature, and stained with Hoechst 33258 (10 μM) for 30 min at 37°C. Stained cells were counted in three randomly chosen low-power fields (× 40).
Cell invasion assay
The chemotactic cell invasion assay was performed using 24-well Transwell units with an 8-μm pore size polycarbonate filter (Corning Incorporated, Costar). The upper side of the polycarbonate filter was coated with Matrigel (5 mg/ml in cold medium) to form a continuous thin layer. Prior to addition of the cell suspension, the dried layer of Matrigel matrix was rehydrated with serum-free RPMI for 3 h at 37°C. Each lower compartment of the Transwell was filled 600 μl RPMI containing with 1 μg/ml S100A8/A9. Serum-starved gastric cancer cells (1 × 105) were suspended in 0.1 ml RPMI and added to the upper compartment of the Transwell unit and incubated for 18 h at 37°C in a humidified atmosphere containing 5% CO2. Cells remaining in the upper compartment were completely removed with gentle swabbing. The number of cells that had crossed the filter to the lower compartment was determined using staining with Hoechst 33258.
Western blotting
Cells were harvested at various times after S100A8/A9 treatment and disrupted in lysis buffer (1% Triton X-100, 1mMEGTA, 1mMEDTA, 10 mM Tris-HCl, pH 7.4 and protease inhibitors). Cell debris was removed by centrifugation at 10, 000 × g for 10 min at 4°C. The resulting supernatants were resolved on a 12% SDS-PAGE under denatured reducing conditions and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat dried milk at room temperature for 30 min and incubated with primary antibodies. The membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody. The signal was visualized using an enhanced chemiluminescence (Amersham, UK).
Nuclear factor-κB (NF-κB) luciferase reporter gene assay
NF-κB transcriptional activity was determined using NF-κB-driven luciferase reporter plasmid (pNF-κB-Luc). Cells were transfected with NF-κB reporter plasmid using lipofectamine according to the instructions from the manufacturer when cells reached 60% confluence. After incubation for indicated time periods, the transfected cells were washed three times with ice-cold PBS, then incubated with 100 μl of passive lysis buffer for 10 min, and harvested. After centrifugation for 5 min, the supernatants were collected for measurements. Luciferase activities were determined with the luciferase reporter assay system according to the instructions of the manufacturers (Promega, USA).
Real-time RT-PCR
Total RNA was extracted from gastric cancer cells, using TRIzol reagent (Invitrogen, Life Technologies, USA) following the manufacturer’s instructions. 2 μg of RNA were reverse transcribed with Superscrip (Invitrogen) in a final volume of 20 μl. The 2 μl of cDNA were amplified with each primer and SYBRGreen (Applied Biosystems, Life Technologies, USA), using the fluorescence reader Corbett Rotor-Gene 6000 (Qiagen Inc., USA). The primers used were the following: GAPDH 5′-TCCATG ACAACTTTGGTATCG-3′, 5′-TGTAGCCAAATTCGTTGTCA-3′; MMP13 5′-TTCGGCTTAGAGGTGACTGG-3′, 5′-CGCAGC AACAAGAAACAAGT-3′; MMP12 5′-TACACATTCAGGAGG CAC-3′, 5′-CCACGGTAGTGACAGCATCA-3′; MMP11 5′-CTT GGCTGCTGTTGTGTGCT-3′, 5′-AGGTATGGAGCGATGTGA CG-3′; MMP9 5′-GAGCACGGAGACGGGTAT-3′, 5′-GCAGG CGGAGTAGGATTG; MMP8 5′-GCATCACCTCTCATCTTCA CC-3′, 5′-AGCATCTCCTCCAATACCTTG-3′; MMP7 5′-GGA ACAGGCTCAGGACTATCTC-3′, 5′-CAACATCTGGCACTCC ACA-3′; MMP3 5′-GATGCCCACTTTGATGATGA-3′, 5′-AGGT TCTGGAGGGACAGGTT-3′; MMP2 5′-GCCTCTCCTGACAT TGACCT-3′, 5′-AACACAGCCTTCTCCTCCTG-3′; MMP1 5′-GATGTGGAGTGCCTGATGTG-3′, 5′-TGATGTCTGCTTGAC CCTCA-3′. The number of cycles in the PCR was determined for each gene and ranged from 25 to 35. Data were normalized to GAPDH, and mRNA abundance was calculated using the 2−ΔΔCT method. The PCR products were also confirmed by mobility on gel electrophoresis.
