Abstract
There is increasing evidence suggesting that dysregulation of certain microRNAs (miRNAs) may contribute to tumor progression and metastasis. Previous studies have shown that miR-409-3p is dysregulated in some malignancies, but its role in bladder cancer is still unknown. Here, we find that miR-409-3p is down-regulated in human bladder cancer tissues and cell lines. Enforced expression of miR-409-3p in bladder cancer cells significantly reduced their migration and invasion without affecting cell viability. Bioinformatics analysis identified the pro-metastatic gene c-Met as a potential miR-409-3p target. Further studies indicated that miR-409-3p suppressed the expression of c-Met by binding to its 3′-untranslated region. Silencing of c-Met by small interfering RNAs phenocopied the effects of miR-409-3p overexpression, whereas restoration of c-Met in bladder cancer cells bladder cancer cells overexpressing miR-409-3p, partially reversed the suppressive effects of miR-409-3p. We further showed that MMP2 and MMP9 may be downstream effector proteins of miR-409-3p. These findings indicate that miR-409-3p could be a potential tumor suppressor in bladder cancer.
Keywords: bladder cancer, c-Met, metastasis, microRNA-409-3p
INTRODUCTION
Bladder cancer ranks ninth in worldwide cancer incidence (Ploeg et al., 2009), with an expected 386,300 newly-diagnosed cases and 150,200 deaths in 2008 (Jemal et al., 2011). Tumor progression and metastasis remains the most common cause of cancer-related deaths. To significantly alter the course of disease, improvements should be made in the understanding, diagnosis, and treatment of highly-aggressive forms of metastatic bladder cancer.
MicroRNAs (miRNAs) are small, endogenous, noncoding RNAs with a length of ∼22 nucleotides, comprising a novel class of gene regulators (Bartel, 2004). They induce post-transcriptional gene repression by translational suppression and/or promoting target mRNA cleavage (Bartel and Chen, 2004), and are thus involved in various cellular processes, such as development, differentiation, signal transduction, cell maintenance and cancer (Bartel and Chen, 2004; Bartel, 2009). It is estimated that over 1,000 miRNAs are encoded in the human genome, and they target approximately 60% of protein-coding genes (Friedman et al., 2009). A growing body of evidence indicates that aberrant regulation of miRNAs is associated with bladder cancer progression and metastasis. For example, multiple miRNAs including miR-34a, miR-133, miR-493, miR-582, miR-1280, and miR-1826 have been implicated in the suppression of metastasis by functioning as tumor-suppressor genes (Hirata et al., 2012; Majid et al., 2012; Uchino et al., 2013; Ueno et al., 2012; Zhang et al., 2012; Zhou et al., 2012;). In addition, a microRNA expression ratio may have the ability to distinguish between invasive and noninvasive bladder tumors with high sensitivity and specificity (Neely et al., 2010; Wszolek et al., 2011).
The microRNA-409-3p (miR-409-3p) was first identified as one of the miRNAs that was downregulated in gastric cancer and could regulate cell proliferation, apoptosis, invasion and metastasis by targeting PHF10 and radixin (Li et al., 2012; Zheng et al., 2012). However, its function has not been extensively and deeply studied in different human cancer types. To the best of our knowledge, the biological function of miR-409-3p in bladder cancer is yet to be understood. In the present study, we detect frequent down regulation of miR-409-3p in human bladder cancer tissues and cells. In addition, for the first time, we find that miR-409-3p could suppress migration and invasion of bladder cancer cells via targeting c-Met.
MATERIALS AND METHODS
Cell lines and cell culture
Four human bladder cancer cell lines T24, 5637, J82 and UMUC3 and one normal bladder cell line, SV-HUC-1, were obtained from the Shanghai Institute of Cell Biology (China). The cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum under a humidified atmosphere of 5% CO2 at 37°C.
Tissue samples
Paired bladder cancer tissues and adjacent non-tumorous bladder mucosal tissues were obtained from patients undergoing radical cystectomy. The samples were collected between Jan 2011 and June 2011 at the First Affiliated Hospital of Medical College, Zhejiang University (China) after informed consent and Ethics Committee’s approval. The demographic and clinical pathology data are listed in Table S1. Tissue samples were trimmed and snap frozen in liquid nitrogen until use.
