Abstract
Demyelination is the pathological process by which myelin sheaths are lost from around axons, and is usually caused by a direct insult targeted at the oligodendrocytes in the vertebrate central nervous system (CNS). A demyelinated CNS is usually remyelinated by a population of oligodendrocyte progenitor cells, which are widely distributed throughout the adult CNS. However, myelin disruption and remyelination failure affect the normal function of the nervous system, causing human diseases such as multiple sclerosis. In spite of numerous studies aimed at understanding the remyelination process, many questions still remain unanswered. Therefore, to study remyelination mechanisms in vivo, a demyelination animal model was generated using a transgenic zebrafish system in which oligodendrocytes are conditionally ablated in the larval and adult CNS. In this transgenic system, bacterial nitroreductase enzyme (NTR), which converts the prodrug metronidazole (Mtz) into a cytotoxic DNA cross-linking agent, is expressed in oligodendrocyte lineage cells under the control of the mbp and sox10 promoter. Exposure of transgenic zebrafish to Mtz-containing media resulted in rapid ablation of oligodendrocytes and CNS demyelination within 48 h, but removal of Mtz medium led to efficient remyelination of the demyelinated CNS within 7 days. In addition, the demyelination and remyelination processes could be easily observed in living transgenic zebrafish by detecting the fluorescent protein, mCherry, indicating that this transgenic system can be used as a valuable animal model to study the remyelination process in vivo, and to conduct high-throughput primary screens for new drugs that facilitate remyelination.
Keywords: CNS, demyelination, nfsB, oligodendrocyte, zebrafish
INTRODUCTION
Myelin, a lipid-rich multilamellar membrane generated by oligodendrocytes in the central nervous system (CNS) and by Schwann cells in the peripheral nervous system (PNS), provides electrical insulation by enwrapping the axon in segments that are separated by nodes of Ranvier. The myelin sheath ensures that membrane depolarization can only occur at the nodes, allowing the fast, saltatory movement of nerve impulses in myelinated axons compared with that in unmyelinated axons (Giuliodori and DiCarlo, 2004). Oligodendrocytes are the myelinating glial cells of the CNS and are generated from the precursors in the pMN domain of the ventral spinal cord after motor neuron development (Lu et al., 2002; Park et al., 2002a; Zhou and Anderson, 2002). The pMN precursors produce migratory oligodendrocyte progenitor cells (OPCs), which travel laterally and dorsally to populate the developing CNS before differentiating into myelin-forming oligodendrocytes (Rowitch, 2004). The importance of myelination for nervous system function is underscored by a number of pathological consequences of demyelinating conditions in humans, such as multiple sclerosis (MS) and hereditary myelin diseases (Franklin and Ffrench-Constant, 2008; Scherer and Wrabetz, 2008).
Demyelination is the pathological process by which myelin sheaths are lost from around axons, and it is usually caused by a direct insult targeted at oligodendrocytes. Demyelination affects the normal function of neurons in the CNS; thus the demyelinated CNS is usually remyelinated by a population of OPCs, which are widely distributed throughout the adult CNS (Franklin and Ffrench-Constant, 2008; Patel and Klein, 2011). However, myelin disruption and remyelination failure affects the normal function of the nervous system, causing primary demyelination diseases such as MS (Franklin and Ffrench-Constant, 2008; Scherer and Wrabetz, 2008). Although considerable progress has been achieved in understanding the remyelination process in the demyelinated CNS using several animal models of chemically induced demyelination, it is still unclear why endogenous repair mechanisms fail to remyelinate axons in patients with demyelination diseases.
