Abstract
The actin cytoskeleton plays an important role in cell shape determination, adhesion and cell cycle progression. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 (EBP50), also known as Na+-H+ exchanger regulatory factor 1 (NHERF1), associates with actin cytoskeleton and is related to cell cycle progression. Its Ser279 and Ser301 residues are phosphorylated by cyclin-dependent kinase 2 (cdc2)/cyclin B during the mitosis phase. However, the biological significance of EBP50 phosphorylation mediated by cdc2/cyclin B is not clear. In the present study, MDA-MB-231 cells with low levels of endogenous EBP50 protein were stably transfected with constructs of EBP50 wild type (WT), phospho-deficient (serine 279 and serine 301 mutated to alanine-S279A/S301A) or phospho-mimetic (serine 279 and serine 301 mutated to aspartic acid-S279D/S301D) mutants. Subsequently, multiple phenotypes of these cells were characterized. Failure of cdc2/cyclin B-mediated EBP50 phosphorylation in cells expressing S279A/S301A (AA cells) significantly increased F-actin content, enhanced the adherence of cells to the extracellular matrix, altered cell morphology and caused defects in cytokinesis, as reflected in the formation of giant cells with heteroploid DNA and multinucleation or giant nuclei. Furthermore, knockdown of EBP50 expression in AA cells rescued cell defects such as the cytokinesis failure and abnormal cell morphology. EBP50 S279A/S301A had a weaker binding affinity with actin than EBP50 S279D/S301D, which might explain the increase of F-actin content in the AA cells. The present results suggest that cdc2/cyclin B-mediated EBP50 phosphorylation may play a role in the regulation of various cell functions by affecting actin cytoskeleton reorganization.
Keywords: actin, adhesion, cytokinesis, ezrin-radixin-moesin-binding phosphoprotein 50, multinucleation, phosphorylation
INTRODUCTION
Actin filament remodeling regulates many important cell processes such as cell adhesion, growth, morphological change and cell cycle progression (Castillo-Romero et al., 2009; Chang et al., 2010; Hable et al., 2003; Watabe et al., 1996; White, 1984; Zhuang et al., 2011). Actin remodeling in coordination can ensure proper execution of G2/M checkpoint arrest (Hsu et al., 2010) and is crucial for entry into mitosis (Zhuang et al., 2011). Cortical F-actin (polymerized actin) disappears at the initial stage of mitosis (Yamagishi and Kawai, 2011). The abnormal change in F-actin content can retard or inhibit cytokinesis (Bai et al., 2001; Watabe et al., 1996). Hyperactivation and mislocalized actin polymerization can lead to the formation of binucleated or multinucleated cells, suggesting a failure of cytokinesis (Moulding et al., 2007). Cell shape determination and adhesion are also associated with actin dynamics (Hu et al., 2010; White, 1984). The important roles played by the actin cytoskeleton in multiple cell activities and the significance of the dynamic equilibrium of actin polymerization have triggered interest in the regulation of actin reorganization.
The actin assembly process has been studied extensively. The dynamic equilibrium between G-actin (monomer form) and F-actin (polymeric form) can be regulated by multiple factors, including ATP hydrolysis, etc. The dynamics of the actin cytoskeleton are also controlled by a number of actin-binding proteins (ABPs) (Grzanka et al., 2010). Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 (EBP50), also known as Na+-H+ exchanger regulatory factor 1 (NHERF1), has been shown as a ABP to indirectly associate with the actin cytoskeleton and regulate F-actin dynamics (Bretscher et al., 1997; Mohler et al., 1999; Morales et al., 2004; Murthy et al., 1998; Shenolikar et al., 2004; Short et al., 1998; Sun et al., 2000). The expression level and phosphorylation status of EBP50 affect its interaction with actin and actin reorganization. Disruption of EBP50 binding to platelet-derived growth factor (PDGF) receptor enhances actin filament reorganization (Demoulin et al., 2003). The phosphorylation of EBP50 at Ser77 by protein kinase C (PKC) inhibits its colocalization with the cortical actin cytoskeleton (Voltz et al., 2007). EBP50 is also known to associate with cell cycle progression and suppresses G1-to-S transition in breast cancer cells (Pan et al., 2006), while decreased EBP50 expression causes abnormal breast cancer cell growth (Dai et al., 2004). EBP50 is phosphorylated at Ser279 and Ser301 by cyclin-dependent kinase 2 (cdc2)/cyclin B during the mitosis phase of the cell cycle (He et al., 2001), and the cdc2/cyclin B complex regulates the G2 to M phase transition. Taken together, these findings suggested that EBP50 phosphorylation might play a role in cell division. However, whether the cdc2/cyclin B-mediated phosphorylation of EBP50 affects its interaction with actin and further regulates cell cycle progression have not been reported.
In this study, we over-expressed the full-length cdc2/cyclin B phospho-deficient mutant EBP50 S279A/S301A and phospho-mimicking mutant EBP50 S279D/S301D in MDA-MB-231 cells. MDA-MB-231 cells stably over-expressing the EBP50 S279A/S301A mutant (AA cells) showed abnormal morphology, increased F-actin content, stronger cell matrix adhesion, defect in cytokinesis and lower affinity to actin, suggesting that cdc2/cyclin B-mediated EBP50 phosphorylation plays an important physiological role in maintaining normal functions of MDA-MB-231 cells by affecting actin cytoskeleton reorganization.
