Abstract
The reorganization of brain structures after intracerebral hemorrhage (ICH) insult is crucial to functional outcome. Although the pattern of neuronal rewiring is well-documented after ischemic stroke, the study of brain plasticity after ICH has been focusing on the enhancement of dendritic complexity. Here we hypothesized that functional restoration after ICH involves brain reorganization which may be favorably modulated by stem cell transplantation. In this study, bone marrow stromal cells (BMSCs) were transplanted into the perilesional sites of collagenase-induced ICH in adult rats one day after ICH injury. Forelimb functional recovery was monitored with modified limb placing and vibrissae-elicited forelimb placement tests. Anterograde and retrograde tracing were used to assess the reorganization of bilateral forelimb areas of the sensorimotor cortex. We found that in rats transplanted with BMSCs after ICH injury, axonal sprouting occurred in the contralateral caudal forelimb area of the cortex, and was significantly higher than in ICH rat models that received only the vehicle (P < 0.01). The number of positive neurons in the ipsilateral rostral forelimb area of the cortex of the BMSC group was 1.5– to 4.5-fold greater than in the vehicle group (P < 0.05). No difference was found between the BMSC and vehicle groups in hemispheric atrophy or labeled neurons in the ipsilateral caudal forelimb area (P = 0.193). Scores for improved functional behavior in the BMSC group were in accord with the results from histology. Neuronal plasticity of the denervated corticospinal tract at bilateral forelimb areas of the cortex in the collagenase-induced ICH rat models was significantly enhanced by BMSC transplantation. BMSC transplantation may facilitate functional recovery after ICH injury.
Keywords: bone marrow stromal cell, intracerebral hemorrhage, neuronal plasticity
INTRODUCTION
Intracerebral hemorrhage (ICH), a subtype of stroke, affects more than one million people each year all over the world (Kleindorfer et al., 2006). It is often clinically devastating with severe mortality and morbidity. Although extensive care in a dedicated stroke unit (Candelise et al., 2007) and rehabilitative intervention greatly improves the outcome of patients, management remains mainly supportive. Therefore, contributions to the understanding of brain restoration processes, as well as management strategies, are hugely important.
The most recent modalities for functional imaging of the human brain [e.g., functional magnetic resonance imaging (fMRI)] have revealed that undamaged key motor areas of the bilateral hemispheres in patients with subcortical stroke are more highly activated than those of healthy people. This activation is important to the recovery process (Gerloff et al., 2006; Grefkes et al., 2008). It has been shown that in stroke recovery, neuronal reorganization of the cortical structure is crucial for functional compensation (Murphy and Corbett, 2009). Establishment of compensatory re-innervation in the bilateral hemispheres after brain injury is mainly achieved through axonal sprouting of surviving neurons, new synapse formation, and factors produced by the brain (Wieloch and Nikolich, 2006). In studies using rat models of ICH, post-injury increases in dendritic arborization were found in the bilateral hemispheres (Nguyen et al., 2008), and rehabilitation improved recovery and enhanced dendritic complexity (Auriat et al., 2010). Although restoration after ICH might be due to neuronal plasticity, the exact pattern of restructuring is unknown. Thus, studies examining axonal reinnervation following ICH are necessary to understand mechanisms underlying brain restoration.
Many studies have shown that stem cells and transgenic stem cells from various sources can improve functional recovery of rodents after ICH (Lee et al., 2008; Nagai et al., 2007). Moreover, in a translation study behavioral effects were improved in a primate model as well (Feng et al., 2011). In these studies, transplanted cells took part in inflammatory reactions, neuroprotection, and neurogenesis and also presented some characteristics of nerve cells. The questions is that whether neuronal plasticity is involved in the beneficial effects of stem cell-based therapy.
After ischemic brain injury, axonal sprouting from the contralesional hemisphere and premotor cortex rewiring were shown to be enhanced by stem cells, and neurotrophic factors secreted by stem cells are believed to contribute to this effect (Andres et al., 2011; Liu et al., 2010). However, owing to pathophysiological differences between ischemic stroke and ICH (Xi et al., 2006), one should not assume that the findings in ischemia will be true of ICH. Thus, studies examining the effects of stem cells on axonal reinnervation after ICH, and those that investigate stem-cell-based therapies after ICH, are greatly required to understand the mechanisms underlying brain restoration.
In the present study, we hypothesized that effective connectivity could be re-established in the primary motor cortex [i.e., the caudal forelimb motor area (CFA)] or the supplementary/premotor or secondary motor area [the rostral forelimb area (RFA)], and that this connectivity might be modified by transplantation of bone marrow stromal cells (BMSCs) after brain injury.
MATERIALS AND METHODS
Experimental groups
Fifty-one 4-month-old male Wistar rats weighing 250 to 300 g, and nine 2-week-old female Wistar rats, were used in this study. All animals were purchased from the Center for Experimental Animals, Harbin Medical University, P.R. China, and they received humane care in compliance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources of Harbin Medical University and the National Institutes of Health. Every effort was made to minimize suffering and limit the number of animals used.