RNA isolation and qRT-PCR
MicroRNA was extracted from tissues and cell lines using RNAiso for small RNA (TaKaRa, Japan) and reverse transcribed using One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, Japan), and total RNA was extracted with RNAiso Plus (TaKaRa, Japan) and transcribed into cDNA with Prime-Script RT Reagent Kit (TaKaRa, Japan). The resulting cDNA was quantified by ABI 7500 FAST real-time PCR System (Applied Biosystems, USA) using SYBR Green (Takara, China). The relative expression level of miR-409-3p, c-Met, MMP2, and MMP9 was calculated and quantified with the 2−ΔΔCt method after normalization with the reference U6 small nuclear RNA or GAPDH expression. All primers used are listed in Table 1.
Table 1.
The oligonucleotides used in this study
| Namea | Sequence (5′→ 3′)b |
|---|---|
| miR-409-3p | GAAUGUUGCUCGGUGAACCCCU |
| mimics (sense) | |
| NC (sense) | ACTACTGAGTGACAGTAGA |
| miR-409-3p F | GAATGTTGCTCGGTGAACCCCT |
| U6 F | TGCGGGTGCTCGCTTCGGCAGC |
| c-Met F | TGTCCCGAGAATGGTCATAA |
| c-Met R | AGGGAAGGAGTGGTACAACA |
| MMP2 F | GATACCCCTTTGACGGTAAGGA |
| MMP2 R | CCTTCTCCCAAGGTCCATAGC |
| MMP9 F | TGTACCGCTATGGTTACACTCG |
| MMP9 R | GGCAGGGACAGTTGCTTCT |
| GAPDH F | AAGGTGAAGGTCGGAGTCA |
| GAPDH R | GGAAGATGGTGATGGGATTT |
| c-Met-utr F | TCGAGAGCTCAGGAAATATTGAGGGCTTCT |
| c-Met-utr R | TCGAGTCGACCTATTCGGGAGGCTTGAG |
| c-Met-mut F | GTCATTCACCCATTAGGTATTGTAAGCCTTTT AAATGTTTGTTTGTTTTTTG |
| c-Met-mut R | CAAAAAACAAACAAACATTTAAAAGGCTTACA ATACCTAATGGGTGAATGAC |
F, forward primer; R, reverse primer.
Restriction sites are in bold; Mutated sites are underlined.
Transient transfection of miRNA mimics and small interfering RNAs
The miR-409-3p mimics (dsRNA oligonucleotides), si-c-Met (sense: GGAGGUGUUUGGAAAGAUAdTdT and antisense: UAUCUUUCCAAACACCUCCdTdT) and negative control mimics (NC) were purchased from GenePharma (China). T24 and 5637 cells were seeded into 6-well plates one day before transfection to ensure 60–70% cell confluence at the time of transfection. Transfection was performed using Lipofectamine 2000 (Invitrogen, USA) in accordance with the manufacturer’s instructions. The miRNA mimics were used at a final concentration of 50 nM. Western blots and qRT-PCR were performed at 48 h post-transfection.
Cell proliferation assay
Approximately 4 × 103 T24 cells or 2 × 103 5,637 cells were plated in each well of a 96-well plate. After an overnight incubation, the cells were transfected with the RNAs (miR-409-3p, si-c-Met or control) for 3 days. Subsequently, the medium was removed, cell counting solution (WST-8, Dojindo Laboratories, Japan) was added to each well, and the cells incubated for an additional 2 h. The absorbance of the solution was measured spectrophotometrically at 450 nm with a MRX II absorbance reader (Dynex Technologies, USA).
Cell migration and invasion assay
Transwell chambers (8 μm pore size; Costar, Switzerland) were used to determine tumor cell migration and invasion. The transwells were prepared for an initial equilibrium by addition of 0.6 ml of RPMI-1640 medium with 10% fetal bovine serum into the lower compartment as a chemoattractant. For the invasion assay, the inserts were coated with 1 mg/ml BD Matrigel Matrix (BD Biosciences, USA). After 48 h transfection, 8 × 104 T24 cells or 4 × 105 5,637 cells were suspended in 0.2 ml of fresh medium without fetal bovine serum, added to the inserts, and cultured for 24 h. Subsequently, the cells on the upper surface of the membrane were removed using cotton buds, and cells on the lower surface of the inserts were fixed and stained with 0.1% crystal violet. Five visual fields of × 200 magnification of each insert were randomly selected, and the number of cells was counted under a light microscope.