The zebrafish provides a powerful model system for investigating oligodendrocyte development due to the rapid external development and transparency of the embryos (Kim et al., 2011). Previously, we generated Tg(mbp:egfp) transgenic zebrafish, which express EGFP in mature oligodendrocytes under the control of the mbp promoter, and demonstrated that this transgenic fish is a valuable animal model for studying the myelination process by visualization of oligodendrocytes and myelin sheaths in the embryonic and adult CNS (Jung et al., 2010). Here, to investigate remyelination mechanisms in vivo, we generated an animal model of demyelination using a transgenic zebrafish system in which a bacterial cytotoxin gene is expressed in oligodendrocytes under the control of the mbp and sox10 promoters. To conditionally ablate oligodendrocytes, we used the bacterial gene, nfsB, which encodes a nitroreductase (NTR) enzyme that can convert prodrugs such as metronidazole (Mtz) into cytotoxins (Curado et al., 2007; Pisharath et al., 2007). Exposure of transgenic zebrafish, which express nfsB fused with mCherry specifically in oligodendrocyte lineage cells, to Mtz-containing medium resulted in rapid ablation of oligodendrocytes and demyelination of the CNS in both larvae and adults. Efficient remyelination of the demyelinated CNS was observed 7 days after the removal of Mtz-containing media. In addition, we were able to observe the demyelination and remyelination processes in vivo in live fishes by detecting mCherry fluorescence in oligodendrocytes and their myelin sheaths, indicating that our transgenic system is a valuable animal model for studying the remyelination process in vivo.
MATERIALS AND METHODS
Animals
The zebrafish embryos used in this study were Tg(mbp:egfp) (Jung et al., 2010), Tg(sox10:egfp) (Carney et al., 2006), Tg(uas: nfsB-mCherry) (Davison et al., 2007), and Tg(uas:egfp) (Asakawa et al., 2008) and raised at 28.5°C in egg water or embryo medium (EM; 15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM NH2PO4, 0.7 mM NaHCO3). Embryos were staged according to “days post-fertilization” (dpf), hours post-fertilization (hpf)” and morphological criteria (Kimmel et al., 1995).
Plasmid construction and generation of transgenic lines
To produce Tg(mbp:gal4-vp16) and Tg(sox10:gal4-vp16) fish, a 5′-entry clone was used for the mbp promoter (Jung et al., 2010) and sox10 promoter (Carney et al., 2006), a middle-entry clone was used for gal4-vp16, and a 3′-entry clone was used for poly A using the BP reaction of the Gateway system (Invitrogen). Next, the LR reaction was performed with mbp/sox10-5′-entry, gal4-vp16-middle entry and poly A-3′-entry clones to generate mbp:gal4-vp16 DNA and sox10:gal4-vp16 DNA using LR II clonase (Invitrogen). To generate transgenic zebrafish, fertilized eggs were injected with 1 nl of a mixture containing 25 ng/ul of the mbp/sox10-gal4-vp16 DNA and 25 ng/ul of the transposase mRNA (Kotani et al., 2006).
Metronidazole treatment
Metronidazole (Mtz, M1547, Sigma) was dissolved in EM containing 0.2% DMSO to 10 mM by vigorous agitation. For the ablation of oligodendrocytes, Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae were incubated in 10 mM Mtz for 48 hrs starting at 5 dpf, and Tg(sox10:gal4-vp16;uas:nfsB-mcherry) larvae were incubated in 5 mM Mtz for 12 h starting at 5 dpf. To stop the treatment, transgenic larvae were rinsed at least three times in EM. Adult Tg(mbp:gal4-vp16;uas:nfsB-mCherry) fishes were placed in 1-liter water containing 5 mM Mtz diluted from a 10 mM stock. Fishes were maintained in a black chamber to avoid photodegradation of Mtz.
TUNEL assay
Embryos grown to 7 dpf development stages were fixed with 4% paraformaldehyde overnight and dehydrated in methanol, followed by a 5-min incubation in acetone at −20°C and 10-min rinse in PBST (PBS, 0.2% Triton X-100, Sigma) for further permeability. Embryos were then subjected to a TUNEL assay using In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s instructions.
Immunohistochemistry
Embryos were anesthetized until movement ceased and fixed in 4% paraformaldehyde overnight. Fixed embryos were embedded in 1.5% agar blocks containing 5% sucrose and equilibrated in a 30% sucrose solution. Frozen blocks were sliced into 10-μm sections using a cryostat microtome and mounted on glass slides. For immunohistochemistry, we used following primary antibodies: a rabbit anti-Sox10 (1:250) (Park et al., 2005), a rabbit anti-GFP (1:500, Abcam), and mouse anti-HuC/D (16A11, 1:20, Molecular Probes). For fluorescent detection of antibody labeling, Alexa Fluor 568-conjugated goat anti-mouse IgG, Alexa Fluor 568-conjugated goat anti-rabbit IgG, and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:500, Molecular Probes) were used.
