Abstract
The hyaloid vessel is a transient vascular network that nourishes the lens and the primary vitreous in the early developmental periods. In hyaloid vessels devoid of the support of astrocytes, we demonstrate that tight junction proteins, zonula occludens-1 and occludin, are regularly expressed at the junction of endothelial cells. To figure out the factor influencing the formation of tight junctions in hyaloid vessels, we further progress to investigate the interactions between endothelial cells and pericytes, two representative constituent cells in hyaloid vessels. Interestingly, endothelial cells interact with pericytes in the early postnatal periods and the interaction between two cell types provokes the up-regulation of transforming growth factor β1. Further in vitro experiments demonstrate that transforming growth factor β1 induces the activation of Smad2 and Smad3 and the formation of tight junction proteins. Taken together, in hyaloid vessels, pericytes seem to regulate the formation of tight junctions by the interaction with endothelial cells even without the support of astrocytes. Additionally, we suggest that the hyaloid vessel is a valuable system that can be utilized for the investigation of cell-cell interaction in the formation of tight junctions in developing vasculatures.
Keywords: endothelial cells, hyaloid vasculature, pericytes, tight junction, transforming growth factor β1
INTRODUCTION
Hyaloid vessels indicate a combination of transient vascular networks including hyaloid artery, pupillary membrane, tunica vasculosa lentis (TVL), and vasa hyaloidea propria (VHP), which appears in the early developmental periods of the eye and regresses with the development of mature vasculatures (Ito and Yoshioka, 1999). These vessels provide nutrient and oxygen to the lens and primary vitreous (Saint-Geniez and D’Amore, 2004). Hyaloid artery originates from the embryonic fissure and passes through the optic nerve and the vitreous cavity to the posterior pole of the lens (Hamming et al., 1977). Pupillary membrane independently comes from the annular vessels in the neuroectodermal optic cup; whereas, TVL and VHP are capillary networks formed by branches from hyaloid artery, which mainly nourish the lens and primary vitreous, respectively (Hahn et al., 2005). Most of hyaloid vessels are regressed within 2 weeks after the birth in mice (Kim et al., 2010a; Lang and Bishop, 1993) and during the first month of life in monkeys (Hamming et al., 1977). In spite of robust research on the regression of hyaloid vessels, studies on the maintenance and characteristics of hyaloid vessels during the developmental processes are limited (McLeod et al., 2012).
Interestingly, hyaloid vessels can be differentiated from other vasculatures in the central nervous system (CNS) and the retina by the constituent cell types. Unlike endothelial cells in the brain and the retina which interact with both pericytes and astrocytes to form neurovascular units and demonstrate barrier characteristics (Kim et al., 2006; 2009a), hyaloid vessels are known to lack the coverage of astrocytes and consists of endothelial cells, pericytes, and hyalocytes (Zhu et al., 1999). However, there is no definite leakage of fluorescein dye from the hyaloid artery and its branches including VHP in primates and little evidence of cellular extravasation along hyaloid vessels in human, demonstrating that certain level of endothelial tightness exist in hyaloid vessels (Hamming et al., 1977; Zhu et al., 1999). In other vessels in the CNS and the retina, chronological interactions among endothelial cells, astrocytes, and pericytes affect the formation of tight junctions (Daneman et al., 2010; Kim et al., 2009a). Interestingly, during developmental processes even before astrocyte generation, pericytes can regulate the blood-brain barrier (BBB) including the formation of tight junctions (Daneman et al., 2010). Notably, pericytes are demonstrated to function to regulate the permeability of BBB in a study with pericyte-deficient mouse mutants (Armulik et al., 2010). Taken together, we posed a hypothesis that interactions between endothelial cells and pericytes might affect the formation of tight junctions in hyaloid vessels which were devoid of the support of astrocytes.
In this study, we demonstrate that the expression of tight junction proteins, zonula occludens (ZO)-1 and occludin, is maintained in endothelial cells of hyaloid vessels including TVL and VHP during the early postnatal periods from postnatal day (P) 4 to P12. Furthermore, we provide that these events are coincided by the coverage of endothelial cells in hyaloid vessels by pericytes, not by astrocytes. Interestingly, interactions between endothelial cells and pericytes in early postnatal periods induce the upregulation of transforming growth factor (TGF)-β1 in hyaloid vessels and in vitro culture systems, which provokes the activation of Smad pathway and further expression of tight junction proteins. In addition, our report suggests that hyaloid vessels be a valuable system that can be utilized for the investigation of cell-cell interaction in the formation of tight junctions in developing vasculatures.
MATERIALS AND METHODS
Animals
C57BL/6 mice were purchased from Central Lab. Animal (Korea) and kept in alternate dark-light cycles of 12 h at room temperature (RT). Newborn mice were kept with female mice which could provide breast-feeding. The care, use, and treatment of all animals were in agreement with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and the guidelines established by the Seoul National University Institutional Animal Care and Use Committee.