The rats were divided into 3 experimental groups: BMSC, vehicle, and sham-control. The BMSC group (n = 16) consisted of rats administered BMSCs in phosphate-buffered saline (PBS) on the day after collagenase-induced ICH injury (see below). The treatment in the vehicle group (n = 16) was identical to that of the BMSC group, except the rats were administered PBS only on the day after ICH. The sham-control group (n = 8) underwent the same surgical procedure as for the ICH model, except that the collagenase applied for induction of ICH was replaced with intrastriatal (within the corpus striatum) administration of saline, and rats were administered PBS.
Establishing the rat ICH model
Experimental ICH was induced by intrastriatal administration of bacterial collagenase as previously described (Rosenberg et al., 1990) with modifications. In brief, after being anesthetized by intraperitoneal (IP) 10% chloral hydrate (3 ml/kg), rats were placed in a stereotaxic frame (Stoelting 51600, USA). A burr hole was created through which a 30-gauge needle was inserted into the striatum (1.2 mm posterior, 5.8 mm ventral, and 3.4 mm lateral to bregma). A saline solution (0.4 μl) containing 0.8 U of collagenase (type IV, Gibco, USA) was injected over a period of 5 min. The needle was slowly withdrawn after another 5 min. The hole in the skull was sealed with bone wax and the scalp was sutured. All rats survived the operation.
Isolation and culture of BMSCs
All of the procedures for isolation and culture of BMSCs were performed under aseptic conditions, as described previously (Otero et al., 2011). Briefly, fresh whole bone marrow was collected from the femurs and tibias of nine 2-week-old female Wistar rats and the cava were rinsed with culture medium. After mechanical dissociation, one rat’s cells were collected and resuspended in 15 ml culture medium containing Dulbecco’s modified Eagle’s medium and Ham’s F-12 nutrient mixture (DMEM/F12), 10% fetal bovine serum (Gibco BRL, USA), penicillin 100 U/ml, streptomycin 100 μg/ml, and 25 ng/ml amphotericin B. The cells were seeded in 75 cm2 flasks and the adherent cells were cultured for further procedures. BMSC cultures grew at 37°C in 5% CO2 and the cells appeared as spindle, tightly cuboid, and polygonal shapes. The culture medium was replaced three times per week.
Labeling of BMSCs and transplantation
The BMSCs of the third passage were used for in vivo transplantation. Before transplantation, the cells were labeled with the red fluorescent cell tracking dye PKH26 (Sigma-Aldrich, USA). The labeling procedure was performed in accordance with the manufacturer’s instructions. After washing with PBS, BMSCs were diluted to a concentration of 1 × 105 cells/μl. Twenty-four hours after ICH, 10 μl of this solution were stereo-taxically transplanted into the ipsilesional subcortex at each of three locations (i-iii), specifically at coordinates: (i) anterior-posterior (A-P), +0.3 mm; medial-lateral (M-L), −3.4 mm; dorsal-ventral (D-V) from the skull, −3.8 mm; (ii) A-P, −1.2 mm; M-L, −3.4 mm; D-V from the skull, −3.8 mm; and (iii) A-P, −2.7 mm; ML, −3.4 mm; D-V from the skull, −3.8 mm. An equal volume of PBS was administrated into the vehicle and sham-operated groups. The animals were maintained at 20°C with ad libitum access to food and water.
Behavioral testing
To evaluate functional recovery and reorganization of the cortex areas corresponding to the forelimbs, we adapted the modified limb placing test (MLPT) (Lee et al., 2008) and vibrissae-elicited forelimb placing test (Hua et al., 2002) for assessing forelimb functional behaviors. On days −1, 1, 3, 7, 14, 21, and 28 after inducement of the model, all rats were assessed by 2 individuals blinded to the rats’ treatment status. Normal rats were scored 0 in the MLPT and 10 in the forelimb placing test before the injury.
In the MLPT, each rat was suspended inverted above a table, and the stretch of the contralesional forelimb was evaluated: a normal stretch was scored as 0 (zero); abnormal flexion as 1. Then, the rat was leaned against the edge of the table, with its contralesional forelimb and hindlimb suspended over the edge, and retrieval and placement were scored. Finally, the rat was placed near the table edge to assess the lateral placement of the forelimb. In the latter two phases of the test, withdrawing normally was scored as 0; delayed (> 2 s) was scored as 1; no performance was scored as 2.
The forelimb placing test was performed at the same time points as the MLPT. In this test, the contralesional vibrissae (whiskers) of the rat were swept against the edge of a table as the rat’s body was held gently. When the animal reacted normally, quickly placing the forelimb ipsilateral to the stimulated vibrissae on the table, the score was 1; if this response was absent, the score was 0. Each trial was repeated 10 times in a single test. Normal rats scored 10, and the maximum injury was scored zero. Four rats were excluded on the first day before transplantation because impairment was deficient.
Measurement of hemispheric atrophy
Thirty-five days after ICH, eight rats from each of the BMSC and vehicle groups that had been used for behavioral tests were randomly selected, placed under deep anesthesia with 10% chloral hydrate (8 ml/kg IP), transcardially perfused with 0.9% saline, and subsequently with phosphate buffer (pH 7.4) containing 4% paraformaldehyde. The brains were removed and postfixed in the same fixative, dehydrated with graded alcohols and embedded in paraffin. Seven coronal sections (4 μm) were taken every 300 μm from 0.3 to 2.1 mm posterior to the bregma. These sections were stained with hematoxylin-eosin (H&E).