RESULTS
Expression of nfsB-mCherry in oligodendrocyte lineage cells using Gal4/UAS transgenic systems
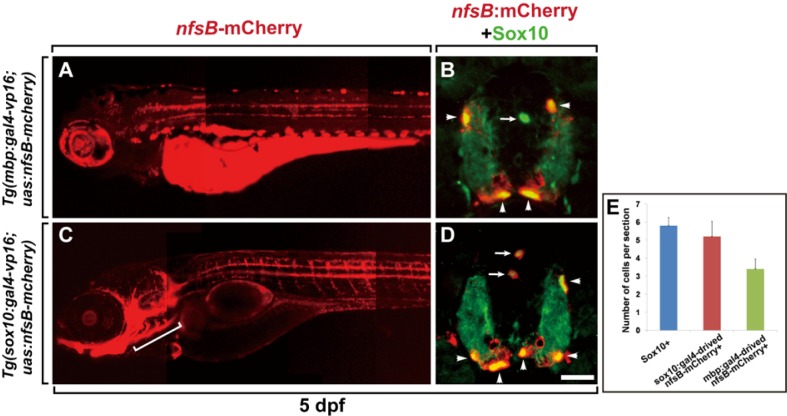
To drive expression of the nfsB-mCherry fusion protein in oligodendrocyte lineage cells, we used two different oligodendrocyte lineage-specific promoters, mbp and sox10. MBP is the second most abundant protein in CNS myelin (Boggs, 2006), and we previously showed that Tg(mbp:egfp) zebrafish, which express EGFP under the control of the mbp promoter, display faithful EGFP expression in mature oligodendrocytes in the embryonic and adult CNS (Jung et al., 2010). Sox10 is a widely used marker for oligodendrocyte lineage cells, including OPCs and mature oligodendrocytes, in zebrafish (Park et al., 2002b; 2005), and Tg(sox10:egfp) embryos that express EGFP under the control of the sox10 promoter drive EGFP expression in neural crest and oligodendrocyte lineage cells in zebrafish (Carney et al., 2006; Chung et al., 2011). We generated Tg(mbp:gal4-vp16) and Tg(sox10: gal4-vp16) to drive Gal4-VP16 transactivator expression in oligodendrocyte lineage cells, and crossed them with Tg(uas:nfsB-mcherry) (Davison et al., 2007) to generate Tg(mbp:gal4-vp16;uas:nfsB-mcherry) and Tg(sox10:gal4-vp16;uas:nfsB-mcherry) zebrafish (Figs. 1A and 1C). Labeling with an anti-Sox10 antibody showed that nfsB-mCherry was specifically expressed in mature oligodendrocytes located in the white matter of the Tg(mbp:gal4-vp16;uas:nfsB-mcherry) spinal cord (Fig. 1B), and in OPCs and mature oligodendrocytes in the Tg(sox10:gal4-vp16; uas:nfsB-mcherry) spinal cord (Fig. 1D). However, as shown in a previous study (Carney et al., 2006), nfsB-mCherry expression was also observed in the branchial arches in Tg(sox10:gal4-vp16; uas:nfsB-mcherry) larvae (Fig. 1C, bracketed area). Together, these data indicate that the Gal4/UAS transgenic system with the mbp and sox10 promoters successfully drives nfsB-mcherry transgene expression in oligodendrocyte lineage cells.
Fig. 1.
Generation of transgenic zebrafish expressing the nfsB-mCherry transgene in oligodendrocyte lineage cells. (A, C) Lateral view of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (A) and Tg(sox10:gal4-vp16; uas:nfsB-mcherry) larvae (C) showing nfsB-mCherry expression in oligodendrocyte lineage cells (anterior to the left). The bracketed area indicates sox10:nfsB-mCherry expression in cranial cartilage in (C). (B, D) Transverse sections of the spinal cord of Tg(mbp: gal4-vp16;uas:nfsB-mcherry) (B) and Tg(sox10:gal4-vp16;uas:nfsB-mcherry) larvae (D) (dorsal at the top). Anti-Sox10 antibody labeling revealed Sox10+/nfsB-mCherry+ mature oligodendrocytes (arrowheads) and Sox10+/nfsB-mCherry− OPCs (arrows) (B) and Sox10+/nfsB-mCherry+ OPCs and mature oligodendrocytes (D). Arrows indicate OPCs in the gray matter, and arrowheads indicate mature oligodendrocytes in the white matter of the spinal cord. (E) Quantification of the number of Sox10+ and NfsB-mCherry+ oligodendrocyte lineage cells prior to Mtz treatment in the spinal cord sections of Tg(mbp/sox10:gal4-vp16;uas:nfsB-mcherry) larvae. Data were obtained from 10 sections from each of 4 transgenic larvae. Scale bar, 20 μm.