Statistical analysis
The data are expressed as means ± S.E.M. and the difference between two groups was evaluated by unpaired Student’s t-test using SPSS v10.1 (SPSS Inc., USA). A probability level of 0.05 was used to establish significance.
RESULTS
S100A8/A9 stimulates invasion and migration of gastric cancer cells
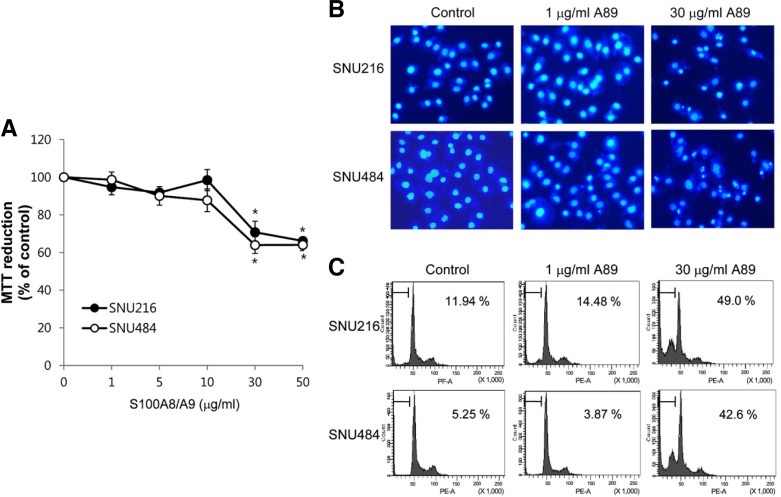
Effect of S100A8/A9 on cell viability was examined in SNU216 and SNU484 gastric cancer cells. SNU216 and SNU484 cells were exposed to various concentrations of S100A8/A9 for 24 h. As shown in Fig. 1A, S100A8/A9 caused loss of cell viability in dose-dependent manner. To delineate whether the S100A8/9-induced cell death was due to the induction of apoptosis, changes in nuclear morphology and cell cycle were investigated. Cells treated with high concentration S100A8/A9 (30 μg/ml) were observed apoptotic-like characteristics such as condensation and fragmentation of nuclei, whereas cells treated with low concentration S100A8/A9 (1 μg/ml) were not shown significant morphological changes (Fig. 1B). The cell cycle analysis also shown that the proportion of the cells in the sub-G1 phase at 30 μg/ml S100A8/A9 was increased (Fig. 1C).
Fig. 1.
Effect of S100A8/A9 on gastric cancer cells. (A) Cells were treated with S100A8/A9 at the indicated concentrations for 24 h and cell death was measured by MMT assay. Data are mean ± SEM of four independent experiments. *P < 0.05 compared with control. After 24 h incubation with 1 or 30 μg/ml S100A8/A9 (A89), Apoptotic cells were evaluated by Hoechst 33258 staining (B) and cell cycle analysis (C). The percentages within box indicate the extent of sub-G 1 phase.
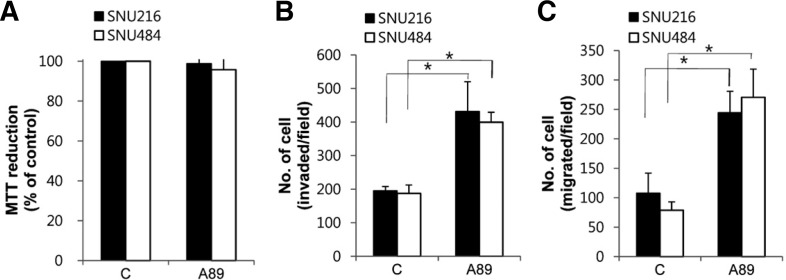
Ang and colleagues showed that S100A8/A9 at 0.4 and 2 μg/ml, respectively, stimulate migration in colorectal and pancreatic cancer cells (Ang et al., 2010). Thus, in subsequent experiments, cells were treated with 1 μg/ml S100A8/A9 to examine the effect on cell proliferation, invasion and migration. Although S100A8/A9 did not affect cell proliferation (Fig. 2A), it significantly increased the invasion and migration activity (Figs. 2B and 2C). These data indicate that exogenous S100A8/A9 induces cell invasion and migration in gastric cancer cells.
Fig. 2.