Lesion volume was quantified using Scion Image J 4.0 (Scion, USA) in accordance with the standard routine method (MacLellan et al., 2008). The volume of hemispheric atrophy was calculated as the average difference in areas between the tissue in the normal hemisphere and that of the injured hemisphere × interval between sections × the number of sections.
Anterograde corticospinal tract tracing
After functional analysis, one μl of a 10% solution of the anterograde tracer biotinylated dextran amine (BDA; 10,000 MW; Molecular Probes, Oregon, USA) in sterile PBS was injected at each of 2 points (i and ii) in the contralateral CFA, specifically at coordinates as described previously (Ramanathan et al., 2006): (i) A-P, +1.0 mm; M-L, −2.5 mm; D-V, −1.5 mm; and (ii) A-P, 0 mm; M-L, −2.5 mm; D-V, −1.5 mm (n = 24). After one week, the brains were processed for immunohistochemistry: after fixation, 10 μm-sections were prepared on a cryostat, fixed with acetone, washed with PBS and incubated with avidin-horseradish peroxidase at 4°C overnight, and then visualized via staining with diaminobenzidine (DAB) and counter-stained with H&E. The lateral ventricle, corpus callosum, striatum, and anterior commissure were used as landmarks for selecting sections, based on the rat stereotaxic atlas.
Digital images were captured through a microscope (Olympus BX60, Japan). Image J software was used for subsequent quantitative measurements of BDA-positive axonal densities (Andres et al., 2011; Liu et al., 2010). Five sections per animal were chosen for quantitative analysis, and at least 5 fields under a magnification of 400× were selected with a measure frame of 0.1 mm × 0.1 mm per field. Separate analyses were made of the cortex, corpus callosum, and striatum. To avoid inter-animal variation in tracing efficiency, axonal densities were normalized with a quotient coefficient calculated as the average axonal density in the contralesional CFA for each individual animal, divided by the total mean density of all animals.
Retrograde corticospinal tract tracing
After the BDA was administrated, the rats were anesthetized and the spinal cord was exposed at level T12, one week before the animals were euthanized. Five microliters of the retrograde tracer fluoro-gold (FG; 4%; Fluorochrome, USA) was injected bilaterally into the spinal cord 0.5 mm lateral to the spinal midline with a microsyringe. Sections were obtained as described above. Five, 10-μm sections from the RFA (3.0 to 3.5 mm rostral to the bregma) and 5, 10-μm sections from the CFA (0 to 1.0 mm rostral to the bregma) (Ramanathan et al., 2006) were taken to detect FG labeling. Labeled pyramidal neurons were analyzed in a blinded manner by counting the number of cell bodies from 6 fields (0.3 mm × 0.3 mm each; magnification, 200×) in the 10 sections of the CFAs and RFAs in each experimental group.
Statistical analyses
Two experimenters blinded to the treatment groups performed the quantitative analyses. All data were analyzed using SPSS 17 software. Data are presented as mean ± standard error of the mean. A value of P < 0.05 was considered significant. The two-sample t-test was used to analyze differences where appropriate. Experimental groups were compared using two-way ANOVA, followed by Tukey’s post-hoc test.
RESULTS
Survival and migration of transplanted BMSCs
We found that polymorphous BMSCs adhered to the bottom of flasks and proliferated constantly in vitro. The third passage was labeled with the fluorescent cell tracking dye PKH26 and appeared orange-red under a fluorescence microscope. Labeled BMSCs were transplanted into the perilesional sites the day after ICH injury. At 35 days post-ICH inoculation (dpi), rats were euthanized to detect the transplanted BMSCs in vivo. No tumor formation or uncontrolled graft growth was observed in the brain tissue sections of the BMSC group.
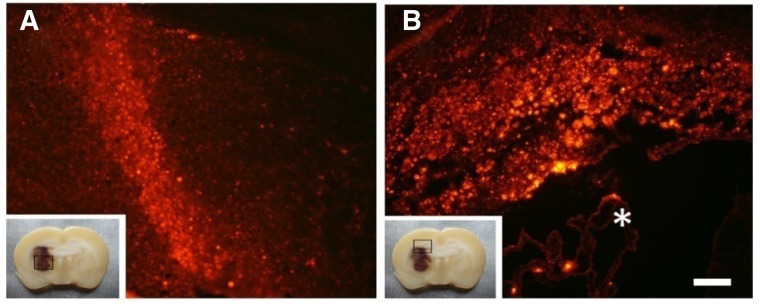
We observed enormous numbers of PKH26-positive cells in the injured hemisphere at 35 dpi (Fig. 1A), and abundant BMSCs had migrated selectively to the hemorrhage lesion sites (Fig. 1A) which were 2 millimeters lower than the injection sites. The majority of positive cells were in the ipsilateral corpus callosum and hippocampus, close to the injection sites (Fig. 1B).
Fig. 1.
BMSCs survived and migrated in the rat brain after ICH. At 35 dpi, PKH26-labeled cells migrated into the lesion site (A) and accumulated in the corpus callosum and hippocampus (B). The box at the left lower side of each image represents low magnification of coronal slices of the ICH rat brain. Scale bar = 300 μm. *The lateral ventricle.