Conditional ablation of oligodendrocytes leads to demyelination in the CNS
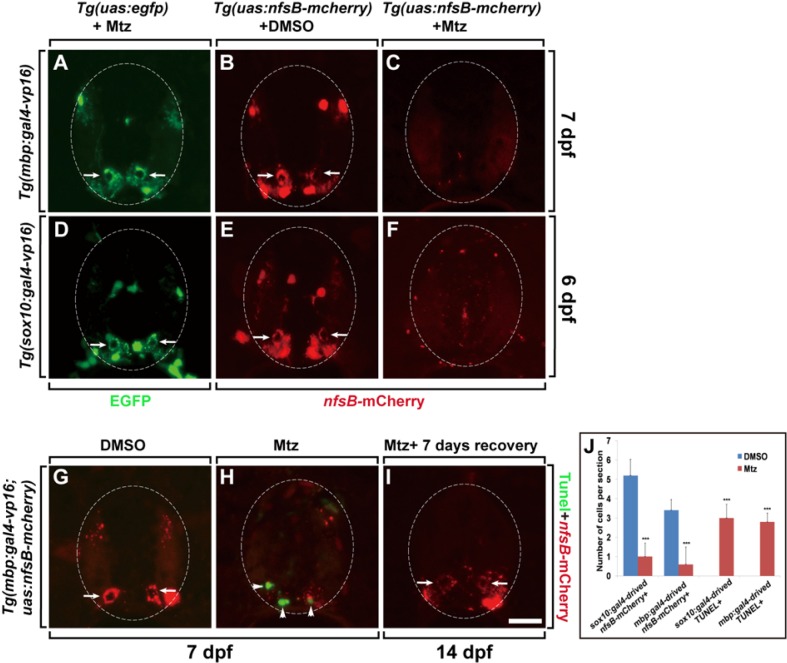
To test the effectiveness of the NTR/Mtz system in inducing demyelination via drug-dependent ablation of oligodendrocyte lineage cells, we crossed Tg(mbp/sox10:gal4-vp16) driver lines with Tg(uas:egfp) and Tg(uas:nfsB-mcherry) fishes and exposed bitransgenic larvae to the prodrug, Mtz, at 5 dpf. In the absence of the nfsB gene, Mtz treatment does not induce oligodendrocyte ablation or demyelination in the spinal cord of Tg(mbp/sox10:gal4-vp16;uas:egfp) larvae; thus the oligodendrocytes and myelin sheaths of the large axons (marked by EGFP expression) in the spinal cord were normal (Figs. 2A and 2D, arrows). Tg(mbp/sox10:gal4-vp16;uas:nfsB-mcherry) larvae treated with DMSO in the absence of Mtz also demonstrated normal oligodendrocyte numbers and myelin sheath formation in the spinal cord (Figs. 2B and 2E, arrows). However, exposure of 5 dpf Tg(mbp/sox10:gal4-vp16;uas:nfsB-mcherry) larvae to Mtz ablated most of the oligodendrocytes and resulted in complete demyelination in the spinal cord (Figs. 2C, 2F, and 2J). Interestingly, incubation of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae for 48 h in 10 mM Mtz ablated most of the oligodendrocytes (Fig. 2C), but a 12 h incubation in 5 mM Mtz was sufficient to ablate the oligodendrocytes in Tg(sox10:gal4-vp16;uas:nfsB-mcherry) larvae (Fig. 2F). This indicates that the ablation efficiency is higher in Tg(sox10:gal4-vp16;uas:nfsB-mcherry) larvae than in Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae.
Fig. 2.