Western blot analysis
Briefly, cells were harvested at 48 h following RNA treatment as described above and then lysed and quantified. Equivalent quantities (30–50 μg) of protein were separated by 10% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% nonfat milk and incubated overnight with the appropriate primary antibody at dilutions specified by the manufacturer. Next, the membranes were washed three times in TBST and incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody at 1:5,000 dilution for 1 h. Bound secondary antibody was detected using an Enhanced Chemiluminescence (ECL) system (Pierce Biotechnology Inc., USA). Primary immunoblotting antibodies were: anti-GAPDH, anti-c-Met, anti-MMP9 (Epitomics, USA) and anti-MMP2 (Santa Cruz Biotechnology, USA).
Vector construction and dual-luciferase reporter assay
The 3′-UTR of c-Met containing miR-409-3p binding sites was cloned downstream of the luciferase reporter in the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA) between the SacI and SalI sites. The mutant 3′-UTR, carrying a mutated sequence in the seeding region of miR-409-3p, was first mutated (primer set in Table 1) within the pMD18-T vector (TaKaRa, Japan) using the MutanBest Kit (TaKaRa, Japan) and then subcloned into the pmirGLO Dual-Luciferase Vector. The insertions were verified by sequencing. HEK 293T cells were plated in 24-well plates and transfected either with 50 nM miRNA mimic or control RNA, along with 100 ng of the luciferase vector (pmirGLO). Cells were harvested 48 h post-transfection and the relative luciferase activity measured by Dual-Glo Luciferase Assay Kit (Promega).
c-Met rescue experiments
The c-Met plasmid was constructed by inserting the human c-Met complementary DNA lacking the 3′-UTR into the pIRES2-EGFP construct (GeneCopoeia). MiR-409-3p or NC was co-transfected with pIRES2-EGFP-c-Met or the pIRES2-EGFP empty vector. The cells were collected 48 h after transfection and analyzed for migration and invasion as described above. The c-Met expression was verified by Western blotting.
Statistical analysis
The results were expressed as the mean ± SD. All analyses were performed using GraphPad Prism version 5 for Windows and a two-tailed value of P < 0.05 was considered statistically significant for the Student’s t-test.
RESULTS
Expression of miR-409-3p is down-regulated in bladder cancer
The expression of miR-409-3p was first evaluated by quantitative reverse transcription-PCR (qRT-PCR) in bladder cancer cell lines and one normal bladder cell line SV-HUC-1. As shown in Fig. 1A, miR-409-3p was significantly down-regulated in all the bladder cancer cell lines compared with SV-HUC-1. We further quantified the expression level of miR-409-3p in 10 pairs of human bladder cancer tissues and adjacent normal mucosal tissues by qRT-PCR (Fig. 1B). The results showed that the expression level of miR-409-3p was generally lower in tumor tissues compared to matched non-tumor tissues. Thus, we speculated that miR-409-3p might be a putative tumor suppressor in bladder cancer.
Fig. 1.
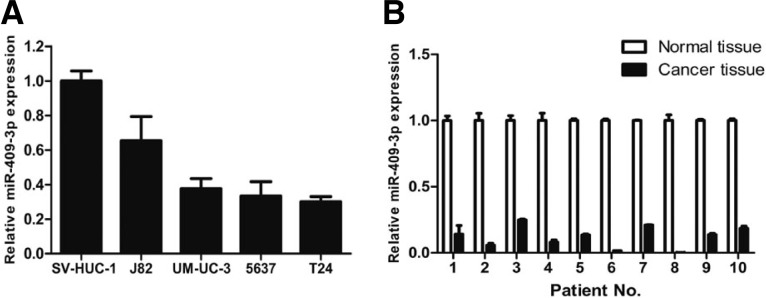
Downregulation of miR-409-3p expression in bladder cancer cell lines and tissues compared with the corresponding controls. (A) Relative expression of miR-409-3p in four bladder cancer cell lines and one normal bladder cell line SV-HUC-1 was determined by qRT-PCR. U6 snRNA was used as internal control. (B) Relative expression of miR-409-3p in 10 surgical specimens of bladder cancer tissues and matched non-tumor tissues was determined by qRT-PCR. U6 snRNA was used as internal control.