Improvement of neurological deficits after BMSC treatment
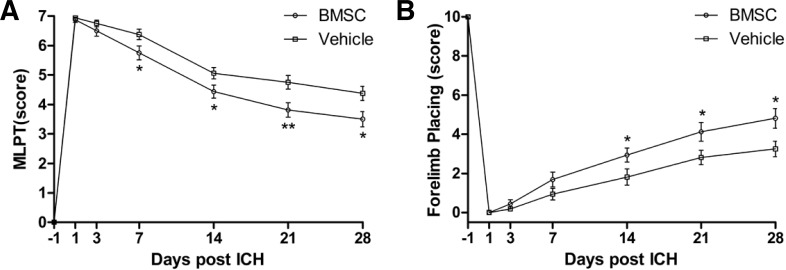
To examine the effect of BMSC transplantation on recovery of motor functions and proprioception of the forelimbs, we performed the MLPT and forelimb placing behavioral tests. In both of the behavioral tests, the rats in the vehicle group exhibited obvious restoration of behavioral functions, which was further enhanced in the rats of the BMSC group.
In the MLPT, the functional behavior of the BMSC group was significantly better than that of the vehicle group at 7 to 28 dpi (P = 0.009 at 21 dpi, P = 0.019 at 28 dpi); the scores in the BMSC group were reduced by 20.7% and 20%, at 21 and 28 dip, respectively, relative to the vehicle group (Fig. 2A), suggesting that BMSCs improved functional recovery. In the vibrissae-elicited forelimb placing test, which assesses the sensorimotor integration of the forelimb, the BMSC group performed more normally after 14 days compared with the vehicle group, and the effects persisted up to 28 days (P = 0.048, 0.038, 0.020 at 14, 21, 28 dpi); the scores of the BMSC group were increased by 1.62-, 1.47- and 1.48-fold, at 14, 21, 28 dpi, respectively, when compared with those of the vehicle group (Fig. 2B).
Fig. 2.
Functional recovery assessed with the MPLT and forelimb placing test. The results showed a significant improvement in the BMSC transplantation group when compared with the vehicle group. (A) The modified limb placing test; (B) The vibrissae-elicited forelimb placing test. *P < 0.05; **P < 0.01.
BMSC transplantation did not ameliorate hemispheric atrophy induced by ICH
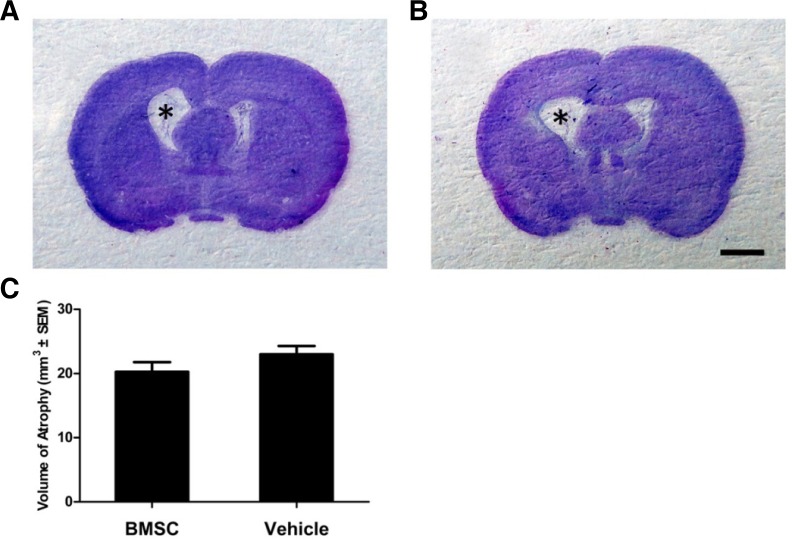
In our rat model of collagenase-induced ICH, damage occurred primarily to the internal capsule, striatum, and thalamus. Hemispheric atrophy was prominent, with ventricular enlargement at 35 dpi in both the vehicle and BMSC groups (Figs. 3A and 3B). Although H&E-based morphometric analysis indicated a smaller atrophic volume in the BMSC group than the vehicle group, the difference was not significant (20.3 ± 4.2 mm3 compared to 23.0 ± 3.7 mm3; P = 0.193; Fig. 3C).
Fig. 3.
Hemispheric atrophy in rat ICH models. (A, B) Representative coronal sections (at A-P = −0.6) of brain tissue stained with H&E at 35 dpi. The atrophy of the injured hemisphere in BMSC (A) and vehicle (B) groups was prominent; BMSC transplantation did not ameliorate atrophy. *Indicates an enlarged lateral ventricle. (C) No statistical difference was found in the volume of hemispheric atrophy between the BMSC and vehicle groups; P = 0.193. Scale bar = 2 mm.