Ablation of oligodendrocytes leads to demyelination of the CNS. All panels show transverse sections of the spinal cord (dorsal at the top). Spinal cords of Tg(mbp:gal4-vp16;uas:egfp) (A) and Tg (sox10:gal4-vp16;uas:egfp) (D) larvae treated with Mtz, and Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (B) and Tg(sox10:gal4-vp16;uas:nfsB-mcherry) (E) larvae treated with DMSO showed a normal number of oligodendrocytes and myelin sheaths. The spinal cords of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (C) and Tg(sox10:gal4-vp16;uas:nfsB-mcherry) (F) larvae treated with Mtz showed ablation of oligodendrocytes and complete demyelination. (G-I) TUNEL staining of spinal cord sections of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae treated with DMSO (G) or Mtz (H-I) for 48 h and recovered for 7 days after removal of Mtz (I). The spinal cord is outlined by the dashed circles. Arrows indicate the myelin sheaths, and arrowheads indicate TUNEL+ cells in the spinal cord. (J) Quantification of the number of NfsB-mCherry+ oligodendrocyte lineage cells and TUNEL+ cells (***p < 0.001) in the spinal cord sections of Tg(mbp/sox10: gal4-vp16;uas:nfsB-mcherry) larvae treated with DMSO and Mtz, respectively. Data were obtained from 10 sections from each of 4 transgenic larvae treated with DMSO and Mtz. Scale bar, 20 μm.
We next tested for the presence of apoptotic cell death using the TUNEL assay. The spinal cord of Mtz-treated Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae demonstrated significantly increased levels of apoptotic cell death, together with complete demyelination (Figs. 2H and 2J) compared with the DMSO-treated control (Fig. 2G). Interestingly, raising the Tg(mbp:gal4-vp16;uas:nfsB-mcherry) zebrafish for 7 days after removal of the Mtz-containing media resulted in the recovery of myelination, indicating that oligodendrocytes were successfully regenerated (Fig. 2I).
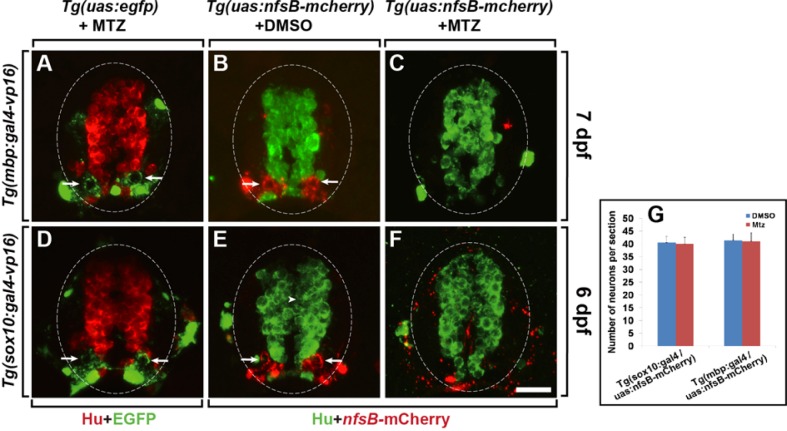
We next tested whether NTR/Mtz-mediated ablation of oligodendrocytes affects the survival of neighboring neurons in the spinal cord. In the spinal cord of Tg(mbp/sox10:gal4-vp16;uas: egfp) larvae treated with Mtz (Figs. 3A and 3D) and Tg(mbp/sox10:gal4-vp16;uas:nfsB-mcherry) larvae treated with DMSO (Figs. 3B and 3E), post-mitotic neurons (marked by anti-Hu antibody labeling)were visualized normally along with oligodendrocytes and the myelin sheath. Hu+ neurons in the spinal cord of Tg(mbp/sox10:gal4-vp16;uas:nfsB-mcherry) larvae treated with Mtz also appeared normal, although most oligodendrocytes were ablated (Figs. 3C, 3F, and 3G), indicating that oligodendrocyte-specific ablation by the NTR/Mtz system does not affect neuronal survival. Together, these data indicate that oligodendrocyte-specific expression of nfsB-mCherry by the Gal4/UAS transgenic system causes drug-dependent ablation of oligodendrocytes and demyelination in the spinal cord of zebrafish larvae.