Effect of S100A8/A9 on gastric cancer cell invasion and migration. (A) Cells were treated with 1 μg/ml S100A8/A9 (A89) for 24 h and cell proliferation was estimated by MTT assay. The cell invasion (B) and migration (C) were measured using a trans well assay and quantified after labeling with Hoechst 33258. Data are mean ± SEM of four independent experiments. *P < 0.05 compared with control (C).
S100A8/A9 activates p38 MAPK and NF-κB signaling
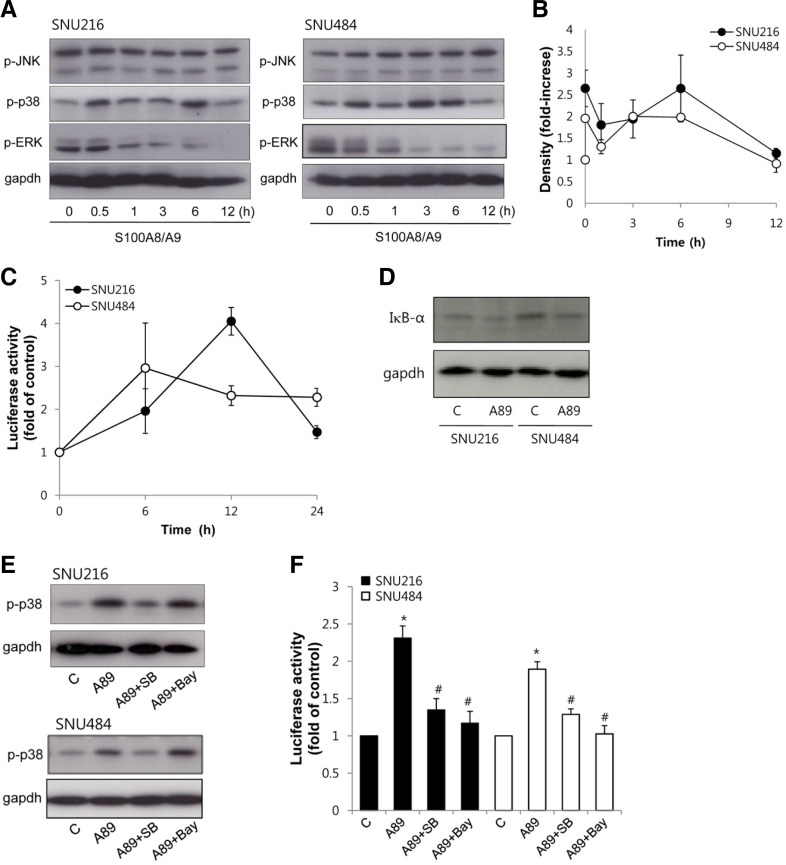
We next determined S100A8/A9-activated signaling pathways in gastric cancer cells. Mitogen-activated protein kinase (MAPK) is a key mediator of intracellular signaling pathway in response to different stimuli and has been reported to be activated by S100A8/A9 in various cancers (Hermani et al., 2006; Ichikawa et al., 2011). To investigate activation of MAPK, the phosphorylation of MAPK subfamilies was estimated by Western blotting in gastric cancer cells. S100A8/A9 stimulated rapid phosphorylation of p38 MAPK in both SNU216 and SNU484 cells within 30 min, with reduced phosphorylation after 12 h. Whereas S100A8/A9 induced an inhibition in phosphorylation of ERK and no detectable phosphorylation of JNK (Figs. 3A and 3B).
Fig. 3.
Effect of S100A8/A9 on MAPK and NF-κB signaling. (A) Lysates of cells treated with 1 μg/ml S100A8/A9 for indicated times were performed Western blotting using antibodies specific against p-ERK1/2, p-p38 MAPK, p-JNK and blot were probed with antibodies against gapd has loading control. (B) S100A8/A9 induced-activation of MAPK was quantified by densitometry. Data are mean ± SEM of three independent experiments. (C) Cells were transfected with pNF-κB-Luc. Transfected cells were treated with 1 μg/ml S100A8/A9 for indicated times and NF-κB transcriptional activity was determined by luciferase assay. (D) Lysates of cells treated with 1 μg/ml S100A8/A9 (A89) for 12 h were performed western blotting using antibodies against IκBα. (E) Cells were treated with 1 μg/ml S100A8/A9 (A89) for 6 h in the presence or absence of 10 μM SB203580 (SB) and 5 μM Bay. Expression of p-p38 MAPK was evaluated by Western blotting. (F) Cells were transfected with pNF-κB-Luc. Transfected cells were treated with 1 μg/ml S100A8/A9 (A89) for 12 h in the presence or absence of 10 μM SB203580 (SB) and 5 μM Bay. NF-κB transcriptional activity was determined by luciferase assay. Data indicated as fold of each control. Data are mean ± SEM of three independent experiments. *P < 0.05 compared with control (C); #P < 0.05 compared with S100A8/A9 (A89) alone.