Transplantation of BMSCs after ICH improved FG-labeled cells in the ipsilateral RFA but did not reverse markedly reduced FG-labeled cells in the CFA
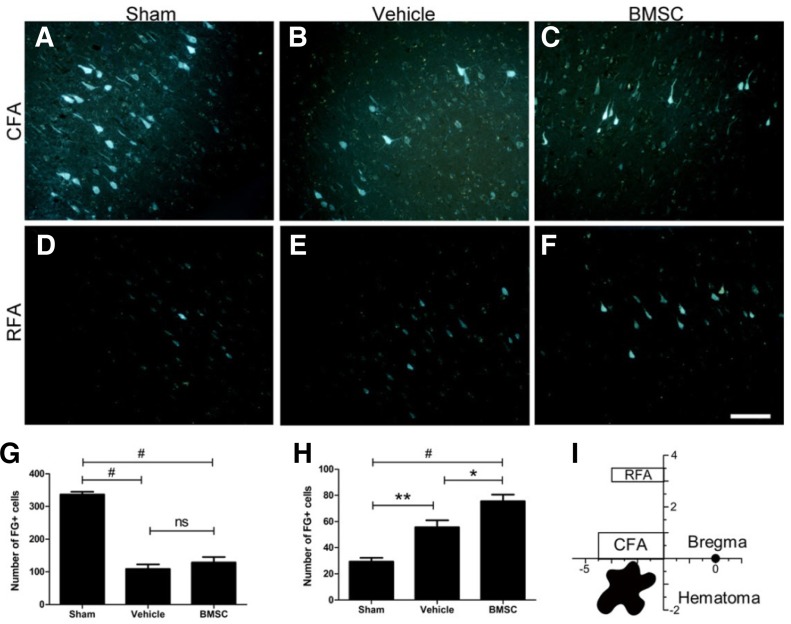
To elucidate functional variance, we investigated the pattern changes within the ipsilesional forelimb areas of rat cortices after ICH and BMSC transplantation by tracing the number of FG-labeled pyramidal cells in the caudal and rostral forelimb areas. After ICH, the magnitude of FG-labeled cells in the CFA of the vehicle group (108.4 ± 40.7) was 3-fold less than that of the sham-operated group (336.5 ± 24.4; P < 0.001; Figs. 4A, 4B, and 4G), indicating that ICH significantly and effectively induced loss of the corticospinal axons which transversed the striatum near the ICH. However, no significant difference in the number of FG-labeled neurons was found between the vehicle (108.4 ± 40.7) and BMSC (128.9 ± 47.4) groups (P = 0.549; Figs. 4B, 4C, and 4G).
Fig. 4.
FG labeling of pyramidal neurons in the ipsilesional cortices of the CFA and RFA. The number of positive pyramidal cells in the CFA was sharply reduced after ICH and was not increased by BMSC transplantation (A-C, and G). By contrast, the number of FG-labeled cells was significantly increased in the RFA after ICH, and the effect was enhanced by BMSC administration (D-F, and H). The schematic drawing of the forelimb areas of the rat brain is shown in (I). *P < 0.05, **P < 0.01, #P < 0.001, ns = not significant. Scale bar = 200 μm.
By contrast, a significantly greater number of FG-labeled cells in the RFA were observed in the vehicle group (55.6 ± 15.0) than in the sham-operated group (29.4 ± 8.2; P = 0.001; Figs. 4D, 4E, and 4H), which was further enhanced by BMSC transplantation (55.6 ± 15.0 and 75.6 ± 14.2, respectively; P = 0.014; Figs. 4E, 4F, and 4H). The cortical forelimb areas and the position of ICH injury in the rat brain are illustrated relative to bregma in Fig. 4I.
The contralateral CFA was involved in neuronal reorganization
To investigate whether reinnervation of the contralateral cortex is changed by hemorrhagic injury and BMSC transplantation, we used BDA to anterogradely label the sensorimotor cortical sprouting originating from the contralateral CFA. We found that compared with the sham-operated group, after ICH injury there were a greater number of BDA-positive fibers crossing the midline of the corpus callosum in the rat brain with BMSC transplantation.