MATERIALS AND METHODS
Cell lines and plasmids
The human breast cancer cell line MDA-MB-231 and COS-7 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco Lab Inc., USA) and Dulbecco’s modified Eagle’s medium (DMEM, Gibco) respectively, supplemented with 10% fetal bovine serum (FBS, Hyclone, USA) and 1% antibiotic-antimycotic agent (Life Technologies, Inc., USA) under standard conditions at 37°C and 5% CO2. The constructs of pBK-CMV-Hemagglutinin (HA)-EBP50 WT, HA-EBP50 S279A/S301A and HA-EBP50 S279D/S301D were kindly provided by Dr. Randy Hall from Emory University (USA). EBP50 knockdown plasmid was generated using the ribozyme transgene method described previously (Yang et al., 2011). Briefly, the secondary structure of human EBP50 was generated using Zucker’s RNA mFold software. The ribozymes that specifically targeted EBP50 were obtained by touchdown PCR with the appropriate primers (sense: CTGCAGCCGGGGAGTCTGGGTCCACTGACC GCTGATGAGTCCGTGAGGA; antisense: ACTAGTCCAAGCCAGGCCAGTTCTTTCGTCCTCACGGAC T). Among this, 5′-CAGCCGGGGAGTCTGGGTCCACTGACCG-3′ ribozyme sequence targeted EBP50 mRNA sequence 773–800 for degradation. The resulting positive inserts were purified and cloned into the pEF6/V5-His-TOPO vector (Invitrogen, USA).
Stable overexpression of EBP50 protein
MDA-MB-231 cells were transfected with constructs of HA-EBP50 WT, HA-EBP50 S279A/S301A (AA), or HA-EBP50 S279D/S301D (DD) respectively with Lipofectamine 2000 (Invitrogen, USA). The cells were split 36 h after transfection, and placed in medium containing G418 (300 μg/ml) to select for transfected cells. After 2 weeks, individual clones were picked up and cultured.
Stable knockdown of EBP50 expression using ribozyme transgenes
To knock down the expression of EBP50 in MDA-MB-231 cells stably expressing the EBP50 S279A/S301A mutant, the pEF6/V5-His-TOPO control vector or pEF6/V5-His-TOPO-EBP50-ribozyme plasmid was transfected using Lipofectamine 2000 and EBP50 stable knockdown cells were obtained by blasticidin S selection at 1.25 μg/ml for 2 weeks.
Western blotting
Protein extracts were run on 8% SDS-polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked in blocking buffer (5% non-fat dry milk in TBST buffer) for 1 h at room temperature and then incubated with primary antibodies against HA, (MBL, Japan) or β-actin (Sigma Chemical Corp., USA) in blocking buffer for 1.5 h at room temperature. The blots were washed three times with TBST buffer and incubated for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody (Zhongshan Goldenbridge Biotechnology Co. Ltd., China) in blocking buffer. Finally, the blots were washed three times with TBST buffer and visualized via enzyme-linked chemiluminescence using the electro-chemiluminescence (ECL) kit (Applygen Technologies Inc., China).
In vitro matrigel adhesion assay
Cells (1 × 105) were seeded into 96-well plates precoated with 5 μg Matrigel basement membrane matrix (BD Biosciences, USA). After 45 min of incubation, non-adherent cells were washed off using a balanced salt solution. The remaining adherent cells were fixed, stained with crystal violet and the absorbance value was determined at 540 nm using a plate reading spectrophotometer (Molecular Devices Corp., USA).
Analysis of cellular DNA content by flow cytometry
A total of 2 × 106 cells were collected, washed in phosphate buffered saline (PBS) solution and fixed by adding 4 ml of absolute ethanol precooled at −20°C. Fixed cells were washed and incubated with 20 μg DNase-free RNaseA at 37°C for 30 min. The cells were then stained with 100 μl of 1 mg/ml propidium iodide (PI, Sigma, USA) at room temperature for 5–10 min. Samples were analyzed with the Epics XL flow cytometer (Beck man-Coulter, USA).
High content screening for cytoskeletal rearrangement
A cytoskeletal rearrangement assay was performed according to the manufacturers’ instructions (Thermo Cellomics® Cytoskeletal Rearrangement Kit, USA). Briefly, Cells on coverslips were washed with PBS, fixed in 3.7% (vol/vol) formaldehyde for 10 min at room temperature, washed again before permeabilization with 0.1% Triton X-100 in PBS for 5 min, and blocked with 1% bovine serum albumin (BSA fraction V) in PBS for 30 min. The cells were stained with rhodamine phalloidin for 30 min at 37°C in the dark to visualize the actin cytoskeleton, and with Hoechst 33258 for 10 min at room temperature in the dark to label the nuclei. Cytoskeletal rearrangement was quantitatively analyzed with the Thermo Scientific Cellomics ArrayScan (Thermo Fisher Scientific Inc., USA).
GST pull-down assay
Glutathione S-transferase (GST)-EBP50 fusion proteins and GST alone were purified using glutathione-agarose beads. The beads were blocked with blocking buffer (containing 3% BSA, 10 mM HEPES, 50 mM NaCl, and 0.1% Tween) for 30 min before being used. COS-7 cells were harvested and lysed in ice-cold lysis buffer (10 mM HEPES, 100 mM NaCl, 0.5% Triton X-100, 5 mM EDTA, and protease inhibitor mixture), and clarified via centrifugation at 13,000 rpm for 15 min. The supernatant was incubated with the same amount of various GSTEBP50 fusion proteins beads with end-over-end rotation at 4°C for 3 h. After five washes with lysis buffer, the pulled-down proteins were eluted from the beads with 1× SDS-PAGE sample buffer, and subjected to western blotting with anti-actin antibody (BD Biosciences, USA).
Statistical analyses
The results of Western blotting were semi-quantitatively analyzed by densitometry using Image J software. All data were presented as means ± SD and statistical significance was analyzed by independent sample T-test. Differences were considered significant when P < 0.05.