Cell culture
Human retinal microvascular endothelial cells (HRMECs) and human placental pericytes were purchased from Applied Cell Biology Research Institute (USA) and PromoCell (Germany), respectively. Maintenance conditions for each cell line were as follows: M199 medium supplemented with 20% fetal bovine serum (FBS) (GIBCO, Life Technologies, USA), basic FGF (3 ng/ml; Millipore, USA), heparin (10 IU/ml; Sigma-Aldrich, USA), and 1% penicillin-streptomycin (GIBCO, Life Technologies) for HRMECs and Pericyte Growth Basal Medium (PromoCell) supplemented with SupplementMix (PromoCell) and 1% penicillin-streptomycin (GIBCO, Life Technologies) for human placental pericytes. For the mixed culture, 2 complete media for each cell line were mixed at a 1 to 1 ratio.
Antibodies
Primary antibodies for immunohistochemistry were as follows: anti-platelet/endothelial cell adhesion molecule-1 (PECAM-1; 1:100, goat, Santa Cruz Biotechnology, USA), anti-ZO-1 (1:100, rabbit, Invitrogen, USA), anti-occludin (1:100, rabbit, Invitrogen), anti-glial fibrillary acidic protein (GFAP; 1:100, rabbit, Dako, Glostrup, Denmark), anti-NG2 (1:100, rabbit, Millipore, USA), anti-platelet derived growth factor-BB (PDGF-BB; 1:100, rabbit, Abcam, UK), anti-platelet derived growth factor receptor-β (PDGFR-β; 1:100, rabbit, Cell Signaling Technology, USA), and anti-transforming growth factor-β1 (TGF-β1; 1:100, rabbit, Santa Cruz Biotechnology). Secondary antibodies were utilized in consideration of species-specificity and visibility in dual staining from following combinations of antibodies: Alexa Fluor® 488 donkey anti-rabbit IgG (Invitrogen), Alexa Fluor® 594 donkey anti-goat IgG (Invitrogen), and Alexa Fluor® 594 donkey anti-rabbit IgG (Invitrogen). Primary antibodies for Western blot were as follows: anti-phospho-Smad2 (p-Smad2; 1:1,000, rabbit, Cell Signaling Technology), anti-phospho-Smad3 (p-Smad3; 1:1,000, rabbit, Cell Signaling Technology), anti-ZO-1 (1:1,000, rabbit, Invitrogen), anti-ZO-2 (1:1,000, rabbit, Invitrogen), anti-claudin-5 (1:1,000, rabbit, Abcam), anti-occludin (1:1,000, rabbit, Invitrogen), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1,000, rabbit, Santa Cruz Biotechnology). Species-specific peroxidase-conjugated secondary antibodies (Pierce, Thermo Scientific, USA) were utilized for Western blot.
Tissue preparation
At P4, P8, and P12, mice (n = 10 per each group) were sacrificed by inhalation of CO2 gas and the eyes were enucleated. The enucleated eyes were fixed with 4% paraformaldehyde and were processed for embedding in paraffin blocks. Sagittal sections were performed at 4 μm thickness from paraffin blocks. The sections were incubated at 60°C for 2 h and processed with deparaffinization and hydrated by sequential immersion in xylene and graded ethyl alcohol solutions. Then, we treated paraffin sections with proteinase K at 37°C for 10 min. The sections were further treated with 0.2% Triton X-100 at RT for 10 min. To minimize nonspecific binding of antibodies, we treated the sections with blocking agent (Invitrogen) for 10 min.
Immunohistochemistry
The sections from paraffin blocks were incubated with primary antibodies in a humidified chamber overnight. After washing 3 times with phosphate-buffered saline (PBS), the sections were incubated with secondary antibodies. The nuclei were stained with 4′,5-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). Then, the slides were mounted with Faramount Aqueous Mounting Medium (Dako) and observed under the fluorescence microscope (BX61; Olympus, Japan). Especially, TVL and VHP were carefully examined for the expression of markers of interest.
Enzyme-linked Immunosorbent assay (ELISA)
After the incubation of cells for indicated duration (24 and 48 h), the culture supernatants were harvested and centrifuged at 13,000 rpm to remove cellular debris. TGF- β1 specific ELISA kit (Bio-Plex Pro™ TGF-β1 set; Bio-Rad Laboratories, USA) was utilized to compare the level of TGF-β1 in the culture supernatants according to the manufacturer’s protocol.
Western blot analysis
HRMECs (5 × 105 cells) were grown on the dish and incubated at 37°C overnight. Then, the culture media were replaced with the culture media supplemented with 1% FBS. After the starvation with low serum media, the cells were treated with TGF-β1 (10 ng/ml; R&D Systems, USA) for indicated duration (0, 0.5, 1, and 6 h). The cells were lysed in a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, and a protease inhibitor cocktail. The lysates (50 μg per lane) were separated by electrophoresis on 10% SDS-PAGE and transferred to nitrocellulose membranes (Amersham Hybond ECL, GE Healthcare Bio-Sciences, USA). The membranes were incubated with primary antibodies at 4°C overnight. Then, the membranes were washed with PBS and incubated with secondary antibodies at RT for 1 h. After meticulous washing with PBS for 1 h, the membranes were treated with the Enhanced Chemiluminescence Detection kit (Pierce, Thermo Scientific) and exposed to the film (Amersham Hyperfilm ECL, GE Healthcare Bio-Sciences). The blots were scanned using a scanner (SCX-3210K, Samsung, Korea) and the intensity of the blots was analyzed using the ImageJ program (1.46r; National Institutes of Health, USA). Intensity values were normalized relative to control values.