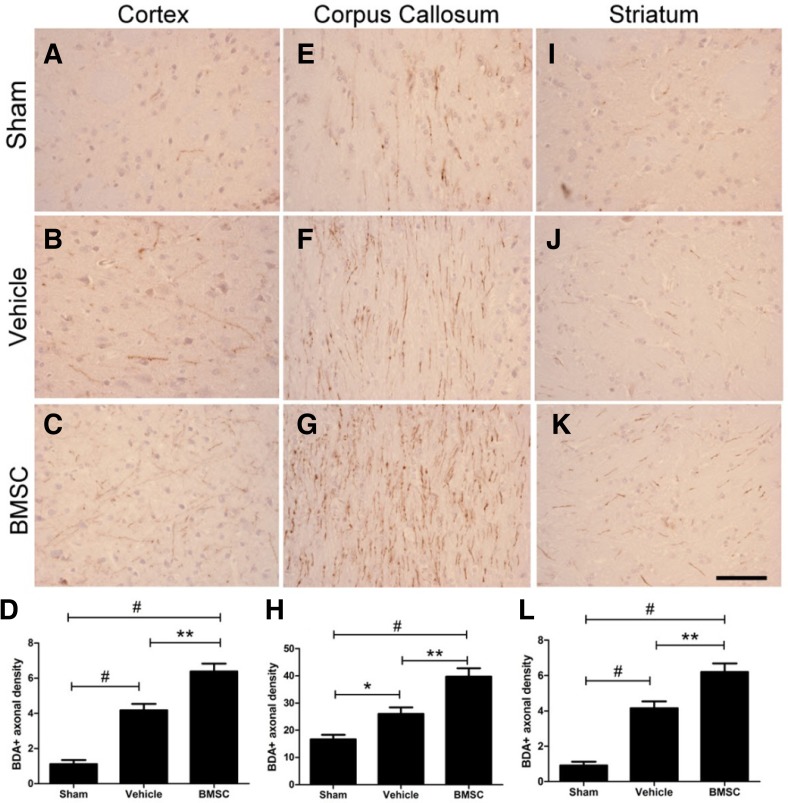
To investigate whether sprouted axons from the contralateral CFA extended into the ipsilateral corticospinal tract, we measured the density of BDA-positive fibers in the defined regions of the ipsilesional CFA, corpus callosum, and striatum. To avoid inter-animal variation in tracing efficiency, the BDA-positive axonal density in the CFA of the contralateral side was calculated to normalize individual differences. Compared with the sham-operated group the density of BDA-labeled fibers crossing the midline significantly increased after ICH injury in the vehicle group in the cortex (1.12 ± 0.65 and 4.18 ± 1.04, respectively; P < 0.001; Figs. 5A, 5B, and 5D), the corpus callosum (16.7 ± 4.87 and 26.0 ± 6.89; P = 0.036; Figs. 5E, 5F, and 5H), and the striatum (0.91 ± 0.59 and 4.15 ± 1.12, P < 0.001; Figs. 5I, 5J, and 5L). In the ipsilateral cortex of the CFA, the density of BDA-labeled fibers was obviously enhanced in the BMSC group over that of the vehicle group in the cortex (6.38 ± 1.28 and 4.18 ± 1.04, respectively; P = 0.001, Figs. 5B, 5C, and 5D), the corpus callosum (39.7 ± 8.62 and 26.0 ± 6.89; P = 0.002, Figs. 5F, 5G, and 5H), and the striatum (6.21 ± 1.34 and 4.15 ± 1.12, P = 0.002; Figs. 5J, 5K, and 5L). Unspecific staining and blood vessels were disregarded when the quantitative analysis was made.
Fig. 5.
BDA tracing showing axonal sprouting into the injured hemisphere. The density of fibers crossing the midline was significantly increased after ICH injury and the density was obviously enhanced by BMSC administration in the ipsilateral CFA (A-D), corpus callosum (E-H), and striatum (I-L). *P < 0.05, **P < 0.01, #P < 0.001. Scale bar = 200 μm.
DISCUSSION
In the present study, we observed complex rewiring of the motor cortex between the bilateral hemispheres of rats after unilateral ICH injury. This evidence augments that of previous reports of ICH-evoked alterations in dendritic arborization (Nguyen et al., 2008) and the reorganization of the corticospinal tract. Moreover, we found that the transplantation of BMSCs enhanced reorganization in the brain, which might correlate with recovery of motor behaviors but had no effect on preservation of tissue volume after brain injury. These data thus provide further understanding of the process of brain restoration, and the value of stem-cell-based therapies.
Previous studies using intracortical microstimulation have verified the existence of an anteroposterior separation between the two forelimb representation areas, CFA and RFA, in the motor cortex of rats (Ramanathan et al., 2006). By comparing the differences in afferent and efferent projections, it has been determined that the CFA corresponds to the primary motor cortex (MI) and the RFA is a second forelimb motor representation (Wang and Kurata, 1998). In keeping with this, using the retrograde corticospinal tract tracing method, we observed that the pattern of FG-labeled cortical cells in the RFA was distinct from that in the CFA. Moreover, ICH induced a significant reduction in FG-labeled cells which the corticospinal tract projects from the CFA, and this damage was not reversed by BMSC transplantation.
This result could explain the failure in preventing loss of ipsilesional brain tissues after BMSC administration. Although several reports have elucidated the anti-atrophy effect of stem cell transplantation after ICH (Otero et al., 2012), our results are inconsistent with Sang-Wuk Jeong and colleagues’ previous study (Jeong et al., 2003), showing that no difference was found in the lesion area of the striatum between the transplanted group and the control group. The discrepancy may be attributed to differences in administration approaches and sources of stem cells. On the other hand, it has been reported that anti-apoptosis is an important characteristic of mesenchymal stromal cells, shown in ICH models after transplantation of mesenchymal stromal cells (Otero et al., 2012).
There is no lack of efficient behavioral tests in abundant studies of neurology. In order to consist with the structural changes of forelimb areas in the section of corticospinal tract tracing, the behavioral tests were selected to focus on forelimb function. In addition to the self-repairing function and the beneficial effect of BMSC transplantation, learning effects might partially account for the functional recovery, because the rats underwent only one training session for the behavioral tests, one day before induction of ICH. Although BMSCs transplantation showed benefits in functional recovery in this study, the beneficial effects are limited. This may attribute to the differences of lesion site. In order to reveal the effect of axonal rewiring, we chose internal capsule as the lesion site to destroy ipsilesional corticospinal tract. Other than that, ICH is a devastating condition and the function of the cerebral cortex is severely destroyed. Thus, ICH hardly recovered from the harmful microenvironment, which influences survival and paracrine factors of the transplanted BMSC. In the present study, however, we adopted general amount of transplanted stem cells and interval after ICH to transplant, but didn’t inspect levels of paracrine factors in the brain. We also considered that the optimal amount of stem cells and interval after ICH to transplant and the paracrine effects may be benefit for treatment of ICH, which will be studied further in our future researches.