Overexpression of miR-409-3p has no apparent effect on bladder cancer cell proliferation
To better understand the biological functions of miR-409-3p, synthetic miR-409-3p mimics and negative control mimics were separately transfected into both T24 and 5,637 cells. The ectopic expression of miR-409-3p was confirmed by qRT-PCR (Fig. 2A). As shown in Fig. 2B, miR-409-3p did not significantly affect bladder cancer cell viability.
Fig. 2.
MiR-409-3p inhibits the migration and invasion of bladder cancer cells without significantly affecting cell viability. T24 and 5637 cells were transfected with miR-409-3p or NC (A-D). (A) The ectopic expression of miR-409-3p was confirmed by qRT-PCR (n = 3). (B) Overexpression of miR-409-3p had no apparent effect on bladder cancer cell viability (n = 3). (C) Representative micrographs of the cell migration assay (top) and quantification (bottom) from three independent experiments (*P < 0.05). (D) Representative micrographs of the cell invasion assay (top) and quantification (bottom) from three independent experiments (*P < 0.05).
Overexpression of miR-409-3p suppresses bladder cancer cell migration and invasion
Next, we explored the potential impact of miR-409-3p in bladder cancer cell migration and invasion. Intriguingly, migration and invasion assay showed that overexpression of miR-409-3p significantly suppressed the migration and invasion of T24 and 5,637 cells compared with the control (Figs. 2B and 2C).
miR-409-3p downregulates c-Met expression by targeting its 3′UTR
A miRNA usually performs its function by reducing the expression of target genes. Thus, our next aim was to investigate the targets of miR-409-3p that contribute to its anti-metastatic function. We searched for putative target genes of miR-409-3p with potential pro-oncogenic functions using several online search tools (e.g., MicroCosm, TargetScan, and Microrna. org). Among these genes, c-Met was observed to decrease significantly at both mRNA and protein levels (Figs. 3C and 3D).
Fig. 3.
The c-Met gene is a target of miR-409-3p. (A) Predicted miR-409-3p-binding sites in the 3′-UTR of c-Met are shown. Alignment between the predicted miR-409-3p target sites and miR-409-3p is marked in gray. (B) HEK293T cells were cotransfected with 50 nM of either miR-409-3p or NC and 100 ng pmirGLO Dual-Luci-ferase miRNA Target Expression Vector comprising Wt or Mut 3′-UTR of c-Met. The relative firefly luciferase activity normalized with renilla luciferase was measured 48 h after transfection. MiR-409-3p significantly suppressed the firefly luciferase activity of construct with Wt 3′-UTR of c-Met. (*P < 0.05). (C) Representative Western blotting image of c-Met protein in miR-409-3p-transfected T24 and 5,637 cells. GAPDH was used as a loading control (n = 3; *P < 0.05). (D) The relative c-Met mRNA levels were determined by real-time RT-PCR in miR-409-3p-ransfected cells (n = 3; *P < 0.05).
We then performed luciferase reporter assays to verify an interaction between miR-409-3p and the 3′UTR of c-Met. The 3′-UTR of c-Met was cloned into down-stream of firefly luci-ferase of pmirGLO Dual-Luciferase miRNA Target Expression Vector. Additionally, a mutated c-Met 3′-UTR (mutated AACA TTC to TTGTAAG) vector was also constructed (Fig. 3A). Co-transfections of either miR-409-3p or NC and luciferase reporter constructs comprising wildtype (Wt) or mutant (Mut) 3′-UTR were conducted. HEK 293T cells transiently transfected with the Wt-3′-UTR-reporter and miR-409-3p showed significantly decreased relative luciferase activity compared with cells transfected with NC. However, the luciferase activity of the reporter carrying 3′-UTR with mutated binding sites was unaffected by a simultaneous transfection with miR-409-3p (Fig. 3B). These results strongly indicate that the 3′-UTR of c-Met carries the binding sites of miR-409-3p.