Fig. 3.
Neurons are unaffected after the ablation of oligodendrocytes. All panels show transverse sections of the spinal cord (dorsal at the top). Spinal cords of Tg(mbp:gal4-vp16;uas:egfp) (A) and Tg(sox10: gal4-vp16;uas:egfp) (D) larvae treated with Mtz and Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (B) and Tg (sox10:gal4-vp16;uas:nfsB-mcherry) (E) larvae treated with DMSO demonstrated normal myelin sheath formation and neuronal numbers. Spinal cords of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (C) and Tg (sox10:gal4-vp16;uas:nfsB-mcherry) (F) larvae treated with Mtz showed ablation of oligodendrocytes and complete demyelination, but a normal number of neurons. Arrows indicate the myelin sheath. (G) Quantification of the number of Hu+ neurons in the spinal cord sections of Tg(mbp/sox10:gal4-vp16;uas: nfsB-mcherry) larvae treated with DMSO and Mtz, respectively. Data were obtained from 10 sections from each of 4 transgenic larvae treated with DMSO and Mtz. Scale bar: 20 μm.
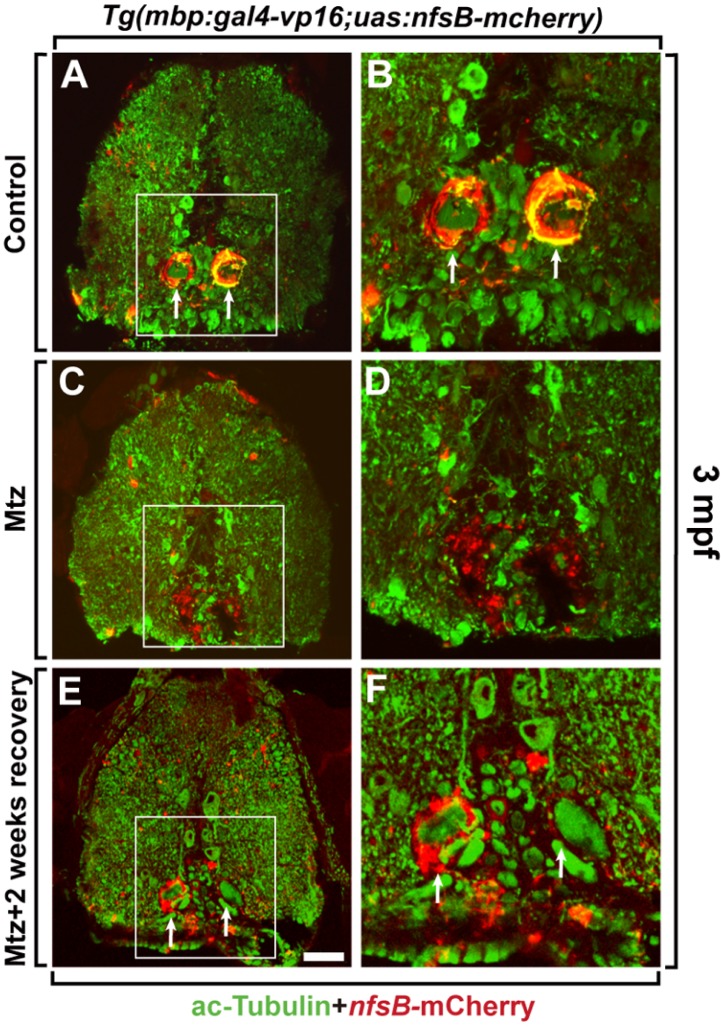
Previously, we have shown that the mbp:egfp transgene is continuously expressed in mature oligodendrocytes within the CNS of adult Tg(mbp:egfp) zebrafish (Jung et al., 2010), indicating that the mbp promoter continuously drives nfsB-mcherry transgene expression in the CNS of the adult Tg(mbp:gal4-vp16;uas:nfsB-mcherry) zebrafish. Therefore, we next labeled transverse spinal cord sections from control and Mtz-treated adult Tg(mbp:gal4-vp16;uas:nfsB-mcherry) zebrafish with an anti-acetylated Tubulin antibody, which labels CNS axon fibers (Fig. 4). As shown in our previous study (Jung et al., 2010), the myelin sheath surrounding large-diameter axons was clearly observed in the ventral spinal cord of DMSO-treated adult Tg(mbp:gal4-vp16;uas:nfsB-mcherry) zebrafish (Fig. 4A, arrows). However, Mtz treatment of adult Tg(mbp:gal4-vp16;uas:nfsB-mcherry) zebrafish resulted in axon demyelination, with only a few myelin sheaths observed in the ventral spinal cord (Fig. 4C). At high magnification, we observed thick myelin sheaths surrounding axons in the ventral spinal cord of control zebrafish (Fig. 4B), but Mtz-treated zebrafish showed scattered debris from the myelin sheaths in the same region of the ventral spinal cord (Fig. 4D), indicating that NTR/Mtz system-mediated oligodendrocyte ablation causes demyelination in the adult spinal cord. Interestingly, raising the adult Tg(mbp:gal4-vp16;uas: nfsB-mcherry) zebrafish for 2 weeks after removal of the Mtz-containing media resulted in the partial recovery of myelination, indicating that oligodendrocytes were successfully regenerated in the adult zebrafish (Figs. 4E and 4F). Taken together, these data show that expression of the nfsB cytotoxin gene in oligodendrocyte lineage cells using the Gal4/UAS transgenic system conditionally ablates oligodendrocytes and induces demyelination in the larval and adult zebrafish CNS upon Mtz treatment.
Fig. 4.
Induction of demyelination in the spinal cord of adult transgenic zebrafish by Mtz treatment. All panels show transverse sections of the spinal cord of 3-month old Tg(mbp:gal4-vp16;uas:nfsB-mcherry) adult fishes (dorsal at the top). Spinal cord sections of DMSO-treated (A, B) and Mtz-treated Tg(mbp:gal4-vp16;uas:nfsB-mcherry) (C-F) adult fishes were labeled with anti-acetylated Tubulin (ac-Tubulin) antibody to detect the axons (green color). (B, D, and F) High-magnification images of the boxed areas in (A), (C), and (E), respectively. (E, F) Spinal cord sections of Tg(mbp:gal4-vp16;uas:nfsB-mcherry) adult fish treated with Mtz for 48 h and recovered for 2 weeks after removal of Mtz. Arrows indicate the myelin sheath. Scale bar: 100 μm in (A), (C), and (E), 25 μm in (B), (D), and (F).