Statistical analysis
Differences between treatment groups were assessed with the Mann-Whitney U-test. All statistical analyses were performed using Prism 5 (GraphPad Software, USA). The mean ± the standard error of the mean (SEM) was shown in figures. We considered P-values less than 0.05 as statistically significant and designated them with asterisk (*) in figures.
RESULTS
Expression of tight junction proteins in hyaloid vessels
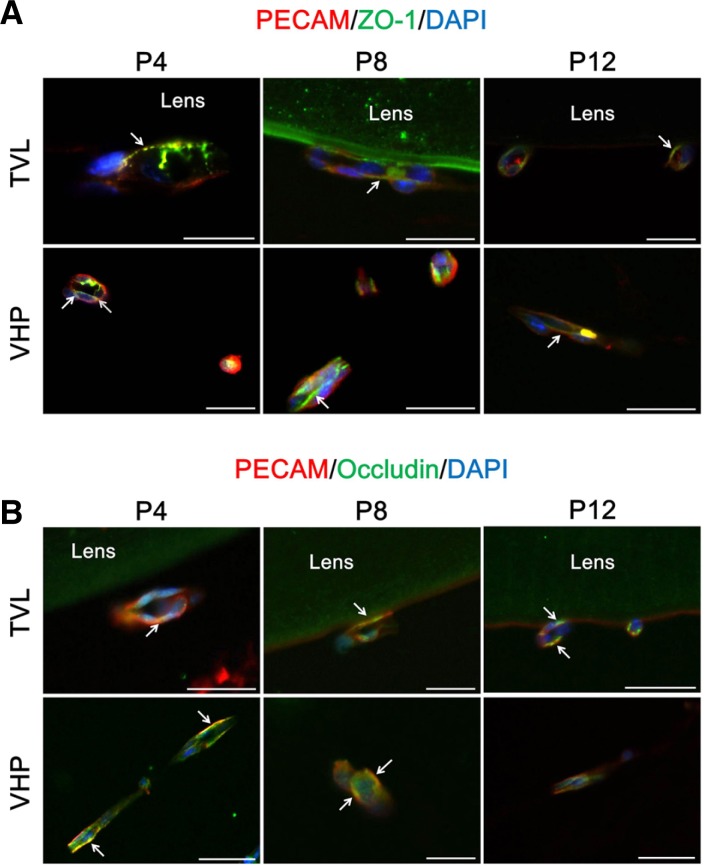
We previously reported that ZO-1, a junctional protein which is located on the cytoplasmic membrane surface at the intercellular tight junction, might demonstrate the tightness of blood-retinal barrier (BRB) in the developing retina and play important roles in the maintenance of BRB in diseased conditions of the retina (Kim et al., 2009a; 2009b; 2010b). To evaluate the expression of ZO-1 in hyaloid vessels, we examined TVL and VHP in the eyes from mice at P4, P8, and P12. We select TVL and VHP for the analysis from hyaloid vessels because both vasculatures represent extensive capillary networks of the hyaloid vascular system. Interestingly, the expression of ZO-1 is maintained in TVL and VHP from P4 to P12 at the surface border of endothelial cells (Fig. 1A). At P4, the expression pattern of ZO-1 in some endothelial cells of TVL is patchy and irregular, demonstrating possible junctional immatureness (Fig. 1A, upper left, designated with an arrow). However, most of endothelial cells of TVL and VHP show regular and constant expression of ZO-1 throughout the early postnatal periods of the eye.
Fig. 1.
Expression of tight junction proteins in hyaloid vessels. At P4, P8, and P12, C57BL/6 mice were sacrificed and the enucleated eyes were processed for further analysis with immunostaining for tight junction proteins, ZO-1 and occludin (n = 10 for each day). ZO-1 and occludin are selected as representative proteins for junctional and transmembrane proteins that constitute tight junctions, respectively. (A) Expression of ZO-1 in hyaloid vessels. Each image is representative of more than 50 vascular lumens of TVL and VHP, respectively. White arrows indicate distinct colocalization sites of PECAM-1 and ZO-1, but the immunoreactivity is not confined to the sites with white arrows. (B) Expression of occludin in hyaloid vessels. Each image is representative of more than 50 vascular lumens of TVL and VHP, respectively. White arrows indicate distinct colocalization sites of PECAM-1 and occludin, but the immunoreactivity is not confined to the sites with white arrows. Scale bars: 50 μm.