Based on our results, it is doubtful that the function of the corticospinal tract of the CFA would be restored by the antiapoptotic effect of MSC transplantation. Nevertheless, increased density of axonal sprouting from the contralateral CFA might enhance the function of the ipsilesional CFA in forelimb movement through axonal reinnervation, at least partially. In addition, it is known that the sensory inputs of the basal forebrain cholinergic system are essential for cortical plasticity (Conner et al., 2005). When damaged after ICH, this could explain the lack of reorganization in the CFA.
On the other hand, the RFA, a premotor area, exhibited obvious rewiring which was significantly enhanced by administration of BMSCs. Although differences in cytoarchitecture and neuronal projections have been found between the RFA and CFA (Wang and Kurata, 1998), the RFA is sufficient to induce movement of the forelimb with intracortical microstimulation (Ramanathan et al., 2006). Furthermore, it was reported that a lesion in the RFA produced more severe and enduring asymmetry dysfunctions in rats, as shown during the bilateral-stimulation task, than a lesion in the CFA (Barth et al., 1990). We postulate that the neuronal plasticity of the RFA may be potentially significant in regard to functional restoration of the forelimb after ICH insult. We also noted the upregulation of positive cells at the secondary motor cortex after ICH, although further data are required (data not shown).
Interhemispheric interactions of the normal human motor cortex are predominantly inhibitory (Ferbert et al., 1992). This is thought to be important in unilateral motor control and influences manual dexterity. However, based on transcranial magnetic stimulation results, interhemispheric inhibition is absent in unilateral cortical stroke patients (Shimizu et al., 2002), and fMRI showed that interhemispheric inhibition was reduced in subcortical ICH patients (Grefkes et al., 2008). This may explain why BMSCs failed to alter the pattern of BDA-labeled transcallosal axons in the corpus callosum after ischemic injury, but significantly increased the density of positive axons (Liu et al., 2010), suggesting the potential benefit of BMSC transplantation in ICH. A study based on observation of subcortical stroke patients showed that high interhemispheric inhibition indicates poor motor recovery and correlates with poor motor performance (Bütefisch et al., 2003). Thus, to improve outcomes after ICH injury it is crucial to explore the mechanisms underlying interhemispheric inhibition and the relevant pathways of regulation.
It is known that BMSCs possess multipotency and a capacity for self-renewal, shown by their abilities to differentiate into various mesodermal layer cells, including bone and muscle (Prockop, 1997). Results of previous studies also indicate that BMSCs differentiate into neural-like cells in a rat brain after ICH insults (Otero et al., 2012). However, as important mechanisms of restoration after brain injury, neuronal development and plasticity are mainly regulated by neurotrophins (Huang and Reichardt, 2001). Mesenchymal stem cells can produce and secrete a variety of trophic factors including vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and basic fibroblast growth factor (bFGF) (Nagai et al., 2007; Wilkins et al., 2009). The effect of these factors on axonal sprouting has been proved both in vitro and in vivo (Cohen-Cory and Fraser, 1995; Reitmeir et al., 2012; Wilkins et al., 2009). Andres and colleagues discovered that the axonal sprouting enhanced by stem cell transplantation was inhibited by neutralization of VEGF. They also found that VEGF enhanced axonal transportation (Andres et al., 2011). Daadi and colleagues have identified several genes which were significantly increased after transplantation of stem cells by using gene microarray analysis, these genes are related to cell survival, neurite outgrowth and neurogenesis (Daadi et al., 2010). The results of these studies indicate that stem-cell-based therapies are a sound strategy for improving functional recovery after ICH through brain reorganization. However, although the corresponding genes of factors associated with axonal sprouting are expressed in the peri-infarct cortex after ischemic stroke (Carmichael et al., 2005), the level changes of expressed factors in the brain after ICH are still not definitively known. These previous studies have provided certain evidence regarding mechanisms underlying brain reorganization after brain injury, it seems that both differentiation and secretion of BMSCs involve axonal plasticity. Nevertheless, the exact mechanisms underlying neuronal plasticity after ICH deserve further investigation.