NF-κB is transcription nuclear factor that could promote tumorigenesis, and is linked to cell invasion and metastasis. S100A8/A9 has also been reported to mediate NF-κB for tumorigenesis (Ghavami et al., 2009; Hermani et al., 2006). Therefore, NF-κB activity was determined by a luciferase reporter assay using an NF-κB reporter construct. S100A8/A9 induced an increase of luciferase activity after 6 h of treatment in both SNU216 and SNU484 cells. NF-κB transcriptional activity was increased to approximately 2-fold of control (Fig. 3C). To further confirm S100A8/A9-induced activation of NF-κB, we examined IκBα degradation. S100A8/A9 also induced degradation of IκBα in both cells (Fig. 3D). These results indicated that the treatment of S100A8/A9 on gastric cancer cells resulted in activation of p38 MAPK and NF-κB signaling pathway.
S100A8/A9-induced p38 MAPK phosphorylation mediates NF-κB activation
The S100A8/A9-induced p38 MAPK activation was totally abolished by the p38 MAPK inhibitor, SB203580, but it was not affected by the NF-κB inhibitor, Bay (Fig. 3E). NF-κB transcription activity was also measured in cells exposed to S100A8/A9 in the present or absence of inhibitors. S100A8/A9-induced NF-κB transcriptional activation was inhibited by addition of SB 203580 or Bay (Fig. 3F). These results indicate that S100A8/9 activated NF-κB through p38 MAPK activation.
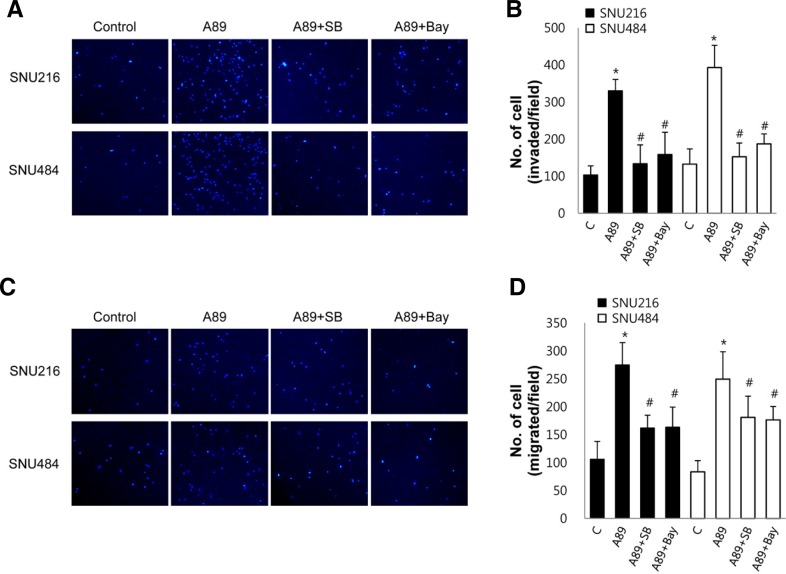
p38 MAPK and NF-κB are involved in S100A8/A9-induced cell invasion and migration
To investigate whether activation of p38 MAPK and NF-κB is involved in the S100A8/A9-induced cell invasion and migration, we examined the effect of inhibitors of p38 MAPK and NF-κB on invasion and migration. The S100A8/A9-induced cell invasion was inhibited by the p38 MAPK inhibitor SB203580 and the NF-κB inhibitor Bay (Figs. 4A and 4B). The S100A8/A9-induced cell migration was also inhibited by SB203580 and Bay (Figs. 4C and 4D). Exposure of cells to SB203580 or Bay alone for 24 h did not affect cell invasion and cell migration (data not shown). These results suggest that activation of p38 MAPK and NF-κB is required for the S100A8/A9-induced cell invasion and migrationin gastric cancer cells.
Fig. 4.