Occludin, a transmembrane protein, is a member of tight junction proteins that constitutes blood-neural barriers including BBB and BRB (Abbott et al., 2010; Kim et al., 2006). Interestingly, like the expression of ZO-1 in hyaloid vessels, that of occludin is evident in TVL and VHP along the junctional border of endothelial cells (Fig. 1B). Particularly, the expression of occludin is more linear and stronger in endothelial cells of TVL at P8 and P12 and VHP through the study period than that of TVL at P4. This phenomenon might imply the dynamic nature of the formation of tight junctions in hyaloid vessels.
Coverage of endothelial cells in hyaloid vessels by pericytes, not by astrocytes
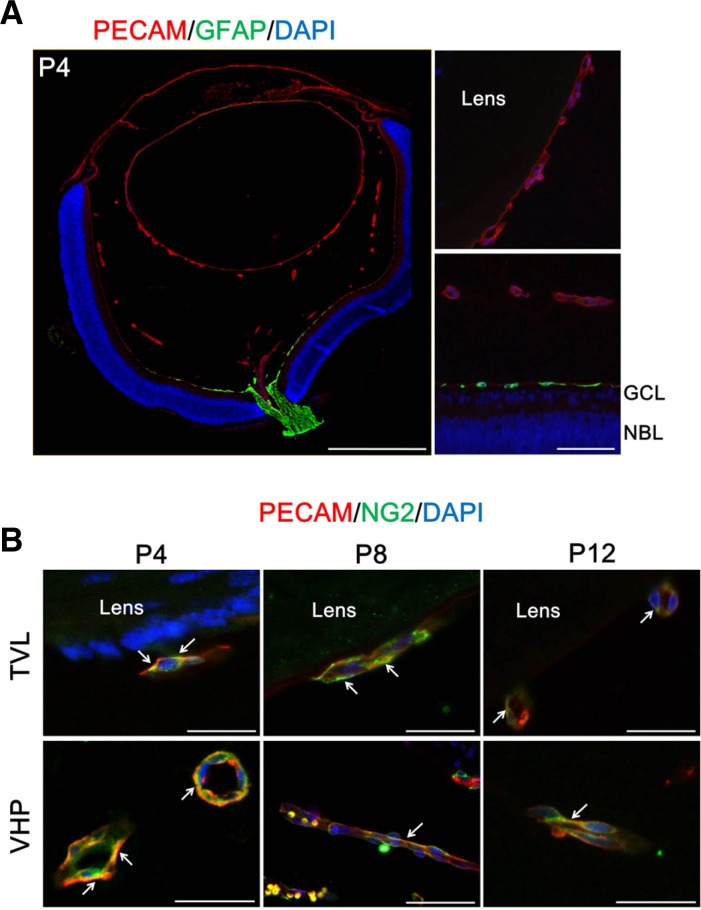
In the development of tight junctions in the early development of the retinal vasculatures, endothelial cells, pericytes, and astrocytes interact with each other in a consecutive order (Kim et al., 2009a). To investigate the involvement of different cell types in the formation of tight junctions in hyaloid vessels, we examined the expression of cell-specific markers for astrocytes and pericytes in hyaloid vessels. As shown in Fig. 2A, the expression of GFAP, an astrocyte marker, is restricted to optic nerve and the superficial retinal layer at P4. There is no evidence of the expression of GFAP in TVL, VHP, and pupillary membrane. As Ling and Stone observed with the cat retina, we also identified minimal extension of GFAP-positive cells along the hyaloid artery (Ling and Stone, 1988). However, definitely, hyaloid vessels are devoid of GFAP-positive cells in the early postnatal periods from P4 to P12.
Fig. 2.
Coverage of endothelial cells in hyaloid vessels by pericytes, not by astrocytes. To figure out the presence of perivascular cells in hyaloid vessels, immunostaining of GFAP, an astrocyte marker, and NG2, a pericytes marker, was performed on the enucleated eyes from mice at P4, P8, and P12. (A) Expression of GFAP in the mouse eye at P4. Magnified views were demonstrated for comparison of the expression patterns of GFAP in between TVL (upper right) and the retina (lower right). Scale bars: 500 μm (the whole eye) and 50 μm (the magnified views). (B) Expression of NG2 in hyaloid vessels. Each image is representative of more than 50 vascular lumens of TVL and VHP, respectively. White arrows indicate distinct colocalization sites of PECAM-1 and NG2, but the immunoreactivity is not confined to the sites with white arrows. Scale bars: 50 μm.
Interestingly, we could identify the expression of a pericyte marker, NG2 in TVL and VHP from P4 to P12 (Fig. 2B). Throughout the early postnatal periods, the expression of NG2 is maintained along the border of endothelial cells, demonstrated by the expression of PECAM-1, in most of vasculatures of TVL and VHP. This coexistence of endothelial cells and pericytes might imply the possible interaction between both cell types.