RESULTS
Generation of a cell pool stably expressing EBP50 WT, EBP50 phospho-deficient or phospho-mimetic mutants
To determine the physiological significance of cdc2/cyclin B-mediated EBP50 phosphorylation, the constructs of EBP50 WT, EBP50 S279A/S301A, S279D/S301D or pBK-CMV-HA vector were stably transfected into MDA-MB-231 cells respectively. MDA-MB-231 cell clones stably expressing HA-EBP50 WT (WT cells), S279A/S301A (AA cells), S279D/S301D (DD cells) mutants or control clones (Vector) were obtained and identified by western blotting. Then, 6–9 clones of each, WT, AA, DD and vector control cells were collected and mixed respectively before further analysis (Fig. 1). The EBP50 protein was expressed at similar levels in AA cells and DD cells.
Fig. 1.
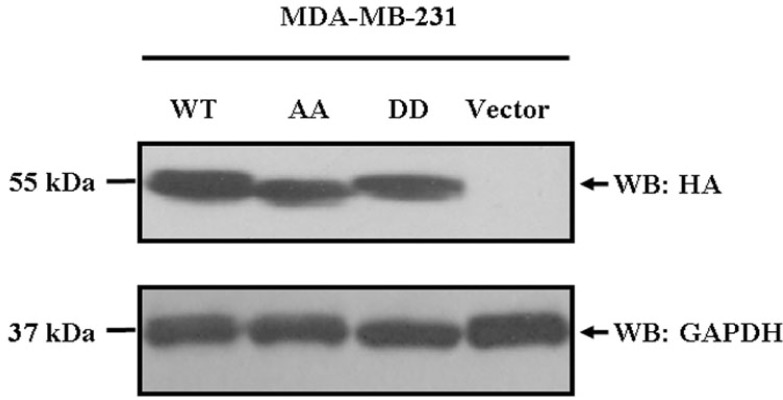
Generation of MDA-MB-231 breast cancer cells stably over-expressing EBP50 WT, or EBP50 phospho-deficient or phospho-mimetic mutants. MDA-MB-231 cells were transfected with pBK-CMV-HA-EBP50 WT, EBP50 phospho-deficient S279A/S301A mutant, phospho-mimetic S279D/S301D mutant expression plasmids or the pBK-CMV-HA vector respectively and screened using G418 sulfate (300 μg/ml). Then, the cells stably expressing these plasmids were named WT, AA, DD or Vector cells respectively. The HA-tag was detected for exogenous EBP50 protein expression. The 6–9 clones of each, WT, AA, DD and vector control cells were mixed respectively before usage. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a protein-loading control.
Cdc2/cyclin B-mediated EBP50 phosphorylation affects actin cytoskeleton polymerization in MDA-MB-231 cells
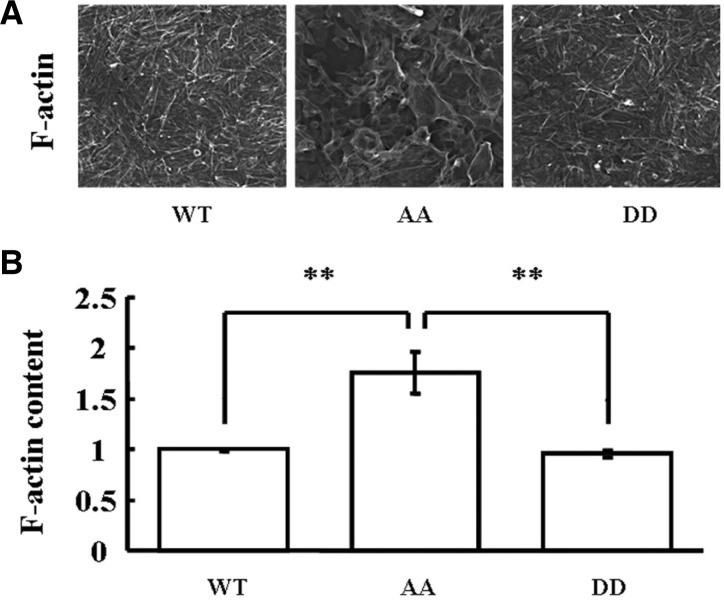
Cytoskeletal reorganization was reported to be regulated by EBP50 and the actin cytoskeleton plays an important role in cell cycle progression. During the mitosis phase of the cell cycle, Ser279 and Ser301 of EBP50 are phosphorylated by the cdc2/cyclin B complex. However, whether EBP50 phosphorylation by the cdc2/cyclin B in mitosis phase could affect cytoskeletal rearrangement was elusive. Therefore, the effect of EBP50 phosphorylation on F-actin organization was analyzed by employing high content screening systems. The results showed a robust increase of F-actin content in AA cells, compared with that in WT or DD cells (Fig. 2, P < 0.01). These results indicated that failure of cdc2/cyclin B-mediated EBP50 phosphorylation may disturb normal cytoskeletal rearrangement in MDA-MB-231 cells.
Fig. 2.
Cdc2/cyclin B-mediated EBP50 phosphorylation affects actin cytoskeleton polymerization in MDA-MB-231 cells. (A) Representative images of F-actin staining in WT, AA, or DD cells. (B) F-actin staining combined with a high content screening assay showed that AA cells had more F-actin content than WT or DD cells (P < 0.01). Error bars indicate SD from triplicate measurements. Data shown are representative of three independent experiments.