c-Met promotes the migration and invasion of bladder cancer cells and the enhanced expression of c-Met can partially rescue miR-409-3p-induced migration and invasion suppression
To determine whether downregulation of c-Met was involved in miR-409-3p-mediated suppression of migration and invasion, we first analyzed the function of c-Met in bladder cancer cells. As shown in Fig. 4A, transfection of small interfering RNA against c-Met into T24 and 5,637 cells led to dramatically decreased c-Met protein expression. Silencing of c-Met significantly suppressed the migration and invasion of bladder cancer cells (Figs. 4C and 4D), which phenocopied the effects of miR-409-3p on migration and invasion of bladder cancer cells. Co-transfection of a construct expressing c-Met and miR-409-3p in T24 cells led to the restoration of c-Met expression, as confirmed by Western blot (Fig. 5A). This restoration of c-Met significantly increased the migratory and invasive capabilities of bladder cancer cells. More importantly, we found that restoration of c-Met could significantly reverse the migration and invasion suppression imposed by miR-409-3p (Figs. 5B and 5C). In summary, these data indicate that inhibition of c-Met expression by miR-409-3p is responsible, at least in part, for the miR-409-3p suppression of cell migration and invasion in human bladder cancer.
Fig. 4.
Knockdown of c-Met reduces the migration and invasion of bladder cancer cells. T24 and 5637 cells were transfected with si-c-Met or NC. (A) Expression of c-Met was detected by Western blotting. (B) Downregulation of c-Met had no apparent effect on bladder cancer cell viability (n = 3). (C) Representative micrographs of the cell migration assay (top) and quantification (bottom) from three independent experiments (*P < 0.05). (D) Representative micrographs of the cell invasion assay (top) and quantification (bottom) from three independent experiments (*P < 0.05).
Fig. 5.
The expression of c-Met partially rescues miR-409-3p-reduced cell migration and invasion. T24 cells were cotransfected with pIRES2-EGFP-c-Met or empty vector and miR-409-3p or NC as indicated. (A) The expression of c-Met analyzed by Western blotting. (B) Representative micrographs of cell migration and invasion assays. (C) The quantification of cell migration and invasion assays from three independent experiments (*P < 0.05).
MMP2 and MMP9 mRNA and protein expressions are significantly reduced in bladder cancer cells transfected with miR-409-3p or si-c-Met
Matrix metalloproteinases (MMPs), especially MMP2 and MMP9, are critical enzymes responsible for the degradation of the basement membrane (BM) and extracellular matrix (ECM), thereby contributing to cancer invasion and metastasis. After transfection of miR-409-3p or si-c-Met, the mRNA and protein levels of MMP2 and MMP9 were significantly reduced in bladder cancer cells (Fig. 6). These results indicate that miR-409-3p might reduce cell migration and invasion partly through indirectly regulating MMP2 and MMP9.
Fig. 6.
Transfection with miR-409-3p (A, B) or si-c-Met (C, D) significantly reduces MMP2 and MMP9 expression in bladder cancer cells. (A) The protein levels of MMP-2 and MMP-9 analyzed by Western blotting with GAPDH used as a loading control. (B) The relative mRNA levels of MMP-2 and MMP-9 determined by real-time RT-PCR (n = 3; *P < 0.05). (C) The protein levels of MMP-2 and MMP-9 analyzed by Western blotting with GAPDH used as a loading control. (D) The relative mRNA levels of MMP-2 and MMP-9 determined by real-time RT-PCR (n = 3; *P < 0.05).
DISCUSSION
In recent years, studies have confirmed the important role of miRNAs in bladder cancer as well and in malignancies. Although miRNA signatures for bladder cancer have been well characterized, elucidation of the roles of dysregulated miRNAs in bladder cancer progression and metastasis remains elusive. MiR-409-3p is a recently discovered miRNA, which has not yet been extensively researched in human cancer. To the best of our knowledge, it has been reported to be dysregulated only in gastric cancer (Li et al., 2012; Zheng et al., 2012) and fibrosarcoma (Weng et al., 2012). In the present study, the miR-409-3p expression level was significantly lower in bladder cancer cells and tissues compared with SV-HUC-1 and corresponding non-tumorous tissues, respectively. Furthermore, gain-of-function analyses of miR-409-3p in vitro suggest that miR-409-3p is able to suppress migration and invasion, and may serve as a metastasis-suppressor gene.