Role of p38 MAPK and NF-κB on S100A8/A9-induced cell invasion and migration. Cell invasion (A, B) and migration (C, D) were measured in cells pretreated with 10 μM SB203580 (SB) and 5 μM Bay for 30 min using a transwell assay. These cells were added to upper chamber and the lower wells of the chamber were filled with medium containing 1 μg/ml S100A8/A9 (A89). After incubation for 18 h, migrated and invaded cells were quantified. Representative and quantitative data were shown. Data are mean ± SEM of three independent experiments. *P < 0.05 compared with control (C); #P < 0.05 compared with S100A8/A9 (A89) alone.
MMP2 and MMP12 are involved in S100A8/A9-induced cell invasion and migration
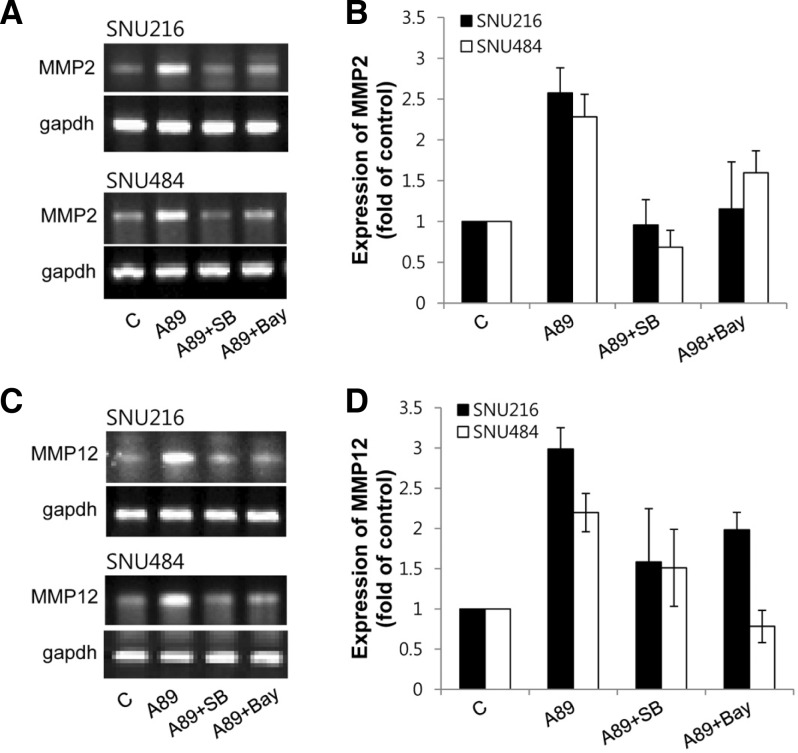
MMPs were shown to play a pivotal role in tumor invasion and metastasis (Roy et al., 2009). To examine whether S100A8/A9 upregulates MMPs expression, we analyzed the mRNA expression levels of MMP1, 2, 3, 7, 8, 9, 11, 12 and 13 by real-time PCR. When the cells were treated with S100A8/A9, mRNA expressions of MMP2 and MMP12 were increased by S100A8/A9 but MMP1, 3, 7, 8, 9, 11 and 13 were not altered (data not shown) in both SNU216 and SNU484 cells. Upregulated MMP2 and MMP12 were inhibited by SB203580 and Bay (Fig. 5). These results indicate that the p38 MAPK and NF-κB are involved in S100A8/A9-induced MMP2 and MMP12 up-regulation in gastric cancer cells.
Fig. 5.
Effect of S100A8/A9 on MMPs expression. Cells were treated with 1 μg/ml S100A8/A9 (A89) for 24 h in the presence or absence of 10 μM SB203580 (SB) and 5 μM Bay and then total RNA of cells extracted. mRNA expression of MMP2 and MMP12 were evaluated by real-time RT-PCR. The PCR products were loaded on 1% agarose gel. Representative (A, C) and quantitative (B, D) data were shown. Data are mean ± SEM of three independent experiments.