Interaction between pericytes and endothelial cells in hyaloid vessels
We previously reported that tight junction could be formed with contact of pericytes to endothelial cells without the prominent involvement of astrocytes in the process of retinal vessel development (Kim et al., 2009a). Furthermore, before the generation of astrocytes, the barrier is developed with recruitment of pericytes to the nascent vessels in the CNS during embryogenesis (Daneman et al., 2010). Because hyaloid vessels are devoid of the support of astrocytes, we investigated the interaction between pericytes and endothelial cells to figure out the roles of cell-cell interaction in the formation of tight junctions in hyaloid vessels.
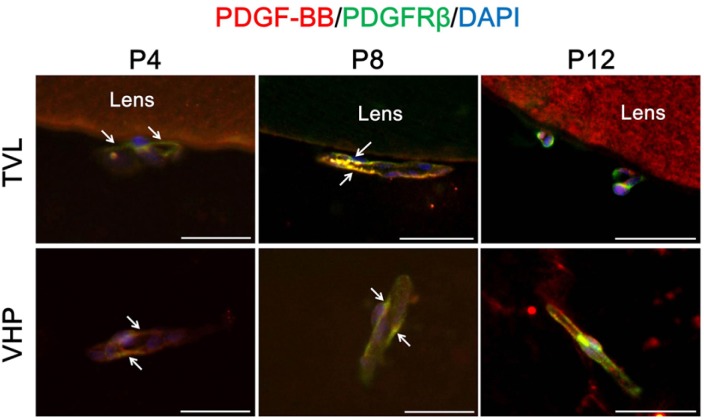
Interestingly, the expression of PDGF-B and PDGFR-β was colocalized at the cell surface of endothelial cells in TVL and VHP at P4 and P8 (Fig. 3). PDGF-B/PDGFR-β signaling determines the expansion of vascular smooth muscle cells and pericytes and leads to recruit pericytes to blood vessels (Bjarnegard et al., 2004; Tallquist et al., 2003). Furthermore, PDGF-deficient mice lack pericytes around the capillaries (Lindahl et al., 1997). In this regard, hyaloid vessels which evidenced to have PDGF-B/PDGFR-β signaling were supposed to recruit pericytes around capillary networks of TVL and VHP and have interactions between endothelial cells and pericytes.
Fig. 3.
Interaction between pericytes and endothelial cells in hyaloid vessels. To figure out the interaction between two types of cells in hyaloid vessels devoid of astrocytes, immunostaining of PDGF-B and PDGFR-β was performed. Each image is representative of more than 50 vascular lumens of TVL and VHP, respectively. White arrows indicate distinct colocalization sites of PECAM-1 and NG2, but the immunoreactivity is not confined to the sites with white arrows. Scale bars: 50 μm.
Upregulation of TGF-β induced by interaction between pericytes and endothelial cells
In this study, we demonstrated the formation of tight junctions and the interaction between pericytes and endothelial cells in hyaloid vessels during the early postnatal periods. Pericyte-derived TGF-β1 is known to contribute the survival of endothelial cells and barrier functions of BBB (Dohgu et al., 2004; Walshe et al., 2009). To figure out the expression of this factor in hyaloid vessels, we performed immunohistochemistry for TGF-β1 in hyaloid vessels. As shown in Fig. 4A, the expression of TGF-β1 is positive at the border of endothelial cells in VHP at P8, at which the expressions of tight junction proteins and PDGF-related signaling molecules were the most prominent.
Fig. 4.
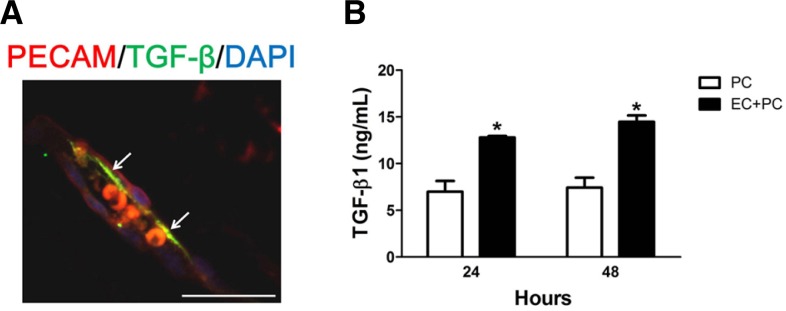
Upregulation of TGF-β induced by interaction between pericytes and endothelial cells. (A) At P8, at which the expressions of tight junction proteins and PDGF-related signaling molecules were the most prominent, the expression of TGF-β1 was examined in TVL. The image is representative of more than 50 vascular lumens of TVL. White arrows indicate distinct colocalization sites of PECAM-1 and TGF-β1, but the immunoreactivity is not confined to the sites with white arrows. Scale bars: 50 μm. (B) The level of TGF-β1 was measured from the culture supernatant by ELISA using the TGF-β1 specific kit. After cultivation of pericytes only and coculture of pericytes and HRMECs, the supernatant was harvested. Each bar represents the mean and the SEM of three independent experiments (*P-value < 0.05). EC, HRMECs; PC, pericytes.