Evidence of miR-409-3p as a tumor growth inhibitor has been reported in gastric cancer and fibrosarcoma cells. In gastric cancer, Li et al. (2012) confirmed that miR-409-3p could suppress cell proliferation and induce apoptosis. Here, our results indicate that miR-409-3p does not serve as a growth inhibitor in bladder cancers. This discrepancy between our results in bladder cancers and that of others may be attributed to the tissue-or cell-specific role of miRNAs. At present, most miRNAs that have been reported to suppress tumor metastasis could also inhibit cell growth. Cell growth may also have an influence on the process of metastasis, for example, by taking part in the final step of metastasis colonization (Gao et al., 2012). We believe that in bladder cancers, miR-409-3p could inhibit tumor metastasis in a way that is independent of the impact on cell proliferation.
Next, we addressed the molecular mechanism of miR-409-3p in suppressing metastasis in bladder cancer cells. In our study, the real-time PCR, Western blot and luciferase assay showed that c-Met is a target of miR-409-3p. And specific c-Met knockdown with siRNA phenocopied the migration and invasion inhibition effect of miR-409-3p over-expression. We also showed that when miR-409-3p-expressing cells resumed c-Met expression, their invasion deficiencies were partly reversed. Therefore, we believe that miR-409-3p plays a role in the suppression of metastasis in bladder cancers by downregulating, at least partially, the protein expression of c-Met. The c-Met oncogene is a well-characterized cell surface receptor tyrosine kinase and commonly up-regulated in a variety of tumors, including human bladder cancer (Cheng et al., 2002). The activation of c-Met through aberrant hepatocyte growth factor paracrine stimulation contributes to tumor invasion and metastasis, with overexpression of c-Met associated with bladder cancer progression and poor prognosis (Cheng et al., 2002; 2005). The microRNAs miR-23b, miR-34a and miR-198 have been reported to inhibit migration and invasion through down-regulation of c-Met expression in human hepatocellular carcinoma cells (Li et al., 2009; Salvi et al., 2009; Tan et al., 2011). Another report indicates that the anti-metastatic miR-340 inhibits breast cancer metastasis by targeting c-Met (Wu et al., 2011). Together with our findings, these results suggest that c-Met could act as a pro-metastatic factor in various cancers and is regulated by miRNAs. Downregulation of these miRNAs in cancer may facilitate the expression of c-Met, leading to enhanced metastasis of the cancer.
Of note, MMP2 and MMP9, two extracellular matrix-degrading enzymes, were also significantly decreased in miR-409-3p-transfected cells, although they were not direct targets of miR-409-3p. Over-expression of MMP-2 and MMP-9 plays an important role in the carcinogenesis and metastasis of human cancer, as an increase of MMP2 and MMP9 may endow cancer cells with a greater ability to penetrate matrix protein barriers (Hazan et al., 2000). Previous studies have indicated that MMP2 and MMP9 activity could be upregulated by c-Met (Baek et al., 2012; Birchmeier et al., 2003). In addition, we also observed that MMP2 and MMP9 expression could be downregulated in c-Met-silenced cells. Our results suggest that MMP2 and MMP9 may be downstream effector proteins of miR-409-3p, which seems to downregulate MMP2 and MMP9 expression indirectly by targeting c-Met and then suppressing the ability to penetrate the extracellular matrix and migrate.
In summary, our study confirms that miR-409-3p is frequently down-regulated in bladder cancer. As a tumor suppressor in bladder cancer cells, miR-409-3p can exert its inhibitory effect on the migration and invasion of cancer cells by targeting c-Met. By identifying the mechanism and function of miR-409-3p as a tumor suppressor, it may be possible to develop miR-409-3p as a novel therapeutic strategy for bladder cancer therapy.