Cdc2/cyclin B-mediated EBP50 phosphorylation plays a role in adherence of MDA-MB-231 cells to the extracellular matrix
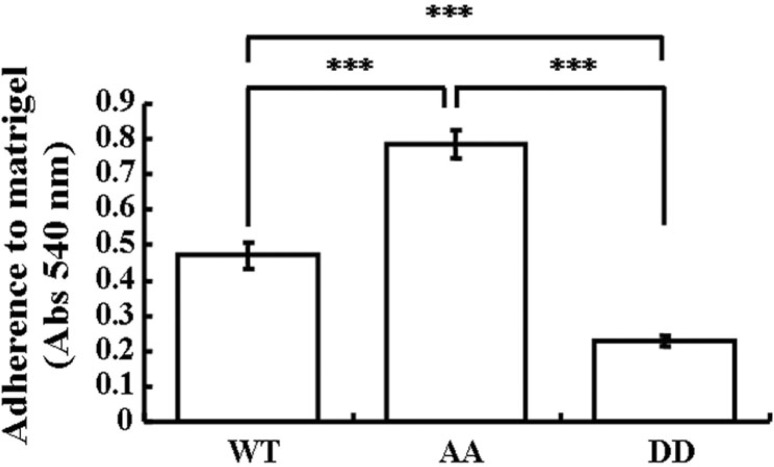
Cell-extracellular matrix (ECM) interactions are directed by the actin cytoskeleton (Moh et al., 2009). Therefore, we further explored the cell-ECM interactions of MDA-MB-231 cells stably expressing a variety of EBP50 constructs. Various cells were seeded in plates coated with ECM matrigel and incubated for 45 min. Non-adherent cells were washed off and adherent cells were determined to identify the adhesive ability of respective cells. The AA cells had significantly stronger ability to adhere to ECM than WT cells or DD cells (Fig. 3, P < 0.001), and DD cells had very weak binding to ECM. These results supported a critical role of EBP50 phosphorylation by cdc2/cyclin B in the regulation of cell adhesion.
Fig. 3.
Cdc2/cyclin B-mediated EBP50 phosphorylation plays a role in adherence of MDA-MB-231 cells to the extracellular matrix. AA cells showed stronger adherence to matrigel than WT or DD cells (P < 0.001), and DD cells had very weak binding to matrigel. Error bars indicate SD from triplicate measurements. Data shown are representative of three independent experiments.
Cdc2/cyclin B-mediated EBP50 phosphorylation participates in cytokinesis
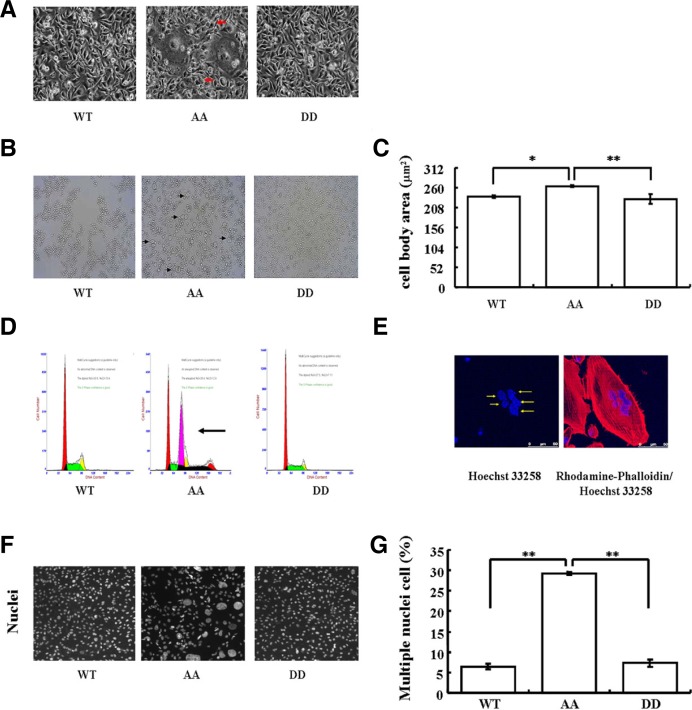
Actin filament remodeling is also known to regulate cellular processes such as determination and maintenance of cell morphology and cytokinesis (Castillo-Romero et al., 2009; Watabe et al., 1996; White, 1984; Zhuang et al., 2011), so we further characterized morphological change of MDA-MB-231 cells, in which a variety of EBP50 mutants stably expressed, and found the formation of cell aggregates in AA cells (Fig. 4A). Cells with a larger size was observed in AA cells through a light microscope, after trypsin treatment, but not in WT or DD cells (Fig. 4B). Consistent with the findings of observation under the light microscope, a proportion of cells with a larger size was observed in AA cells, but not in WT or DD cells with the quantitatively based high-content screening (Fig. 4C, vs. WT P < 0.05 or vs. DD P < 0.01).
Fig. 4.
Cdc2/cyclin B-mediated EBP50 phosphorylation participates in cytokinesis. (A) Morphology of AA cells was altered. (B) Giant cells were observed in AA cells after trypsin treatment under light microscope. (C) F-actin staining combined with a high content screening assay showed that the cell body area (μm2) of AA cells was significantly greater than that of WT (P < 0.05) or DD (P < 0.01) cells. Error bars indicate SD from triplicate measurements. Data shown are representative of three independent experiments. (D) Heteroploid DNA content was also observed in AA cells by flow cytometry. Flow cytometry analysis was performed three times. (E) Confocal microscopy showed the presence of multiple nuclei in AA cells. (F) Representative images of nuclei in WT, AA, or DD cells. (G) High content screening assay results confirmed that the ratio of giant or multinucleated cells among AA cells was significantly greater than that among WT or DD cells (P < 0.01). Error bars indicate SD from triplicate measurements. Data shown are representative of three independent experiments.
Cell size homeostasis in proliferating cells requires a coordination of growth with division, and it could be altered by a nuclear division failure or a cytokinesis failure. Thus, we further monitored the changes in the DNA content by flow cytometry. Results showed the DNA content increased significantly and the heteroploid rate was up to 28.4% in the AA cells, whereas the heteroploid was not detected in either the WT or the DD cells (Fig. 4D). Consistently, cells with either single giant nuclei, or multiple smaller-size nuclei were observed in the AA cells by confocal microscopy (Fig. 4E). Verification of the confocal results with the quantitative high content screening assay also showed that there were a significantly larger proportion of giant or multi-nucleated cells among the AA mutant expressing cells than that among the WT or DD cells (Figs. 4F and 4G, P < 0.01). Large nuclei may result from its cytokinesis failure (Bramwell et al., 1982), and multinucleation is a marker of cytokinesis defects. Taken together, these results suggested that cytokinesis was abnormal in the cells stably expressing the EBP50 phospho-deficient AA mutant, revealing that cdc2/cyclin B-dependent EBP50 phosphorylation is important for cytokinesis.