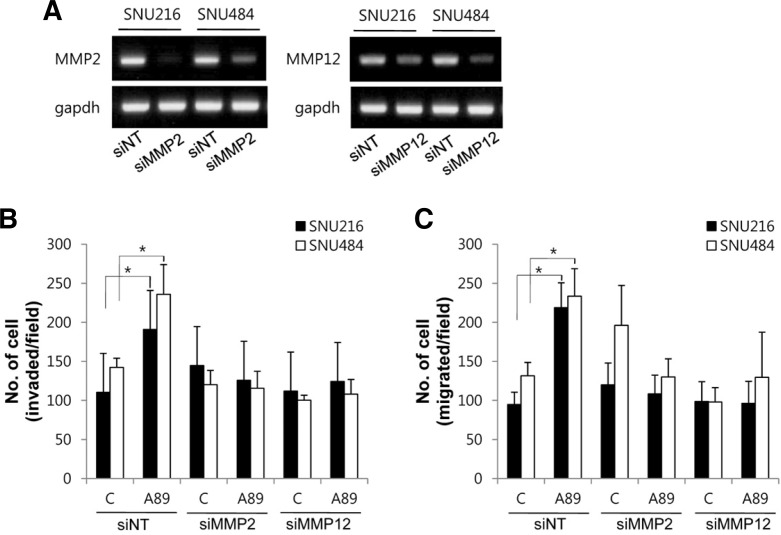
Next, to examine roles of MMP2 and MMP12 in the S100A8/A9-induced cell invasion and migration, cells were transfected with MMP2 siRNA or MMP12 siRNA. MMP2 siRNA or MMP12 siRNA decreased the mRNA level of MMP2 or MMP12 in SNU216 and SNU484 cells, respectively (Fig. 6A). S100A8/A9-induced cell invasion and migration were inhibited by transfection of MMP2 siRNA or MMP12 siRNA (Figs. 6B and 6C). These results suggest that MMP2 and MMP12 are associated with S100A8/A9-induced cell invasion and migration in gastric cancer cells.
Fig. 6.
Role of MMP2 and MMP12 on S100A8/A9-induced cell invasion and migration. (A) Cells were transfected with MMP2 siRNA (siMMP2), MMP12 siRNA (siMMP12) or non-targeted siRNA (siNT) and two days after transfection the efficiency of knockdown of MMP2 and MMP12 were determined by RT-PCR. (B, C) After transfection with siRNA, invasion and migration were measured using a transwell assay and quantified after labeling with Hoechst 33258. Data are mean ± SEM of four independent experiments. *P < 0.05 compared with control (C).
DISCUSSION
The tumor microenvironment plays an important role in tumor progression. A variety of stromal cells in the surrounding environment does not only enhance growth of the primary cancer but also facilitate its metastatic dissemination to distant organs (Joyce and Pollard, 2009). Stromal S100A8- and S100/A9-positive myeloid cells in a cancer microenvironment have been characterized (Ichikawa et al., 2011; Sheikh et al., 2007; Turovskaya et al., 2008). Previously, we have also observed the enhanced S100A8 and S100A9 expression in infiltrating cells in the stroma of gastric cancers (Choi et al., 2012). These observations prompted us to examine the role of exogenous S100A8/A9. The present study showed that exogenous S100A8/A9 induces migration and invasion of the gastric cancer cell lines SNU216 and SNU484 (Fig. 2), indicating that extracellular S100A8/A9 may play an important role in the migration and invasion of gastric cancer cells. Consistent with this, S100A8/A9 have been reported to play a role in guidance for the adhesion and invasion of disseminating malignant cells in lung cancer (Hiratsuka et al., 2006) and colon cancer (Ichikawa et al., 2011). However, we previously demonstrated that endogenous S100A8 and S100A9 might be negative role on migration and invasion (Choi et al., 2012), indicating that endogenous S100A8/A9 play a role that is different to that of exogenous S100A8/A9. These results suggest that extracellular S100A8/A9 promote migration and invasion through interaction with gastric cancer cells.
Although S100A8/A9 protein has been shown to exert apoptotic effect against various tumor cells including colon carcinoma cells (Ghavami et al., 2004) and breast cancer cells and neuroblastoma cells (Ghavami et al., 2008a), the effect of S100A8/A9 on cell viability has been reported to be dependent on concentration exposed. S100A8/A9 resulted in significantly reduced cell viability at concentration above 50 μg/ml, while it at concentration lower than 25 μg/ml promotes proliferation and migration of various cancer cells (Ghavami et al., 2008b; Hermani et al., 2006; Moon et al., 2008). We observed that S100A8/A9 at high concentration (30 μg/ml) exerts apoptotic effects in gastric cancer cells (Fig. 1), whereas at low concentration (1 μg/ml) promotes migration and invasion without significant changes in cell proliferation (Fig. 2). This concentration (1 μg/ml) was chosen for migration and invasion because S100A8/A9 at 0.4 and 2 μg/ml, respectively, increase migration in colorectal and pancreatic cancer cells (Ang et al., 2010), and because higher concentrations (over 1 μg/ml) of S100A8/A9 did not significantly increase migration and invasion compared to 1 μg/ml (data not shown).