Previously, Dohgu et al. (2004) demonstrated that coculture of brain capillary endothelial cells with brain pericytes increased the expression of TGF-β1 Mrna. Interestingly, the ELISA study demonstrated that the production of TGF-β1 was increased by coculture of retinal microvascular endothelial cells with pericytes in our study (Fig. 4B). These in vivo and in vitro results showed that the interaction between endothelial cells and pericytes might also affect the production of TGF-β1, a molecule that could affect the formation of tight junctions in hyaloid vessels.
Activation of Smad pathway and expression of tight junction proteins induced by TGF-β
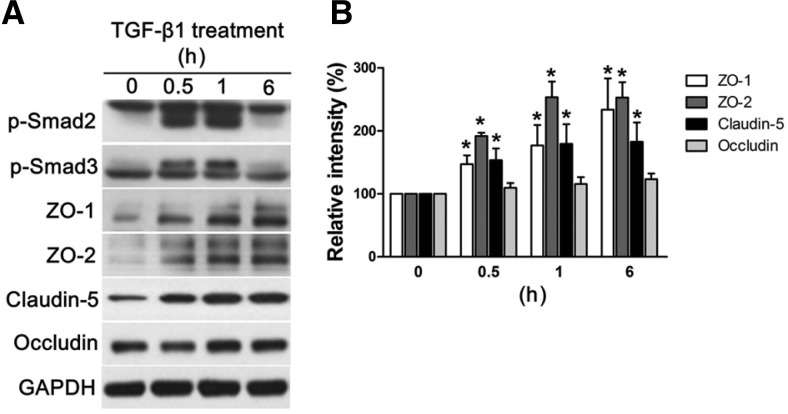
To figure out the role of pericytes-derived TGF-β1 in the formation of tight junctions, we further investigated the alterations in endothelial cells induced by the treatment with TGF-β1. TGF-β1 induced phosphorylation of Smad2 and Smad3, and further production of tight junction proteins including a transmembrane protein, claudin-5, and junctional proteins such as ZO-1 and ZO-2 at the concentration of 10 ng/ml (Fig. 5A). Quantification of signal intensities of blots also demonstrated significant increase in the relative density of ZO-1, ZO-2, and claudin-5 with the treatment of TGF-β1 compared to controls (Fig. 5B).
Fig. 5.
Activation of Smad pathway and expression of tight junction proteins induced by TGF-β. HRMECs were treated with TGF-β1 for indicated periods of time and prepared for further analysis with Western blot. (A) Each figure represents three independent experiments. (B) Quantitative analysis was performed by measuring the band intensity of the protein expression in the treatment groups compared to those of control. Each bar represents the mean and the SEM of three independent experiments (*P-value < 0.05). p-Smad2, the phosphorylated form of Smad2 protein; p-Smad3, the phosphorylated form of Smad3 protein.
DISCUSSION
In this study, the formation of tight junctions of hyaloid vessels in the early postnatal periods was in relation with the interaction between endothelial cells and pericytes, which led to upregulation of TGF-β1, a molecule that played an important role in the regulation of tight junction proteins in endothelial cells. Hyaloid vessels demonstrate characteristic anatomical distribution in the early developmental processes of the eye. First of all, the hyaloid artery originates from the embryonic fissure and passes through the vitreous cavity to the lens (Hahn et al., 2005; McLeod et al., 2012). Pupillary membrane comes from the annular vessels in the rim of the neuroectodermal optic cup (Hahn et al., 2005). In patients with persistent pupillary membrane, this vascular structure fails to regress to possibly obscure the vision (Lee and Yu, 2004). We selected TVL and VHP for the analysis of the expression of various markers in hyaloids vessels because these vessels represent extensive capillary networks that nourish the lens and primary vitreous, which are the major targets of hyaloid vessels.
Interestingly, hyaloid vessels in mice undergo regression which starts at around P4 in some of vessels and further progresses to demonstrate almost complete regression at around P16 (Kim et al., 2010a). As for primates, the vessel walls of hyaloids vessels become acellular and demonstrate no definite evidence of blood flow by the fourth week after the birth (Daneman et al., 2010). On the other hand, most of hyaloids vessels are regressed within two weeks in mice of different strains (Brown et al., 2005; Kim et al., 2010a; Lang and Bishop, 1993). In this regard, we examined TVL and VHP of mice from P4 to P12 with the purpose of investigation of the dynamic nature of the characteristics of hyaloids vessels before complete regression of them.