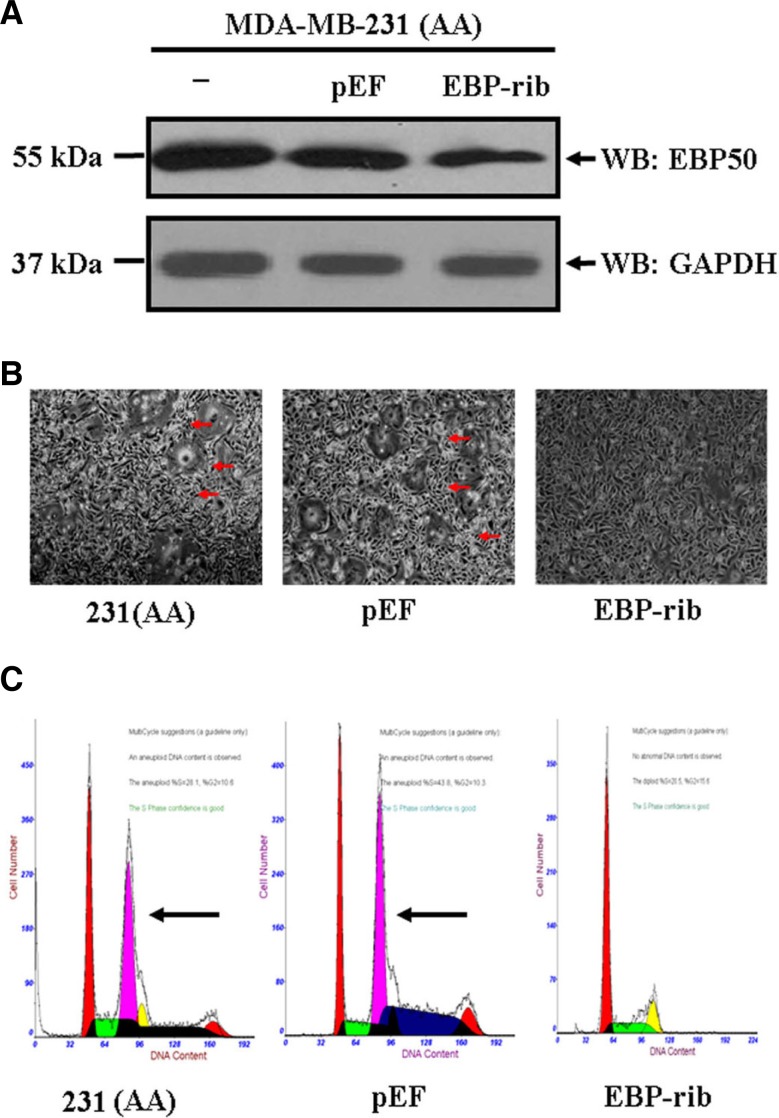
To further confirm the results that cdc2/cyclin B-dependent EBP50 phosphorylation affects cytokinesis, we knocked down EBP50 expression in the AA cells to rescue the morphological changes resulted from overexpressing the EBP50 phospho-deficient mutant. EBP50 protein expression was knocked down up to 55% with EBP50 ribozyme system (Fig. 5A). As we expected, the cell morphology such as the cell aggregation, cell size and DNA content were rescued by EBP50 knockdown. Meanwhile, control vector transfection had no effect on these phenotypes (Figs. 5B and 5C), indicating that morphological changes and heteroploid DNA content resulted from the expression of the AA mutant and EBP50 phosphorylation was required for normal cytokinesis.
Fig. 5.
Knockdown of EBP50 expression in MDA-MB-231 cells stably expressing the EBP50 S279A/S301A mutant rescues the formation of cell aggregates and heteroploid DNA content. (A) pEF6/V5-His-TOPO control vector or pEF6/V5-His-TOPO-EBP50-ribozyme plasmid was transfected using Lipofectamine 2000 and EBP50 stable knockdown cells were obtained by blasticidin S selection at 1.25 μg/ml. Stable knock-down clones for EBP50 (EBP-rib) or control vector (pEF) were picked up and mixed. EBP50 protein expression was detected. GAPDH was used as a protein-loading control. EBP50 was knocked down to 45% as revealed by Western blotting. Data shown are representative of three independent experiments. (B) Knockdown of EBP50 in AA cells reversed the formation of cell aggregates, and control vector expression can not have an effect on the cell aggregates. (C) Flow cytometry analysis showed that knockdown of EBP50 in AA cells reversed the DNA content from heteroploid to normal, while the control vector expression had no such effect. The data in each panel were obtained from three individual experiments.