The present study showed that S100A8/A9 incresed phosphorylation of p38 MAPK, but inhibited phosphorylation of ERK and did not affect phosphorylation of JNK (Fig. 3). This is consistent with the finding that S100A8/A9 provoked p38 MAPK while JNK phosphorylation was unaffected in human prostate and breast cancer cells (Ghavami et al., 2008b; Hermani et al., 2006). In contrast, S100A8/A9 activates ERK and JNK but not p38 MAPK in colon cancer cells (Ichikawa et al., 2011). Therefore, the effect of S100A8/A9 on MAPK signaling may depend on the cancer cell types.
In the present study, S100A8/A9 also caused NF-κB activation, inhibited by the p38 MAPK inhibitor SB203580, but S100A8/A9-induced phosphorylation of p38 MAPK was not affected by the NF-κB inhibitor, Bay (Fig. 3). These data indicate that p38 MAPK mediates the NF-κB activation induced by S100A8/A9. These results are consistent with reports that S100A8/A9 promotes activation of MAP kinase and NF-κB signaling pathway in breast cancer, leukemia, neuroblastoma, prostate cancer and colon cancer cells (Ghavami et al., 2008b; Hermani et al., 2006; Ichikawa et al., 2011). In addition, we found that activation of p38 MAPK and NF-κB was associated with the S100A8/A9-induced cell migration and invasion as evidenced by attenuation of the migration and invasion by the SB203580 and Bay (Fig. 4). Our data are in line with previous reports that the activation of NF-κB downstream of MAPK pathway is involved in numerous pathological processes, such as cancer cell adhesion, invasion, metastasis, and angiogenesis (Aggarwal, 2004; Takade et al., 2004).
Enzymatic degradation of extracellular matrix is one of the crucial steps in cancer invasion and metastasis. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes and capable of degrading ECM proteins. Abnormal expression of MMPs has been found to be involved in tumor invasion, metastasis, and angiogenesis (Egeblad and Werb, 2002; Roy et al., 2009). As shown in Fig. 5, S100A8/A9 increased the expression level of MMP2 and MMP12 but not MMP1, MMP3, MMP7, MMP8, MMP9, MMP11 and MMP13 (data not shown). Upregulated MMP2 and MMP12 were inhibited by SB203580 and Bay. These results suggest that S100A8/A9 induced an increase of MMP2 and MMP12 through activation of p38 MAPK and NF-κB. Role of MAPK pathway in regulation of MMPs is well known (Rao, 2003). The transcription of MMPs is regulated by upstream sequences, including motifs corresponding to NF-κB (Silva, 2004). We further found that knockdown of MMP2 or MMP12 by transfection of each siRNA decrease the S100A8/A9-induced invasion and migration of gastric cancer cells. MMP2 has been shown to be related to invasiveness in various cancer including hepato-cellular carcinoma (Li et al., 2011), colorectal cancer (Dong et al., 2011) and gastric cancer (Zhao et al., 2010). It also has been reported that MMP12 correlates with metastasis in hepatocellular carcinoma (Ng et al., 2011), squamous cell carcinoma (Kerkerla et al., 2002) and non-small cell lung cancer (Hofmann et al., 2005). Thus, these two MMPs may play important roles in S100A8/A9-induced invasion and migration of gastric cancer cells.
In summary, exogenous S100A8/A9 promotes cell migration and invasion through activation of p38 MAPK and NF-κB followed by up-regulation of MMP2 and MMP12 in gastric cancer cells. To our knowledge, this is the first report to examine involvement of p38 MAPK, NF-κB and MMPs signaling pathway on S100A8/A9-induced migration and invasion in gastric cancer. These results suggest that extracellular S100A8/A9 plays an important role in regulating processes such as tumor cell migration and invasion. Thus, the molecular pathways mediated by S100A8/A9 could be an attractive target for pharmacological interventions.