Acknowledgments
This study was supported by grants from the National Key Clinical Specialty Construction Project of China, Key medical disciplines of Zhejiang province, Combination of traditional Chi-nese and Western medicine key disciplines of Zhejiang Province (2012-XK-A23), Health sector scientific research special project (201002010), National Natural Science Foundation of China (Grant No. 30900552) and Zhejiang Provincial Natural Science Foundation of China (Z2090356 and LY12H05006).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Baek JH, Birchmeier C, Zenke M, Hieronymus T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J Immunol. 2012;189:1699–1707. doi: 10.4049/jimmunol.1200729. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Trink B, Tzai TS, Liu HS, Chan SH, Ho CL, Sidransky D, Chow NH. Overexpression of c-met as a prognostic indicator for transitional cell carcinoma of the urinary bladder: a comparison with p53 nuclear accumulation. J Clin Oncol. 2002;20:1544–1550. doi: 10.1200/JCO.2002.20.6.1544. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY, Chang TY, Ho CL, Tzai TS, Chow NH. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. Br. J Cancer. 2005;92:1906–1914. doi: 10.1038/sj.bjc.6602593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C, Li H, Shi DB. The molecular mechanism of micro-RNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2012;32:491–501. doi: 10.1038/onc.2012.61. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Ueno K, Shahryari V, Tabatabai ZL, Dahiya R. MicroRNA-1826 targets VEGFC, beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer. Carcinogenesis. 2012;33:41–48. doi: 10.1093/carcin/bgr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y, Zheng X. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei M, Ju J, Yu Y, Yan M, et al. MicroRNA-409-3p regulates cell proliferation and apoptosis by targeting PHF10 in gastric cancer. Cancer Lett. 2012;320:189–197. doi: 10.1016/j.canlet.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Majid S, Dar AA, Saini S, Shahryari V, Arora S, Zaman MS, Chang I, Yamamura S, Chiyomaru T, Fukuhara S, et al. MicroRNA-1280 inhibits invasion and metastasis by targeting ROCK1 in bladder cancer. PLoS One. 2012;7:e46743. doi: 10.1371/journal.pone.0046743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely LA, Rieger-Christ KM, Neto BS, Eroshkin A, Garver J, Patel S, Phung NA, McLaughlin S, Libertino JA, Whitney D, et al. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol Oncol. 2010;28:39–48. doi: 10.1016/j.urolonc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi A, Sabelli C, Moncini S, Venturin M, Arici B, Riva P, Portolani N, Giulini SM, De Petro G, Barlati S. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J. 2009;276:2966–2982. doi: 10.1111/j.1742-4658.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- Tan S, Li R, Ding K, Lobie PE, Zhu T. miR-198 inhibits migration and invasion of hepatocellular carcinoma cells by targeting the HGF/c-MET pathway. FEBS Lett. 2011;585:2229–2234. doi: 10.1016/j.febslet.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Uchino K, Takeshita F, Takahashi RU, Kosaka N, Fujiwara K, Naruoka H, Sonoke S, Yano J, Sasaki H, Nozawa S, et al. Therapeutic effects of microRNA-582-5p and -3p on the inhibition of bladder cancer progression. Mol Ther. 2013;21:610–619. doi: 10.1038/mt.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Hirata H, Majid S, Yamamura S, Shahryari V, Tabatabai ZL, Hinoda Y, Dahiya R. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer Ther. 2012;11:244–253. doi: 10.1158/1535-7163.MCT-11-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C, Dong H, Chen G, Zhai Y, Bai R, Hu H, Lu L, Xu Z. miR-409-3p inhibits HT1080 cell proliferation, vascularization and metastasis by targeting angiogenin. Cancer Lett. 2012;323:171–179. doi: 10.1016/j.canlet.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Wszolek MF, Rieger-Christ KM, Kenney PA, Gould JJ, Silva Neto B, Lavoie AK, Logvinenko T, Libertino JA, Summerhayes IC. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol. 2011;29:794–801. e791. doi: 10.1016/j.urolonc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, Mao SS, Zhang GH, Xu XC, Zhang N. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117:2842–2852. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H, Wang B, Ouyang J, Zhang X. Inhibitory effects of microRNA-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting Notch1. J Huazhong Univ Sci Technol Med Sci. 2012;32:375–382. doi: 10.1007/s11596-012-0065-z. [DOI] [PubMed] [Google Scholar]
- Zheng B, Liang L, Huang S, Zha R, Liu L, Jia D, Tian Q, Wang Q, Wang C, Long Z, et al. MicroRNA-409 suppresses tumour cell invasion and metastasis by directly targeting radixin in gastric cancers. Oncogene. 2012;31:4509–4516. doi: 10.1038/onc.2011.581. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wu D, Tao J, Qu P, Zhou Z, Hou J. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand J Urol [Epub ahead of print] 2012 doi: 10.3109/00365599.2012.748821. [DOI] [PubMed] [Google Scholar]