Cdc2/cyclin B-dependent EBP50 phosphorylation regulates the interaction of EBP50 with actin
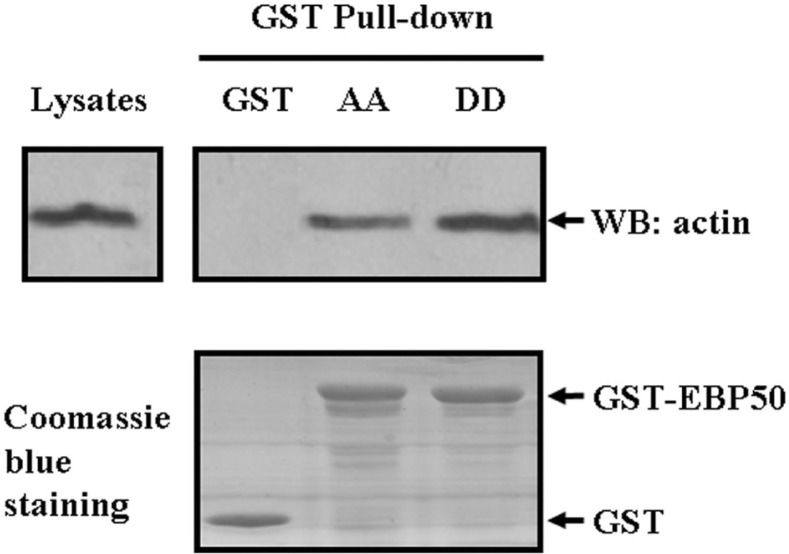
The actin cytoskeleton remodeling is a dynamic process that participates in many fundamental cellular functions such as cell adhesion, cell morphology change and cell cycle progression (Hu et al., 2010; Kunda and Baum, 2009; Pollard and Cooper, 2009). EBP50 is known to interact with the actin cytoskeleton and regulate actin reorganization (Bretscher et al., 1997; Murthy et al., 1998; Shenolikar et al., 2004; Sun et al., 2000). Hence, to further explore the underlying molecular mechanisms by which cdc2/cyclin B-mediated EBP50 phosphorylation participates in the regulation of cellular functions such as cell adhesion, cell shape and cytokinesis, the binding affinity of actin with EBP50 AA and EBP50 DD was studied by GST pull-down assay. EBP50 protein is phosphorylated by cdc2/cyclin B kinase, which is present in all known eukaryotes, but absent in bacteria, so in E. coli expression system, wild type EBP50 is unphospho-rylated and the same as EBP50 phospho-defective mutant (He et al., 2001). In this study, we expressed GST-tagged fusion protein in E. coli. Hence, we only explored the binding difference with actin between phospho-defective and phospho-mimetic EBP50 mutants. As the results were shown in Fig. 6, GST-EBP50 AA pulled down 50% less actin than the GST-EBP50-DD mutant (P < 0.05, n = 3), indicating that cdc2/cyclin B kinase-mediated EBP50 phosphorylation affected its interaction with actin.
Fig. 6.
Cdc2/cyclin B-mediated EBP50 phosphorylation regulates the interaction of EBP50 with actin. COS-7 cells were harvested and lysed in ice-cold lysis buffer, and supernatants were incubated with 50 μl of GST, GST-EBP50 S279A/S301A (AA) or GST-EBP50 S279D/S301D (DD) fusion proteins adsorbed onto glutathione agarose. Pulled down proteins were eluted from the beads with 1× SDS-PAGE sample buffer and subjected to Western blot analysis. The GST-EBP50 AA pull-down contained less actin than that of the GST-EBP50 DD mutant from COS-7 cell lysates. Coomassie blue staining showed equal loading of GST-EBP50 AA and GST-EBP50 DD. The GST pull-down assay was carried out three times independently.
DISCUSSION
Actin dynamics regulate many vital cell functions such as cell shape, cell adhesion, motility, cytokinesis, endocytosis and membrane trafficking (Bretscher, 1991; Lee et al., 2010; Moulding et al., 2007; Nestor et al., 2011; Shao et al., 2010; White, 1984; White et al., 2001). The dysregulation of actin organization can lead to defects in mitosis and cytokinesis (Moulding et al., 2007). EBP50 is an actin-binding protein and required for actin cytoskeleton reorganization (Demoulin et al., 2003; Mohler et al., 1999; Murthy et al., 1998; Reczek et al., 1997; Voltz et al., 2007). EBP50 can be phosphorylated at Ser279 and Ser301 during mitosis phase by cdc2/cyclin B (He et al., 2001). In the present study, we first observed an increase of the F-actin content in AA cells, suggesting that a defect in EBP50 phosphorylation may disturb actin cytoskeleton reorganization dynamics and turnover during the mitosis phase. Further investigation revealed that these changes were related to a lower affinity of the EBP50 phospho-deficient mutant to actin. AA cells showed altered morphology, enhanced adherence of cells to ECM and cytokinesis defect as reflected in bigger cell size and multinucleation or giant nuclei, accompanied by increased F-actin content. Knockdown of EBP50 AA reversed the defects such as heteroploid DNA and altered morphology of the cells, further suggesting that EBP50 phosphorylation deficiency-triggered functional abnormalities was closely correlated with the increase of F-actin content. The phosphorylation of S279 and S301 of EBP50 mediated by cdc2/cyclin B was found to play a key role in modulating EBP50 association with actin. EBP50 WT has the same binding affinity with actin as EBP50 AA in prokaryotic cell. However, in eukaryotic cell with the progression of cell cycle, EBP50 WT oscillated its phosphorylation level, i.e. it is phosphorylated during M phase. Hence, EBP50 WT will have different affinity with actin from EBP50 AA in M phase, resulting in the different phenotypes between WT cells and AA cells. The present results suggest a novel mechanism by which EBP50 phosphorylation enhance its binding affinity with actin, and decrease the F-actin content, furthermore may regulate functions of breast cancer cells.
Post-translational modification of EBP50 could affect protein conformation and regulate the interaction between EBP50 and its binding proteins. The intramolecular interaction between the second PDZ domain (PDZ2, amino acids 150–237) and the C-terminal 242–358 fragment of EBP50 plays an autoinhibitory role, preventing complex formation between EBP50 and other proteins (Cheng et al., 2009). EBP50 phosphorylation at S162 of PDZ2 by PKC reduces the affinity of EBP50 for the cystic fibrosis transmembrane conductance regulator (CFTR) (Raghuram et al., 2003). EBP50 phosphorylation at S339 and S340 by PKC has been shown to inhibit the tail-PDZ2 intramolecular interaction and enhance binding to CFTR (Li et al., 2007). This study result showed EBP50 phosphorylation at S279 and S301 by cdc2/cyclin B kinase might also affect protein conformation and regulate the interaction between EBP50 and actin, and further play a role in actin reorganization.