For tracing BMSCs in vivo, we chose a red membrane-labeling fluorescent dye (PKH26) as a tracer. PKH26 is nontoxic and has been found useful for in vivo cell tracking applications (Jin et al., 2011). It can stably incorporate the fluorescent dye into the long aliphatic tails of lipid regions of the cell membrane and does not change the morphology of the cells. Therefore it was an ideal dye for in vivo cell tracking in this study, as described in a previous paper (Horan and Slezak 1989). In the present study, transplanted BMSCs could survive more than 35 days in vivo, indicating that the allogeneic transplanted BMSCs had low immunogenicity. This agrees with the results of previous studies (Otero et al., 2011). Here we administrated BMSCs mainly into the ipsilesional corpus callosum and hematoma debris. The ability to migrate is a well-documented characteristic of BMSCs (Karp and Leng 2009), mainly attributed to upregulated chemokines (Ries et al., 2007) and homing receptors (Borlongan et al., 2011) in the destructed tissue. Interestingly, and similar to other reports (Nagai et al., 2007), we also observed that BMSCs accumulated extensively in the corpus callosum. During the transplantation of the BMSCs, the transverse fibers of the corpus callosum could have blocked a portion of the dose so that there was a backflow along the route of the injection needle. If so, this could lead to the distribution of several transplanted cells around the corpus callosum. However, neither the site of injection nor the anatomical structure of the corpus callosum can explain the distribution of the majority of the BMSCs throughout the entire area and the complete transplanted half of the corpus callosum; it may be that other factors such as tissue affiliation or receptors are involved in this phenomenon. Whether the transplanted cells promote axonal sprouting from the contralesional hemisphere through contact with the fibers of the corpus callosum is still unknown, and further studies are required to identify the relevant signals and molecular mechanisms. While neuronal plasticity was obvious in our collagenase induced-ICH model, dendritic atrophy lasted more than 60 days in an ICH model of blood infusion (MacLellan et al., 2010), indicating the necessity for verification of brain reorganization in different models. However, it is worthwhile to note that although the collagenase injection model of ICH is commonly used, it has limitations such as a profound and long-lasting inflammatory response induced by this model. Moreover, pathophysiologically its relevance as a model of the ICH condition is doubtful.
Collectively our results suggest that extended neuronal plasticity contributed to the rewiring of the denervated corticospinal tract in our collagenase-induced ICH model, which was significantly enhanced by the transplantation of BMSCs. This strongly implies that BMSCs may facilitate functional recovery after ICH injury. Here we demonstrated that the RFA and contralesional sensorimotor cortex are involved in neuronal restoration after ICH injury, and therefore are important mechanisms of rehabilitation and an avenue for stem cell-based therapies after stroke.
Acknowledgments
We thank Prof. Liu Enzhong, and Ms. Huang Qi for technical assistance, Dr. Lv Manhua for reagents given generously, and Medjaden Bioscience for assisting in the preparation of this manuscript. This study was supported by grants from the National Natural Science Foundation of China (No. 81041082), Natural Science Foundation of Heilongjiang Province (D200922), the Foundation of the First Affiliated Hospital of Harbin Medical University (2011BS004), and the China Postdoctoral Science Foundation (2011M501059).
REFERENCES
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriat AM, Wowk S, Colbourne F. Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behav Brain Res. 2010;214:42–47. doi: 10.1016/j.bbr.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Netz J, Wessling M, Seitz RJ, Hömberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Candelise L, Gattinoni M, Bersano A, Micieli G, Sterzi R, Morabito A, PROSIT Study Group Stroke-unit care for acute stroke patients: an observational follow-up study. Lancet. 2007;369:299–305. doi: 10.1016/S0140-6736(07)60152-4. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth- associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Zhu H, Zhu Z, Wei J, Lu S, Li Q, Zhang N, Li G, Li F, Ma W, et al. Serial 18F-FDG PET demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J Nucl Med. 2011;52:90–97. doi: 10.2967/jnumed.110.080325. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebath JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Küst J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Horan PK, Slezak SE. Stable cell membrane labelling. Nature. 1989;340:167–168. doi: 10.1038/340167a0. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- Jin SZ, Meng XW, Sun X, Han MZ, Liu BR, Wang XH, Pei FH. Hepatocyte growth factor promotes liver regeneration induced by transfusion of bone marrow mononuclear cells in a murine acute liver failure model. J Hepatobiliary Pancreat Sci. 2011;18:397–405. doi: 10.1007/s00534-010-0343-8. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, Moomaw CJ, Schneider A, Miller R, Kissela B. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37:2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41:S95–S98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behavior. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AP, Huynh HD, Sjovold SB, Colbourne F. Progressive brain damage and alterations in dendritic arborization after collagenase-induced intracerebral hemorrhage in rats. Curr Neurovasc Res. 2008;5:171–177. doi: 10.2174/156720208785425710. [DOI] [PubMed] [Google Scholar]
- Otero L, Zurita M, Bonilla C, Aguayo C, Vela A, Rico MA, Vaquero J. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy. 2011;13:562–571. doi: 10.3109/14653249.2010.544720. [DOI] [PubMed] [Google Scholar]
- Otero L, Zurita M, Bonilla C, Aguayo C, Rico MA, Rodríguez A, Vaquero J. Allogeneic bone marrow stromal cell transplantation after cerebral hemorrhage achieves cell transdifferentiation and modulates endogenous neurogenesis. Cytotherapy. 2012;14:34–44. doi: 10.3109/14653249.2011.608349. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc. Natl. Acad. Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitmeir R, Kilic E, Reinboth BS, Guo Z, ElAli A, Zechariah A, Kilic U, Hermann DM. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol. 2012;123:273–284. doi: 10.1007/s00401-011-0914-z. [DOI] [PubMed] [Google Scholar]
- Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kurata K. Quantitative analyses of thalamic and cortical origins of neurons projecting to the rostral and caudal forelimb motor areas in the cerebral cortex of rats. Brain Res. 1998;781:137–147. doi: 10.1016/s0006-8993(97)01223-7. [DOI] [PubMed] [Google Scholar]
- Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16:258–264. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]