DISCUSSION
MS is a chronic, inflammatory demyelinating disease and is the most common neurological disorder in young adults in the Western hemisphere. Several chemically induced animal models of demyelination have been established and used to study the potential mechanisms of MS pathology and for the drug discovery process (Merrill, 2009). Cuprizone is the most commonly used chemical toxin to induce demyelination in animal models. Cuprizone is administered via the feed, and demyelination is evident in mice 3 weeks after starting a cuprizone diet. Remyelination occurs within 4 weeks of replacing the cuprizone diet with normal food (Matsushima and Morell, 2001). Ethidium bromide (EtBr) solutions can induce complete demyelination after 2 weeks, but must be administered by injecting it into specific regions of the CNS. Additionally, because EtBr is a DNA-intercalating agent, EtBr treatment damages all nucleated cells around the injected area, causing unexpected cell death in non-oligodendroglial cells (Merrill, 2009).
In contrast to these chemically-induced models of demyelination, our own model, based on a genetically engineered transgenic zebrafish system, has several advantages. First, compared with mouse models, both ablation of oligodendrocytes and demyelination are easy to observe because the mbp/sox10-driven Gal4-VP16 transactivator induces NfsB cytotoxin expression together with that of the mCherry fluorescent protein. Thus, we can easily detect oligodendrocytes and myelin sheaths under a fluorescent microscope. Furthermore, the remyelination process can be easily detected by monitoring the recovery of fluorescence in the CNS. Second, demyelination can be induced conditionally. Compared with animal models generated using a cuprizone diet or the injection of an EtBr solution, the simple exposure of bitransgenic zebrafish to Mtz-containing medium induces consistent oligodendrocyte ablation and demyelination. This advantage permits the generation of a large number of demyelination models, which is essential for the large-scale screening assays performed when searching for new drugs using libraries of small molecules. Finally, the process of generating the demyelination and remyelination models is much faster using the transgenic zebrafish system. The transgenic zebrafish model requires only 12 h to 2 days to induce demyelination, and 1 week for remyelination of the demyelinated CNS. However, in contrast to the rapid remyelination (within 1 week) observed in demyelinated Tg(mbp:gal4-vp16;uas:nfsB-mcherry) larvae after removal of Mtz, the process took longer in demyelinated Tg(sox10:gal4-vp16;uas: nfsB-mcherry) larvae; sometimes, Mtz treatment caused the unexpected death of the transgenic larvae. Since the sox10 promoter drives nfsB-mCherry expression in cartilage cells as well as oligodendrocytes (Fig. 1C, bracketed area), we reasoned that Mtz treatment also induces cell death in neural crest-derived cartilage cells.