Cytokinesis, the final step of cell division, is mediated by the formation of a contractile ring composed of actin and myosin to partition one cell into two. Cytokinesis occurs at the point of minimum cortical F-actin content (Wong et al., 1997) and actin depolymerization is required for cytokinesis (Hotulainen et al., 2005). Unregulated actin synthesis or turnover leads to polyploidization of human megakaryocytes, which in turn possibly abrogates the formation of the actin cleavage furrow in telophase (Baatout et al., 1998). The enhanced and delocalized actin polymerization of whole cell may cause defects of mitosis and cytokinesis in X-linked neutropenia (Bai et al., 2001; Castillo-Romero, 2009; Moulding et al., 2007). Actin-polymerization and microfilament-stabilization can also alter cell morphology (Bai et al., 2001; Castillo-Romero, 2009). The failure of EBP50 phosphorylation by cdc2/cyclin B caused a significant increase in F-actin content, further leading to altered cell morphology and cytokinesis failure as reflected in bigger cell size and multinucleation or giant nuclei. EBP50 knockdown restored heteroploid DNA and morphological changes in cells over-expressing the EBP50 phosphor-deficient mutant, revealing EBP50 phosphorylation in S279 and S301 was required for cytokinesis.
EBP50 is phosphorylated in M phase of HeLa cells, while HeLa cells decrease its adhesive ability to cell matrix, detach from matrix, become round and prepare to divide. The weak adhesive state of M phase was consistent with cells undergoing cytokinesis (Murphy-Ullrich, 2001). DD cells had the weakest adhesive ability to matrix, which was consistent with cell behavior during mitosis phase. While during interphase, adherent cells have stronger adhesive ability. The strong adhesive state to matrix is characteristic of a differentiated, quiescent cell (Murphy-Ullrich, 2001). AA cells showed strongest adhesive ability to matrix compared with other cells, which was consistent with cell behavior during interphase. This study also revealed increased adhesive ability of AA cells was positively correlated with its increased F-actin content. Actin polymerization can increase cell adhesion (Lee et al., 2010), so it is possible that the increased F-actin content caused by EBP50 dephosphorylation enhanced the adhesive ability of cells. The increased adhesive ability of AA cells might also be the reason of cell aggregates.
In summary, EBP50 phosphorylation at Ser279 and Ser301 during the mitosis phase of the cell cycle is important for normal cell cycle progression in addition to inhibiting self-oligomerization (He et al., 2001). Changes in the phosphorylation status of EBP50 during the different phases of the cell cycle may influence cell morphology, adhesion and cell cycle progression by changing its binding affinity for actin and further affecting actin polymerization. This study provides critical information concerning the unique physiological function of the EBP50 protein and its involvement in cell division and adhesion, which may help to better understand the role of EBP50 in tumor development. These data also provide insight into the regulation of the final stages of cell division. As cytokinesis defects can cause chromosomal instability, knowledge of the processes that regulate normal cytokinesis may help to understand the events that lead to tumorigenesis.
Acknowledgments
This work was supported by the National Natural Science Foundation of the People’s Republic of China (No. 811272887), Beijing Natural Science Foundation (No.7131003), the Foundation of Beijing Educational Committee (KM201110025002) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (CIT&TCD201304187).
REFERENCES
- Baatout S, Chatelain B, Staquet P, Symann M, Chatelain C. Induction and enhancement of normal human megakaryocyte polyploidization are concomitant with perturbation in the actin metabolism. Eur J Clin Invest. 1998;28:845–855. doi: 10.1046/j.1365-2362.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- Bai R, Verdier-Pinard P, Gangwar S, Stessman CC, McClure KJ, Sausville EA, Pettit GR, Bates RB, Hamel E. Dolastatin 11, a marine depsipeptide, arrests cells at cytokinesis and induces hyperpolymerization of purified actin. Mol Pharmacol. 2001;59:462–469. doi: 10.1124/mol.59.3.462. [DOI] [PubMed] [Google Scholar]
- Bramwell VH, Crowther D, Gallagher J, Stoddart RW. Studies of lectin binding to normal and neoplastic lymphoid tissues. I. Normal nodes and Hodgkin’s disease. Br J Cancer. 1982;46:568–581. doi: 10.1038/bjc.1982.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337–374. doi: 10.1146/annurev.cb.07.110191.002005. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110:3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- Castillo-Romero A, Leon-Avila G, Perez Rangel A, Cortes Zarate R, Garcia Tovar C, Hernandez JM. Participation of actin on Giardia lamblia growth and encystation. PLoS One. 2009;4:e7156. doi: 10.1371/journal.pone.0007156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Heo KS, Woo CH, Lee H, Le NT, Thomas TN, Fujiwara K, Abe J. MK2 SUMOylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting MK2 kinase and HSP27 phosphorylation. Blood. 2010;117:2527–2537. doi: 10.1182/blood-2010-08-302281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Li J, Fazlieva R, Dai Z, Bu Z, Roder H. Autoinhibitory interactions between the PDZ2 and C-terminal domains in the scaffolding protein NHERF1. Structure. 2009;17:660–669. doi: 10.1016/j.str.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JL, Wang L, Sahin AA, Broemeling LD, Schutte M, Pan Y. NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene. 2004;23:8681–8687. doi: 10.1038/sj.onc.1207962. [DOI] [PubMed] [Google Scholar]