In this study, the expression of ZO-1 and occludin in hyaloid vessels were relatively constant at the junctional borders of endothelial cells marked by the expression of PECAM-1 from P4 to P12. Both markers were selected to be representative of junctional and transmembrane proteins, respectively. Bloodneural barriers, including BBB and BRB, are composed of endothelial tight junctions (Abbott et al., 2010; Kim et al., 2006). Tight junctions of endothelial cells contain transmembrane proteins such as occludin, claudin-5 and junctional proteins such as ZO-1, -2, and -3 (Antonetti et al., 1999). Interestingly, the expression of these proteins is closely related with the barrier characteristics of endothelial cells in the brain parenchyma and the retina. Particularly, ZO-1, a junctional protein which links the transmembrane proteins such as occluding and claudin-5 to the actin cytoskeleton of endothelial cells, plays important roles in the formation of tight junctions in the developing and diseased retina (Kim et al., 2006). In the developing retina, ZO-1 can determine the permeability of retinal vessels even with weak expression (Kim et al., 2009a). Furthermore, the expression of ZO-1 in retinal endothelial cells and the retina is affected by the condition that disrupts the endothelial tight junctions such as ischemia and the treatment with permeability factor including vascular endothelial growth factor (VEGF) (Kim et al., 2007; 2009b). Occludin is also closely related to the barrier function of retinal endothelial cells and retinal vasculatures (Antonetti et al., 1999; Kim et al., 2007; 2009b). Interestingly, intraocular injection of VEGF affects not only the expression but also the phosphorylation of occludin in the retina (Antonetti et al., 1999). Furthermore, VEGF mediates vascular leakage from retinal vessels with the loss of tight junction proteins including occludin in them (Kim et al., 2009b).
In general, blood-neural barriers including BBB and BRB are characterized by these tight junction proteins which are prerequisites for the maintenance of barrier function and affected by interactions among constituent cells such as endothelial cells, pericytes, and astrocytes (Kim et al., 2006; 2009a). In the development of retinal vasculatures, endothelial cells behind the leading front of retinal vessels have a contact with pericytes first to form the weak expression of ZO-1 (Kim et al., 2009a). Subsequently, the foot processes of astrocytes enclose the retinal vessels and lead to the maturation of tight junctions. Interestingly, even with the weak expression of ZO-1 induced by the interaction between pericytes and endothelial cells, retinal vessels exhibit no definite vascular leakage demonstrated by perfusion with 30 kDa fluorescein-conjugated dextran (Kim et al., 2009a). In contrast, hyaloid-retinal vessels of zebrafish which lack the expression of ZO-1 and occludin show easy leakage of dyes of 2,000 kDa fluorescein dextran (Kim et al., 2011).
In this study, we demonstrated that endothelial cells of hyaloid vessels in mice were devoid of the support of astrocytes. This phenomenon follows the findings from other mammals including primates and cats (Ling and Stone, 1988; Zhu et al., 1999). GFAP-positive cells are observed in Bergmeister’s papilla in primates and the very short portion along the hyaloid artery from the optic disc in cats from previous studies. We also demonstrated minimal extension of GFAP-positive cells along the hyaloid artery at P4 in mice in this study; however, absolute majority of hyaloid vessels were definitely devoid of astrocytes. On the other hand, hyaloid vessels were covered by pericytes demonstrated by immunoreactivity with NG2, which is invariably expressed by the mural cell component independent of the developmental mechanisms and is a valuable marker for identification of pericytes in the developmental processes (Ozerdem et al., 2001). In this regard, we further progressed to figure out the interaction between endothelial cells and pericytes in hyaloid vessels. Interestingly, hyaloid vessels were evidenced to have interactions between two types of cells demonstrated by colocalized expression of PDGF-B from endothelial cells and PDGFR-β on pericytes. Particularly, the immunoreactivity of both markers was more prominent in TVL and VHP at P4 and P8.
Is it possible for hyaloid vessels to express tight junction proteins with the interaction between pericytes and endothelial cells? Previous studies demonstrate that pericytes regulate the barrier characteristics of BBB and pericyte coverage determines vascular permeability of vessels of the CNS even before the generation of astrocytes (Armulik et al., 2010; Daneman et al., 2010). We also reported that pericytes induce the barrier characteristics of BRB in developing retinal vasculatures even without the ensheathment of foot processes of astrocytes (Kim et al., 2009a). Interestingly, pericyte-derived TGF-β1 induced by the interaction between pericytes and endothelial cells contributes to the induction of vascular barrier function of BBB and BRB (Dohgu et al., 2004; Walshe et al., 2009). In this study, we also demonstrated the upregulation of TGF-β1 at the border of endothelial cells of hyaloid vessels. Furthermore, the coculture of pericytes and HRMECs induced the production of TGF-β1 demonstrated by ELISA.