Recently, it has been shown that targeted ablation of oligodendrocytes in Xenopus laevis transgenic lines, which express NfsB cytotoxin under the control of mbp promoter, induces demyelination efficiently (Kaya et al., 2012). In contrast to Xenopus model, targeted ablation of oligodendrocytes in transgenic mouse exhibited normal levels of CNS myelin but induced axonal pathology (Oluich et al., 2012), suggesting that different animal models with different genetic strategies used to induce oligodendrocyte ablation could show distinct phenotypes. Together, our data suggest that Mtz treatment of Tg(mbp: gal4-vp16;uas:nfsB-mcherry) zebrafish maybe a valuable animal model for studying the remyelination process in vivo, and for conducting high-throughput primary screens of new drugs using libraries of small molecules.
Acknowledgments
This research was supported by the Brain Research Program (2011-0019233) and Basic Science Research Program (2009-0075120) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
REFERENCES
- Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, Kawakami K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci USA. 2008;105:1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- Chung AY, Kim S, Kim H, Bae YK, Park HC. Microarray screening for genes involved in oligodendrocyte differentiation in the zebrafish CNS. Exp Neurobiol. 2011;20:85–91. doi: 10.5607/en.2011.20.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Giuliodori MJ, DiCarlo SE. Myelinated vs. unmyelinated nerve conduction: a novel way of understanding the mechanisms. Adv Physiol Educ. 2004;28:80–81. doi: 10.1152/advan.00045.2003. [DOI] [PubMed] [Google Scholar]
- Jung SH, Kim S, Chung AY, Kim HT, So JH, Ryu J, Park HC, Kim CH. Visualization of myelination in GFP-transgenic zebrafish. Dev Dyn. 2010;239:592–597. doi: 10.1002/dvdy.22166. [DOI] [PubMed] [Google Scholar]
- Kaya F, Mannioui A, Chesneau A, Sekizar S, Maillard E, Ballagny C, Houel-Renault L, Dupasquier D, Bronchain O, Holtzmann I, et al. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J Neurosci. 2012;32:12885–12895. doi: 10.1523/JNEUROSCI.2252-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Chung AY, Kim D, Kim YS, Kim HS, Kwon HW, Huh TL, Park HC. Tcf3 function is required for the inhibition of oligodendroglial fate specification in the spinal cord of zebrafish embryos. Mol Cells. 2011;32:383–388. doi: 10.1007/s10059-011-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kotani T, Nagayoshi S, Urasaki A, Kawakami K. Transposon-mediated gene trapping in zebrafish. Methods. 2006;39:199–206. doi: 10.1016/j.ymeth.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE. In vitro and in vivo pharmacological models to assess demyelination and remyelination. Neuropsychopharmacology. 2009;34:55–73. doi: 10.1038/npp.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluich LJ, Stratton JA, Xing YL, Ng SW, Cate HS, Sah P, Windels F, Kilpatrick TJ, Merson TD. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J Neurosci. 2012;32:8317–8330. doi: 10.1523/JNEUROSCI.1053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev Biol. 2002a;248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- Park HC, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev Biol. 2002b;248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- Park HC, Boyce J, Shin J, Appel B. Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function. J Neurosci. 2005;25:6836–6844. doi: 10.1523/JNEUROSCI.0981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Klein RS. Mediators of oligodendrocyte differentiation during remyelination. FEBS Lett. 2011;585:3730–3737. doi: 10.1016/j.febslet.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosc. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–1589. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]