- Demoulin JB, Seo JK, Ekman S, Grapengiesser E, Hellman U, Ronnstrand L, Heldin CH. Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem J. 2003;376:505–510. doi: 10.1042/BJ20030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem. 2001;276:9206–9213. doi: 10.1074/jbc.M008745200. [DOI] [PubMed] [Google Scholar]
- Grzanka D, Marszalek A, Gagat M, Izdebska M, Gackowska L, Grzanka A. Doxorubicin-induced F-actin reorganization in cofilin-1 (nonmuscle) down-regulated CHO AA8 cells Folia Histochem. Cytobiol. 2010;48:377–386. doi: 10.2478/v10042-010-0072-5. [DOI] [PubMed] [Google Scholar]
- Hable WE, Miller NR, Kropf DL. Polarity establishment requires dynamic actin in fucoid zygotes. Protoplasma. 2003;221:193–204. doi: 10.1007/s00709-002-0081-0. [DOI] [PubMed] [Google Scholar]
- He J, Lau AG, Yaffe MB, Hall RA. Phosphorylation and cell cycle-dependent regulation of Na+/H+ exchanger regulatory factor-1 by Cdc2 kinase. J Biol Chem. 2001;276:41559–41565. doi: 10.1074/jbc.M106859200. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FF, Lin TY, Chen JY, Shieh SY. p53-Mediated transactivation of LIMK2b links actin dynamics to cell cycle checkpoint control. Oncogene. 2010;29:2864–2876. doi: 10.1038/onc.2010.40. [DOI] [PubMed] [Google Scholar]
- Hu S, Biben T, Wang X, Jurdic P, Geminard JC. Internal dynamics of actin structures involved in the cell motility and adhesion: modeling of the podosomes at the molecular level. J Theor Biol. 2010;270:25–30. doi: 10.1016/j.jtbi.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009;19:174–179. doi: 10.1016/j.tcb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Lee GH, Ahn T, Kim DS, Park SJ, Lee YC, Yoo WH, Jung SJ, Yang JS, Kim S, Muhlrad A, et al. Bax inhibitor 1 increases cell adhesion through actin polymerization: involvement of calcium and actin binding. Mol Cell Biol. 2010;30:1800–1813. doi: 10.1128/MCB.01357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Poulikakos PI, Dai Z, Testa JR, Callaway DJ, Bu Z. Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J Biol Chem. 2007;282:27086–27099. doi: 10.1074/jbc.M702019200. [DOI] [PubMed] [Google Scholar]
- Moh MC, Tian Q, Zhang T, Lee LH, Shen S. The immunoglobulin-like cell adhesion molecule hepaCAM modulates cell adhesion and motility through direct interaction with the actin cytoskeleton. J Cell Physiol. 2009;219:382–391. doi: 10.1002/jcp.21685. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci USA. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulding DA, Blundell MP, Spiller DG, White MR, Cory GO, Calle Y, Kempski H, Sinclair J, Ancliff PJ, Kinnon C, et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J Exp Med. 2007;204:2213–2224. doi: 10.1084/jem.20062324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, Gusella J, Ramesh V. NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem. 1998;273:1273–1276. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- Nestor MW, Cai X, Stone MR, Bloch RJ, Thompson SM. The actin binding domain of betaI-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS One. 2011;6:e16197. doi: 10.1371/journal.pone.0016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Wang L, Dai JL. Suppression of breast cancer cell growth by Na+/H+ exchanger regulatory factor 1 (NHERF1) Breast Cancer Res. 2006;8:R63. doi: 10.1186/bcr1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram V, Hormuth H, Foskett JK. A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc. Natl. Acad. Sci USA. 2003;100:9620–9625. doi: 10.1073/pnas.1633250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Wang JH, Pollak MR, Wells A. α-actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS One. 2010;5:e13921. doi: 10.1371/journal.pone.0013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology. 2004;19:362–369. doi: 10.1152/physiol.00020.2004. [DOI] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem. 2007;282:33879–33887. doi: 10.1074/jbc.M703481200. [DOI] [PubMed] [Google Scholar]
- Watabe S, Wada S, Saito S, Matsunaga S, Fusetani N, Ozaki H, Karaki H. Cellular changes of rat embryonic fibroblasts by an actin-polymerization inhibitor, bistheonellide A, from a marine sponge. Cell Struct Funct. 1996;21:199–212. doi: 10.1247/csf.21.199. [DOI] [PubMed] [Google Scholar]
- White JG. Arrangements of actin filaments in the cytoskeleton of human platelets. Am J Pathol. 1984;117:207–217. [PMC free article] [PubMed] [Google Scholar]
- White SR, Williams P, Wojcik KR, Sun S, Hiemstra PS, Rabe KF, Dorscheid DR. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:282–294. doi: 10.1165/ajrcmb.24.3.3995. [DOI] [PubMed] [Google Scholar]
- Wong GK, Allen PG, Begg DA. Dynamics of filamentous actin organization in the sea urchin egg cortex during early cleavage divisions: implications for the mechanism of cytokinesis. Cell Motil Cytoskeleton. 1997;36:30–42. doi: 10.1002/(SICI)1097-0169(1997)36:1<30::AID-CM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Kawai H. Cytoskeleton organization during the cell cycle in two stramenopile microalgae, Ochromonas danica (Chrysophyceae) and Heterosigma akashiwo (Raphidophyceae), with special reference to F-actin organization and its role in cytokinesis. Protist. 2011;163:686–700. doi: 10.1016/j.protis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang Y, Chen P, Hu J, Xiong Y, Feng D, Liu H, Zhang H, Yang H, He J. Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) is required for the estradiol-dependent increase of phosphatase and tensin homolog (PTEN) protein expression. Endocrinology. 2011;152:4537–4549. doi: 10.1210/en.2011-1207. [DOI] [PubMed] [Google Scholar]
- Zhuang C, Tang H, Dissanaike S, Cobos E, Tao Y, Dai Z. CDK1-mediated phosphorylation of Abi1 attenuates Bcr-Abl-induced F-actin assembly and tyrosine phosphorylation of WAVE complex during mitosis. J Biol Chem. 2011;286:38614–38626. doi: 10.1074/jbc.M111.281139. [DOI] [PMC free article] [PubMed] [Google Scholar]