To investigate the alterations of tight junctions induced by TGF-β1 in in vitro systems, we examined the expression of various tight junction proteins and demonstrated the increase of ZO-1, ZO-2, and claudin-5 with the treatment of TGF-β1. Clau-din-5 is also an important component of tight junctions between retinal endothelial cells, critically related with the barrier function of the retinal vasculature (Koto et al., 2007). An unexpected result was the constant expression of occludin. We speculated that there could be two explanations for this result: 1) Junctional localization of occludin is related with the barrier characteristics of endothelial cells induced by TGF-β1 (Garcia et al., 2004). Interestingly, we demonstrated that occludin was localized at the junctional border of endothelial cells of hyaloid vessels in mice. As shown in hyaloid vessels, occludin might be translocated to the junctional border of endothelial cells with the action of TGF-β1. 2) Phosphorylation of occludin is also one of the determinants for the vascular permeability and barrier characteristics (Antonetti et al., 1999). In this regard, the constant expression of occludin did not rule out the relation of TGF-β treatment with the formation of tight junctions in endothelial cells. Furthermore, the treatment of TGF-β1 effectively induced the expression of ZO-1, ZO-2, and claudin-5 in our in vitro experiments. This phenomenon corresponds to previous reports that TGF-β1 lowers the endothelial permeability of brain capillary endothelial cells and induces the formation of BBB and BRB (Dohgu et al., 2004; 2005; Walshe et al., 2009). The interaction between astrocytes and endothelial cells which occurs in the development of retinal and brain vasculatures also shares the same mechanism of TGF-β1 induced formation of tight junctions (Garcia et al., 2004).
In conclusion, the interaction between pericytes and endothelial cells can regulate the formation of tight junctions in hyaloid vessels devoid of astrocytes. We demonstrate the expression of tight junction proteins, ZO-1 and occludin, in hyaloid vessels during the early postnatal periods. Pericyte-derived TGF-β induced by the interaction between pericytes and endothelial cells might involve the formation of tight junction proteins in hyaloid vessels. These findings have interesting implications for investigating the characteristics of hyaloid vessels, a unique vascular system which appears only at the early postnatal periods and regresses with the development of mature vasculatures. In addition, we suggest that hyaloid vessels be a good illustration showing that cell-cell interaction determines the formation of tight junctions in developing vasculatures.
Acknowledgments
We thank to Ms. Esther Yang for the technical help on immunofluorescence staining. This study was supported by the Seoul National University Brain Fusion Program Research Grant (800-20120453), the Bio-Signal Analysis Technology Innovation Program (2009-0090895), the Mid-Career Researcher Program (2012-0004931), the Pioneer Research Program (2012-0009544) and NRF/MEST (2010-0005109).
REFERENCES
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Brown AS, Leamen L, Cucevic V, Foster FS. Quantitation of hemodynamic function during developmental vascular regression in the mouse eye. Invest Ophthalmol Vis Sci. 2005;46:2231–2237. doi: 10.1167/iovs.04-0848. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol. 2004;24:491–497. doi: 10.1023/B:CEMN.0000022776.47302.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties Brain. Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hahn P, Lindsten T, Tolentino M, Thompson CB, Bennett J, Dunaief JL. Persistent fetal ocular vasculature in mice deficient in bax and bak. Arch Ophthalmol. 2005;123:797–802. doi: 10.1001/archopht.123.6.797. [DOI] [PubMed] [Google Scholar]
- Hamming NA, Apple DJ, Gieser DK, Vygantas CM. Ultrastructure of the hyaloid vasculature in primates. Invest Ophthalmol Vis Sci. 1977;16:408–415. [PubMed] [Google Scholar]
- Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol. 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW. Blood-neural barrier: intercellular communication at gliovascular interface. J Biochem Mol Biol. 2006;39:339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Kim KW, Min BH. The role of clusterin in in vitro ischemia of human retinal endothelial cells. Curr Eye Res. 2007;32:693–698. doi: 10.1080/02713680701487871. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009a;87:653–659. doi: 10.1002/jnr.21884. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YM, Ahn EM, Kim KW, Yu YS. Decursin inhibits VEGF-mediated inner blood-retinal barrier breakdown by suppression of VEGFR-2 activation. J Cereb Blood Flow Metab. 2009b;29:1559–1567. doi: 10.1038/jcbfm.2009.78. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Mun JY, Kim KW. Autophagy-induced regression of hyaloid vessels in early ocular development. Autophagy. 2010a;6:922–928. doi: 10.4161/auto.6.8.13306. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Min BH, Kim KW. Protective effect of clusterin on blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010b;51:1659–1665. doi: 10.1167/iovs.09-3615. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Kim KW, Kim JH. Investigation of barrier characteristics in the hyaloid-retinal vessel of zebrafish. J Neurosci Res. 2011;89:921–928. doi: 10.1002/jnr.22607. [DOI] [PubMed] [Google Scholar]
- Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Lee SM, Yu YS. Outcome of hyperplastic persistent pupillary membrane. J. Pediatr. Ophthalmol. Strabismus. 2004;41:163–171. doi: 10.3928/0191-3913-20040501-09. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Ling TL, Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988;44:73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Hasegawa T, Baba T, Grebe R, Galtier d’Auriac I, Merges C, Edwards M, Lutty GA. From blood islands to blood vessels: morphologic observations and expression of key molecules during hyaloid vascular system development. Invest Ophthalmol Vis Sci. 2012;53:7912–7927. doi: 10.1167/iovs.12-10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D’Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Provis JM, Penfold PL. The human hyaloid system: cellular phenotypes and inter-relationships. Exp Eye Res. 1999;68:553–563. doi: 10.1006/exer.1998.0632. [DOI] [PubMed] [Google Scholar]