Acknowledgments
This study was supported by a grant 0920050 from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
REFERENCES
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ang CW, Nedijadi TA, Sheikh AM, Tweedle E, Tonack S, Honap SE, Jenkins R, Park BK, Schwarte-Waldhoff I, Khattak I, et al. Smad4 loss is associated with fewer S100A8-positive monocytes in colorectal tumors and attenuated response to S100A8 in colorectal and pancreatic cancer cells. Carcinogenesis. 2010;31:1541–1551. doi: 10.1093/carcin/bgq137. [DOI] [PubMed] [Google Scholar]
- Choi JH, Shin NR, Moon HJ, Kwon CH, Kim GH, Song GA, Jeon TY, Kim DH, Park DY. Identification of S100A8 and S100A9 as negative regulators for lymph node metastasis of gastric adenocarcinoma. Histol Histopathol. 2012;27:1439–1448. doi: 10.14670/HH-27.1439. [DOI] [PubMed] [Google Scholar]
- Dong W, Li H, Zhang Y, Yang H, Guo M, Li L, Liu T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim Biophys Sin. 2011;43:840–848. doi: 10.1093/abbs/gmr085. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–175. doi: 10.1189/jlb.0903435. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Chazin WJ, Kadkhoda K, Xiao W, Zuse A, Hashemi M, Eshraghi M, Schulze-Osthoff K, Klonisch T, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008a;1783:297–311. doi: 10.1016/j.bbamcr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Rashedi I, Dattilo BM, Eshraghi M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C, Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukocyte Biol. 2008b;83:1484–1492. doi: 10.1189/jlb.0607397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, Kerkhoff C. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625:73–83. doi: 10.1016/j.ejphar.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Hermani A, De Servi B, Medunganin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–197. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburantani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- Hofmann HS, Hansen G, Richter G, Taege C, Simm A, Silber RE, Burdach S. Matrixproteinase-12 expression correlates with local recurrence and metastatic desease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–1092. [PubMed] [Google Scholar]
- Ichikawa M, Williams R, Wang L, Vogl T, Srikrichna G. S100A8/A9 acrivate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkela E, Ala-aho R, Klemi P, Grenman S, Shapiro SD, Kahari VM, Saarialho-Kere U. Metalloelastease (MMP-12) expression by tumour squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J Pathol. 2002;198:258–269. doi: 10.1002/path.1198. [DOI] [PubMed] [Google Scholar]
- Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interlukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A, Yong HY, Song JI, Cukovic D, Salagrama S, Kaplan D, Putt D, Kim H, Dombkowski A, Kim HR. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol Cancer Res. 2008;6:1544–1553. doi: 10.1158/1541-7786.MCR-08-0189. [DOI] [PubMed] [Google Scholar]
- Nacken W, Kerkhoff C. The hetero-oligomeric complex of the S100A8/A9 protein is extremely protease resistant. FEBS Lett. 2007;581:5127–5130. doi: 10.1016/j.febslet.2007.09.060. [DOI] [PubMed] [Google Scholar]
- Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representative of the S100: protein family ac prominent players in innate immunity. Microsc Res Tech. 2003;60:569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- Ng KT, Qi X, Kong KL, Cheung BY, Lo CM, Poon RT, Fan ST, Man K. Overexpression of matrix metalloproteinase-12 (MMP-12) correlates with poor prognosis of hepatocellular carcinoma. Eur. J Cancer. 2011;47:2299–2305. doi: 10.1016/j.ejca.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Roth J, Goebeler M, Sorg C. S100A8 and S100A9 in inflammatory diseases. Lancet. 2001;257:1041. doi: 10.1016/S0140-6736(05)71610-X. [DOI] [PubMed] [Google Scholar]
- Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutophilchemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, Shekouh A, Campbell F, Dodson A, Prime W, Crnogorac-Jurcevic T, et al. The expression of S100A8 in pancreatic cancer-associated monocyte is associated with the Smad4 status of pancreatic cancer cells. Proteomics. 2007;7:1929–1940. doi: 10.1002/pmic.200700072. [DOI] [PubMed] [Google Scholar]
- Silva D. Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets. 2004;4:327–336. doi: 10.2174/1568009043332961. [DOI] [PubMed] [Google Scholar]
- Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Singh S, Aggarwal BB. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- Turovskaya O, Foell D, Shiha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhus A, et al. RAGE, carboxylatedglycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong HY, Moon A. Roles of calcium-binding proteins, S100A8 and S100A9, in invasive phenotype of human gastric cancer cells. Arch Pharm Res. 2007;30:75–81. doi: 10.1007/BF02977781. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]